Abstract
Electrogenic sodium-dependent glucose transport along the length of the intestine was compared between the omnivorous Nile tilapia (Oreochromis niloticus) and the carnivorous rainbow trout (Oncorhynchus mykiss) in Ussing chambers. In tilapia, a high-affinity, high-capacity kinetic system accounted for the transport throughout the proximal intestine, midintestine, and hindgut segments. Similar dapagliflozin and phloridzin dihydrate inhibition across all segments support this homogenous high-affinity, high-capacity system throughout the tilapia intestine. Genomic and gene expression analysis supported findings by identifying 10 of the known 12 SLC5A family members, with homogeneous expression throughout the segments with dominant expression of sodium-glucose cotransporter 1 (SGLT1; SLC5A1) and sodium-myoinositol cotransporter 2 (SMIT2; SLC5A11). In contrast, trout’s electrogenic sodium-dependent glucose absorption was 20–35 times lower and segregated into three significantly different kinetic systems found in different anatomical segments: a high-affinity, low-capacity system in the pyloric ceca; a super-high-affinity, low-capacity system in the midgut; and a low-affinity, low-capacity system in the hindgut. Genomic and gene expression analysis found 5 of the known 12 SLC5A family members with dominant expression of SGLT1 (SLC5A1), sodium-glucose cotransporter 2 (SGLT2; SLC5A2), and SMIT2 (SLC5A11) in the pyloric ceca, and only SGLT1 (SLC5A1) in the midgut, accounting for differences in kinetics between the two. The hindgut presented a low-affinity, low-capacity system partially attributed to a decrease in SGLT1 (SLC5A1). Overall, the omnivorous tilapia had a higher electrogenic glucose absorption than the carnivorous trout, represented with different kinetic systems and a greater expression and number of SLC5A orthologs. Fish differ from mammals, having hindgut electrogenic glucose absorption and segment specific transport kinetics.
Keywords: gastrointestinal tract, glucose absorption, SLC5A, sodium-dependent glucose transporters, tilapia, trout
INTRODUCTION
Glucose is the major molecular constituent of carbohydrate digestion, entering the intestinal enterocytes via the apically located sodium-dependent glucose transporters (SGLTs) (1, 5, 10, 11, 32). These transporters are members of the SLC5A (solute carrier family member 5A) family, simultaneously transporting glucose and sodium, creating a measurable current during absorption (6, 9). The transporters exist in both omnivorous and carnivorous species and represent the first ports of entry for glucose into the intestine (23, 46, 50, 70).
Since their initial discovery, a total of 12 SLC5A isoforms have been identified in the omnivorous human genome and are expressed in various tissues (25, 84). Ten of these genes are sodium-coupled substrate transporters for solutes like glucose, myoinositol, and anions, whereas the other two genes have different coupling ions and functions (57, 84). The stoichiometry of this transport is dependent on the family member (57, 62). Generally, the SGLTs are characterized kinetically in two categories. First is a high-affinity (Ha) transporter, with sensitivity to low concentrations of glucose and low-capacity (Lc), saturating at low concentrations of glucose (17, 21a, 32, 35, 57, 62). Second is a low-affinity (La) transporter with sensitivity to glucose at higher concentrations and high-capacity (Hc) transporter, saturating at high concentrations of glucose (17, 21a, 32, 35, 57, 62). This classification developed due to the initial difference in kinetics between the first SGLTs discovered (1, 17, 32, 34, 45, 82, 89). The SGLT isoform 1 (SLC5A1) transporter demonstrated a Ha/Lc transport, and sodium-glucose cotransporter 2 (SGLT2; SLC5A2) exhibited La/Hc transport (1, 6, 32, 42, 51). Physiological studies identifying two transport systems supported the existence of both a Ha/Lc and La/Hc for glucose in the intestine of both omnivorous and carnivorous mammals, including cat, rat, pig, human, and cattle (5, 9, 21a, 36, 45, 49, 89). Only a few of these studies have segregated the Ha/Lc to the proximal small intestine in omnivores and carnivores, but gene association is minimal or lacking (32, 34, 62). However, defining specific physiological attributes to members of the SLC5A family has been challenging due to a previous lack of genomic information, substrate promiscuity, species differences, and tissue-specific regulation (34, 60). In fish, this is particularly true, with the identification of SGLTs being minimal and their functions mostly presumed from sequence identity with mammalian SGLTs, despite sequence differences (1, 61, 91).
Studies comparing intestinal glucose absorption kinetics and association with SLC5A gene family between omnivores and carnivores are lacking in mammalian literature and unknown in fish, despite the generally accepted notion that omnivores can absorb larger amounts of glucose than carnivores (8, 13, 16, 18, 21, 35, 77). This gap in the literature is particularly salient in fish, which have a lower importance for carbohydrates in their natural diet, but have known differences between omnivorous and carnivorous utilization of glucose (71). Here, using ex vivo intestinal segments mounted in Ussing chambers, we measured the sodium-coupled electrogenic absorption of glucose along the gastrointestinal tract of omnivorous Nile tilapia (Oreochromis niloticus) and carnivorous rainbow trout (Oncorhynchus mykiss). Differences and absences of intestinal segmental kinetic segregation and pharmacological inhibition were successfully compared with expression of specific SLC5A family members with previously described functions, some supporting known glucose absorption. Tilapia demonstrated similar kinetics throughout all of its intestinal segments, which was defined as a one-kinetic homogeneous system. Specifically, tilapia has a single Ha/Hc sodium-dependent glucose transport system along the entirety of its intestinal tract. In contrast, trout demonstrated different transport kinetics in the pyloric ceca, midgut, and hindgut intestinal segments. This was defined as a three-kinetic heterogeneous system, with a Ha/Lc, sHa/Lc, and La/Lc transport occurring in the pyloric ceca, midgut, and hindgut, respectively. Overall, the data presented here define a Hc one-kinetic homogenous system in tilapia, and a Lc three-kinetic heterogeneous system of sodium-dependent glucose transport in trout, supported by SLC5A gene expression.
MATERIALS AND METHODS
Maintenance of Animals
All fish were maintained in accordance with the guidelines of the Canadian Council on Animal Care (2005) (15). All animal protocols were approved by the Animal Care Committee at the University of Saskatchewan (AUP no. 19980142).
Nile tilapia.
Nile tilapia were obtained from AmeriCulture (Animas, NM) as fingerlings. They were housed in 360-liter tanks filtered via a biological filtration system. Photoperiod was kept constant at 14:10-h light-dark cycle, and the water temperature was maintained at 27 ± 2°C. Fish were fed a standard ration of commercial feed by hand twice per day to visual satiety. The average weight of fish at the time of study was 500 g.
Rainbow trout.
Female rainbow trout were obtained as wild-type, fertilized eggs from Trout Lodge (Sumner, WA). After hatching, the fish were reared in standard 1,000- to 4,000-liter density tanks, provided with biologically filtered recirculation systems until 2 yr of age, when used for this study. They were housed in municipal, dechlorinated water at temperatures between 11 ± 2°C, with a photoperiod at 12:12-h light-dark cycle. They were fed a standard ration of commercial feed at 2–5% of their body weight. At the time of study, the average weight of fish used was 400 g.
Ex Vivo Tissue Collection
Fish were euthanized by blunt trauma, and the entire intestinal section was dissected out of both fish. In Nile tilapia, the intestine was much longer than that of the trout, and it contained no pyloric ceca. Its intestinal section was separated as proximal intestine (2 in. distal from the stomach), midintestine (5 in. distal from the stomach), and hindgut (5–6 in. distal from the stomach). In rainbow trout, the intestine was separated according to the pyloric ceca region (located directly distal to the stomach), midgut (located right after the pyloric ceca, 2 in. from the stomach), and hindgut (5–6 in. from the stomach). The pyloric ceca is visually distinct from the midgut section. Similarly, the hindgut is visually distinct from the midgut, and it was represented as a thicker, larger diameter tissue, darker in pigment, along with visual differences in musculature (14).
Electrophysiology
Ussing Chamber technique.
The fish intestinal sections were examined in Ussing chambers using techniques adapted from this group (58, 59, 73, 80). The Ussing chamber system used in this study was an EasyMount Ussing Chamber System (Physiologic Instruments, San Diego, CA). Sections were washed in teleost saline (in mM): 118 NaCl, 2.9 KCl, 2.0 CaCl2·2H2O, 1.0 MgSO4·7H2O, 0.1 NaH2PO4·H2O, 2.5 Na2HPO4, and 1.9 NaHCO3 at pH 7.9 (78). This physiological buffer was used for both tilapia and trout. No glucose was added to the buffer. The lumen of the intestine for both fish was also washed with buffer using an 18-gauge needle, to clean the luminal membrane of any residual chyme.
The dissected tissue sections were exposed as a flat sheet between two sliders of inserts (slider no. P2304, Physiologic Instruments). The available tissue area for tilapia and trout was constant and equal to 0.3 cm2. The teleost saline buffer solutions bathing the apical and basal sides of the intestine had a buffer volume of 5 ml and were continuously gassed throughout the experiment with 1% CO2 and 99% O2 for the fish (19).
The transepithelial voltage and passing current set across the tissue was measured via agar bridges and Ag/AgCl reference electrodes. These electrodes were connected to leads that lead to the voltage/current clamp. The short-circuit current was measured by the computer (in µA), and this was a result of the tissue current opposing the current induced by the electrodes. The pulse was kept constant at 0.001 V. A recirculating bath capable of chilling and heating controlled the temperature of the jacketed chambers, with tissues examined at fish housing temperatures. The temperature used for tilapia was between 25 and 26°C and for trout was between 11 and 12°C. Needle valves were also present for the adjustment of gas flow into the chambers.
Once mounted, the tissue was allowed to reach a steady baseline current for 30–40 min before 1 mM increments of glucose were added to the apical side and the short-circuit current were measured in microamperes. An equivalent amount of d-mannitol was added to the basal side to prevent the development of osmotic gradients from the addition of glucose. Also, mannitol would not be transported across the epithelium, which presents no interference with the measurement of glucose transport. Before each addition of glucose and mannitol, a wait period of 3–4 min was allowed for equilibration of the current.
Chemicals.
Dapagliflozin is a competitive inhibitor of glucose for mammalian SGLT2 (La/Hc) transporters, with EC50 values around 1.12 nM for human SGLT2 (17, 39, 51). Phloridzin dihydrate is a competitive inhibitor against mammalian sodium-glucose cotransporter 1 (SGLT1) (Ha/Lc) transporters, with a Ki around 1 µM for renal and intestinal sodium-glucose cotransporters (1, 79, 86). For pharmacological characterization, 0.001, 0.01, 0.1, 1, 10, 100, 200, and 300 µM of dapagliflozin, and 1, 10, and 100 µM of phloridzin dihydrate (AdooQ Bioscience, Irvine, CA) were used in sequential order in the Ussing chamber as final concentrations. The inhibitors were added after each tissue segment reached a final glucose and mannitol concentration of 50 mM. For tilapia, dose responses of dapagliflozin, followed by phloridzin dihydrate addition were performed. Additionally, a dose response of phloridzin dihydrate by itself was conducted in tilapia after final glucose and mannitol concentrations of 50 mM was reached. In trout, dose responses of dapagliflozin followed by phloridzin dihydrate were used.
RNA extraction and cDNA synthesis using RT-PCR.
Approximately 1-mg samples of tissue were obtained from the dissection of the intestinal tract, and stored in RNAlater RNA Stabilization Solution at −80°C. RNA was isolated using the TRIzol reagent, and cDNA was synthesized through reverse transcription PCR using the qScript cDNA SuperMix (Quanta Biosciences). The reaction was run as incubation for 5 min at 25°C, then 30 min at 42°C, and the final 5 min at 85°C. The cDNA samples were stored at −80°C for subsequent use in quantitative PCR (qPCR).
Genomic identification of SLC5A genes.
A detailed BLAST+ (Basic Local Alignment Search Tool) application was used to identify all of the annotated SLC5A transporters in both tilapia and trout. The 12 human SLC5A transporter sequences retrieved from the National Center for Biotechnology Information website (https://www.ncbi.nlm.nih.gov/) were used in a “blastn” command to search for similar mRNA sequences in the tilapia and trout genome (90). The expect value (e-value) was used to assess the significance of the match, where an e-value close to 0 was considered significant and an e-value higher than 10−15 was considered as a nonsignificant match. Once SLC5A transporters were identified from tilapia and trout, phylogenetic trees were generated using the alignment program CLUSTAL W and MEGA7 (https://www.megasoftware.net/) software.
Gene transcript expression levels by quantitative polymerase chain reaction.
qPCR was performed on the RT-PCR reaction using the GoTaq qPCR Master Mix containing SYBRGreen1 (Promega, Madison, WI). The Bio-Rad qPCR system was used to perform these reactions. The total volume of the PCR reaction was 12.5 µl. A total of 40 cycles of qPCR were performed, with each cycle consisting of GoTaq Hot Start Polymerase activation at 95°C for 2 min, and then denaturation at 95°C for 15 s, followed by annealing/extension at the primer’s hybridization temperature (59°C) for 1 min. The dissociation step was at 60–95°C. Serial dilutions of cDNA were also used to generate a standard curve for each target gene, where the efficiency of each primer was calculated. The efficiencies for the tilapia primers ranged from 1.9 to 2.1, and similar range of efficiencies was determined for trout primers as well. EFα1 (α-elongation factor 1) was the housekeeping gene, and it was used to normalize the relative expression levels in tilapia and trout. The method of analysis used to represent the relative expression levels was calculated using ΔCT, where the amount of the gene of interest was normalized to the amount of EFα1 in that sample (75).
The primers for tilapia and trout SLC5A genes and EFα1 were designed using the Integrated DNA Technologies website (https://www.idtdna.com/pages), where the sequences and their accession numbers are presented in Tables 1 and 2, respectively.
Table 1.
Tilapia primer sequences used for quantitative PCR
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Accession No. |
|---|---|---|---|
| SGLT1 | CCCGAGTACTTGAAGAAGAG | GCAATAACAGCGAGGTAGA | XM_019361130.1 |
| SGLT2 | AGGAGGATGAGGGAGAATAA | ACACTGGGTCCTCACTAA | XM_013273846.2 |
| SMIT1 | CAACGCCAAGTACAGAAAC | CTCTGCGATTCTCCCTTATAG | XM_019353114.1 |
| SGLT4 | ATGGTGGTTGGAATCTGG | GTCCCTGCTAGACCAATAAA | XM_005475685.3 |
| NIS | TCCATGTCCTACCTCTACTT | GACCCTCCACTTTCTTCTT | XM_005453978.3 |
| SMCT1 | TCAGTGTTGAGTGAGAAGG | AGAGCGAACACCACATAG | XM_013271289.2 |
| SGLT5 | CACCGTGGACTCTGATTT | TACATCTGCGTGTGAAGAG | XM_003455975.4 |
| SMIT2 | GAGACGGAAGAAGGAAGATG | GCCCAGTAACCAATGATAAAG | XM_005461087.3 |
| SMIT | GCTGTCTGTGGATCTCTATT | GAACTGTGTCCGTGTAGAT | XM_019352280.1 |
| SMVT | CAGGAGGAATAGCAGAAGTC | CTTGGTTCACACCGTACA | XM_005460242.3 |
| EFα1 | CTCTTCTACCGTCGGATTAC | ATTGACTCCCTCGTAGAAAC | XM_005476483.3 |
EFα1, α-elongation factor 1; NIS, sodium-iodide cotransporter; SGLT1, SGLT2, SGLT4, SGLT5, sodium-glucose cotransporters 1, 2, 4, and 5, respectively; SMCT1, sodium-monocarboxylate cotransporter 1; SMIT, SMIT1, SMIT2, sodium-myoinositol cotransporter and cotransporters 1 and 2, respectively; SMVT, sodium-multivitamin cotransporter.
Table 2.
Trout primer sequences used for quantitative PCR
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Accession No. |
|---|---|---|---|
| SGLT1 | CTAAGTGTCACGGCTCTATAC | GCGTCTGGAAGTTCTCATAA | XM_021591066.1 |
| SGLT2 | ATTGGTAGTGGTCACTTTGT | AAACAGCCAGCCCAATAG | NM_001124432 |
| SGLT4 | GTGCTGGTGTTTGTGTATG | AGATGGTGCTCTGGAATG | XM_021604024.1 |
| NIS | GGCTTGTGTCTCGTTGTA | TGTCCAGCACCAAGTATG | XM_021573060.1 |
| SMIT2 | CAAGATCCTGCCCTTCTT | GTAGCTTCATGACCAGTTTG | XM_021592865.1 |
| SMVT | ATCTTACCAGCGCTTACC | CCCATAGATCAAAGCCAGTA | XM_021573745.1 |
| EFα1 | AGCGAGCTCAAGAAGAAG | GACCAAGAGGAGGGTATTC | NM_001124339.1 |
EFα1, α-elongation factor 1; NIS, sodium-iodide cotransporter; SGLT1, SGLT2, SGLT4, sodium-glucose cotransporters 1, 2, and 4, respectively; SMIT2, sodium-myoinositol cotransporter 2; SMVT, sodium-multivitamin cotransporter.
Statistical analyses.
Maximal velocity (Vmax; represented in µA/cm2) and Michaelis-Menten contant (Km; represented in mM) values were determined for each intestinal section in each species using GraphPad Prism 8 (GraphPad Software) (44, 53, 64, 66). Here, the Km values for both fish species represent the concentration at 50% maximum rate of the transporter, and the assumption is made that the rate of transport (catalysis) and rate of binding are similar. Tilapia intestinal segments followed Sigmoidal kinetics, whereas trout pyloric ceca and midgut followed Michaelis-Menten kinetics, and the kinetics in trout hindgut fit Sigmoidal Hill kinetics (F-test, P < 0.05, Figs. 1 and 4). The equations used for the Sigmoidal Hill kinetics and Michaelis-Menten fits were the following:
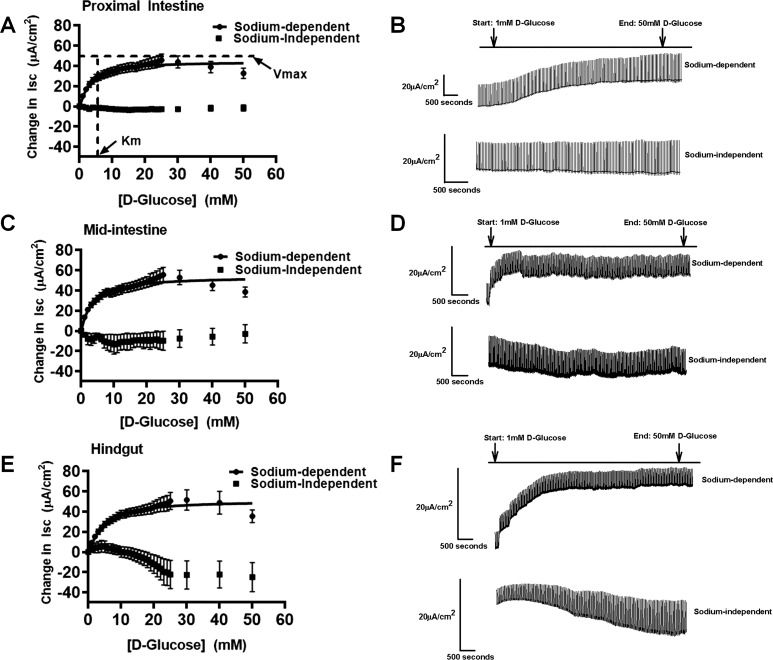
Fig. 1.
Sigmoidal Hill kinetics for sodium-dependent and sodium-independent electrogenic glucose transport for tilapia in proximal intestine (A), illustrated with representative trace on the right side (B) (sodium-dependent fish: n = 26; sodium-independent fish: n = 7); midintestine (C), illustrated with representative trace on the right side (D) (sodium-dependent fish: n = 30; sodium-independent fish: n = 6); and hindgut (E), illustrated with representative trace on the right side (F) (sodium-dependent fish: n = 31; sodium-independent fish: n = 6). The maximal velocity (Vmax) and Michaelis-Menten constant (Km) are represented in the proximal intestine (A) for illustration. Values are means ± SE. Isc, short-circuit current.
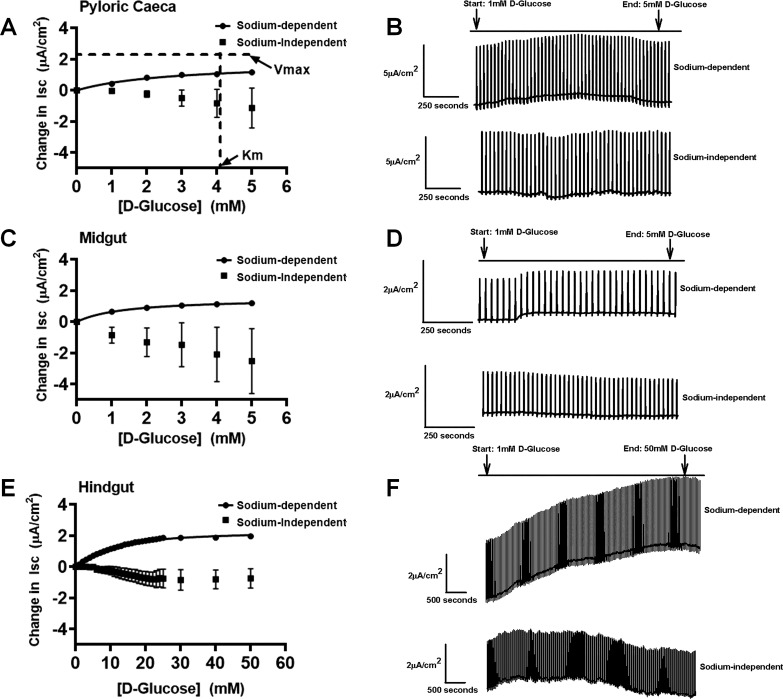
Fig. 4.
Michaelis-Menten and Sigmoidal Hill kinetics for sodium-dependent and sodium-independent electrogenic glucose transport for trout in pyloric ceca (A), illustrated with representative trace on the right side (B) (sodium-dependent fish: n = 12; sodium-independent fish: n = 5); midgut (C), illustrated with representative trace on the right side (D) (sodium-dependent fish: n = 18; sodium-independent fish: n = 4); and hindgut (E), illustrated with representative trace on the right side (F) (sodium-dependent fish: n = 24; sodium-independent fish: n = 4). The maximal velocity (Vmax) and Michaelis-Menten constant (Km) are represented in pyloric ceca (A) for illustration. Values are means ± SE. Isc, short-circuit current.
A one-way ANOVA was performed on the three intestinal sections of each species to determine whether the Vmax and Km values for each gut section was significantly different (92). All data met parametric assumptions, being normally distributed and exhibited homogeneity of variance, so ANOVA analyses were used (92). After ANOVA, pairwise comparisons were made using Tukey’s posteriori tests, as appropriate. A P < 0.05 was considered significantly different. To compare the Vmax values between tilapia and trout, Student’s t-test was performed to confirm significance. For the inhibitor dapagliflozin, the percent activity remaining was calculated from the division of the 50 mM increment short-circuit current (the concentration where the transporter is 100% saturated and it is 100% uninhibited) and the resultant drop in current from the addition of each drug concentration. For phloridzin dihydrate, the percent activity remaining was calculated from the division of the 300 μM concentration of dapagliflozin (concentration where Ha/Lc transporter is uninhibited) and the resultant drop in current from the addition of the drug. Parametric two-way ANOVAs (two factors: intestinal sections and dosage of the drug) were performed on the three intestinal sections of each species and the dosages of each inhibitor to determine significance (92). The Ki values for the dapagliflozin response and the phloridzin dihydrate-only responses were calculated for tilapia using the “One-Site Fit logIC50” from GraphPad Prism 8 (GraphPad Software). This fit follows the equation: . Parametric one-way ANOVA was performed on the three intestinal sections to determine significance. For the qPCR analyses, fold differences were determined by the Bio-Rad system, where the threshold cycle was established using the housekeeping gene, EFα1. A two-way ANOVA (two factors: intestinal location and gene) was conducted on the gene expressions, followed by Tukey’s posteriori test for pairwise comparisons. All statistical analyses were performed on SigmaPlot (Systat).
RESULTS
Nile Tilapia
Ha/Hc electrogenic glucose absorption kinetics.
In tilapia, glucose gradients performed in an Ussing chamber measured short-circuit current and demonstrated electrogenic sodium-dependent glucose absorption that followed Sigmoidal Hill kinetics in all intestinal segments, with Hill coefficients > 1 (proximal intestine, midintestine, and hindgut). All three intestinal sections saturated around 30 mM glucose in tilapia (Fig. 1). Additionally, the calculated Vmax and Km values were consistent with that of a Ha/Hc system in all segments (Fig. 1 and Table 3). No statistically significant differences in Vmax and Km values were found among the different intestinal segments in tilapia (Table 3; Vmax: P = 0.5, Km: P = 0.4), demonstrating a single Ha/Hc system throughout the tilapia gastrointestinal tract. Generally, low Km values around 4–6 mM and high Vmax values around 47–60 µA/cm2 were found throughout tilapia gut. Sodium dependency of glucose transport was confirmed by replacement of sodium with potassium, creating a sodium-free teleost saline Ussing chamber buffer, which eliminated the glucose-stimulated current (Fig. 1). Similarly, a sodium-free teleost saline using N-methyl-d-glucamine as a substitute for sodium was performed, which eliminated the glucose-stimulated current as well (data not shown).
Table 3.
Vmax and Km values for Nile tilapia and rainbow trout
| Nile Tilapia |
Rainbow Trout |
|||||
|---|---|---|---|---|---|---|
| Tilapia (Vmax)/Trout (Vmax), % | Tissue | Vmax, µA/cm2 | Km, mM | Tissue | Vmax, µA/cm2 | Km, mM |
| Proximal | 47.0 ± 6.7 | 4.2 ± 0.7 | Pyloric ceca | 2.3 ± 0.4a,b | 4.2 ± 1.0a | 2,043d |
| Midintestine | 59.6 ± 7.0 | 6.0 ± 1.0 | Midgut | 1.7 ± 0.3a | 1.8 ± 0.3b | 3,506d |
| Hindgut | 56.2 ± 9.3 | 5.7 ± 1.1 | Hindgut | 2.4 ± 0.2b | 12.3 ± 1.6c | 2,342d |
Values are means ± SE. Maximal velocity (Vmax; µA/cm2) and Michaelis-Menten constant (Km; mM) are represented for each tissue type in Nile tilapia and rainbow trout. Area of intestinal segment was 0.3 cm2. There were no significant differences in Vmax and Km values between tissues for tilapia. In trout, significant differences between Vmax values are as follows:
pyloric ceca vs.
hindgut, and a midgut vs. bhindgut, with no significant differences between pyloric ceca and midgut (Tukey’s test after one-way ANOVA, P < 0.05). Significant differences between Km values in trout are as follow: apyloric ceca vs. bmidgut, apyloric ceca vs.
Significant Vmax values between tilapia and trout (Student’s t-test, P < 0.05).
Inhibition of Ha/Hc kinetics.
Dapagliflozin, a selective inhibitor for SGLT2 (SLC5A2) or La/Hc sodium-dependent glucose transport, and phloridzin dihydrate, an SGLT1 (SLC5A1) or Ha/Lc system inhibitor, were used to help decipher some of the kinetics (51, 28, 65). Fig. 2 illustrates the percent activity remaining in each intestinal tissue after the cumulative addition of increasing dosages of inhibitors dapagliflozin and then phloridzin, as well as phloridzin addition alone.
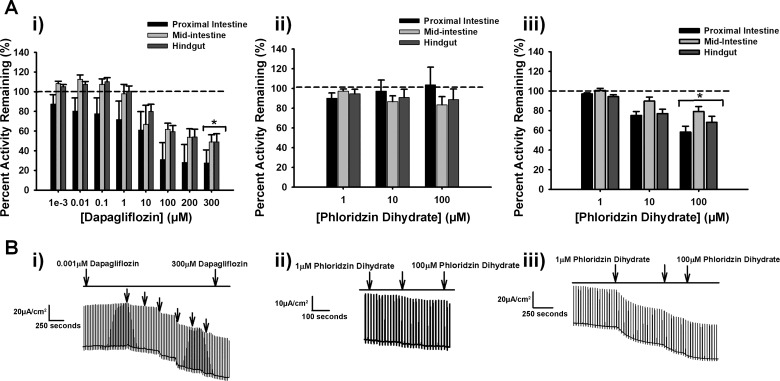
Fig. 2.
A: percent activity remaining of short-circuit current in Nile tilapia of dapagliflozin (0.001, 0.01, 0.1, 10, 100, 200, and 300 µM; i) (all tissues: n = 25), following addition of phloridzin dihydrate (1, 10, 100 µM; ii) (all tissues: n = 25) and phloridzin dihydrate (1, 10, 100 µM; iii) without previous addition of dapagliflozin (proximal: n = 22, midintestine: n = 18, and hindgut: n = 20) on intestinal tissues. B: representative traces for proximal tissue of dapagliflozin addition (i), phloridzin dihydrate addition following dapagliflozin (ii), and phloridzin dihydrate-only addition (iii). Values are means ± SE. *Significance from 100% transporter activity before inhibition, which is presented as the dashed line (two-way ANOVA, P < 0.05).
The three intestinal sections in tilapia were similarly inhibited by dapagliflozin in a dose-dependent manner (Fig. 2Ai, P > 0.5 for tissue differences), with ~50–60% activity remaining at a final 300 µM dose in all intestinal segments (Fig. 2Ai, P < 0.05 for inhibitor effect). The Ki (inhibition at 50%) values for the proximal intestine was 7.7 ± 1.5 µM, midintestine was 7.4 ± 1.7 µM, and the hindgut was 5.7 ± 1.6 µM. These values were not significantly different from each other (P = 0.6). No significant inhibition was found on addition of phloridzin dihydrate added sequentially after dapagliflozin (Fig. 2Aii, P > 0.5). However, addition phloridzin by itself resulted in significant inhibition (Fig. 2Aiii, P < 0.05 for inhibitor effect) at a final concentration of 100 µM, with ~60–80% activity remaining, again with no significant differences found between intestinal segments (Fig. 2Aiii, P > 0.5 for tissue differences). Additionally, the Ki values for the proximal intestine was 11.1 ± 0.002 µM, the midintestine was 11.6 ± 0.002 µM, and the hindgut was 7.6 ± 0.002 µM. The Ki values between the intestinal segments were not significantly different from each other (P = 0.4).
SLC5A gene profiling affirms kinetics.
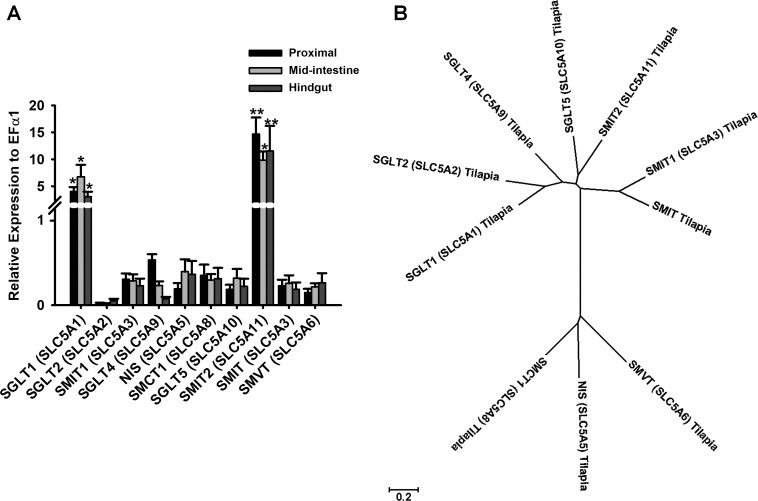
To identify the genes responsible for the observed current, tilapia genomic analysis was performed, which found 10 of 12 known SLC5A family members. The SLC5A transporters identified were SGLT1 (SLC5A1), SGLT2 (SLC5A2), SMIT1 (SLC5A3, sodium-myoinositol cotransporter 1), SGLT4 (SLC5A9, sodium-glucose cotransporter 4), NIS (SLC5A5, sodium-iodide cotransporter), SMCT1 (SLC5A8, sodium-monocarboxylate cotransporter 1), SGLT5 (SLC5A10, sodium-glucose cotransporter 5), SMIT2 (SLC5A11, sodium-myoinositol cotransporter 2), SMIT (SLC5A3, sodium-myoinositol cotransporter), and SMVT (SLC5A6, sodium-multivitamin cotransporter) in the genome using the BLAST+ application (Table 1). The expression levels of each gene were then compared among the different tissues using quantitative real-time PCR.
All intestinal tissues in tilapia had similar gene profiling with no significant differences in expression from each other, supporting the similarity in physiological responses of the three segments (Fig. 3A, P > 0.5 for tissue differences). Overall, SGLT1 (SLC5A1) and SMIT2 (SLC5A11) expression were significantly higher than the expression of the other genes in all tissues (Fig. 3A, P < 0.001). The proximal intestine and hindgut had significantly higher SMIT2 (SLC5A11) expression than SGLT1 (SLC5A1) (Fig. 3A, P < 0.05 for transporter type within each tissue location), whereas the midintestine had statistically similar SGLT1 (SLC5A1) and SMIT2 (SLC5A11) expression (Fig. 3A, P > 0.5 for transporter type in midintestine). Sequence similarities in identified tilapia SLC5A transporters were compared in a phylogenetic tree (Fig. 3B). In a CLUSTAL W analysis, the nucleotide identities of SLC5A family members found in the genome relative to tilapia SGLT1 (SLC5A1) were 60.6% SGLT2 (SLC5A2), 47.9% SMIT1 (SLC5A3), 57.5% SGLT4 (SLC5A9), 35.5% NIS (SLC5A5), 33% SMCT1 (SLC5A8), 53.8% SGLT5 (SLC5A10), 55.3% SMIT2 (SLC5A11), 48.3% SMIT (SLC5A3), and 37.4% SMVT (SLC5A6).
Fig. 3.
A: relative expression levels of SLC5A transporters in Nile tilapia (proximal intestine: n = 8–10, midintestine: n = 6–10, hindgut: n = 6–10) using quantitative PCR. Expression levels were normalized against EFα1 (α-elongation factor 1) housekeeping gene. Values are means ± SE. *There were no significant differences between tissues for SGLT1 (SLC5A1): proximal vs. midintestine vs. hindgut. **Significant differences were found between genes SGLT1 (SLC5A1) and SMIT2 (SLC5A11) for proximal and hindgut (two-way ANOVA, P < 0.05). B: phylogenetic tree of the 10 members of the SLC5A family of cotransporters. The alignment program CLUSTAL W and the phylogenetic display program MEGA7 were used to generate the tree. NIS, sodium-iodide cotransporter; SGLT1, SGLT2, SGLT4, and SGLT5, sodium-glucose cotransporters 1, 2, 4, and 5, respectively; SMCT1, sodium-monocarboxylate cotransporter 1; SMIT, SMIT1, SMIT2, sodium-myoinositol cotransporter and cotransporters 1 and 2, respectively; SMVT, sodium-multivitamin cotransporter.
Rainbow Trout
Electrogenic glucose absorption reveals a three-kinetic system.
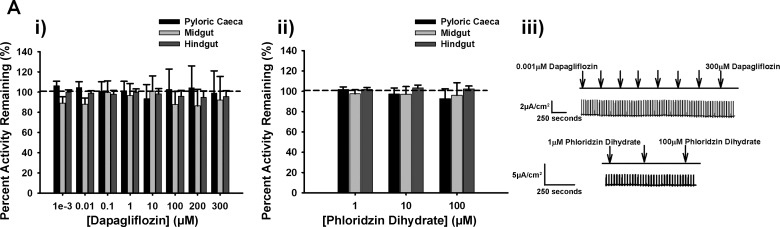
A glucose gradient performed in the Ussing chamber using the pyloric ceca, midgut, and hindgut intestinal segments of trout resulted in short-circuit current. The pyloric ceca and midgut both followed Michaelis-Menten kinetics, whereas the hindgut followed Sigmoidal Hill kinetics (Fig. 4). The pyloric ceca and midgut both saturated around 5 mM glucose, with a significantly higher Km value in the pyloric ceca (4.2 mM) compared with the midgut (1.8 mM) (Table 3, P < 0.05), but not significantly different Vmax values between the two trout tissues (2.3 vs. 1.7 µA/cm2) (Table 3, P > 0.5). These values characterize a Ha/Lc system in the pyloric ceca and a super-high-affinity (sHa)/Lc system in the midgut of trout (Fig. 4 and Table 3). The trout hindgut saturated around 50 mM glucose, but with a significantly higher Km value than the midgut and pyloric ceca (Fig. 4 and Table 3, P < 0.05). The Hill coefficient was > 1 for the hindgut. The Vmax value in the trout hindgut was slightly higher than that of the midgut, but was similar to the pyloric ceca and not significantly different from each other (Fig. 4 and Table 3, P > 0.5). The observed Vmax (2.4 µA/cm2) and higher Km (12.3 mM) values in the hindgut in comparison to the proximal sections of the trout intestine support a La/Lc system. Thus trout presents with three different kinetic systems along their gastrointestinal tract, with large differences in affinity for glucose and an overall Lc for glucose transport compared with tilapia. Specifically, tilapia Vmax values were significantly (2,043–3,506% or ~20−35 times, P < 0.05 in Student’s t-test) higher compared with trout (Table 3). Finally, sodium dependency of glucose transport was demonstrated in a sodium-free buffer (Fig. 4). Additionally, a sodium-free teleost saline using N-methyl-d-glucamine as a substitute for sodium was performed, which eliminated the glucose-stimulated current as well (data not shown). Interestingly, in trout, there was no significant inhibition in any of the kinetic systems in response to both dapagliflozin and phloridzin dihydrate (Fig. 5A, i and ii, P > 0.5 for inhibitor effect in two-way ANOVA).
Fig. 5.
A: percent activity remaining in rainbow trout of dapagliflozin (0.001, 0.01, 0.1, 10, 100, 200, and 300 µM; i) (all tissues: n = 12), following addition of phloridzin dihydrate (1, 10, 100 µM; ii) (all tissues: n = 12) and representative trace for pyloric ceca of dapagliflozin and phloridzin dihydrate additions (iii). Values are means ± SE. Dashed line represents 100% transporter activity before inhibition.
SLC5A gene profiling affirms kinetics.
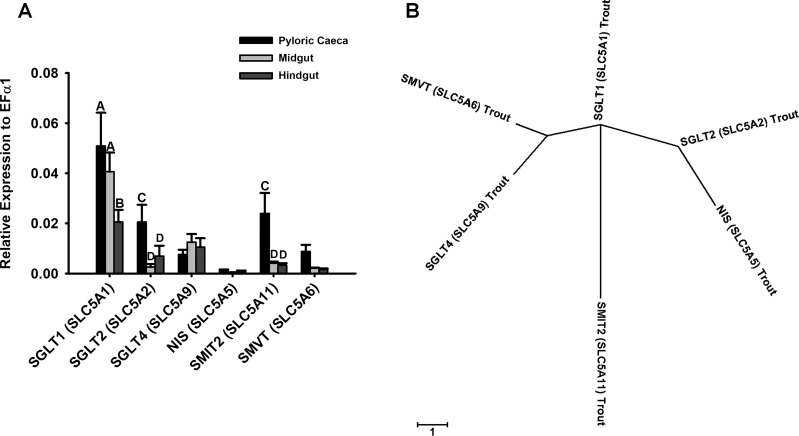
To identify the genes responsible for the observed kinetics, trout genomic analysis was performed and identified 5 of 12 known SLC5A family members. Trout SGLT1 (SLC5A1), SGLT2 (SLC5A2), SGLT4 (SLC5A9), NIS (SLC5A5), SMIT2 (SLC5A11), and SMVT (SLC5A6) were identified in the trout genome after using the BLAST+ application (Table 2). The expression levels of each gene were compared among the different tissues using quantitative real-time PCR.
In trout, SGLT1 (SLC5A1) expression in the pyloric ceca and midgut were significantly higher than the expression levels of the other genes, supporting the higher affinity and Lc kinetics in both tissue segments (Fig. 6A, P < 0.001). In addition, SGLT2 (SLC5A2) and SMIT2 (SLC5A11), two La transporters, had significantly higher expression in the trout pyloric ceca than the midgut, supporting the Ha to sHa paradigm between pyloric ceca and midgut (Fig. 6A, P < 0.05). A significant drop in SGLT1 (SLC5A1) was found in the trout hindgut, potentially explaining the reduced affinity in this segment (Fig. 6A, P < 0.05). However, significant drops in SGLT2 (SLC5A2) and SMIT2 (SLC5A11) were also noted, leaving the observed glucose absorbing capacity unexplained (Fig. 6A, P < 0.05). The phylogenetic tree identifying sequence similarities between trout SLC5A transporters are illustrated in Fig. 6B. In a CLUSTAL W analysis, the nucleotide identities relative to trout SGLT1 (SLC5A1) were 62.3% SGLT2 (SLC5A2), 56.5% SGLT4 (SLC5A9), 38.3% NIS (SLC5A5), 59.4% SMIT2 (SLC5A11), and 40.5% SMVT (SLC5A6).
Fig. 6.
A: relative expression levels of SLC5A transporters in rainbow trout (pyloric ceca: n = 6–10, midgut: n = 10, hindgut: n = 8–10) using quantitative PCR. Expression levels were normalized against EFα1 (α-elongation factor 1) housekeeping gene. Values are means ± SE. A,B,C,D Significant differences were found between tissues for SGLT1 (SLC5A1) for A pyloric ceca vs. B hindgut and for A midgut vs. B hindgut (two-way ANOVA, P < 0.05); there were no significant differences between pyloric ceca and midgut. Significant differences were found between tissues for SGLT2 (SLC5A2) and SMIT2 (SLC5A11) for C pyloric ceca vs. D midgut and for C pyloric ceca vs. D hindgut (two-way ANOVA, P < 0.05); there were no significant differences between midgut and hindgut. B: phylogenetic tree of the six members of the SLC5A family of cotransporters. The alignment program CLUSTAL W and the phylogenetic display program MEGA7 were used to generate the tree. NIS, sodium-iodide cotransporter; SGLT1, SGLT2, SGTLT4, sodium-glucose cotransporters 1, 2, and 4, respectively; SMIT2, sodium-myoinositol cotransporter 2; SMVT, sodium-multivitamin cotransporter.
DISCUSSION
This study demonstrated that tilapia intestine possesses higher electrogenic glucose absorption and greater expression of glucose transporter orthologs than trout intestine. Differences between the two fish species portray tilapia as a homogeneous, one-kinetic glucose absorption system (Ha/Hc) throughout the gastrointestinal tract. In contrast, trout have heterogeneous, three-kinetic glucose absorption systems (Ha/Lc, sHa/Lc, and La/Lc) corresponding to the three different intestinal segments. Comparative studies between omnivore and carnivore sodium-dependent glucose absorption has been minimally explored in mammals and birds, with studies lacking in fish (22, 26, 43). Additionally, the existence of two different segment-specific, sodium-dependent glucose transport systems along the small intestine associated with SLC5A transporters have been shown in mammals, but has not been described in fish (49, 61, 89). In the present study, we present fundamental differences that exist between the omnivorous tilapia and carnivorous trout in terms of sodium-dependent glucose transport along the gastrointestinal tract. Additionally, both fish species have hindgut sodium-dependent glucose absorption, suggesting a divergent adaptation from mammals, where colonic glucose absorption is lacking.
One-Kinetic SLC5A-Associated Homogeneous Glucose Absorption in Tilapia
Tilapia, an omnivorous fish, would generally consume a higher carbohydrate diet in their natural environment and has been shown to better tolerate a higher glucose load than trout in terms of its clearance from the plasma (76). In captivity, tilapia are generally fed a higher carbohydrate-inclusion diet (up to 50% dietary carbohydrate), which is correlated with good growth (52, 64, 69, 76, 87). In contrast, trout can tolerate up to 20% carbohydrate in their diet, before exhibiting poor growth (25, 87). Both dietary carbohydrate levels in fish are generally lower than in mammalian diets, which are up to 60% inclusion (16). Our study adds to these known nutritional physiological differences by demonstrating that tilapia have ~20–35 times (~2,000–3,500%, Table 3) higher capacity for sodium-dependent glucose transport than corresponding segments in trout. The higher absorption in tilapia is driven by a single unique Ha/Hc kinetic system, which is demonstrated by low Km and high Vmax values along the entirety of the intestinal tract, which would enhance their ability to absorb more glucose, a characteristic of omnivores (Table 3) (87). Similarly, a study carried out in the omnivorous freshwater fish black bullhead (Ictalurus melas) using isolated enterocytes found brush-border transport of 3-O-methyl-d-glucose (nonmetabolizable form of d-glucose) to saturate at ~40 mM (79).
Further characterization with pharmacological inhibitors supported the homogenous Ha/Hc kinetic system in tilapia. In mammals, these inhibitors have been used to differentiate the kinetic systems (Ha/Lc and La/Hc) of SGLTs (27, 34, 51, 67, 86). Specifically, the recently developed dapagliflozin and the traditionally used phloridzin dihydrate have been used to inhibit SGLT2 (SLC5A2), a La/Hc transporter and SGLT1 (SLC5A1), a Ha/Lc transporter, respectively, in the kidney of rats, pigs, and humans (27, 51, 84, 85). Additionally, phloridzin dihydrate has been used with studies in fish using techniques like brush-border membrane vesicles exhibiting inhibition of sodium-dependent glucose transport (1, 61, 79, 81, 88). Here, dapagliflozin was first applied, to selectively inhibit La/Hc transporter kinetics, followed by phloridzin dihydrate to inhibit any further Ha/Lc kinetics.
Dapagliflozin resulted in inhibition, with all intestinal segments responding similarly, with sequential addition of phloridzin dihydrate not resulting in any further inhibition. In contrast, phloridzin addition by itself resulted in similar significant inhibition in all segments. All segments reacting the same to all inhibitors with similar Ki values exhibited throughout the intestine support a one-kinetic, homogeneous system throughout tilapia gut. The lack of sequential inhibition of phloridzin after dapagliflozin suggest that 1) dapagliflozin can inhibit Ha/Lc transporters (SGLT1-like) in tilapia; or 2) the unique Ha/Hc system is created by a single SGLT, which is sensitive to both drugs. Arguing against the latter possibility is the fact that genomic and gene expression analysis suggested the former.
Bioinformatic analysis identifying 10 SLC5A transporters exhibited similar gene expressions across all three intestinal segments in tilapia. Such similar expression would explain homogenous segmental sodium-dependent glucose kinetics and inhibition. More specifically, the similar, but significantly higher, levels of SGLT1 (SLC5A1) and SMIT2 (SLC5A11) expression in all three segments compared with all of the other SLC5A genes, support two different transporters contributing to the Ha/Hc system. The elevated expression of SGLT1 (SLC5A1), a well characterized Ha/Lc transporter, supports the high-affinity kinetics and phloridzin inhibition (9). Interestingly, although SMIT2 (SLC5A11) had the highest expression compared with all of the other genes, it is known to primarily transport inositols (90). However, it is previously known as SGLT6 and was characterized as a very La glucose transporter in mammals (3, 7, 20). The high capacity of glucose transport was demonstrated with rabbit SMIT2 (SLC5A11) cRNA injected into Xenopus oocytes and detected glucose transport at ~50 mM (57). In the phylogenetic tree (Fig. 3B), SMIT2 (SLC5A11) is divergent from SGLT1 (SLC5A1) with only 55.3% homology, suggesting differences in function. Nonetheless, the putative high-capacity glucose transporter could explain Hc kinetics found in each of the tilapia intestinal segments. Altogether, the combined dominant expression and function of these two transporters are further supported by a Sigmoidal Hill fit. Together, this suggests that the heterogeneous expression in tilapia of 10 different SLC5A transporters along all areas of gastrointestinal tract, with a dominance of SGLT1 (SLC5A1) and SMIT2 (SLC5A11) expression, produces the Ha/Hc kinetics and the pharmacological inhibition observed. Such a Ha/Hc system would be advantageous for an omnivorous fish to absorb high levels of glucose presented to the gut from the diet (87).
Three-Kinetic SLC5A-Associated Glucose Absorption in Trout
The segregation of three-kinetic, sodium-dependent glucose transport systems was found along the gastrointestinal tract of trout. In all of the trout gut segments, the capacity for glucose transport was low in comparison to tilapia. However, the overall affinity was higher in the proximal segments of trout pyloric ceca and midgut, but drastically decreased as it reached the distal hindgut. The low capacity in all three segments of trout would protect the fish from rapid glucose absorption. Exogenous glucose ingestion in carnivorous fish has been shown to induce the process of gluconeogenesis, further increasing the plasma glucose levels (54, 68). This can lead to persistent hyperglycemia (high plasma glucose levels) and high levels of plasma insulin, which can hinder growth and increase liver size (48, 72). The differences in affinity explain how the proximal sections are sensitive to glucose transport when luminal glucose concentrations are low, around 1–5 mM, but the hindgut is sensitive to glucose transport only when the intestinal luminal glucose concentration is much higher, >5mM.
The differences in affinity for glucose between the trout pyloric ceca and midgut may assist glucose absorption as the concentration of glucose decreases in the chyme as it moves through the intestine (1). The Ha/Lc transport system characterized in the present study in the pyloric ceca would be efficient at absorbing most of the available glucose in the trout chyme. However, as the chyme moves to the midgut with decreasing glucose concentrations, the sHa/Lc transport system would become advantageous. A related study examining proximal intestinal glucose absorption in the carnivorous, seawater, pacific copper rockfish demonstrate similar kinetic segmental differences (1). The uptake of d-[3H]glucose in brush-border membrane vesicles of pyloric ceca and upper intestine (anatomically similar to midgut) exhibited Michaelis-Menten kinetics of sodium-dependent glucose absorption (1). The mucosal-to-serosal glucose influx (Vmax) was similar between both organs (pyloric ceca and upper intestine), but the Km values were different (high Km for pyloric ceca and low Km for upper intestine) (1). The differences in the Km values indicate that pyloric ceca has lower affinity for glucose concentration than the upper intestine (midgut) (1). Similarly, we found differences in Km values between pyloric ceca and midgut, where the midgut demonstrated a sHa for glucose compared with the pyloric ceca, with no differences in the Vmax. Ahearn et al. (1) that, since the pyloric ceca has first access to the food, it absorbs more of the nutrients at higher concentrations. In contrast, the chyme that is released to the subsequent midgut section has lower nutrient content, thus needing a higher affinity transport system (1). Here we took trout intestinal segmental contributions further by characterizing glucose uptake in the hindgut as well. Interestingly, the above assertion does not hold for the trout hindgut with its Sigmoidal Hill fit and La and Hc than the midgut, suggesting physiological roles for this system other than nutrient absorption. Finally, the use of known mammalian SGLT inhibitors suggests a lack of contribution from trout SGLT1 (SLC5A1) and SGLT2 (SLC5A2) to the kinetic systems. However, given that the sequence similarity of trout SGLT1 (SLC5A1) and SGLT2 (SLC5A2) to human is only 58% and 66%, respectively, this finding was not too surprising. Alternatively, the lack of inhibition may be attributed to the lower temperature used for trout kinetic measurements. Such temperature sensitivity of glucose transport inhibitors has previously been described (29). Thus the three-kinetic trout system was further characterized by genomic and gene expression analysis.
In trout, 5 of the known 12 SLC5A transporters where found and exhibited high SGLT1 (SLC5A1, Ha/Lc) expression in the pyloric ceca and midgut compared with the other SLC5A transporters. These data are correlated with the Michaelis-Menten Vmax values for the trout pyloric ceca and midgut segments that exhibit Lc transport. However, the trout pyloric ceca SGLT2 (SLC5A2) and SMIT2 (SLC5A11), which are La transporters, were more prominently expressed than in the midgut, possibly explaining the comparatively lower affinity detected in the pyloric ceca segment (3, 7, 20). Therefore, the kinetics of Ha/Lc in the trout pyloric ceca is likely due to the combined expression of SGLT1 (SLC5A1), SGLT2 (SLC5A2), and SMIT2 (SLC5A11), whereas the sHa/Lc kinetics in the midgut is due to a decrease in SGLT2 (SLC5A2) and SMIT2 (SLC5A11). In comparison, the decrease in trout hindgut SGLT1 (SLC5A1) expression was associated with a drastic reduction in affinity to glucose in this segment, again suggesting that the Ha and sHa of the pyloric ceca and midgut is driven by SGLT1 (SLC5A1). The expression of the other SLC5A genes in the trout hindgut, which do not significantly differ from the midgut then may account for the Lc kinetics. Although our analysis of SLC5A gene expression does account for the kinetics defined in this study, there is still the possibility of another unidentified SGLT-like transporter that may explain the Lc paradigm in trout hindgut. Overall, our results confirm that the carnivorous trout has SGLT1 (SLC5A1), SGLT2 (SLC5A2), and SMIT2 (SLC5A11) transporters in the pyloric ceca likely driving an observed Ha/Lc system, and an increase in proportional SGLT1 (SLC5A1) expression to SGLT2 (SLC5A2) and SMIT2 (SLC5A11) driving a sHa/Lc system in the midgut. A La/Lc kinetic system in the trout hindgut is supported by low expression of all trout SLC5A genes and a significant drop in SGLT1 (SLC5A1).
Fish Hindgut Glucose Absorption: Different from Mammals
The presence of sodium-dependent glucose absorption in the hindgut of tilapia and trout was surprising in comparison to the mammalian models, where it is nonexistent in this location (32, 34, 35, 49). However, for aquatic animals, the regulation of ionic and osmotic balances are extremely important, utilizing ionic and substrate transporters in their gills, kidneys, and intestines to maintain this balance (38, 83). The gut plays a role in osmoregulation by controlling the intake of dietary ions, using glucose transporters along with dietary substrates to passively contribute to ionic and osmotic balances (63, 83). Interestingly, the presence of active d-glucose uptake in the rectum was found in frugivorous avian species and was considered important to offset the fast digesta transit through their short gut (55, 56). However, the omnivorous chicken with its longer gut demonstrated high hindgut glucose uptake (30). This suggest functions of this glucose absorption other than nutrient uptake. One possibility would be osmoregulation, especially given the proximity of the rectum and cloaca (2, 31). Together, our characterization of sodium-dependent glucose transport and associated SGLTs in the hindguts of these fish may be an unexplored mechanism that may contribute to ionic and osmotic regulation in tilapia and trout.
Omnivorous and Carnivorous Comparisons Between Fish and Mammals
The homogenous Ha/Hc kinetic system of sodium-dependent glucose absorption that exists throughout the omnivorous tilapia differs from previously characterized omnivorous systems, such as the dog, cattle, human, rat, and pig (27, 32, 34, 35, 45, 74). Generally, two types of intestinal segmental differences in sodium-dependent glucose absorption kinetic systems were identified, with Ha/Lc kinetics in the jejunum, La/Hc in the ileum, and the colon being void of transport (27, 32, 34, 35, 45, 74). Thus tilapia have evolved to use their entire intestinal tract for Ha/Hc sodium-dependent glucose absorption, whereas omnivorous mammals only utilized their small intestinal sections (jejunum and ileum) for either Ha/Lc or La/Hc, but never show Ha/Hc sodium-dependent glucose absorption (5, 12, 32, 49). This fundamental difference suggests that tilapia may be more efficient than these other omnivorous mammals at absorbing glucose. Comparatively, trout differ from the carnivorous cat, with the cat having similar intestinal kinetic segmental segregation to omnivorous animals with Ha/Lc and La/Hc kinetics (89). The trout differs with three-kinetic, sodium-dependent glucose transport systems (Ha/Lc, sHa/Lc, La/Lc), that is overall Lc. This suggests that trout have a very limited capacity to absorb glucose compared with other omnivorous and carnivorous mammals.
Limitations
There are a few limitations in this study based on the techniques used. The assumption of mRNA expression of the SLC5A transporters directly correlating with protein function does not always hold. However, identifying these fish SLC5A proteins using Western blots is not currently possible as there are no available fish SLC5A antibodies. Another technical limitation in this study involves the possibility of contamination of other tissues when collecting whole intestinal segments for RT-qPCR. Avoiding contamination by scraping cells off the epithelial lumen was not possible, given the very thin intestinal tissue, which would tear upon scraping, thus not completely eliminating contamination. Another limitation addresses the higher absorptive capacity seen in tilapia, which could be due to other structural and functional adaptations. The present study expressed absorptive capacity in terms of intestinal segment area exposed by the insert in the Ussing chamber (0.3 cm2). However, while the tissue area was constant, the epithelial surface area may have differed between the two species, depending on the size of villi and microvilli structures. Additionally, the higher SGLT1 expression in tilapia compared with trout may contribute to the higher Vmax values, as the coupling ratio of sodium to glucose for SGLT1 is known to be 2:1 (6, 9, 23, 24, 32–34, 37, 49). Finally, the definition of Km in this study is collectively termed as affinity, where the assumption is made that the rate of transport and binding is similar. This assumption is supported by the fact that our technique requires the simultaneous binding and transport of glucose to generate a current. These limitations further address the fact that tilapia and trout may have other adaptive mechanisms in place for the observed differences, but studying these mechanisms/functions would be beyond the scope of the present study.
In conclusion, in tilapia, electrogenic sodium-dependent glucose absorption was characterized as a segmentally homogenous Ha/Hc kinetic system. These finding were supported by the high expression of SGLT1 (SLC5A1) and SMIT2 (SLC5A11), and similar sensitivities to dapagliflozin and phloridzin dihydrate throughout the gastrointestinal tract. In trout, a Ha/Lc system in the pyloric ceca was supported by the expression of SGLT1 (SLC5A1), SGLT2 (SLC5A2), and SMIT2 (SLC5A11), whereas the midgut demonstrated an sHa/Lc kinetic system, supported by the SGLT1 (SLC5A1) expression and a decrease in SGLT2 (SLC5A2) and SMIT2 (SLC5A11) expression compared with pyloric ceca. Finally, the hindgut demonstrated a La/Lc kinetic system supported by a decrease in SGLT1 (SLC5A1) expression and a general expression of SLC5A genes. Overall, tilapia and trout demonstrate differences in transport kinetics and intestinal segmental contribution associated with SLC5A family members. Additionally, these findings highlight segmental kinetic differences between the gastrointestinal tract of fish and mammals.
Perspectives and Significance
Here, we present the first report comparing the kinetic characterization of electrogenic sodium-dependent glucose transport in the omnivorous Nile tilapia and the carnivorous rainbow trout gastrointestinal tract. The differences found suggest that omnivorous and carnivorous fish may have unique transport systems for glucose. This may have evolved to allow each species to maximize its glucose uptake from its natural diet. However, this omnivore-carnivore paradigm needs to be further tested in other mammalian and aquatic systems. Finally, in comparison to mammals, the discovery here of hindgut sodium-dependent glucose absorption in fish suggests that this process may play a greater role in osmoregulation, a homeostatic mechanism vital to aquatic species.
GRANTS
This research was supported by Natural Sciences and Engineering Research Council of Canada Discovery Grant 371364–2010 (M. E. Loewen), Natural Sciences and Engineering Research Council of Canada CRD CRDPJ: 446912–13, and the Saskatchewan Pulse Crop Development Board (M. E. Loewen and L. P. Weber).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.E.L. conceived and designed research; M.S. and M.E.L. performed experiments; M.S. and M.E.L. analyzed data; M.S., L.P.W., and M.E.L. interpreted results of experiments; M.S. and M.E.L. prepared figures; M.S. and M.E.L. drafted manuscript; M.S., L.P.W., and M.E.L. edited and revised manuscript; M.S., L.P.W., and M.E.L. approved final version of manuscript.
REFERENCES
- 1. Ahearn GA, Behnke RD, Zonno V, Storelli C. Kinetic heterogeneity of Na-D-glucose cotransport in teleost gastrointestinal tract. Am J Physiol Regul Integr Comp Physiol 263: R1018–R1023, 1992. doi: 10.1152/ajpregu.1992.263.5.R1018. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GL, Braun EJ. Postrenal modification of urine in birds. Am J Physiol Regul Integr Comp Physiol 248: R93–R98, 1985. doi: 10.1152/ajpregu.1985.248.1.R93. [DOI] [PubMed] [Google Scholar]
- 3.Aouameur R, Da Cal S, Bissonnette P, Coady MJ, Lapointe JY. SMIT2 mediates all myo-inositol uptake in apical membranes of rat small intestine. Am J Physiol Gastrointest Liver Physiol 293: G1300–G1307, 2007. doi: 10.1152/ajpgi.00422.2007. [DOI] [PubMed] [Google Scholar]
- 5.Arai T, Washizu T, Sagara M, Sako T, Nigi H, Matsumoto H, Sasaki M, Tomoda I. D-glucose transport and glycolytic enzyme activities in erythrocytes of dogs, pigs, cats, horses, cattle and sheep. Res Vet Sci 58: 195–196, 1995. doi: 10.1016/0034-5288(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 6.Aschenbach JR, Steglich K, Gäbel G, Honscha KU. Expression of mRNA for glucose transport proteins in jejunum, liver, kidney and skeletal muscle of pigs. J Physiol Biochem 65: 251–266, 2009. doi: 10.1007/BF03180578. [DOI] [PubMed] [Google Scholar]
- 7.Augustin R, Mayoux E. Mammalian sugar transporters. : Glucose Homeostasis, edited by Szablewski L. London: IntechOpen, 2014, p. 3–36. [Google Scholar]
- 8.Axelsson E, Ratnakumar A, Arendt M-L, Maqbool K, Webster MT, Perloski M, Liberg O, Arnemo JM, Hedhammar A, Lindblad-Toh K. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495: 360–364, 2013. doi: 10.1038/nature11837. [DOI] [PubMed] [Google Scholar]
- 9.Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I. Revised immunolocalization of the Na+-d-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol 295: C475–C489, 2008. doi: 10.1152/ajpcell.00180.2008. [DOI] [PubMed] [Google Scholar]
- 10.Blanco AM, Bertucci JI, Ramesh N, Delgado MJ, Valenciano AI, Unniappan S. Ghrelin facilitates GLUT2-, SGLT1- and SGLT2-mediated intestinal glucose transport in goldfish (Carassius auratus). Sci Rep 7: 45024, 2017. doi: 10.1038/srep45024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breves G, Kock J, Schröder B. Transport of nutrients and electrolytes across the intestinal wall in pigs. Livest Sci 109: 4–13, 2007. doi: 10.1016/j.livsci.2007.01.021. [DOI] [Google Scholar]
- 12.Brot-Laroche E, Dao MT, Alcalde AI, Delhomme B, Triadou N, Alvarado F. Independent modulation by food supply of two distinct sodium-activated D-glucose transport systems in the guinea pig jejunal brush-border membrane. Proc Natl Acad Sci USA 85: 6370–6373, 1988. doi: 10.1073/pnas.85.17.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buddington RK, Chen JW, Diamond JM. Dietary regulation of intestinal brush-border sugar and amino acid transport in carnivores. Am J Physiol Regul Integr Comp Physiol 261: R793–R801, 1991. doi: 10.1152/ajpregu.1991.261.4.R793. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G. The morphology of the gut of the Brown trout (Salmo trutta). Q J Microsc Sci 100: 183–198, 1959. [Google Scholar]
- 15.Canadian Council on Animal Care Guidelines On: The Care and Use of Fish in Research, Teaching and Testing. Ottawa, ON, Canada: Canadian Council on Animal Care, 2005. [Google Scholar]
- 16.Carciofi AC, Takakura FS, de-Oliveira LD, Teshima E, Jeremias JT, Brunetto MA, Prada F. Effects of six carbohydrate sources on dog diet digestibility and post-prandial glucose and insulin response. J Anim Physiol Anim Nutr (Berl) 92: 326–336, 2008. doi: 10.1111/j.1439-0396.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 17.Chao EC, Henry RR. SGLT2 inhibition–a novel strategy for diabetes treatment. Nat Rev Drug Discov 9: 551–559, 2010. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 18.Charalampopoulos D, Rastall R. () Prebiotics and Probiotics Science and Technology. New York: Springer, 2009, p 1. [Google Scholar]
- 19.Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 296: G1151–G1166, 2009. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coady MJ, Wallendorff B, Gagnon DG, Lapointe JY. Identification of a novel Na+/myo-inositol cotransporter. J Biol Chem 277: 35219–35224, 2002. doi: 10.1074/jbc.M204321200. [DOI] [PubMed] [Google Scholar]
- 21.de-Oliveira LD, Carciofi AC, Oliveira MC, Vasconcellos RS, Bazolli RS, Pereira GT, Prada F. Effects of six carbohydrate sources on diet digestibility and postprandial glucose and insulin responses in cats. J Anim Sci 86: 2237–2246, 2008. doi: 10.2527/jas.2007-0354. [DOI] [PubMed] [Google Scholar]
- 21a.Delezay O, Verrier B, Mabrouk K, van Rietschoten J, Fantini J, Mauchamp J, Gerard C. Characterization of an electrogenic sodium/glucose cotransporter in a human colon epithelial cell line. J Cell Physiol 163: 120–128, 1995. doi: 10.1002/jcp.1041630114. [DOI] [PubMed] [Google Scholar]
- 22.Diamond JM, Karasov WH, Cary C, Enders D, Yung R. Effect of dietary carbohydrate on monosaccharide uptake by mouse small intestine in vitro. J Physiol 349: 419–440, 1984. doi: 10.1113/jphysiol.1984.sp015165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Díez-Sampedro A, Eskandari S, Wright EM, Hirayama BA. Na+-to-sugar stoichiometry of SGLT3. Am J Physiol Renal Physiol 280: F278–F282, 2001. doi: 10.1152/ajprenal.2001.280.2.F278. [DOI] [PubMed] [Google Scholar]
- 24.Dyer J, Al-Rammahi M, Waterfall L, Salmon KS, Geor RJ, Bouré L, Edwards GB, Proudman CJ, Shirazi-Beechey SP. Adaptive response of equine intestinal Na+/glucose co-transporter (SGLT1) to an increase in dietary soluble carbohydrate. Pflügers Arch 458: 419–430, 2009. doi: 10.1007/s00424-008-0620-4. [DOI] [PubMed] [Google Scholar]
- 25.Enes P, Panserat S, Kaushik S, Oliva-Teles A. Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol Biochem 35: 519–539, 2009. doi: 10.1007/s10695-008-9259-5. [DOI] [PubMed] [Google Scholar]
- 26.Ferraris RP, Ahearn GA. Intestinal glucose transport in carnivorous and herbivorous marine fishes. J Comp Physiol 152: 79–90, 1983. doi: 10.1007/BF00689731. [DOI] [Google Scholar]
- 27.Ferraris RP, Diamond JM. A method for measuring apical glucose transporter site density in intact intestinal mucosa by means of phlorizin binding. J Membr Biol 94: 65–75, 1986. doi: 10.1007/BF01901014. [DOI] [PubMed] [Google Scholar]
- 28.Ferraris RP, Diamond JM. Use of phlorizin binding to demonstrate induction of intestinal glucose transporters. J Membr Biol 94: 77–82, 1986. doi: 10.1007/BF01901015. [DOI] [PubMed] [Google Scholar]
- 29.Forsling ML, Widdas WF. The effect of temperature on the competitive inhibition of glucose transfer in human erythrocytes by phenolphthalein, phloretin and stilboestrol. J Physiol 194: 545–554, 1968. doi: 10.1113/jphysiol.1968.sp008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garriga C, Rovira N, Moretó M, Planas JM. Expression of Na+-d-glucose cotransporter in brush-border membrane of the chicken intestine. Am J Physiol Regul Integr Comp Physiol 276: R627–R631, 1999. doi: 10.1152/ajpregu.1999.276.2.R627. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein DL, Braun EJ. Contributions of the kidneys and intestines to water conservation, and plasma levels of antidiuretic hormone, during dehydration in house sparrows (Passer domesticus). J Comp Physiol B 158: 353–361, 1988. doi: 10.1007/BF00695334. [DOI] [PubMed] [Google Scholar]
- 32.Halaihel N, Gerbaud D, Vasseur M, Alvarado F. Heterogeneity of pig intestinal d-glucose transport systems. Am J Physiol Cell Physiol 277: C1130–C1141, 1999. doi: 10.1152/ajpcell.1999.277.6.C1130. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann J, Möller N, Lange P, Breves G. Different phlorizin binding properties to porcine mucosa of the jejunum and ileum in relation to SGLT1 activity. J Anim Sci 94, Suppl 3: 238–242, 2016. doi: 10.2527/jas.2015-9702. [DOI] [Google Scholar]
- 34.Herrmann J, Schröder B, Klinger S, Thorenz A, Werner AC, Abel H, Breves G. Segmental diversity of electrogenic glucose transport characteristics in the small intestines of weaned pigs. Comp Biochem Physiol A Mol Integr Physiol 163: 161–169, 2012. doi: 10.1016/j.cbpa.2012.05.204. [DOI] [PubMed] [Google Scholar]
- 35.Hirayama BA, Lostao MP, Panayotova-Heiermann M, Loo DD, Turk E, Wright EM. Kinetic and specificity differences between rat, human, and rabbit Na+-glucose cotransporters (SGLT-1). Am J Physiol Gastrointest Liver Physiol 270: G919–G926, 1996. doi: 10.1152/ajpgi.1996.270.6.G919. [DOI] [PubMed] [Google Scholar]
- 36.Hoenig M, Clark M, Schaeffer DJ, Reiche D. Effects of the sodium-glucose cotransporter 2 (SGLT2) inhibitor velagliflozin, a new drug with therapeutic potential to treat diabetes in cats. J Vet Pharmacol Ther 41: 266–273, 2018. doi: 10.1111/jvp.12467. [DOI] [PubMed] [Google Scholar]
- 37.Horiba N, Masuda S, Takeuchi A, Takeuchi D, Okuda M, Inui K. Cloning and characterization of a novel Na+-dependent glucose transporter (NaGLT1) in rat kidney. J Biol Chem 278: 14669–14676, 2003. doi: 10.1074/jbc.M212240200. [DOI] [PubMed] [Google Scholar]
- 38.Hwang PP, Lee T-H, Lin L-Y. Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301: R28–R47, 2011. doi: 10.1152/ajpregu.00047.2011. [DOI] [PubMed] [Google Scholar]
- 39.Isaji M. SGLT2 inhibitors: molecular design and potential differences in effect. Kidney Int Suppl 79: S14–S19, 2011. doi: 10.1038/ki.2010.511. [DOI] [PubMed] [Google Scholar]
- 42.Kanwal A, Singh SP, Grover P, Banerjee SK. Development of a cell-based nonradioactive glucose uptake assay system for SGLT1 and SGLT2. Anal Biochem 429: 70–75, 2012. doi: 10.1016/j.ab.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Karasov WH, Duong P, Diamond JM, Carpenter FL. Food passage and intestinal nutrient absorption in hummingbirds. Auk 103: 453–464, 1986. [Google Scholar]
- 44.Karasov WH, Martínez del Rio C, Caviedes-Vidal E. Ecological physiology of diet and digestive systems. Annu Rev Physiol 73: 69–93, 2011. doi: 10.1146/annurev-physiol-012110-142152. [DOI] [PubMed] [Google Scholar]
- 45.Kaunitz JD, Wright EM. Kinetics of sodium D-glucose cotransport in bovine intestinal brush border vesicles. J Membr Biol 79: 41–51, 1984. doi: 10.1007/BF01868525. [DOI] [PubMed] [Google Scholar]
- 46.Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol 531: 585–595, 2001. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirchner S, Panserat S, Lim PL, Kaushik S, Ferraris RP. The role of hepatic, renal and intestinal gluconeogenic enzymes in glucose homeostasis of juvenile rainbow trout. J Comp Physiol B 178: 429–438, 2008. doi: 10.1007/s00360-007-0235-7. [DOI] [PubMed] [Google Scholar]
- 49.Klinger S, Lange P, Brandt E, Hustedt K, Schröder B, Breves G, Herrmann J. Degree of SGLT1 phosphorylation is associated with but does not determine segment-specific glucose transport features in the porcine small intestines. Physiol Rep 6: e13562, 2018. doi: 10.14814/phy2.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koepsell H, Spangenberg J. Function and presumed molecular structure of Na(+)-D-glucose cotransport systems. J Membr Biol 138: 1–11, 1994. doi: 10.1007/BF00211064. [DOI] [PubMed] [Google Scholar]
- 51.Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Li L, Pfister M. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 85: 520–526, 2009. doi: 10.1038/clpt.2008.251. [DOI] [PubMed] [Google Scholar]
- 52.Krogdahl Å, Hemre GI, Mommsen TP. Carbohydrates in fish nutrition: digestion and absorption in postlarval stages. Aquacult Nutr 11: 103–122, 2005. doi: 10.1111/j.1365-2095.2004.00327.x. [DOI] [Google Scholar]
- 53.Kuehl GE, Lampe JW, Potter JD, Bigler J. Glucuronidation of nonsteroidal anti-inflammatory drugs: identifying the enzymes responsible in human liver microsomes. Drug Metab Dispos 33: 1027–1035, 2005. doi: 10.1124/dmd.104.002527. [DOI] [PubMed] [Google Scholar]
- 54.Legate NJ, Bonen A, Moon TW. Glucose tolerance and peripheral glucose utilization in rainbow trout (Oncorhynchus mykiss), American eel (Anguilla rostrata), and black bullhead catfish (Ameiurus melas). Gen Comp Endocrinol 122: 48–59, 2001. doi: 10.1006/gcen.2001.7620. [DOI] [PubMed] [Google Scholar]
- 55.Levey DJ, Duke GE. How do frugivores process fruit? Gastrointestinal transit and glucose absorption in Cedar Waxwings (Bombycilla cedrorum). Auk 109: 722–730, 1992. doi: 10.2307/4088148. [DOI] [Google Scholar]
- 56.Levey DJ, Karasov WH. Digestive modulation in a seasonal frugivore, the American robin (Turdus migratorius). Am J Physiol Gastrointest Liver Physiol 262: G711–G718, 1992. doi: 10.1152/ajpgi.1992.262.4.G711. [DOI] [PubMed] [Google Scholar]
- 57.Lin X, Ma L, Fitzgerald RL, Ostlund RE Jr. Human sodium/inositol cotransporter 2 (SMIT2) transports inositols but not glucose in L6 cells. Arch Biochem Biophys 481: 197–201, 2009. doi: 10.1016/j.abb.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loewen ME, Bekar LK, Walz W, Forsyth GW, Gabriel SE. pCLCA1 lacks inherent chloride channel activity in an epithelial colon carcinoma cell line. Am J Physiol Gastrointest Liver Physiol 287: G33–G41, 2004. doi: 10.1152/ajpgi.00023.2004. [DOI] [PubMed] [Google Scholar]
- 59.Loewen ME, Smith NK, Hamilton DL, Grahn BH, Forsyth GW. CLCA protein and chloride transport in canine retinal pigment epithelium. Am J Physiol Cell Physiol 285: C1314–C1321, 2003. doi: 10.1152/ajpcell.00210.2003. [DOI] [PubMed] [Google Scholar]
- 60.Mackenzie B, Panayotova-Heiermann M, Loo DD, Lever JE, Wright EM. SAAT1 is a low affinity Na+/glucose cotransporter and not an amino acid transporter. A reinterpretation. J Biol Chem 269: 22488–22491, 1994. [PubMed] [Google Scholar]
- 61.Maffia M, Acierno R, Cillo E, Storelli C. Na+-d-glucose cotransport by intestinal BBMVs of the Antarctic fish Trematomus bernacchii. Am J Physiol Regul Integr Comp Physiol 271: R1576–R1583, 1996. doi: 10.1152/ajpregu.1996.271.6.R1576. [DOI] [PubMed] [Google Scholar]
- 62.Malo C. Separation of two distinct Na+/D-glucose cotransport systems in the human fetal jejunum by means of their differential specificity for 3-O-methylglucose. Biochim Biophys Acta 1022: 8–16, 1990. doi: 10.1016/0005-2736(90)90394-4. [DOI] [PubMed] [Google Scholar]
- 63.McGowan KM, Long SD, Pekala PH. Glucose transporter gene expression: regulation of transcription and mRNA stability. Pharmacol Ther 66: 465–505, 1995. doi: 10.1016/0163-7258(95)00007-4. [DOI] [PubMed] [Google Scholar]
- 64.Newsome SD, Fogel ML, Kelly L, del Rio CM. Contributions of direct incorporation from diet and microbial amino acids to protein synthesis in Nile tilapia. Funct Ecol 25: 1051–1062, 2011. doi: 10.1111/j.1365-2435.2011.01866.x. [DOI] [Google Scholar]
- 65.Obermeier M, Yao M, Khanna A, Koplowitz B, Zhu M, Li W, Komoroski B, Kasichayanula S, Discenza L, Washburn W, Meng W, Ellsworth BA, Whaley JM, Humphreys WG. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dispos 38: 405–414, 2010. doi: 10.1124/dmd.109.029165. [DOI] [PubMed] [Google Scholar]
- 66.Obi IE, Sterling KM, Ahearn GA. Transepithelial D-glucose and D-fructose transport across the American lobster, Homarus americanus, intestine. J Exp Biol 214: 2337–2344, 2011. doi: 10.1242/jeb.055095. [DOI] [PubMed] [Google Scholar]
- 67.Osorio H, Bautista R, Rios A, Franco M, Arellano A, Vargas-Robles H, Romo E, Escalante B. Effect of phlorizin on SGLT2 expression in the kidney of diabetic rats. J Nephrol 23: 541–546, 2010. [PubMed] [Google Scholar]
- 68.Palmer TN, Ryman BE. Studies on oral glucose intolerance in fish. J Fish Biol 4: 311–319, 1972. doi: 10.1111/j.1095-8649.1972.tb05680.x. [DOI] [Google Scholar]
- 69.Pieper A, Pfeffer E. Studies on the comparative efficiency of utilization of gross energy from some carbohydrates, proteins and fats by rainbow trout (Salmo Gairdneri, R.). Aquaculture 20: 323–332, 1980. doi: 10.1016/0044-8486(80)90093-9. [DOI] [Google Scholar]
- 70.Polakof S, Alvarez R, Soengas JL. Gut glucose metabolism in rainbow trout: implications in glucose homeostasis and glucosensing capacity. Am J Physiol Regul Integr Comp Physiol 299: R19–R32, 2010. doi: 10.1152/ajpregu.00005.2010. [DOI] [PubMed] [Google Scholar]
- 71.Polakof S, Panserat S, Soengas JL, Moon TW. Glucose metabolism in fish: a review. J Comp Physiol B 182: 1015–1045, 2012. doi: 10.1007/s00360-012-0658-7. [DOI] [PubMed] [Google Scholar]
- 72.Polakof S, Soengas JL. Evidence of sugar sensitive genes in the gut of a carnivorous fish species. Comp Biochem Physiol B Biochem Mol Biol 166: 58–64, 2013. doi: 10.1016/j.cbpb.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 73.Polentarutti BI, Peterson AL, Sjöberg AK, Anderberg EK, Utter LM, Ungell AL. Evaluation of viability of excised rat intestinal segments in the Ussing chamber: investigation of morphology, electrical parameters, and permeability characteristics. Pharm Res 16: 446–454, 1999. doi: 10.1023/A:1018890106045. [DOI] [PubMed] [Google Scholar]
- 74.Poulsen SB, Fenton RA, Rieg T. Sodium-glucose cotransport. Curr Opin Nephrol Hypertens 24: 463–469, 2015. doi: 10.1097/MNH.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sasaki T, Mori IC, Furuichi T, Munemasa S, Toyooka K, Matsuoka K, Murata Y, Yamamoto Y. Closing plant stomata requires a homolog of an aluminum-activated malate transporter. Plant Cell Physiol 51: 354–365, 2010. doi: 10.1093/pcp/pcq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shiau SY. Utilization of carbohydrates in warmwater fish—with particular reference to tilapia, Oreochromis niloticus × O. aureus. Aquaculture 151: 79–96, 1997. doi: 10.1016/S0044-8486(96)01491-3. [DOI] [Google Scholar]
- 77.Shiau S-Y, Peng C-Y. Protein-sparing effect by carbohydrates in diets for tilapia, Oreochromis niloticus × O. aureus. Aquaculture 117: 327–334, 1993. doi: 10.1016/0044-8486(93)90329-W. [DOI] [Google Scholar]
- 78.Small SA, Farrell AP. Vascular reactivity of the coronary artery in steelhead trout (Oncorhynchus mykiss). Comp Biochem Physiol C 97: 59–63, 1990. doi: 10.1016/0742-8413(90)90172-6. [DOI] [PubMed] [Google Scholar]
- 79.Soengas JL, Moon TW. Transport and metabolism of glucose in isolated enterocytes of the black bullhead Ictalurus melas: effects of diet and hormones. J Exp Biol 201: 3263–3273, 1998. [DOI] [PubMed] [Google Scholar]
- 80.Tarran R, Loewen ME, Paradiso AM, Olsen JC, Gray MA, Argent BE, Boucher RC, Gabriel SE. Regulation of murine airway surface liquid volume by CFTR and Ca2+-activated Cl− conductances. J Gen Physiol 120: 407–418, 2002. doi: 10.1085/jgp.20028599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teerijoki H, Krasnov A, Pitkänen TI, Mölsä H. Monosaccharide uptake in common carp (Cyprinus carpio) EPC cells is mediated by a facilitative glucose carrier. Comp Biochem Physiol B Biochem Mol Biol 128: 483–491, 2001. doi: 10.1016/S1096-4959(00)00346-8. [DOI] [PubMed] [Google Scholar]
- 82.Turner RJ, Moran A. Further studies of proximal tubular brush border membrane D-glucose transport heterogeneity. J Membr Biol 70: 37–45, 1982. doi: 10.1007/BF01871587. [DOI] [PubMed] [Google Scholar]
- 83.Verri T, Barca A, Pisani P, Piccinni B, Storelli C, Romano A. Di- and tripeptide transport in vertebrates: the contribution of teleost fish models. J Comp Physiol B 187: 395–462, 2017. doi: 10.1007/s00360-016-1044-7. [DOI] [PubMed] [Google Scholar]
- 84.Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, Radović N, Jadrijević S, Aleksic I, Walles T, Sauvant C, Sabolić I, Koepsell H. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch 467: 1881–1898, 2015. doi: 10.1007/s00424-014-1619-7. [DOI] [PubMed] [Google Scholar]
- 85.White JR. Apple trees to sodium glucose co-transporter inhibitors: a review of SGLT2 inhibition. Clin Diabetes 28: 5–10, 2010. doi: 10.2337/diaclin.28.1.5. [DOI] [Google Scholar]
- 86.Wielert-Badt S, Lin J-T, Lorenz M, Fritz S, Kinne RKH. Probing the conformation of the sugar transport inhibitor phlorizin by 2D-NMR, molecular dynamics studies, and pharmacophore analysis. J Med Chem 43: 1692–1698, 2000. doi: 10.1021/jm9905460. [DOI] [PubMed] [Google Scholar]
- 87.Wilson RP. Utilization of dietary carbohydrate by fish. Aquaculture 124: 67–80, 1994. doi: 10.1016/0044-8486(94)90363-8. [DOI] [Google Scholar]
- 88.Wittenberger C, Coprean D, Morar L. Studies on the carbohydrate metabolism of the lateral muscles in carp (influence of phloridzin, insulin and adrenaline). J Comp Physiol 101: 161–172, 1975. doi: 10.1007/BF00694156. [DOI] [Google Scholar]
- 89.Wolffram S, Eggenberger E, Scharrer E. Kinetics of D-glucose transport across the intestinal brush-border membrane of the cat. Comp Biochem Physiol A 94: 111–115, 1989. doi: 10.1016/0300-9629(89)90793-7. [DOI] [PubMed] [Google Scholar]
- 90.Wright EM. Glucose transport families SLC5 and SLC50. Mol Aspects Med 34: 183–196, 2013. doi: 10.1016/j.mam.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Wright JR Jr, O’Hali W, Yang H, Han XX, Bonen A. GLUT-4 deficiency and severe peripheral resistance to insulin in the teleost fish tilapia. Gen Comp Endocrinol 111: 20–27, 1998. doi: 10.1006/gcen.1998.7081. [DOI] [PubMed] [Google Scholar]
- 92.Zar JH. Biostatistical Analysis (5th Ed). London: Pearson, 2010. [Google Scholar]