Abstract
Paraneoplastic polyarthritis is an inflammatory arthritis, is usually seronegative, and has a temporal and pathophysiological relationship with an underlying malignancy. Although head and neck tumors may be a cause of paraneoplastic polyarthritis, its association with tongue carcinoma has not been previously reported. We present the case of a 69-year-old man who was a former smoker and presented with polyarthritis since 2 months in the wrists, proximal interphalangeal joints, knees, and elbows, with increased levels of acute-phase reactants; negativity for rheumatoid factor, anticitrullinated cyclic peptide antibody, and antinuclear antibody; and negative results for crystals and microorganisms in the synovial fluid. Cervical computed tomography and posterior rhinoscopy were performed, which detected an asymptomatic lesion on the base of the tongue, whose biopsy was compatible with nonkeratinizing squamous cell carcinoma. Polyarthritis did not respond to glucocorticoids at medium doses (oral prednisone 20 mg/day) but progressively resolved after the initiation of antineoplastic therapy.
Keywords: Paraneoplastic polyarthritis, paraneoplastic syndrome, tongue carcinoma, head and neck neoplasms
Introduction
Paraneoplastic polyarthritis (PP) or carcinomatous polyarthritis is an inflammatory arthritis, is usually seronegative, and has a temporal and pathophysiological relationship with an underlying malignancy, which usually corresponds to solid tumors, and less frequently, to hematological neoplasms (1, 2). Although head and neck tumors may be a cause of PP (3, 4), its association with tongue carcinoma (TC) has not been reported. We present a case of PP as an initial manifestation of CL.
Case Presentation
A 69-year-old man consulted for pain and swelling in the right wrist of sudden onset and 2 months of evolution, which extended to contralateral wirst, proximal interphalangeal (PIP) joints 2, 3, and 5 of the right hand, knees, and elbows and was associated with asthenia, hyporexia, and weight loss of 10 kg. His history included smoking (20 cigarettes/day) and alcohol consumption until 50 years of age, JAK2-negative essential polyglobulia, and prostatectomy at 68 years of age due to prostatic adenocarcinoma, with undetectable prostate-specific antigen (PSA) after the procedure. Examination revealed synovitis in the wrists, right PIP joints 2, 3, and 5 (Figure 1), and knees, with moderate effusion in the left knee. He presented palpable lymph nodes of 1–2 cm in diameter, which were mobile and did not indurate or cause pain in the left cervical and supraclavicular regions; no lesions were observed in the oral cavity. Laboratory tests showed hypochromic microcytic anemia; mild thrombocytosis; C-reactive protein level of 95 mg/L; erythrocyte sedimentation rate of 51 mm/h; PSA level of 0 ng/mL; negative results for rheumatoid factor (RF), anticitrullinated cyclic peptide antibody, and antinuclear antibody; and tumor markers levels (carcinoembryonic antigen, CA 19-9, α-fetoprotein, and β2-microglobulin) within normal ranges. Left knee arthrocentesis was performed, revealing inflammatory fluid leukocyte count of 46000/μL (91% neutrophils), without crystals and negative cultures. Oral prednisone was started (10 mg/day), but polyarthritis was persistent. X-ray examination of the hands showed increased soft tissue in the wrists and absence of erosions. Given the suspicion of a paraneoplastic condition, thoracic-abdominal-pelvic computed tomography (CT) and colonoscopy were performed, but they did not detect alterations. However, cervical CT showed pathological mucous thickening of lingual tonsils and regional adenopathies (Figure 2). Posterior rhinoscopy showed an exophytic/papillomatous lesion on the tongue base and lingual aspect of the epiglottis, whose biopsy was compatible with nonkeratinizing squamous cell carcinoma (Figure 3). The patient was diagnosed with PP secondary to TC. The dose of oral prednisone was increased to 20 mg/day, without noticing changes. Subsequently, he received 2 cycles of local radiotherapy and 5 cycles of chemotherapy, with gradual resolution of polyarthritis within the month of initiation of antineoplastic treatment. The patient has not presented new episodes of arthritis during a 6-month follow-up period.
Figure 1.
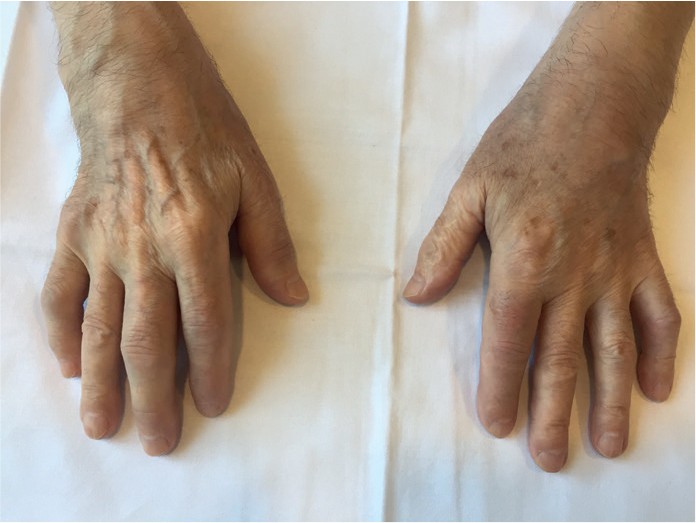
Swelling in the wrists and right proximal interphalangeal joints 2, 3 and 5 in relation to synovitis in these locations
Figure 2.
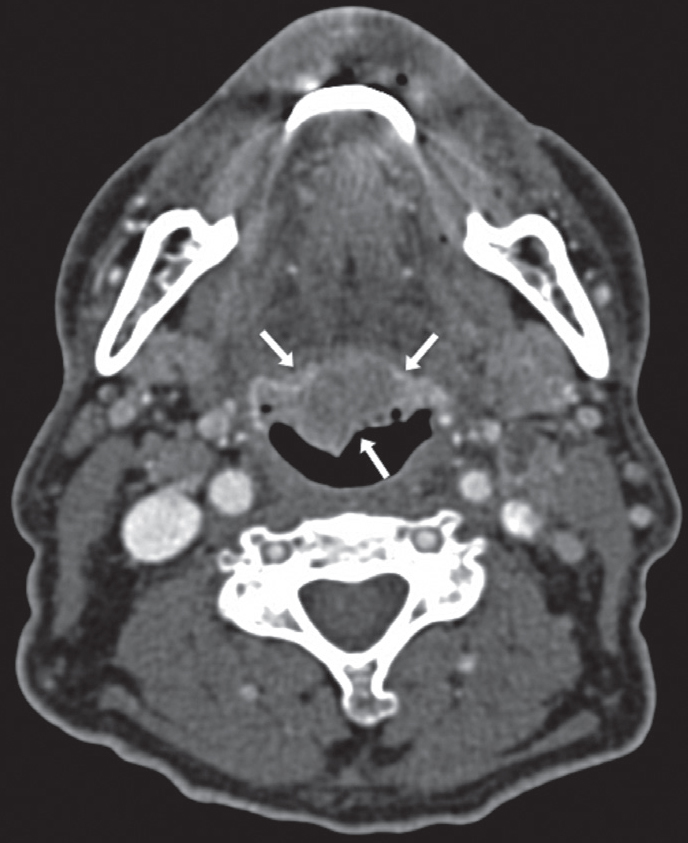
Axial slice of cervical CT showing a pathological mucous thickening of lingual tonsils, well delimited and of homogeneous density (arrows)
Figure 3.
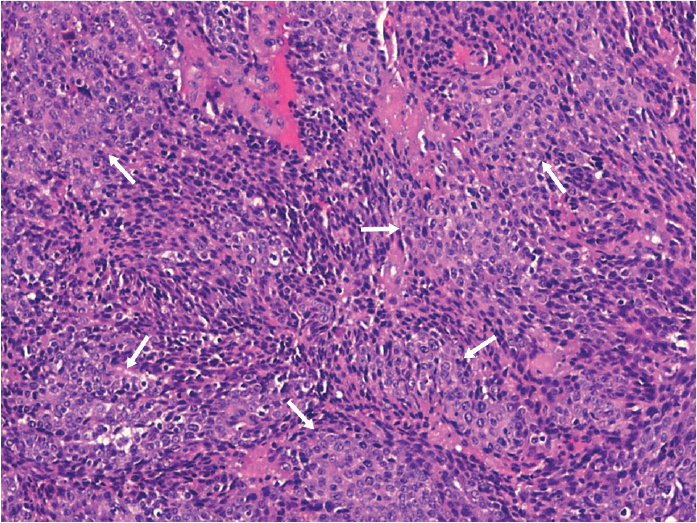
Microscopic image of the tongue lesion corresponding to a non-keratinizing squamous cell carcinoma. A solid growth of squamous cells with nuclear atypia and mitosis (arrows) is observed. The cells are medium sized and do not present cytoplasmic keratinization (hematoloxilin-eosin 20X)
Written informed consent was obtained from the patient.
Discussion
Paraneoplastic syndrome (PS), including PP, is characterized by the following: 1) occurs during the course of a malignancy or precedes it, 2) symptoms do not arise from tumor invasion or compression, and 3) the clinical picture improves with the treatment of the underlying neoplasm (5). Caldwell and McCallum identified the key features of PP, which include a close temporal relationship arthritis/neoplasia (12 months), advanced age, asymmetric joint involvement, sudden onset, predominance of lower extremities respecting hands and wrists, absence of RF, rheumatoid nodules and radiographic erosions, absence of a family history of rheumatic disease, and nonspecific synovial histopathology (6), all of which were present in our case, except symmetry and hand involvement, which have also been described in some patients with PP (2). Its physiopathology is unknown, suggesting the participation of immunocomplexes and T lymphocytes with cross-reactivity against synovial antigens (1). PP responds poorly to treatment with nonsteroidal anti-inflammatory drugs, glucocorticoids, and disease-modifying antirheumatic drugs, generally achieving its resolution with the treatment of the underlying neoplasm, although tumor recurrence is not usually associated with reappearance of arthritis (1, 2). PP has been reported as a manifestation of head and neck tumors, including laryngeal carcinoma (6), hypopharyngeal (4), and laryngopharyngeal (7), but not TC, and this neoplasm has been associated with other PSs, such as dermatomyositis, autoimmune retinopathy, subacute cerebellar degeneration, thrombophlebitis, and hypercalcemia (8–10). In our patient, the suspicion of PP led to the diagnosis of TC because it did not manifest direct symptoms of the tumor and was not accessible on physical examination; thus, it was detected in the complementary tests.
Conclusion
Head and neck tumors, including TC, may be a cause of PP and may be the initial manifestation in some cases. However, these neoplasms are not always evident on physical examination; hence, they must be considered, especially in patients with key characteristics of PP.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.B.F., W.A.S.G., R.C.G., J.L.M.H.; Design - A.B.F., W.A.S.G., R.C.G., J.L.M.H.; Supervision - A.B.F., W.A.S.G., R.C.G., J.L.M.H.; Resources - A.B.F., W.A.S.G., R.C.G., J.L.M.H.; Materials - A.B.F., W.A.S.G., R.C.G., J.L.M.H.; Data Collection and/or Processing - A.B.F., W.A.S.G., R.C.G., J.L.M.H.; Analysis and/or Interpretation - A.B.F., W.A.S.G., R.C.G., J.L.M.H.; Literature Search - A.B.F., W.A.S.G., R.C.G., J.L.M.H.; Writing Manuscript - A.B.F., W.A.S.G., R.C.G., J.L.M.H.; Critical Review - A.B.F., W.A.S.G., R.C.G., J.L.M.H.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Manger B, Schett G. Paraneoplastic syndromes in rheumatology. Nat Rev Rheumatol. 2014;10:662–70. doi: 10.1038/nrrheum.2014.138. [DOI] [PubMed] [Google Scholar]
- 2.Zupancic M, Annamalai A, Brenneman J, Ranatunga S. Migratory polyarthritis as a paraneoplastic syndrome. J Gen Intern Med. 2008;23:2136–9. doi: 10.1007/s11606-008-0794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlito A, Elsheikh MN, Manni JJ, Rinaldo A. Paraneoplastic syndromes in patients with primary head and neck cancer. Eur Arch Otorhinolaryngol. 2007;264:211–22. doi: 10.1007/s00405-006-0217-5. [DOI] [PubMed] [Google Scholar]
- 4.Baijens LW, Manni JJ. Paraneoplastic syndromes in patients with primary malignancies of the head and neck. Four cases and a review of the literature. Eur Arch Otorhinolaryngol. 2006;263:32–6. doi: 10.1007/s00405-005-0942-1. [DOI] [PubMed] [Google Scholar]
- 5.Shneider BS, Manalo A. Paraneoplastic syndromes: Unusual manifestation of malignant disease. DM. 1979;2:1–60. doi: 10.1016/s0011-5029(79)80013-9. [DOI] [PubMed] [Google Scholar]
- 6.Eggelmeijer F, Macfarlane JD. Polyarthritis as the presenting symptom of the occurrence and recurrence of a laryngeal carcinoma. Ann Rheum Dis. 1992;51:556–7. doi: 10.1136/ard.51.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandoval M, Girons Bonells J, Foglia Fernández M, Amilibia Cabeza E. Paraneoplastic syndrome as a manifestation of cancer of the hypopharynx. Acta Otorrinolaringol Esp. 1999;50:405–9. [PubMed] [Google Scholar]
- 8.Sugiyama T, Nakagawa T, Inui M, Tagawa T. Tongue carcinoma in a young patient with dermatomyositis: a case report and review of the literature. J Oral Maxillofac Surg. 2001;59:925–8. doi: 10.1053/joms.2001.25033. [DOI] [PubMed] [Google Scholar]
- 9.Chapireau D, Adlam D, Cameron M, Thompson M. Paraneoplastic syndromes in patients with primary oral cancers: a systematic review. Br J Oral Maxillofac Surg. 2010;48:338–44. doi: 10.1016/j.bjoms.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Gavrila A, Hasinski S, Rose LI. Hypercalcemia associated with squamous cell carcinoma of tongue. Endocr Pract. 2001;7:459–62. doi: 10.4158/EP.7.6.459. [DOI] [PubMed] [Google Scholar]


