Abstract
Objective
The Periodic fever syndromes (PFS) are a group of disorders of the innate immune system. We investigated patients diagnosed with PFS at the Dartmouth Hitchcock Pediatric Rheumatology Clinic.
Methods
Case acquisition was performed by reviewing ICD 9/10 coded records for familial Mediterranean fever (ICD 9 277.31), laboratory test records for PFS genetic screening, and clinic records between 1/1/2011 and 12/31/2017.
Results
Twenty-seven cases had clinical evaluations including PFS genetic screening. Clinical diagnoses included familial Mediterranean fever (FMF) (10 cases), Muckle-Wells (2 cases), tumor necrosis factor receptor associated periodic syndrome (TRAPS) (4 cases), hyper IgD syndrome (HIDS) (1 case), Crohn’s Disease (1 case), systemic onset juvenile idiopathic arthritis (SoJIA) (1 case), fever of unknown origin (FUO) (1 case), periodic fever adenitis pharyngitis aphthous ulcer (PFAPA) (6 cases), and cold-induced urticaria (1 case). Fifteen cases were associated with a genetic cause. Seven of the 10 FMF cases were confirmed genetically and were either heterozygous or compound heterozygotes. Both cases of Muckle-Wells had either a compound heterozygote for CIAS 1 or a NOD gene mutation. Both TRAPS cases presented atypically with patients developing systemic lupus erythematosus (SLE) or being asymptomatic. Two patients had novel syndromes. One FMF patient had a TRNT1 gene mutation who responded to intravenous immunoglobulin (IVIg) and colchicine after failing multiple treatments. The other had SoJIA with a LPIN 2 gene mutation but responded to colchicine. Only one of the 15 genetically proven cases had classical presentation and genetics (HIDS secondary to a mevalonate kinase (MVK) gene mutation).
Conclusion
PFS screening was helpful in over half of the cases to develop therapeutic treatment plans. Given the atypical clinical presentations seen with genetically determined PFS, extensive genetic testing is indicated for all patients presenting with a PFS, excluding classical PFAPA syndrome.
Keywords: Innate immunity, familial Mediterranean fever, hereditary autoinflammatory diseases, cryopyrin-associated periodic syndromes
Introduction
Periodic fever syndromes (PFS) are a group of disorders that primarily affect the innate immune system many of which (but not all) are genetic defects in the genes coding for the proteins that compose the inflammasome. Many times, these disorders are associated with mutations in a specific gene-MEFV with familial Mediterranean fever (FMF), NLRP3 with Muckle-Wells syndrome (MWS), TNFRSF1A with tumor necrosis factor (TNF)-associated periodic syndrome (TRAPS), MVK with hyperimmunoglobulinemia D with periodic fever syndrome (HIDS), and LPIN2 with Majeed syndrome (1). However, many patients with a PFS do not have a recognized genetic defect. In our series, about one third of our PFS patients had PFAPA, one third had a recognized genetic defect or a variant of unknown significance in a recognized gene coding for a protein in the innate immune system, and one third had no genetic abnormality but did not fit PFAPA.
There have been case reports that show autoinflammatory diseases presenting atypically, either clinically or genetically (2–4). In one case report of FMF, the patient presented with episodes of fever, diarrhea, and abdominal pain that were suspicious of Crohn’s disease. Further testing showed a mutation in the FMF gene, and the patient was subsequently diagnosed with FMF. However, symptoms similar to Crohn’s disease are not expected in FMF patients (5).
Familial Mediterranean fever is an autosomal recessive disorder characterized by mutations in the MEFV gene which encodes pyrin. Most of the mutations occur on exon 10, with the most common being M694V. Pyrin is a 781-aa protein that works to interact with apoptosis-associated speck-like protein which contains a caspase recruitment domain (ASC) to modulate IL-1β processing and inhibit NF-κB activation and apoptosis. The effects of mutated pyrin continue to be controversial. It is believed that a mutated pyrin leads to inflammation mediated by IL-1 and continuing leukocytic apoptosis; however, there are studies that contradict this current belief. Colchicine is the accepted treatment for FMF that with daily dosing, can cause remission or reduction of attacks (6). The mechanism of action of colchicine is controversial, but the protein pyrin is associated with microtubules so perhaps the disruption of microtubules prevents the action of pyrin on the generation of caspase activity.
Muckle-Wells syndrome is part of cryopyrin-associated periodic syndromes. It is an autosomal dominant disorder that presents with febrile episodes lasting 24–48 hours accompanied by abdominal pain, urticaria, arthritis, conjunctivitis, progressive deafness, and development of amyloidosis (7). It is associated with the NLRP3 gene (also is known as CIAS1) which encodes cryopyrin. This protein is part of the NALP3 inflammasome cascade. This cascade activates IL-1β and IL-18 through caspase 1. The NALP3 inflammasome is also activated by macrophages which increases the inflammation (8, 9).
Tumor necrosis factor receptor associated periodic syndrome is an autosomal dominant disease. It manifests as fever episodes lasting 1–3 weeks, conjunctivitis, periorbital edema, arthralgia, myalgia, an erythematous migratory plaque, and possible polyserositis. The TNFRSF1A gene encodes a transmembrane TNF receptor, which is bound by TNF proteins involved with the inflammation process. The exact pathophysiology is unknown (10). In theory, TRAPS should respond to etanercept since it is effectively replacement therapy; however, in many cases, IL-1 blockade is more efficacious.
Hyperimmunoglobulinemia D with periodic fever syndrome is an autosomal recessive disorder that is associated with the MVK gene. HIDS manifests as fever episodes with arthralgia, gastrointestinal symptoms, rash, and lymphadenopathy. MVK mutation causes underactivity of mevalonate kinase, which leads to increased mevalonic acid (11). How mevalonic acid affects inflammation and causes a periodic fever is unknown.
Majeed syndrome presents with chronic recurrent multifocal osteomyelitis, inflammatory dermatosis, and congenital dyserythropoietic anemia. It is an autosomal recessive disorder at the LPIN2 gene (3). The lipin-2 protein may be involved with oxidative stress response and mitosis (12), but it appears to be independent of the inflammasome.
In Mendelian genetics, it is accepted that autosomal dominant syndromes only require one gene abnormality. Autosomal recessive syndromes require two gene mutations on complementary alleles. There have been reports of abnormal genetics with PFS that differ from the current understanding. We performed a retrospective study to examine cases of difficult-to-control PFS and explore the genetics of each case.
Methods
Data was identified and obtained from at a single hospital center. We identified cases by reviewing ICD 9/10 coded records containing familial Mediterranean fever (ICD 9 277.31) and reviewing laboratory test records for PFS genetic screening with Medical Neurogenetics Next Gen PFS Panel. Institutional Review Board approval was obtained to complete a de-identified retrospective case analysis. Clinic records between 1/1/2011 and 12/31/2017 were reviewed for this case analysis.
Results
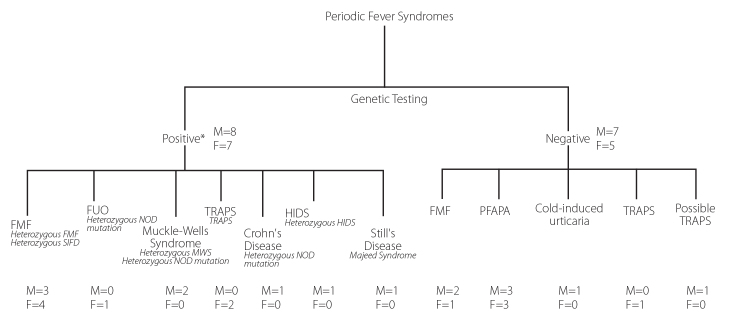
Genetic testing and clinical presentation identified potential periodic fever syndromes
From our institution, we identified 27 patients (12 female and 15 male) with clinical signs of PFS excluding those with classical PFAPA. Genetic screening showed 15 patients testing positive for a PFS gene and 12 patients testing negative for a PFS gene (figure 1). Clinical diagnoses included FMF (10 cases), MuckleWells (2 cases), TRAPS (4 cases), and HIDS (1 case). Other diagnoses included Crohn’s disease (1 case), SoJIA (1 case) FUO (1 case), PFAPA (6 cases), and cold-induced urticaria (1 case).
Figure 1.
Genetic screening showed 15 patients testing positive for a PFS gene and 12 patients testing negative for a PFS gene
PFS can present with classic clinical symptoms but have atypical genetic mutations
Of the 15 cases that were associated with a genetic cause, the majority of the genetics and presenting clinical presentation were atypical (table 1). Of the 10 FMF cases, 7 cases were confirmed genetically to be either heterozygous or compound heterozygous. The two cases of MWS presented with non-classical genetics including one as compound heterozygote for CIAS 1 and the other for the NOD gene. The two genetically confirmed cases of TRAPS presented atypically, with one asymptomatic and the other developing SLE. There were also two novel syndrome presentations. One had a mutation in the TRNT1 gene who eventually remitted with IVIg and colchicine after failing multiple treatments. The other had SoJIA with a mutation in the LPIN 2 gene, but responded to colchicine.
Table 1.
Characteristics of the 15 patients who were clinically diagnosed with PFS and who tested positive with PFS genetic screening
| Case | Clinical Presentation | Genetics | Takeaway | Clinical Diagnosis | Genetic Diagnosis | Therapeutic intervention |
|---|---|---|---|---|---|---|
| 1 | Severe Crohn’s disease associated with fevers, fistula, polyarthralgias, subcutaneous nodules, bilateral knee effusions, and GI bleeding; other symptoms include Raynaud’s disease, cold-induced urticaria | heterozygous NOD2; (c.3017dupC) | heterozygous NOD2 mutation seen with Crohn’s disease | Crohn’s disease | heterozygous NOD mutation | Failed tx: mercaptopurine, mesalamine, plaquenil, pentasa, methotrexate, metronidazole, steroids Current tx: sulfasalazine, certolizumab pegol |
| 2 | Periodic fevers every two to five weeks with joint, abdominal, and chest pain | heterozygous E148Q, P369S, R408Q mutations | Three independent mutations at the MEFV gene conferring FMF symptoms | FMF | compound heterozygous FMF | Self-resolves |
| 3 | Periodic fevers with bilateral neck pain followed by abdominal pain and migraines; past medical history includes common variable immune deficiency | Heterozygous A-G 694 MEFV |
heterozygous mutation at MEFV gene conferring phenotypic expression of FMF symptoms | FMF | heterozygous FMF | Failed tx: none Current tx: colchicine |
| 4 | Past medical history includes FMF; episodic, self-resolving abdominal pain with recurrent respiratory tract illnesses | heterozygous E148Q | heterozygous mutation at MEFV gene conferring phenotypic expression of FMF symptoms | FMF | heterozygous FMF | Failed tx: none Current tx: colchicine |
| 5 | Recurrent episodes of cold-induced urticaria with arthralgias, edema, tenderness, and fever | heterozygous 1591T MEFV |
heterozygous mutation at MEFV expressing atypical FMF presentation | FMF | heterozygous FMF | Failed tx: methotrexate Current tx: colchicine, canakinumab |
| 6 | Recurrent fever, arthralgias, abdominal pain, and pretibial erysipelas rash triggered by stress, cold, exertion, and cessation of colchicine | heterozygous M694V and M694I | Two heterozygous MEFV mutations seen with FMF symptoms |
FMF | compound heterozygous FMF | Failed tx: none Current tx: partial response to colchicine |
| 7 | Recurrent fevers, GI bleeding, skin manifestations, frequent bronchitis, history of cognitive deficits, behavioral changes and motor disturbances | heterozygous TRNT1 | heterozygous mutation at TRNT1 gene conferring FMF symptoms | FMF | heterozygous SIFD | Failed tx: anakinra, Previous successful tx: IVIg Current tx: partial response to colchicine |
| 8 | Recurrent fever since age 2 with elevated inflammatory markers. Late abdominal and joint pain. Neg anti-nuclear antibodies, normal anti-neutrophil cytoplasmic antibody and proteinase 3. Development of Crohn’s disease | heterozygous M694V exon 10 | FMF in heterozygote | FMF | heterozygous FMF | Current tx: infliximab, colchicine, azathioprine |
| 9 | Periodic fevers every two to four weeks with irritability, insomnia, nausea, vomiting, anorexia, and articular complaints | heterozygous NOD2 (c 2017C→T) | heterozygous NOD2 mutation seen with fever of unknown origin | FUO | heterozygous NOD mutation | Current tx: steroids |
| 10 | Periodic fevers every 28 days with accompanying downward progressing pustular, erythematous rash starting on abdomen; severe arthralgias on hands and feet; other symptoms include abdominal pain, oral ulcers, lower extremity weakness, and dysuria | heterozygous V3771 MVK |
heterozygous mutation at MVK gene conferring expression of HIDS | HIDS | heterozygous HIDS | Failed tx: anakinra Current tx: None |
| 11 | Flares with urticaria, abdominal pain, and fever; intact audiology exam | heterozygous V200M CIAS1 and heterozygous E148Q MEFV |
Two independent heterozygous mutations at CIAS1 and MEFV genes presents with hybrid MWS symptoms | Muckle-Wells syndrome | compound heterozygous | Current tx: canakinumab |
| 12 | Recurrent fevers associated with sinus congestion and multiple sclerosis flares; history of photosensitive rash, childhood hive episodes, and progressive deafness | heterozygous c2407C>A NOD2 | heterozygous NOD2 mutation usually with Crohn’s disease or Blau syndrome presenting as Muckle-Wells syndrome | Muckle-Wells syndrome | heterozygous NOD mutation | Failed tx: hydoxyurea Current tx: ruxolitinib for essential thrombocytosis |
| 13 | Episodic fevers responsive to steroids with elevated inflammatory markers lasting one to six weeks associated with night sweats, shoulder, back, and hand pain without edema and occasional non-pruritic erythematous macular rash on hands, conjunctivitis, and glossitis; episodes evolved to include headaches, twice daily fevers, arthralgias and myalgias, macular rash on hands and feet, and pharyngitis | heterozygous LPIN2 | heterozygous LPIN2 mutation confers symptoms consistent with Still’s disease | Still’s disease | Majeed syndrome | Current tx: colchicine; steroids |
| 14 | Genetic, but not tissue transglutaminase positive celiac disease; no fever, abdominal pain or conjunctivitis | heterozygous R121Q in TNFRSF1A | heterozygous TNFRSF1A mutation with unusual presentation of TRAPS | TRAPS | TRAPS | Current tx: none |
| 15 | Episodic flares lasting three to five days including numbness, paresthesias, and weakness in extremities, constipation, oral ulcers, low grade fever, chest, joint, abdominal, and back pains. Development of SLE |
heterozygous R121Q in TNFRSF1A | heterozygous R92 conferring unusual presentation of TRAPS | TRAPS | TRAPS | Failed tx: methotrexate, adalimumab, anakinra, infliximab, hydroxychloroquine, colchicine, tocilizumab Current tx: golimumab |
GI: gastrointestinal, FMF: familial Mediterranean fever, IVIg: intravenous immunoglobulin, NOD2: nucleotide-binding oligomerization domain-containing protein 2, MEFV: familial Mediterranean fever gene, MVK: mevalonate kinase, HIDS: hyper IgD syndrome, TRAPS: tumor necrosis factor receptor associated periodic syndrome, FUO: fever of unknown origin, SIFD: sideroblastic anemia, B-cell immunodeficiency, periodic fevers, and developmental delay
Of the 15 genetically confirmed cases, only 1 had a classical presentation and classical genetics-HIDS secondary to a mutation in the MVK gene.
PFS can present with a negative genetic screen
There were 12 patients who presented with symptomatic PFS but who tested negative genetically for PFS. Of the 10 FMF cases, three cases did not screen positive for PFS genetics. The six PFAPA cases expectedly screened negative for PFS. There was one case of cold-induced urticaria with negative genetics and two cases of TRAPS negative for PFS genetics.
Phenotypic expression of autonomic recessive syndromes can occur with heterozygous and compound heterozygous mutations
We observed four cases of heterozygous mutations in the MEFV gene and one case of a heterozygous mutation in the TRNT1 gene that led to the expression of symptomatic PFS. There were two cases with compound heterozygous mutations in the MEFV gene that resulted in phenotypic expression of FMF.
In these seven cases, patients presented with symptoms that resembled FMF. There were periodic fevers and joint and abdominal pains. One patient had erysipelas rashes. In the majority of these cases, there was a response to colchicine.
Muckle-Wells syndrome can present with non-classical genetics
MWS was diagnosed clinically in two patients. These patients presented with a history of urticaria and periodic fevers. One patient had abdominal pain during episodes, and the other patient had progressive deafness. Patients responded to interleukin-1 receptor antagonists. While these patients have symptoms that resemble MWS, genetic analysis revealed a heterozygous mutation in NOD2 or compound heterozygous mutations in the CIAS1 and MEFV genes. Here, the genetic diagnosis is not consistent with the clinical diagnosis.
NOD mutations have a variety of different phenotypic expressions
We observed different expressions of the NOD gene mutation. It can present as fever of unknown origin. This patient presents with periodic fevers every two to four weeks with gastrointestinal and articular symptoms. These symptoms are responsive to steroids. The NOD gene mutation also presented as severe, refractory Crohn’s disease with fistula formation, polyarthralgias, and subcutaneous nodules. The patient failed 6-MP, mesalamine, plaquenil, pentasa, methotrexate, metronidazole and steroids, but is responsive to sulfasalazine and certolizumab pegol.
Novel syndromes involving the TNFRSF-1A gene
We report a novel presentation of TRAPS involving a heterozygous R121Q mutation in the TNFRSF-1A gene. The patient does not have a rash, fevers, abdominal or chest pain, or conjunctivitis. The patient does have musculoskeletal pain secondary to hypermobility, gastric reflex, Raynaud’s syndrome, genetic celiac disease, and growth failure.
Novel syndromes involving the LPIN2 gene
Another novel presentation involves a heterozygous mutation of the LPIN2 gene manifesting as adult-onset Still’s disease (SoJIA). The patient had a history of episodic fevers as a child with non-pruritic erythematous macular rashes, conjunctivitis, glossitis, night sweats, and arthralgias. These episodes are responsive to prednisone and colchicine. His presentation is most consistent with adult-onset Still’s disease rather than Majeed syndrome.
Discussion
PFS are difficult to diagnose given their rarity and pleomorphic presentations. This spectrum of disease is typically diagnosed clinically and often not subjected to genetic testing. Many of these diseases, other than PFAPA and adult-onset Still’s disease, are associated with genetic mutations. In our retrospective clinical observations, we observed cases where the treatment based on clinical presentation failed. In over half of the cases, we found that by using the genetic diagnoses to direct treatment plans, the patients responded.
Many of the cases presented with non-classical Mendelian genetics. FMF is believed to be an autosomal recessive syndrome. However, we observed four cases (cases 3, 4, 5, 8) of one heterozygous mutation in the MEFV gene conferring FMF symptoms. There was one case (case 2) with three heterozygous mutations in the MEFV gene, and one case (case 6) with two heterozygous mutations in the MEFV gene. Whether these mutations are in cis or trans cannot be determined from the data available. In general, autosomal recessive diseases require two mutations for a phenotype to be expressed on complementary alleles. However, there have been reports as early as the 1940s of phenotypic expressions with one mutation in a recessive disorder. Ataxia telangiectasia (AT) is an autosomal recessive disorder. Carriers, with one mutation in the ataxia telangiectasia mutated (ATM) gene, display a predisposition to cancer. Smirnov and Cheung demonstrated that AT carriers and patients (who have two mutations in the ATM gene) can share more similar gene expression patterns than compared to AT carriers and noncarriers. They used microarray analyses to find 29 mRNAs and quantitative real-time PCR to find 7 miRNAs that show expression patterns of AT carriers that are closer to that of patients in radiation exposed lymphoblastoid cells (13).
There have been reports of compound heterozygous mutations in the literature. Caglayan, et. al looked at the frequency of compound heterozygous mutations at their institution in Turkey and found 66 Turkish patients with compound heterozygous mutations who displayed symptoms typical of FMF. They also found that the most common MEFV mutation was M694V, which was found in 32% of the alleles. Out of 66 patients, 41 had a compound heterozygous mutation involving M694V. The next most common mutations found were E148Q at 20.6%, V726A at 17% and M680I at 14.5%. M694I is a rare mutation and seen 0.7% to 5.3% of the time (14). Treatment with colchicine was effective in 83% of patients (2).
We observed a case (case 2) of three compound heterozygous mutations. The patient’s family is a carrier of three compound heterozygous mutations. The mother is homozygous for E148Q and two other mutations on the same chromosome. She reports chronic joint symptoms, fevers of unknown origin, and an episode of pleurisy in the past alleviated by colchicine. His older brother tested positive for FMF and has symptoms of fever and chest and abdominal pain responsive to colchicine. E148Q was originally believed to be a nonpathologic mutation (15). However, homozygous mutations display symptoms and require treatment with colchicine (16). There are also reports of E148Q causing FMF in compound heterozygous mutations. Fukushima et al. reported a case of 3 adult family members of Japanese descent, one mother and her two children, with a compound heterozygosity for L110P/E148Q/M694I. The husband/father of the family members had no mutations. The mother had symptoms of fever and abdominal, chest, and joint pains since 30 years old. The older child had symptoms of fever and joint and abdominal pain 3–4 times a year since she was a child. The younger child had fever and abdominal pain once a month since the age of 30. Colchicine helped in all three cases (4).
We observed a case (case 7) where the genetic mutation did not normally correspond to the clinical diagnosis. There was a patient with FMF that had a heterozygous TRNT1 mutation, which normally manifests as sideroblastic anemia with B-cell immunodeficiency, periodic fevers, and developmental delay (SIFD). SIFD is an autosomal recessive disease that responds to IVIg, regular blood transfusions, and iron chelation (17). Not only did the patient respond to IVIg, but also high dose colchicine. This challenges the way we think about linking specific genes to diseases. It suggests that there are more downstream effects and mutations that may not yet be detectable by current genetic testing. Even though certain genetics are seen mostly with specific diseases, there could be other genes or proteins that cause specific symptoms to manifest.
NOD2 mutations have a broad presentation. They are associated with inflammatory diseases such as Crohn’s disease and Blau syndrome. In one of our reported cases (case 9), we observed a NOD2 mutation associated with fever of unknown origin, which has not been reported in the literature. Our patient’s specific variant could be a benign mutation resulting in a mild presentation of PFS. Other epigenetic changes could have affected the expression of the mutated protein. The patient could also have other genomic mutations that were not detected in the genomic screen that could be affecting the expression of the NOD2 mutation. The NOD2 mutation is also linked to severe presentations of Crohn’s disease (18). We observed another case (case 1) where the patient had failed multiple Crohn’s disease therapies. We observed a third case (case 12) where the NOD2 mutation was associated with MWS. These three cases illustrate how different mutations in the NOD2 gene can phenotypically be expressed differently. A substitution of cysteine for threonine was seen with fever of unknown origin. A duplicate cysteine was seen with severe Crohn’s disease. A substitution of cysteine for alanine was seen with MWS. These cases suggest that the different mutated proteins that occur with these mutations have drastically different functions in the human body. The correct NOD2 gene expression is likely to be very important in the inflammation cascade.
The CIAS1or NLRP3 gene is associated with MWS (19). It is inherited in an autosomal dominant pattern. We observed a case (case 11) where the patient presented with two separate heterozygous mutations in the CIAS1 gene and the MEFV gene. These mutations are associated with an abnormal presentation of MWS that includes urticarial and abdominal pain with periodic fevers seen with FMF. Although FMF is an autosomal recessive disorder, this case illustrates how a heterozygous mutation in an autosomal recessive disorder can be co-expressed with an autosomal dominant disorder. Both gene products, cyropyrin from CIAS1 and pyrin from MEFV, are involved with the assembly of the inflammasome, a molecular complex that helps trigger and regulate the inflammatory process. The V200M mutation in CIAS1 gene leading to a gain of function in cryopyrin and the E148Q mutation in MEFV gene leading to a dysfunctional or reduced pyrin could both be contributing to an inappropriately prolonged inflammatory response in the patient, manifesting as recurrent episodes of fever, abdominal pain, urticaria, and joint tenderness. While FMF is an autosomal recessive condition requiring a homozygous mutation, it still does not preclude the possibility that the patient’s presentation is a hybrid between MWS and FMF, given that their respective gene mutations may interact in the inflammasome. There has been another case report of a hybrid CIAS1 and MEFV gene mutations (20). The case reported periodic fevers with intact hearing. Episodes were controlled with IL-1 receptor antagonist, colchicine, and prednisone. In both our case and the previous case report, the hybrid presentation of MWS and FMF was responsive to treatment with an IL-1 receptor antagonist.
LPIN2 gene mutation is believed to be associated with Majeed syndrome; however, we observed a case (case 13) that was more consistent with adult-onset Still’s disease. The patient presented with daily fevers, joint pain, and a macular rash, which are symptoms consistent with adult-onset Still’s disease (21). There is currently no known gene to cause adult-onset Still’s disease. However, genetic screening showed a mutation in the LPIN2 gene. Majeed syndrome is an autosomal recessive syndrome, and according to Mendelian genetics, Majeed syndrome symptoms should not be expressed. This case suggests that abnormal lipin-2 protein may be involved in an inflammatory cascade that is abnormal in adult-onset Still’s disease.
We observed two different presentations of the same mutation in the TNFRSF1A gene at R121Q. TRAPS is an autosomal dominant disease. One patient (case 14) was asymptomatic while the other patient (case 15) had episodes of fevers, chest, abdominal, and joint pains, oral ulcers, and numbness, paresthesias, and weakness in the extremities. These observations raise questions whether there are more genes, proteins, and pathways involved in TRAPS. These two cases have the genetics of TRAPS, and we would expect that the patients present with TRAPS symptoms. However, we see two abnormal presentations of TRAPS despite having the expected genetic mutations.
In our retrospective case studies, we observe multiple examples where the clinical diagnoses do not correspond with the genetic diagnoses. We found that genetic testing was helpful in guiding some treatment plans. In case 7, the patient was phenotypically diagnosed with FMF while genotypically diagnosed with SIFD. The patient’s partial response to IVIg may be explained by the genetics.
There were also many examples of non-classical Mendelian genetics, and it challenges the way we think, approach, and provide genetic counseling to families with PFS. We suggest that extensive genetic testing be used in all patients presenting with a PFS other than classic presentations of PFAPA (22, 23).
Acknowledgements
We also acknowledge and appreciate the contributions of Dr. Stephanie Mathew.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Institutional Review Board of Geisel School of Medicine at Dartmouth.
Informed Consent: Written informed consent was obtained from subjects who participated in this study.
Peer-review: Externally peer-reviewed
Author Contributions: Concept - D.A.A., T.K.H.; Design - D.A.A., T.K.H.; Supervision - D.A.A., T.K.H.; Data Collection and/or Processing - D.A.A., T.K.H.; Analysis and/or Interpretation - D.A.A., T.K.H.; Literature Search - D.A.A., T.K.H.; Writing Manuscript D.A.A., T.K.H.; Critical Review - D.A.A., T.K.H.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Durrant K, Ignacio Arostegui J. Comparison Chart of Systemic Autoinflammatory Diseases (SAID) Autoinflammatory Alliance. 2013 http://www.nomidalliance.org/downloads/comparative_chart_front.pdf. [Google Scholar]
- 2.Caglayan A, Demiryilmaz F, Ozyazgan I, Gumus H. MEFV gene compound heterozygousmutations in familial Mediterranean fever phenotype: a retrospective clinical and molecularstudy. Nephrol Dial Transplant. 2010;25:2520–3. doi: 10.1093/ndt/gfp632. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson P, Chen S, Tayeh M, Ochoa L, Leal S, Pelet A, et al. Homozygous mutations in LPIN2are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J Med Genet. 2005;42:551–7. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukushima Y, Obara K, Hirata H, Sugiyama K, Fukuda T, Takabe K. Three Japanese patients(mother and two children) with familial Mediterranean fever associated with compoundheterozygosity for L110P/E148Q/M694I and anautosomal true dominant inheritance pattern. Asian Pac J Allergy Immunol. 2013;31:325–9. doi: 10.12932/AP0244.31.4.2013. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto S, Urayoshi S, Yoshida Y. FamilialMediterranean fever in which Crohn’s diseasewas suspected: a case report. BMC Res Notes. 2014;7:678. doi: 10.1186/1756-0500-7-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onen F. Familial Mediterranean fever. Rheumatol Int. 2006;26:489–96. doi: 10.1007/s00296-005-0074-3. [DOI] [PubMed] [Google Scholar]
- 7.Cuisset L, Drenth J, Berthelot J, Meyrier A, Vaudour G, Watts R, et al. Genetic linkage of theMuckle-Wells Syndrome to chromosome 1q44. Am J Hum Genet. 1999;65:1054–9. doi: 10.1086/302589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, et al. The NLRP3 inflammasome is released as aparticulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15:738–48. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- 9.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, et al. The adaptor ASC hasextracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol. 2014;15:727–37. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantarini L, Lucherini O, Muscari I, Frediani B, Galeazzi M, Brizi M, et al. Tumour necrosis factorreceptor-associated periodic syndrome (TRAPS):State of the art and future perspectives. Autoimmun Rev. 2012;12:38–43. doi: 10.1016/j.autrev.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Houten S, Kuis W, Duran M, de Konig T, vanRoyen-Kerkhof A, Romeijin G, et al. Mutationsin MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic feversyndrome. Nat Genet. 1999;22:175–7. doi: 10.1038/9691. [DOI] [PubMed] [Google Scholar]
- 12.Herlin T, Fiirgaard B, Bjerre M, Kerndrup G, Hasle H, Bing X, et al. Efficacy of anti-IL-1 treatmentin Majeed syndrome. Ann Rheum Dis. 2013;72:410–3. doi: 10.1136/annrheumdis-2012-201818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smirnov D, Cheung V. ATM gene mutations result in both recessive and dominant expression phenotypes of genes and microRNAs. Am JHum Genet. 2008;83:243–53. doi: 10.1016/j.ajhg.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershoni-Baruch R, Brik R, Shinawi M, Livneh A. The differential contribution of MEFV mutant alleles to the clinical profile of familial Mediterranean fever. Eur J of Hum Genet. 2002;10:145–9. doi: 10.1038/sj.ejhg.5200776. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Chetrit E, Lerer I, Malamud E, Domingo C, Abeliovich D. The E148Q mutation in the MEFV gene: is it a disease-causing mutation or a se-quence variant? Hum Mutat. 2000;15:385–6. doi: 10.1002/(SICI)1098-1004(200004)15:4<385::AID-HUMU22>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 16.Topaloglu R, Ozaltin F, Yilmaz E, Ozen S, Balci B, Besbas N, et al. E148Q is a disease-causingMEFV mutation: a phenotypic evaluation inpatients with familial Mediterranean fever. Ann Rheum Dis. 2005;64:750–2. doi: 10.1136/ard.2004.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiseman D, May A, Jolles S, Connor P, Powell C, Heeney M, et al. A novel syndrome of congenital sideroblastic anemia, B-cell immunodeficiency, periodic fevers, and developmentaldelay (SIFD) Blood. 2013;122:112–23. doi: 10.1182/blood-2012-08-439083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weersma R, Stokkers P, van Bodegraven A, vanHogezand R, Verspaget H, de Jong D, et al. Molecular prediction of disease risk and severity in a large Dutch Crohn’s disease cohort. Gut. 2009;58:388–95. doi: 10.1136/gut.2007.144865. [DOI] [PubMed] [Google Scholar]
- 19.Dode C, Le Du N, Cuisset L, Letourneur F, Berthelot J, Vaudour G, et al. New mutationsof CIAS1 that are responsible for Muckle-WellsSyndrome and Familial Cold Urticaria: A novelmutation underlies both syndromes. Am J Hum Genet. 2002;70:1498–506. doi: 10.1136/gut.2007.144865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolas-Sanchez F, Pinol-Ripoll G, Arostegui-Gorospe J, Grau-Junyent J, Sarrat-Nuevo R, Malgarejo Moreno P. Polyradiculoneuritis,cryopyrin-associated periodic syndromes, andfamilial Mediterranean fever. Neurologia. 2015;30:315–7. doi: 10.1016/j.nrl.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Efthimiou P, Paik P, Bielory L. Diagnosis andmanagement of adult onset Still’s disease. AnnRheum Dis. 2006;65:564–72. doi: 10.1136/ard.2005.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvarez-Errico D, Vento-Tormo R, Ballestar E. Genetic and epigenetic determinants in autoinflammatory diseases. Front Immunol. 2017;8:1–8. doi: 10.3389/fimmu.2017.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman HM, Broderick L. The role of the inflammasome in patients with autoinflammatory diseases. J Allergy Clin Immunol. 2016;138:3–14. doi: 10.1016/j.jaci.2016.05.001. [DOI] [PubMed] [Google Scholar]