Abstract
Acute kidney injury (AKI) is an increasing clinical problem that is associated with chronic kidney disease progression. Cannabinoid receptor 2 (CB2) activation has been shown to mitigate some of the deleterious tubular effects due to AKI, but its role on the renal vasculature has not been fully described. In this study, we investigated the effects of our novel CB2 receptor agonist, SMM-295, on renal vasculature by assessing cortical perfusion with laser Doppler flowmetry and changes in luminal diameter with isolated afferent arterioles. In this study, intravenously infused SMM-295 (6 mg/kg) significantly increased cortical renal perfusion (13.8 ± 0.6%; P < 0.0001; n = 7) compared with vehicle (0.1 ± 1.5%; n = 10) normalized to baseline values in anesthetized C57BL/6J mice. This effect was not dependent upon activation of the CB1 receptor (met-anandamide; 6 mg/kg iv) and was predominantly abolished in Cnr2 knockout mice with SMM-295 (6 mg/kg iv). Ablation of the renal afferent nerves with capsaicin blocked the SMM-295-dependent increase in renal cortical perfusion, and the increased renal blood flow was not dependent upon products synthesized by cyclooxygenase or nitric oxide synthase. The increased renal perfusion by CB2 receptor activation is also attributed to a direct vascular effect, since SMM-295 (5 μM) engendered a significant 37 ± 7% increase (P < 0.0001; n = 4) in luminal diameters of norepinephrine-preconstricted afferent arterioles. These data provide new insight into the potential benefit of SMM-295 by activating vascular and nonvascular CB2 receptors to promote renal vasodilation, and provide a new therapeutic target to treat renal injuries that impact renal blood flow dynamics.
Keywords: agonist, cannabinoids, CB2 receptor, isolated afferent arterioles, renal perfusion, SMM-295
INTRODUCTION
Acute kidney injury (AKI) is defined by an immediate loss in renal function due to a number of factors, including the onset of sepsis, exposure to nephrotoxins, and organ hypoperfusion due to ischemia (2). Ischemia-reperfusion injury (IRI) is one of the most common forms of AKI and involves a complex series of cellular changes that can lead to lethal tubular damage and loss of renal function in the most severe cases. The regulation of renal blood flow dynamics to preserve glomerular filtration rate during AKI induced by IRI is critical in the recovery of the kidney. To date, our understanding regarding the mechanisms involved in regulating vascular function and hemodynamics in the kidney during AKI needs further investigation.
Natural and synthetic cannabinoids have emerged in the past few years as a topic of interest during normal and diseased states in the kidney, including AKI. Studies show that the complete endocannabinoid system can be identified in various cells within the kidney (29). The endocannabinoid system comprises endogenous molecules, N-arachidonoylethanolamine (AEA) and 2-acylglycerol, both of its cognate G protein-coupled receptors, cannabinoid receptor 1 (CB1) and 2 (CB2), and the enzymes involved in their biosynthesis and catabolism (29). Moreover, there is impetus to understand the biological activity of the cannabinoid receptors because of increased acceptance of the medicinal purposes of cannabis and related compounds. The majority of studies to date have focused upon the role of the CB1 receptors in the central and peripheral organs due in large part to its psychotropic and pain management effects. In the kidney, however, the CB1 receptors consistently demonstrate deleterious effects on glomerular and tubular function in various acute and chronic forms of injury. The role of the cannabinoid receptors on the regulation of renal hemodynamics, even under normal conditions remains poorly understand. Early rat studies implicated that anandamide (AEA) increased renal blood flow with a concomitant reduction in glomerular filtration rate by preferential vasodilation of the efferent arteriole via CB1 receptor activation (10). Consistent with this finding, Deutsch et al. (6) demonstrated that segments of the renal and arcuate arteries were capable of dilating in the presence of AEA through CB1 receptor stimulation. Conversely, a synthetic CB1 receptor agonist demonstrated renal vasoconstriction (8). In addition, there are emerging new developments in selective CB2 receptor targeting, whereby their role in the kidney generally opposes the effects by the CB1 receptor to protect renal function in the glomerulus and renal tubules (16). As part of these findings, our group demonstrated that the novel CB2 agonist SMM-295 protects the renal tubular epithelial cells from severe damage following renal IRI by reducing proapoptotic signaling (21). To date, no one has used this compound to study the effects of CB2 receptor activation in terms of its potential to modulate renal vascular function.
For this reason, the present study was designed to evaluate the functional effects of a novel selective CB2 receptor agonist to control the renal vasculature through the use of laser Doppler flowmetry and changes in luminal diameters from isolated perfused afferent arterioles.
METHODS
Animals.
C57BL/6J and Cnr2 knockout (B6.129P2-Cnr2tm1Dgen/J; stock #5786) mice aged 10–12 wk were obtained from the Jackson Laboratory (Bar Harbor, ME). Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center before any procedures were performed. All mice were provided ad libitum access to food and water before and after the surgical procedures and were kept on a 12 h/12 h light/dark cycle during the course of their housing in the animal facilities.
Chemicals and assay kits.
Indomethacin (cat. #I7378), capsaicin (cat. #M2028), and Nw-nitro-l-arginine (LNNA; cat #N5501) were obtained from Millipore Sigma (St. Louis, MO). Cremophor ELP was purchased from BASF. SMM-295 was synthesized by Dr. Bob Moore as previously described by our group (21). Mouse calcitonin gene-related peptide (CGRP) ELISA (cat. #E0056Mo) was obtained from Bioassay Technology Laboratory (Shanghai, China) and performed according to the manufacturer’s protocol.
Animal surgeries.
A total of 72 mice were used in this study, which utilizes both survival (bilateral IRI) and nonsurvival surgical techniques (terminal anesthetized hemodynamic and CGRP analysis) using protocols that have been well established and published by our laboratories (20–22, 27, 32).
Hemodynamic analysis.
Renal cortical perfusion and mean arterial blood pressure measurements were conducted with techniques published by our group (17, 18). A total of 72 mice were anesthetized with a combination of ketamine (100 mg/kg ip)/xylazine (10 mg/kg ip) mixture and readministered as needed throughout the procedure to maintain a constant level of anesthesia. Catheters were placed into the jugular vein for infusion of drugs with an infusion pump and into the carotid artery to measure arterial blood pressure (ADInstruments, Colorado Spring, CO). Laser Doppler probes were calibrated with a standard calibration device using a motility standard (Perimed, Järfälla, Sweden) such that 1 perfusion unit (PU) corresponds to an analog output of 10 mV. The kidney was isolated, and a Doppler probe is placed on the surface of the kidney attached to a holder to measure cortical renal blood flow. Cortical renal blood flow, measured and analyzed with a PowerLab data acquisition system and LabChart software (ADInstruments), was allowed to come to a steady baseline level before any pharmacological manipulations. Indomethacin (1 mg/kg ip) and LNNA (10 mg/kg ip) were administered. Fifteen minutes before infusion of SMM-295 or vehicle [EtOH (5%):Cremophor ELP (5%):saline (90%)]. Capsaicin (50 mM) was applied by a cotton-tipped applicator for 15 min to the isolated renal vein, and the animal rested for 90 min following capsaicin treatment. Subsequently, the vessels and surrounding tissues were washed with saline solution and allowed to rest for an additional 60 min before SMM-295 infusion as previously described by Peixoto-Neves et al. (18). SMM-295 or vehicle was administered via infusion pump with a volume of 1 ml over a 10 min period. At the end of the experiment, plasma was collected by cardiac puncture with a 23-gauge needle and 1 ml syringe.
Calcitonin gene-related peptide ELISA.
A total of 12 mice were anesthetized with a mixture of ketamine (100 mg/kg)/xylazine (10 mg/kg). An indwelling catheter was placed into the jugular vein for infusion with SMM-295 (n = 6) or the vehicle solution (n = 6) via an infusion pump at a rate of 0.1 ml/min for 10 min. We collected blood from the inferior vena cava after clamping it with microisolator clips immediately above the renal veins. The detection for CGRP was performed with a commercially available mouse CGRP ELISA kit.
Isolated perfused afferent arteriole measurement of luminal diameters.
Measurements of the afferent arteriole luminal diameter were performed on isolated perfused mouse afferent arterioles as previously described (7, 11, 31, 34, 35). In brief, the afferent arterioles with attached glomeruli were isolated and transferred to a temperature-regulated chamber mounted on an inverted microscope in media. A set of micropipettes held the glomerulus and cannulated the afferent arterioles, holding the intraluminal pressure at 60 mmHg. The arteriole preparations were then incubated with norepinephrine (NE; 1 µM) and/or SMM-295 (5 µM) for 15 min periods during the measurement of the intraluminal diameter.
Bilateral ischemia-IRI.
A total of 18 mice were anesthetized with pentobarbital (50–80 mg/kg ip) before IRI was performed. Flank incisions were made on both sides of the mice, the kidneys were isolated, and the renal hilus was clamped. The renal ischemia was maintained for 24.5 min, and immediately upon removal of the microisolator clamps to initiate reperfusion, either CB2 receptor agonist SMM-295 (6 mg/kg ip; n = 6) or vehicle (Cremophor; n = 6) was administered. The kidneys were subsequently monitored for restoration of blood perfusion before closure of animal. All surgeries were conducted under aseptic conditions, and all mice were administered buprenorphine for pain management and antibiotics, if needed, after the surgery and during the remainder of the experimental period. After 24 h, another dose of vehicle or SMM-295 (6 mg/kg ip) was administered to each of the mice. Plasma was collected every 24 h following IRI. Forty-eight hours after injury, laser Doppler flow measurements were performed with the mice under terminal anesthesia, the mice were euthanized.
Statistics and study design.
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (5a). All values are shown as means ± SE from GraphPad Prism software (version 6.0; GraphPad, La Jolla, CA). An unpaired t-test or one-way analysis of variance was performed with Bonferroni post hoc analysis to confirm significant differences (P < 0.05) between animal groups. Post hoc analysis was performed only in the groups where F achieved P < 0.05 and there was no significant variance in homogeneity. The ARRIVE guidelines were used to design animal experiments as a standardized approach to ensure validity and reproducibility of results.
RESULTS
SMM-295 effect on cortical renal perfusion.
Laser Doppler flowmetry was performed to detect changes in renal cortical perfusion in anesthetized C57BL/6J mice following the administration of a novel CB2 receptor agonist, SMM-295 (see chemical structure in Fig. 1A). Administration of SMM-295 (6 mg/kg) or vehicle solution was infused into an indwelling jugular vein catheter over a 10 min period at a rate of 0.1 ml/min. Renal perfusion was monitored for a 30 min period; vehicle treatment led to an insignificant increase in the renal perfusion by 1.1 ± 10.6 PU (0.1 ± 1.5% from baseline levels) (Fig. 1B). Infusion of SMM-295 (6 mg/kg) resulted in a significant increase (P < 0.0001) of 136.1 ± 8.9 PU (or 13.8 ± 0.6%) from the baseline levels. Mean arterial pressure following administration of SMM-295 (6 mg/kg iv) resulted in a significant reduction of 19.0 ± 2.8 mmHg (P < 0.0001; n = 7) compared with vehicle (−1.3 ± 1.1 mmHg; n = 10) (Fig. 1C). Cnr2 knockout mice only reduced blood pressure by 9.1 ± 2.3 mmHg (n = 6) similar to the changes measured with met-anandamide (−7.3 ± 2.6; n = 6; data not shown).
Fig. 1.
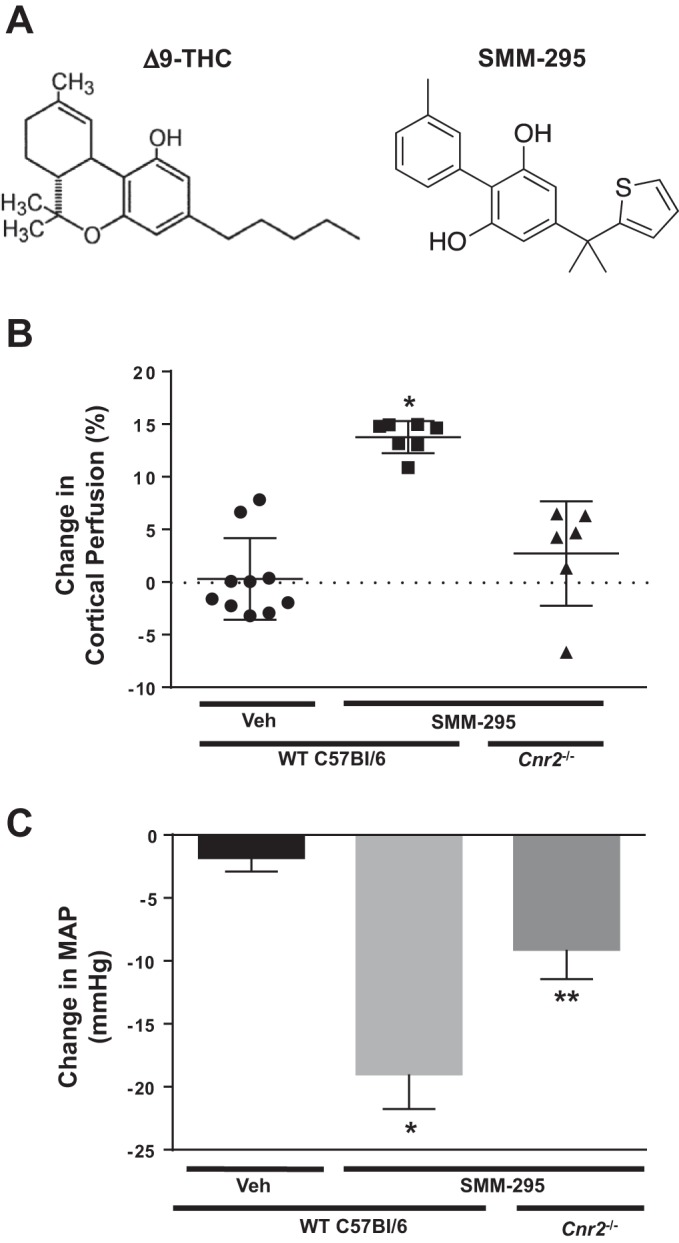
Cannabinoid receptor 2 (CB2) agonist SMM-295 promotes increased renal cortical perfusion. A: chemical structure of Δ9-THC and SMM-295. Measurement of renal cortical perfusion by laser Doppler flowmetry (B) and mean arterial pressure (MAP, C) following intravenous infusion of vehicle (Veh; n = 10) or SMM-295 (6 mg/kg; n = 7) in anesthetized C57BL/6J mice, and SMM-295 (6 mg/kg; n = 6) in Cnr2 knockout (KO) mice. Data are graphed as a percent change from baseline levels in each mouse. *P < 0.0001 significant difference in cortical perfusion for both groups (B, C) and only vehicle group for MAP (C); **P < 0.05 significant difference between wild-type (WT) and Cnr2 KO mouse.
To exclude the possibility of the CB1 receptor playing a role in this effect, a metabolically stable CB1 receptor agonist, met-anandamide, was infused at the same dose (6 mg/kg) into a separate group of anesthetized mice (Fig. 2). In these mice, there was nearly no change in the renal cortical perfusion following administration of met-anandamide (Δ in PU = −3.6 ± 12.0 or −0.7 ± 1.9% from the baseline levels; n = 6 mice) compared with the baseline levels. In addition, we examined the effect of SMM-295 in a genetic Cnr2 knockout mouse to confirm the selectivity of the CB2 receptor agonist (Fig. 1B). Indeed, the change in cortical perfusion following intravascular administration of SMM-295 into the Cnr2 knockout mice resulted in an insignificant increase in PU (24.0 ± 21.7) or 2.7 ± 2.0% change from baseline levels (n = 6 mice). HU-308 (6 mg/kg), a selective CB2 agonist, was found to have a modest increase in renal cortical blood flow by 6.9 ± 1.5% (P < 0.05; n = 6), demonstrating the role of the CB2 receptor in blood perfusion.
Fig. 2.
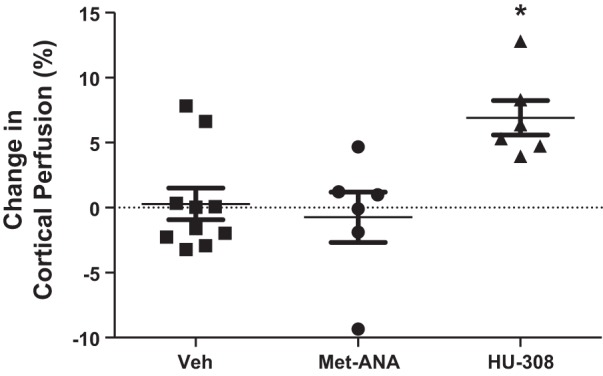
Increased cortical renal perfusion is not through the CB1 receptor. Measurement of renal cortical perfusion by laser Doppler flowmetry following intravenous infusion of met-anandamide (Met-ANA; 6 mg/kg; selective CB1 receptor agonist; n = 6) and HU-308 (6 mg/kg; selective CB2 receptor agonist; n = 6) in anesthetized C57BL/6J mice. Data for the vehicle (control) were regraphed from Fig. 1 and are shown as a percent change from baseline levels in each mouse. *P < 0.05 significant different from control group.
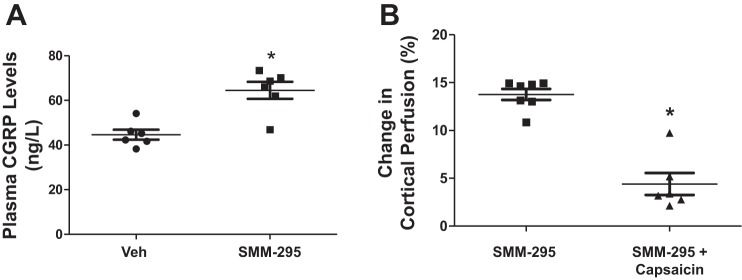
CGRP levels following SMM-295 infusion.
Plasma was isolated from blood collected from the renal vein to measure CGRP levels by ELISA (Fig. 3A). In vehicle-treated mice, SMM-295 induced a significant increase (P = 0.0068) in plasma CGRP by 44% to 64.5 ± 3.9 ng/l (n = 6) from 44.7 ± 2.2 ng/l (n = 6) in the vehicle-treated mice.
Fig. 3.
Renal afferent nerve activation is involved in calcitonin gene-related peptide (CGRP) release and increased cortical renal perfusion. A: plasma CGRP levels was measured by ELISA from C57BL/6J mice infused with either vehicle or SMM-295 (6 mg/kg). n = 6 mice per group. *P = 0.0068 significant difference between groups. B: measurement of renal cortical perfusion by laser Doppler flowmetry during control and SMM-295 (6 mg/kg) administration in mice treated with capsaicin to ablate renal afferent nerve activity. n = 6 mice per group. *P < 0.0001 significant difference between groups.
To block the effects of CGRP release from afferent nerve terminals, capsaicin was applied to the renal nerves before the administration of SMM-295 into the anesthetized mice. Renal cortical perfusion was significantly lower (P < 0.0001) at 38 ± 9.7 PU (or 4.4 ± 1.1% from baseline period; n = 6 mice) following administration of SMM-295 (6 mg/kg) into the jugular vein compared with noncapsaicin-treated mice (Fig. 3B).
Role of cyclooxygenase and nitric oxide synthase in CB2 receptor renal perfusion.
To test whether cyclooxygenase products were involved in promoting the increase in cortical perfusion following stimulation of the CB2 receptor, we administered a single dose of indomethacin (1 mg/kg ip) 20 min into the anesthetized mouse before injection of SMM-295 (6 mg/kg). The increase in cortical perfusion (14.4 ± 1.5% from baseline; n = 6) by SMM-295 was not different in the presence of indomethacin compared with the SMM-295 without pretreatment (Fig. 4). As an alternate mode of action, we investigated whether nitric oxide could play a role in controlling renal perfusion due to SMM-295. Similarly, blockade of nitric oxide synthase (NOS) was achieved with LNNA pretreatment 20 min before the administration of SMM-295 (6 mg/kg). The increase in the cortical perfusion promoted by SMM-295 was not reduced by the inhibition of NOS (n = 7), but it was significantly higher (P < 0.01) than SMM-295-treated mice with either vehicle or indomethacin pretreatment.
Fig. 4.
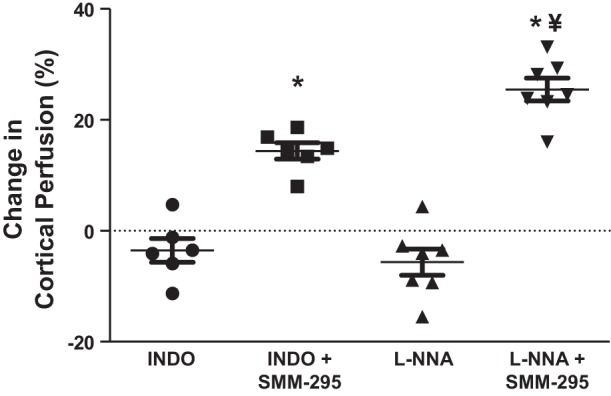
Cyclooxygenase and nitric oxide synthase are not involved with the increase in cortical renal perfusion following SMM-295 administration. Change in cortical renal perfusion units using SMM-295 (6 mg/kg iv) as graphed by percent change from baseline levels following pretreatment with indomethacin (INDO; 1 mg/kg ip; n = 6) or LNNA (10 mg/kg ip; n = 7) in C57BL/6J mice. ¥P < 0.01 significant difference between LNNA and the other two groups; *P < 0.0001 significant difference between the inhibitor treatment period with SMM-295 treatment.
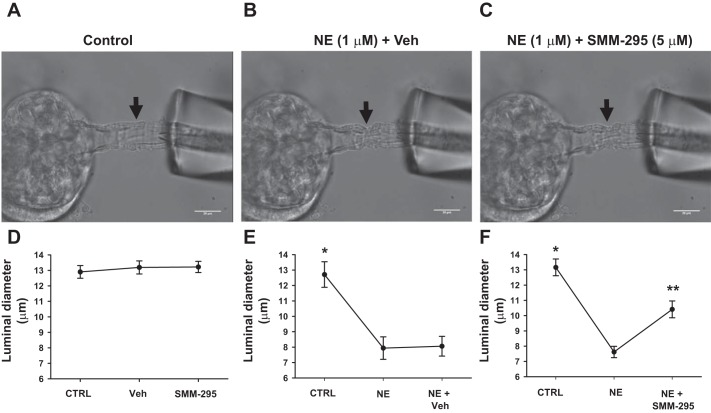
SMM-295 increases luminal diameter of afferent arterioles.
Afferent arterioles were isolated from the mouse and placed into a bath solution for perfusion to pressurize at 60 mmHg. Under these basal conditions, the luminal diameters were measured in the presence of the control solution at 12.9 ± 0.41 μm (Fig. 5, A and D). To determine whether SMM-295 promoted a vasoconstriction on the isolated afferent arterioles, SMM-295 (5 μM) was applied to the bath solution; it did not result in any changes in the luminal diameters during the two separate, but consecutive 15 min incubation periods. Both of the luminal diameters remained similar to the control period (13.19 ± 0.42 and 13.22 ± 0.36 μm, respectively; n = 4).
Fig. 5.
SMM-295 increases luminal diameter of preconstricted isolated perfused afferent arterioles. Afferent arterioles were isolated from C57BL/6 mice and perfused to measure luminal diameter changes at baseline and after treatment with norepinephrine (NE; 1 µM) and SMM-295 (5 µM). Representative images of the isolated afferent arterioles at baseline (control) (A) and with NE (B), and NE + SMM-295 (C) treatment. Graphical analyses of the luminal diameter changes, if any, during the following conditions: SMM-295 incubation during 2 consecutive periods (15 min each period) (D); NE incubation during 2 consecutive periods (15 min each period) (E); NE incubation during the first period, and NE + SMM-295 during the second period (F). n = 4 arterioles per group. *P = 0.0019 significant difference between NE treatment with or without vehicle; **P < 0.001 significant difference between NE alone and NE + SMM-295 groups. Black arrows indicate where the luminal diameter was measured.
To test whether SMM-295 could dilate afferent arterioles, the vessels were preconstricted with NE (1 μM) for 15 min, followed by addition of either SMM-295 (5 μM) in the bath solution (Fig. 5, C and F) or vehicle (Fig. 5, B and E) in the presence of NE (1 μM). As shown in Fig. 5, B and E, the luminal diameters of the afferent arterioles were significantly decreased (P = 0.0019) from 12.71 ± 0.83 μm to 7.94 ± 0.73 μm following application of NE (n = 4). The afferent arteriole remained constricted 8.06 ± 0.64 μm in a subsequent 15 min period with the vehicle. In the presence of SMM-295 (5 μM), afferent arteriolar diameter significantly increased by 37 ± 7% (P < 0.0001) to 10.41 ± 0.55 μm from the NE-preconstricted state of 7.62 ± 0.37 μm (Fig. 5F). All of the changes in the luminal diameter occurred within 5 min after the addition of each individual drug or combination of drugs.
SMM-295 effect on cortical renal perfusion following IRI.
In a separate group of C57BL/6J mice, bilateral IRI was initiated, and either vehicle or SMM-295 (6 mg/kg ip) was administered immediately after removal of the clamps to allow for reperfusion of the ischemic kidneys (Fig. 6). Daily injections were made with either vehicle or SMM-295 for a period of 48 h at which point the mice were anesthetized to measure the cortical perfusion. As shown in Fig. 6, the sham-operated (no IRI) mice for a period of 48 h exhibited cortical perfusion of 979.6 ± 73.3 PU (n = 6), which was considerably higher (P = 0.0002) than the IRI-treated mice administered vehicle (604.8 ± 31.1 PU; n = 6). Administration of SMM-295 (6 mg/kg ip once a day) was measured to have cortical perfusion at similar to the sham-operated mouse kidneys measurements (1,005.9 ± 62 PU; n = 6).
Fig. 6.
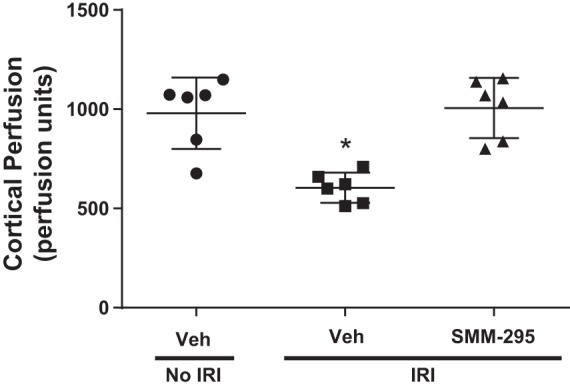
Daily administration of SMM-295 prevented the reduction in renal cortical perfusion following bilateral renal ischemia-reperfusion injury (IRI). Renal cortical perfusion was measured by laser Doppler flowmetry in normal and IRI-treated C57BL/6J mice (n = 6 mice/group) administered vehicle or SMM-295 (6 mg/kg ip) every 24 h for 2 days. n = 6 mice per group. *P = 0.0002 significant difference between the other two groups.
DISCUSSION
This is the first study to demonstrate that SMM-295, a novel selective CB2 receptor agonist, has the capability to increase renal cortical blood flow and also promote direct vasodilation of isolated perfused afferent arterioles. Due to the absence of an effective and selective neutral CB2 receptor antagonist, our pharmacological findings were confirmed by evaluating our CB2 agonist in a genetic Cnr2 knockout mouse model on the C57BL/6J background.
Previous studies have primarily investigated the effects of synthetic and endocannabinoids to control renal tubular and excretory function, but vascular effects have been little investigated (16). Early studies evaluating the biological function of the endocannabinoid AEA show a putative vasodilator effect in the kidney leading to reduced renal vascular resistance (6, 10). These studies demonstrate that the primary receptor responsible for this effect was CB1 (6, 10), which is similar to other published studies using similar endogenous and synthetic cannabinoid receptor analogs to evaluate central and peripheral vascular bed regulation through the CB1 receptor (28). Alternatively, there is evidence that AEA increased renal blood flow changes and urine excretion through a non-CB1-dependent mechanism (23). Conversely, there are conflicting studies that provide evidence that CB1 can have an opposite effect to produce vasoconstriction in the kidney (8) or no effect on renal hemodynamics (12). Similar to our results, Li and Wang (12) showed that renal cortical and medullary blood flow was nonresponsive to direct intramedullary administration of a highly selective CB1 agonist, met-anandamide. Moreover, Koura et al. (10) could not fully explain the increased vasodilation of the afferent arteriole solely to the CB1 receptor, but this may have involved a non-CB1 receptor. This would be consistent with our findings where CB2 receptor stimulation with SMM-295 increased luminal diameter of the NE-preconstricted isolated perfused afferent arterioles. The direct vasodilator effects by CB2 receptors have also been reported in other vascular beds, including the mesenteric arteries (13) and cerebral vasculature (4). It may be possible that other cannabinoid binding proteins, such as TRPV1, GPR18, or GPR55, could also indirectly be activated to increase their function to promote changes in the systemic and regional vasculature either alone or in concert with the non-CB1 receptors (1, 9, 19). However, GPR55 has no effect on the anandamide-dependent vasodilation, at least in mesenteric arteries (9).
Using the intact kidney in the anesthetized mouse, we confirmed that there is increased renal cortical blood flow mediated by SMM-295 through the activation of the CB2 but not the CB1 receptor. This effect may be attributed to dilation of peripheral blood vessels on CB2 receptors, including those in the kidney, since a marked reduction in mean arterial pressure was associated with the increased renal perfusion mediated by SMM-295. Moreover, there was a lesser increase in renal perfusion by SMM-295 in Cnr2 knockout mice with reduced change in blood pressure. In this and previous studies by our laboratory, the CB1 receptor stimulation reduces blood pressure using specific agonists by either direct vascular effect or vagal stimulation (12, 15), but there is no change in renal cortical perfusion (12).
Moreover, the ability of SMM-295 to mediate this effect may not be solely due to its effects within the kidney; sensory nerve activation outside of the kidney may also contribute. Prior studies showed that nonselective CB1/CB2 receptor agonists, such as △9-THC and WIN 55,212-2, can augment release of CGRP (30, 33), which can promote rat mesenteric arterial bed dilation (33). Other studies showed that CB2 receptors can mediate the release CGRP for attenuation of osteoarthritic knee joint pain and in the rat spinal cord (3, 25), but these effects may not be universally confirmed (14). In our study, SMM-295 produced an increase in CGRP levels from blood collected immediately flowing out of the renal vein. CGRP is known to be a potent vasodilator and released at the nerve terminals of sensory nerves to regulate blood vessel tone (5, 24). The mechanisms by which CGRP can promote vasorelaxation remain to be fully understood, but it has been suggested that the primary mode of action is through a cAMP-dependent activation of protein kinase A to open potassium ion channels (5, 24). The subsequent hyperpolarization of the cell membrane leads to dilation of the smooth muscle cells surrounding the blood vessels. This may be the mode of action in the kidney, since we were unable to determine any reduction in blood flow following administration of SMM-295 by pretreating the mice with either indomethacin or LNNA to prevent the production of vasodilator prostaglandins and nitric oxide, respectively. Previous studies have documented that CGRP may activate other second messenger systems to increase the production and release of nitric oxide (5, 24). Further studies will be needed to ascertain the mechanism by which CB2 receptor activation of the sensory nerves leading to the kidneys regulates cortical and possibly medullary hemodynamics.
Perspectives and Conclusion
With the increasing societal acceptance of drugs that activate cannabinoid receptors for medicinal use, it is essential to better understand the biological function of these receptors, particularly the CB2 receptor in the control of renal function. In the kidney, there is increasing data to demonstrate that activation of the CB2, and not CB1 receptor, can play a crucial role in protecting renal tubular and vascular cells during acute injury or chronic disease states (16). For this reason, the development of CB2 receptor agonists remains an area of intense research. While there are many commercially available CB2 agonists, only a handful of these were concluded to be considered as “gold standard” compounds in terms of their selectivity and efficacy according to a recently published review (26). However, even the most optimal CB2 receptor agonists have their limitations in terms of their selectivity, high metabolism in vivo, and lack of testing for their efficacy in animal models in vivo. Our recent work using our newly synthesized CB2 receptor agonist, SMM-295, demonstrated a protective effect that reduced renal tubular epithelial cells damage following AKI by decreasing apoptotic signaling (21). The current study builds upon our knowledge about SMM-295 by showing its ability to increase renal cortical perfusion and promote afferent arteriolar dilation. Further studies are needed, however, to better understand the role of CB2 receptors in the regulation of blood flow distribution in the kidney between the renal cortex and medulla, and also whether there are direct effects to control GFR.
In conclusion, our study has provided the first evidence for CB2 receptor activation to promote increased renal perfusion; this receptor should be further evaluated in preclinical studies for its beneficial effects in other acute and chronic disease models of the kidney.
GRANTS
Funding for the work presented in this study was provided by University of Tennessee Office of Research (F. Park) and National Institutes of Health Grants DK-099276 (R. Liu), HL-137987 (R. Liu), and DK-101668 (A. Adebiyi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.P., H.S., B.M.M., A.A., and F.P. conceived and designed research; J.D.P., H.S., S.J., J.W., and B.M.M. performed experiments; J.D.P., H.S., S.J., J.W., R.L., and F.P. analyzed data; J.D.P., H.S., S.J., J.W., R.L., A.A., and F.P. interpreted results of experiments; J.D.P., J.W., and F.P. prepared figures; J.D.P. and F.P. drafted manuscript; J.D.P., H.S., B.M.M., A.A., and F.P. edited and revised manuscript; J.D.P., H.S., S.J., J.W., R.L., B.M.M., A.A., and F.P. approved final version of manuscript.
REFERENCES
- 1.Akerman S, Kaube H, Goadsby PJ. Anandamide acts as a vasodilator of dural blood vessels in vivo by activating TRPV1 receptors. Br J Pharmacol 142: 1354–1360, 2004. doi: 10.1038/sj.bjp.0705896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci 23: 1530–1538, 2006. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 4.Benyó Z, Ruisanchez É, Leszl-Ishiguro M, Sándor P, Pacher P. Endocannabinoids in cerebrovascular regulation. Am J Physiol Heart Circ Physiol 310: H785–H801, 2016. doi: 10.1152/ajpheart.00571.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84: 903–934, 2004. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 5a.Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA, Gilchrist A, Hoyer D, Insel PA, Izzo AA, Lawrence AJ, MacEwan DJ, Moon LD, Wonnacott S, Weston AH, McGrath JC. Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471, 2015. doi: 10.1111/bph.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest 100: 1538–1546, 1997. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y, Hall JE, Lu D, Lin L, Manning RD Jr, Cheng L, Gomez-Sanchez CE, Juncos LA, Liu R. Aldosterone blunts tubuloglomerular feedback by activating macula densa mineralocorticoid receptors. Hypertension 59: 599–606, 2012. doi: 10.1161/HYPERTENSIONAHA.111.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardiner SM, March JE, Kemp PA, Bennett T. Regional haemodynamic responses to the cannabinoid agonist, WIN 55212-2, in conscious, normotensive rats, and in hypertensive, transgenic rats. Br J Pharmacol 133: 445–453, 2001. doi: 10.1038/sj.bjp.0704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue TL, Brown AJ, Morrison AD, Douglas SA. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol 152: 825–831, 2007. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koura Y, Ichihara A, Tada Y, Kaneshiro Y, Okada H, Temm CJ, Hayashi M, Saruta T. Anandamide decreases glomerular filtration rate through predominant vasodilation of efferent arterioles in rat kidneys. J Am Soc Nephrol 15: 1488–1494, 2004. doi: 10.1097/01.ASN.0000130561.82631.BC. [DOI] [PubMed] [Google Scholar]
- 11.Lai EY, Wang Y, Persson AE, Manning RD Jr, Liu R. Pressure induces intracellular calcium changes in juxtaglomerular cells in perfused afferent arterioles. Hypertens Res 34: 942–948, 2011. doi: 10.1038/hr.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Wang DH. Differential mechanisms mediating depressor and diuretic effects of anandamide. J Hypertens 24: 2271–2276, 2006. doi: 10.1097/01.hjh.0000249706.42230.a8. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Dyck E, Andrade-Urzua F, Elizalde A, Ferrer-Villada T, Dagnino-Acosta A, Huerta M, Osuna-Calleros Z, Rangel-Sandoval C, Sanchez-Pastor E. ACPA and JWH-133 modulate the vascular tone of superior mesenteric arteries through cannabinoid receptors, BKCa channels, and nitric oxide dependent mechanisms. Pharmacol Rep 69: 1131–1139, 2017. doi: 10.1016/j.pharep.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Milne M, Ashton JC. Effect of cannabinoids on CGRP release in the isolated rat lumbar spinal cord. Neurosci Lett 614: 39–42, 2016. doi: 10.1016/j.neulet.2015.12.060. [DOI] [PubMed] [Google Scholar]
- 15.Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park F, Potukuchi PK, Moradi H, Kovesdy CP. Cannabinoids and the kidney: effects in health and disease. Am J Physiol Renal Physiol 313: F1124–F1132, 2017. doi: 10.1152/ajprenal.00290.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park F, Zou AP, Cowley AW Jr. Arginine vasopressin-mediated stimulation of nitric oxide within the rat renal medulla. Hypertension 32: 896–901, 1998. doi: 10.1161/01.HYP.32.5.896. [DOI] [PubMed] [Google Scholar]
- 18.Peixoto-Neves D, Soni H, Adebiyi A. CGRPergic Nerve TRPA1 Channels Contribute to Epigallocatechin Gallate-Induced Neurogenic Vasodilation. ACS Chem Neurosci 10: 216–220, 2019. doi: 10.1021/acschemneuro.8b00493. [DOI] [PubMed] [Google Scholar]
- 19.Penumarti A, Abdel-Rahman AA. The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J Pharmacol Exp Ther 349: 29–38, 2014. doi: 10.1124/jpet.113.209213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pressly JD, Hama T, Brien SO, Regner KR, Park F. TRIP13-deficient tubular epithelial cells are susceptible to apoptosis following acute kidney injury. Sci Rep 7: 43196, 2017. doi: 10.1038/srep43196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pressly JD, Mustafa SM, Adibi AH, Alghamdi S, Pandey P, Roy KK, Doerksen RJ, Moore BM Jr, Park F. Selective Cannabinoid 2 Receptor Stimulation Reduces Tubular Epithelial Cell Damage after Renal Ischemia-Reperfusion Injury. J Pharmacol Exp Ther 364: 287–299, 2018. doi: 10.1124/jpet.117.245522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regner KR, Nozu K, Lanier SM, Blumer JB, Avner ED, Sweeney WE Jr, Park F. Loss of activator of G-protein signaling 3 impairs renal tubular regeneration following acute kidney injury in rodents. FASEB J 25: 1844–1855, 2011. doi: 10.1096/fj.10-169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritter JK, Li C, Xia M, Poklis JL, Lichtman AH, Abdullah RA, Dewey WL, Li PL. Production and actions of the anandamide metabolite prostamide E2 in the renal medulla. J Pharmacol Exp Ther 342: 770–779, 2012. doi: 10.1124/jpet.112.196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94: 1099–1142, 2014. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuelert N, Zhang C, Mogg AJ, Broad LM, Hepburn DL, Nisenbaum ES, Johnson MP, McDougall JJ. Paradoxical effects of the cannabinoid CB2 receptor agonist GW405833 on rat osteoarthritic knee joint pain. Osteoarthritis Cartilage 18: 1536–1543, 2010. doi: 10.1016/j.joca.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Soethoudt M, Grether U, Fingerle J, Grim TW, Fezza F, de Petrocellis L, Ullmer C, Rothenhäusler B, Perret C, van Gils N, Finlay D, MacDonald C, Chicca A, Gens MD, Stuart J, de Vries H, Mastrangelo N, Xia L, Alachouzos G, Baggelaar MP, Martella A, Mock ED, Deng H, Heitman LH, Connor M, Di Marzo V, Gertsch J, Lichtman AH, Maccarrone M, Pacher P, Glass M, van der Stelt M. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun 8: 13958, 2017. doi: 10.1038/ncomms13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soni H, Kaminski D, Gangaraju R, Adebiyi A. Cisplatin-induced oxidative stress stimulates renal Fas ligand shedding. Ren Fail 40: 314–322, 2018. doi: 10.1080/0886022X.2018.1456938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanley CP, Hind WH, Tufarelli C, O’Sullivan SE. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc Res 107: 568–578, 2015. doi: 10.1093/cvr/cvv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam J. The emerging role of the endocannabinoid system in the pathogenesis and treatment of kidney diseases. J Basic Clin Physiol Pharmacol 27: 267–276, 2016. doi: 10.1515/jbcpp-2015-0055. [DOI] [PubMed] [Google Scholar]
- 30.Tumati S, Yamamura HI, St John PA, Vanderah TW, Roeske WR, Varga EV. Sustained cannabinoid agonist treatment augments CGRP release in a PKA-dependent manner. Neuroreport 20: 815–819, 2009. doi: 10.1097/WNR.0b013e32832be50b. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Shen C, Liu H, Wang S, Chen X, Roman RJ, Juncos LA, Lu Y, Wei J, Zhang J, Yip KP, Liu R. Shear stress blunts tubuloglomerular feedback partially mediated by primary cilia and nitric oxide at the macula densa. Am J Physiol Regul Integr Comp Physiol 309: R757–R766, 2015. doi: 10.1152/ajpregu.00173.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White SM, North LM, Haines E, Goldberg M, Sullivan LM, Pressly JD, Weber DS, Park F, Regner KR. G-protein βγ subunit dimers modulate kidney repair after ischemia-reperfusion injury in rats. Mol Pharmacol 86: 369–377, 2014. doi: 10.1124/mol.114.092346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson JD, Kendall DA, Ralevic V. Delta 9-tetrahydrocannabinol inhibits electrically-evoked CGRP release and capsaicin-sensitive sensory neurogenic vasodilatation in the rat mesenteric arterial bed. Br J Pharmacol 152: 709–716, 2007. doi: 10.1038/sj.bjp.0707448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yip KP, Balasubramanian L, Kan C, Wang L, Liu R, Ribeiro-Silva L, Sham JSK. Intraluminal Pressure Triggers Myogenic Response via Activation of Calcium Spark and Calcium-Activated Chloride Channel in Rat Renal Afferent Arteriole. Am J Physiol Renal Physiol 315: F1592–F1600, 2018. doi: 10.1152/ajprenal.00239.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Lin L, Lu Y, Liu H, Duan Y, Zhu X, Zou C, Manning RD Jr, Liu R. Interaction between nitric oxide and superoxide in the macula densa in aldosterone-induced alterations of tubuloglomerular feedback. Am J Physiol Renal Physiol 304: F326–F332, 2013. doi: 10.1152/ajprenal.00501.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]