Abstract
This pilot study examined whether ischemic conditioning (IC), a noninvasive, cost-effective, and easy-to-administer intervention, could improve gait speed and paretic leg muscle function in stroke survivors. We hypothesized that 2 wk of IC training would increase self-selected walking speed, increase paretic muscle strength, and reduce neuromuscular fatigability in chronic stroke survivors. Twenty-two chronic stroke survivors received either IC or IC Sham on their paretic leg every other day for 2 wk (7 total sessions). IC involved 5-min bouts of ischemia, repeated five times, using a cuff inflated to 225 mmHg on the paretic thigh. For IC Sham, the cuff inflation pressure was 10 mmHg. Self-selected walking speed was assessed using the 10-m walk test, and paretic leg knee extensor strength and fatigability were assessed using a Biodex dynamometer. Self-selected walking speed increased in the IC group (0.86 ± 0.21 m/s pretest vs. 1.04 ± 0.22 m/s posttest, means ± SD; P < 0.001) but not in the IC Sham group (0.92 ± 0.47 m/s pretest vs. 0.96 ± 0.46 m/s posttest; P = 0.25). Paretic leg maximum voluntary contractions were unchanged in both groups (103 ± 57 N·m pre-IC vs. 109 ± 65 N·m post-IC; 103 ± 59 N·m pre-IC Sham vs. 108 ± 67 N·m post-IC Sham; P = 0.81); however, participants in the IC group maintained a submaximal isometric contraction longer than participants in the IC Sham group (278 ± 163 s pre-IC vs. 496 ± 313 s post-IC, P = 0.004; 397 ± 203 s pre-IC Sham vs. 355 ± 195 s post-IC Sham; P = 0.46). The results from this pilot study thus indicate that IC training has the potential to improve walking speed and paretic muscle fatigue resistance poststroke.
NEW & NOTEWORTHY This pilot study is the first to demonstrate that ischemic conditioning can improve self-selected walking speed and reduce paretic muscle fatigue in stroke survivors. Ischemic conditioning has been shown to be safe in numerous patient populations, can be accomplished at home or at the bedside in only 45 min, and requires no specialized training. Future larger studies are warranted to determine the efficacy of ischemic conditioning as a neurorehabilitation therapy poststroke.
Keywords: fatigue, gait, ischemic conditioning, motor function, rehabilitation, stroke
INTRODUCTION
Approximately 20–25% of chronic stroke survivors are unable to walk without full physical assistance (26). Furthermore, mainstream therapies to address motor deficits poststroke have resulted in only moderate changes in walking velocity (18, 36, 39, 51, 52), which are not likely to impact community ambulation or quality of life. Therefore, there is still a need for therapies, or adjuncts to traditional therapies, that can promote paretic leg muscle performance and improve walking in stroke survivors. Recently, ischemic conditioning (IC) has emerged as a potential stimulus to enhance neural plasticity and motor performance (58) in addition to its known beneficial effects on cardiovascular protection [recently reviewed by Heusch et al. (27)].
IC was first described in 1986 as a vascular stimulus to protect vital organs from ischemic injury (40) and has been extensively studied for cardioprotection. Subsequent studies have shown that IC, when performed on the arm or leg in the absence of concurrent exercise or therapy, improves muscle performance. For example, in healthy individuals, brief, repeated 5-min bouts of limb ischemia (using a blood pressure cuff inflated to 225 mmHg on the arm or leg) improve 5-km running time (1a), maximal power output (13), and motor learning (6, 7). It has also been shown that IC alone can reduce neuromuscular fatigue in healthy humans as task duration during handgrip exercise improves following a single session of IC (2). Although the mechanisms by which IC can improve muscle performance and reduce fatigue are complex and remain elusive, it is likely that local mechanisms at the level of the muscle, remote neural factors, and circulating humoral factors all contribute. Although not all studies that have examined the effects of IC on muscle and athletic performance have shown benefit, all studies to date have been performed on young, healthy individuals, where a ceiling effect likely exists (30, 48). To our knowledge, our group was the first to report improved motor function after a single session of IC in a clinical population with functional deficits, specifically chronic stroke survivors with leg weakness (28).
Whereas our previous work primarily focused on short-term changes in leg strength following IC, in this pilot study we sought to determine whether 2 wk of IC training on the paretic leg of chronic stroke survivors, in the absence of gait training or therapy, could result in clinically meaningful improvements in self-selected walking speed, paretic leg strength, and fatigue resistance. To our knowledge, the effects of IC on gait have not been measured in either healthy individuals or clinical populations. As leg weakness and increased neuromuscular fatigue contribute to walking deficits poststroke, we felt it a worthy pursuit to examine the effects of IC on all three measures.
In the field of cardioprotection, it has clearly been shown that there are short (0–12-h) and long (24–72-h) windows of protection following IC and these windows are mediated by separate mechanisms (31a). Our previous proof-of-principle study showed that a single IC session immediately improves paretic muscle strength (28), but it has been suggested by several studies that the delayed, or long-term, phase of IC may be more robust, especially as applied to neural plasticity (16, 44, 31a). The ability to walk is vital in activities of daily living and maintaining independence (47), self-selected walking speed is a valid, sensitive, and specific measure (22), and restoring gait is considered a hallmark of success in poststroke rehabilitation (15). Thus, in this study we sought to determine whether 2 wk of IC training would provide increases in self-selected walking speed compared with sham controls. Our secondary hypotheses were that compared with sham controls, IC would also improve muscle strength and promote neuromuscular fatigue resistance.
METHODS
General methods.
This study was a prospective, single-blinded, randomized, sham-controlled pilot study with paired analysis. The trial is registered at https://www.clinicaltrials.gov/ with the unique identifier NCT03095755. All study procedures were approved by the institutional review boards at the Medical College of Wisconsin and Marquette University. Written informed consent was obtained from all individual participants included in the study. All study participants were studied twice, with ~2 wk between test sessions. Inclusion criteria were 1) history of a single, unilateral stroke >1 yr before study enrollment as confirmed by standard-of-care MRI and 2) residual hemiparesis as determined by the presence of both paretic knee extensor weakness (compared with nonparetic leg, as assessed with the Biodex dynamometer) and visually appraised asymmetric gait (as assessed by a physical therapist) during the familiarization session (see below). Exclusion criteria were 1) history of multiple strokes, 2) brain-stem stroke, 3) lower extremity contractures, 4) stage II hypertension (>160/100 mmHg), 5) inability to follow two-step commands, 6) deep vein thrombosis, 7) peripheral arterial grafts in the lower extremity, and 8) any condition in which lower extremity tissue ischemia is contraindicated.
Study recruitment.
On the basis of our previous work in 10 stroke survivors that showed that a single session of IC improved leg strength (28) and other studies that examined the effects of IC on motor performance in healthy individuals with group sizes of 10–15 individuals (2, 7, 13, 41, 54), we aimed to enroll 11 study participants per group in this pilot study to have a complete data set on 10 individuals per group (accounting for an ~10% attrition rate). Potential study participants were screened and recruited from two Health Insurance Portability and Accountability Act (HIPAA)-compliant databases managed by A. S. Hyngstrom and M. J. Durand. The first source for study recruitment was a HIPAA-compliant database of 40 stroke survivors managed by A. S. Hyngstrom. The second source was a HIPAA-compliant, secure, online REDCap database managed by the Stroke Rehabilitation Center of Southeast Wisconsin and M. J. Durand. The database contains the names and contact information of stroke survivors interested in participating in stroke research and draws from ~300 new stroke cases and 400 patients with chronic stroke treated annually at Froedtert Hospital and the Medical College of Wisconsin. From the database, the study team had access to MRI DICOM images and radiology reports to confirm lesion location, as well as 184 discrete fields related to physical function and medical history to prescreen eligible participants.
Eligible study participants were contacted by a member of the study team either over the phone or via email. To ensure that potentially eligible participants could perform all study procedures and to familiarize study participants with all study procedures, a “familiarization” session was conducted with all participants after they issued written informed consent. During the familiarization sessions, study participants performed a series of muscle contractions on the Biodex dynamometer with their paretic and nonparetic leg to demonstrate their ability to follow multistep directions and volitionally activate their knee extensor muscles. Gait asymmetry was also confirmed by a physical therapist. To avoid carryover effects, the familiarization session was conducted ≥7 days before the baseline testing session, and no cuff inflations were performed (see below).
Ten-meter walk test.
Self-selected walking speed is one of the most well-validated clinical measures in stroke rehabilitation. Consistent with other studies (12, 17), participants walked 10 m at their comfortable speed in a hallway and could use whatever assistive device was necessary while being guarded by a member of the study team. The time it took to walk the middle 6 m was recorded, the test was done in triplicate, and the average of the three trials was recorded in meters per second. Following self-selected walking speed assessment, study participants were told to walk as fast as they could to assess fast walking speed, and values were recorded in the same manner as for self-selected walking speed.
Knee extensor maximum voluntary contractions and fatigability.
A Biodex dynamometer (Biodex Medical Systems) was used to measure isometric knee extension torques during maximum voluntary contractions (MVCs; strength) and during a submaximal contraction fatiguing protocol (task endurance) as we have previously described (19, 28). Custom-written LabVIEW (National Instruments) programs were used to acquire all data. Torque signals were low-pass filtered and sampled at 1,000 Hz using a data acquisition card and personal computer. The MVC task involved an increase in knee extension torque from zero to maximum over 2–3 s, and study participants were verbally encouraged to achieve maximal torque. The MVC was defined as the peak torque generated during a given 2- to 3-s effort. Study participants rested for 60 s between trials. Trials were repeated until peak torques from two of three trials were within 5% of each other (usually 3–5 trials). The peak torque generated was used as the MVC. The vertical and horizontal position of the dynamometer chair and the length and position of the lever used to measure knee extensor torque were recorded during the pretest session to ensure that study participants were seated in the same position for posttesting.
After the MVC was determined, study participants performed a sustained, submaximal, isometric contraction of their paretic knee extensors until task failure. The target torque for the fatigue task was equal to 30% of the paretic leg’s knee extensor MVC. Task failure was defined as the time at which participants could not maintain the knee extension target torque within a 10% window of error for 3 s or had >5 deviations of the window of error within a 10-s time window. Each study participant was given visual feedback and verbal encouragement of their performance (23). Task duration serves as a metric of neuromuscular fatigue (21). A single MVC was performed at the end of the fatigue protocol to verify that an acute decrease in maximal torque generation (i.e., muscle fatigue) was achieved.
To determine the effects of IC on muscle contractile properties, changes in the magnitude of the resting muscle twitch amplitude were quantified as we have done before (28). A constant-current stimulator (DS7AH; Digitimer, Welwyn Garden City, UK) delivered a 100-µs pulse at an intensity 10% above that required to elicit a maximal resting twitch (no more than 500 mA).
Ischemic conditioning.
IC treatments were performed on the paretic thigh in accordance with other studies that have used IC as an intervention (1, 1a, 13) and consistent with our previous work (28). We specifically chose to perform IC on the paretic leg so that local effects of IC (i.e., the release of vasoactive metabolites from the vascular endothelium or improved mitochondrial bioenergetics and oxygen consumption due to transient ischemia) would benefit the affected limb. Also, in light of previous work that has suggested that the magnitude of the IC response is directly proportional to the volume of tissue made ischemic (34), we chose to perform IC on the leg versus the arm.
In supine position with legs level with the heart, a thigh cuff (Hokanson SC-12 thigh cuff) was placed around the proximal, paretic thigh, inflated slowly (over 3–5 s) to 225 mmHg for 5 min using the Hokanson rapid cuff inflator, and then released rapidly (~0.3 s) for a 5-min recovery period. An inflation pressure of 225 mmHg is consistent with other studies in which IC was performed on the leg with cuff inflation pressures between 200 and 250 mmHg (1, 9, 13, 24, 59), and given that our study participants both were normotensive and had normal body mass index, this pressure would be expected to cause complete arterial occlusion. Five cycles of inflation-recovery were performed.
Sham ischemic conditioning.
For the group receiving IC Sham, the same physical setup was used, but the cuff was only inflated to 10 mmHg. This level of inflation was chosen because participants still perceive some cuff tightness but the inflation pressure is not high enough to produce ischemia. The participants were blinded to the purpose of the sham or typical IC protocols. This approach is consistent with studies that use IC as an intervention (10, 11, 41). All posttesting occurred 24–48 h after the last IC or IC Sham session was completed. Study participants were randomized using an online number generator.
Statistical analysis.
All data are presented as means ± SD, and all statistical analyses were performed using IBM SPSS Statistics version 24. Differences in subject demographics, time since stroke, self-selected walking speed, Berg balance score, Fugl-Meyer score, MVC, and paretic-to-nonparetic MVC ratio between the IC and IC Sham group were compared using an unpaired Student’s t-test or Mann-Whitney rank sum test, when appropriate. Differences in self-selected walking speed, fast walking speed, MVC, and time to muscle fatigue were compared using a mixed-effects analysis of variance (ANOVA) with one within-subjects variable (time: pre vs. post) and one between-subjects factor (group: IC vs. IC Sham) after testing the data for normal distribution using the Shapiro-Wilk test. Main effects of time and interaction effects of time × group were determined. A post hoc Bonferroni test was used to test for differences between individual means. Pre-post changes to self-selected and fast walking speed, MVC, and time to fatigue in the IC and IC Sham groups were compared using an unpaired Student’s t-test or Mann-Whitney rank sum test as appropriate. To determine the effect size of either IC or IC Sham on self-selected walking speed, fast walking speed, time to muscle fatigue, and MVC, Cohen’s d was calculated using the following equation: d = (M2 − M1)/SDpooled, where M1 and M2 are the means of the two samples and SDpooled is the pooled standard deviation of the two samples. P < 0.05 was considered statistically significant for all analyses.
RESULTS
Study participants.
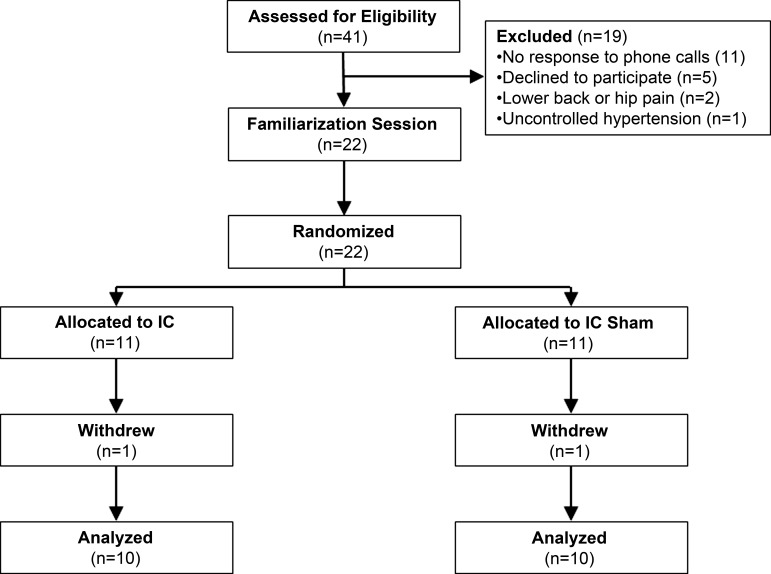
Forty-one chronic stroke survivors were contacted via phone or email, and 22 individuals participated in this 2-wk training study (see Fig. 1 for study design). Two participants withdrew from the study (see below). The average age of all study participants was 56 ± 18 yr, and the average time since stroke was 9 ± 9 yr. As shown in Table 1, there were no differences in demographics or the level of physical function (as assessed by baseline self-selected walking speed, lower extremity Fugl-Meyer score, Berg balance score, paretic leg strength, or paretic-to-nonparetic MVC ratio) between the 10 participants in the IC and IC Sham groups who completed the study. The time since stroke was less for study participants in the IC Sham group (15 ± 10 vs. 4 ± 3 yr in IC group; P = 0.005); however, no participants in either group were actively undergoing physical therapy or exercise training.
Fig. 1.
Diagram of the clinical trial. IC, ischemic conditioning.
Table 1.
Characteristics of study participants
| IC | IC Sham | P Value | |
|---|---|---|---|
| n | 10 | 10 | NA |
| Age, yr | 54 ± 20 | 58 ± 18 | 0.66 |
| Sex | |||
| Male, n | 3 | 3 | NA |
| Female, n | 7 | 7 | NA |
| Height, cm | 169 ± 11 | 167 ± 11 | 0.70 |
| Weight, kg | 74 ± 14 | 79 ± 19 | 0.51 |
| Body mass index, kg/m2 | 26 ± 5 | 28 ± 5 | 0.37 |
| Systolic blood pressure, mmHg | 118 ± 8 | 123 ± 10 | 0.25 |
| Diastolic blood pressure, mmHg | 78 ± 10 | 81 ± 5 | 0.50 |
| Heart rate, beats/min | 78 ± 11 | 72 ± 13 | 0.30 |
| Time since stroke, yr | 15 ± 10 | 4 ± 3 | 0.005 |
| Type of stroke | |||
| Ischemic, n | 6 | 8 | NA |
| Hemorrhagic, n | 2 | 2 | NA |
| Unknown, n | 2 | 0 | NA |
| Affected side | |||
| Left, n | 3 | 5 | NA |
| Right, n | 7 | 5 | NA |
| Assistive device | |||
| Yes, n | 5 | 5 | NA |
| No, n | 5 | 5 | NA |
| Self-selected walking speed, m/s | 0.87 ± 0.21 | 0.91 ± 0.42 | 0.81 |
| Fast walking speed, m/s | 1.18 ± 0.26 | 1.12 ± 0.54 | 0.74 |
| Lower extremity Fugl-Meyer score (0–34) | 29 ± 4 | 26 ± 5 | 0.26 |
| Berg balance score (0–56) | 50 ± 4 | 51 ± 8 | 0.87 |
| MVC | |||
| Paretic, N·m | 103 ± 57 | 103 ± 59 | 0.99 |
| Nonparetic, N·m | 147 ± 79 | 153 ± 72 | 0.87 |
| Paretic-to-nonparetic MVC ratio | 0.72 ± 0.18 | 0.67 ± 0.25 | 0.55 |
Values are means ± SD; n = no. of participants. IC, ischemic conditioning; MVC, maximum voluntary contraction; NA, not applicable.
Two participants withdrew from the study for reasons unrelated to the study procedures. One person in the IC group mentioned during their first testing session (before any IC) that they had been having persistent but mild abdominal pain, which worsened over the 2-wk training and resulted in hospitalization. One person in the IC Sham group suffered a fall at home and chose to withdraw from the study because of soreness from the fall. No participants in the IC group reported excessive pain or asked to withdraw from the study because of pain related to the IC procedure (cuff inflation to 225 mmHg on the proximal paretic thigh). There was no difference in the number of sessions performed between the IC and IC Sham groups (7 ± 1 vs. 7 ± 2 sessions, respectively; P = 0.90). The average number of days between pretest and posttest sessions was also not different between the groups (18 ± 7 days IC vs. 17 ± 8 days IC Sham, P = 0.44).
Self-selected walking speed.
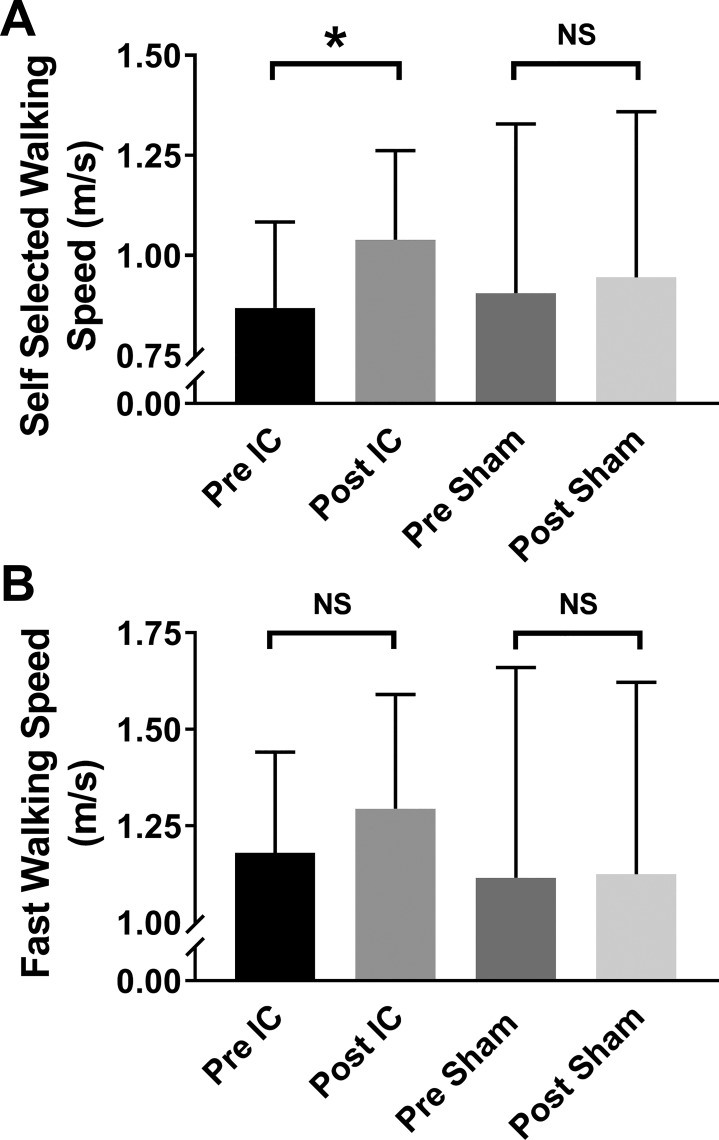
Gait speed during the 10-m walk test was measured in 20 chronic stroke survivors before and after 2 wk of IC training (n = 10) or IC Sham (n = 10). As shown in Fig. 2A, there was a significant interaction effect of time × group (P = 0.012). Pairwise multiple comparisons of group means indicated that IC significantly improved self-selected walking speed from pretest values (0.86 ± 0.21 to 1.04 ± 0.22 m/s; P < 0.001), whereas there was no difference observed in participants in the IC Sham group (0.92 ± 0.47 m/s pretest vs. 0.96 ± 0.46 m/s posttest; P = 0.25). The minimal clinically important difference (MCID) in self-selected walking speed in stroke survivors has been estimated to between 0.10 and 0.16 m/s (43, 55). Taken as the mean of each individual’s change in self-selected walking speed (change from pretest to posttest), participants in the IC study group increased their self-selected walking speed by 0.17 ± 0.04 m/s, whereas there was a negligible change in the IC Sham group (0.04 ± 0.06 m/s; P = 0.01 vs. IC, unpaired t-test). This improvement in the IC group exceeds the MCID in this patient population. Finally, of the 10 individuals in the IC group, 9 had improved self-selected walking speed after 2 wk of IC.
Fig. 2.
Changes in self-selected (A) and fast (B) walking speed, as assessed by the 10-m walk test, in chronic stroke survivors after 2 wk of either ischemic conditioning (IC) or IC Sham training on the paretic leg. In the IC-training group, self-selected walking speed increased from 0.86 ± 0.21 to 1.04 ± 0.22 m/s, whereas no change was observed in the IC Sham group (0.92 ± 0.47 m/s pre-IC Sham vs. 0.96 ± 0.46 m/s post-IC Sham). Fast walking speed also tended to increase in the IC group (1.18 ± 0.26 m/s pretest vs. 1.30 ± 0.30 m/s posttest) with no change in the IC Sham group (1.14 ± 0.57 m/s pretest vs. 1.14 ± 0.53 m/s posttest), but this difference did not reach statistical significance (group × time interaction P value = 0.105). Data are shown as means ± SD. NS, not significant. *P < 0.05 pre-IC vs. post-IC.
As shown in Fig. 2B, there was a trend for improvement in fast walking speed in our study participants following IC (1.18 ± 26 m/s pre-IC vs. 1.30 ± 0.30 m/s post-IC; mean change = 0.11 ± 0.16 m/s). However, the interaction effect of time × group did not reach statistical significance (P = 0.105). The mean of each individual’s pre-post change also did not reach statistical significance between the IC and IC Sham groups (IC mean change = 0.11 ± 0.17 m/s and IC Sham mean change = 0.01 ± 0.07 m/s; P = 0.19, Mann-Whitney rank sum test).
Paretic leg knee extensor strength and muscle fatigability.
We measured paretic leg knee extensor MVCs in all 20 study participants before and after 2 wk of either IC or IC Sham training. We also measured paretic muscle fatigability in 8 of 10 participants in the IC group and 10 of 10 in the IC Sham group. Given the moderate stress of performing the muscle fatigue protocol on the cardiovascular system (increased blood pressure and heart rate), the study team chose not to perform the protocol on one person in the IC group because of a resting systolic blood pressure of 160 mmHg. Another study participant elected not to perform the fatiguing task.
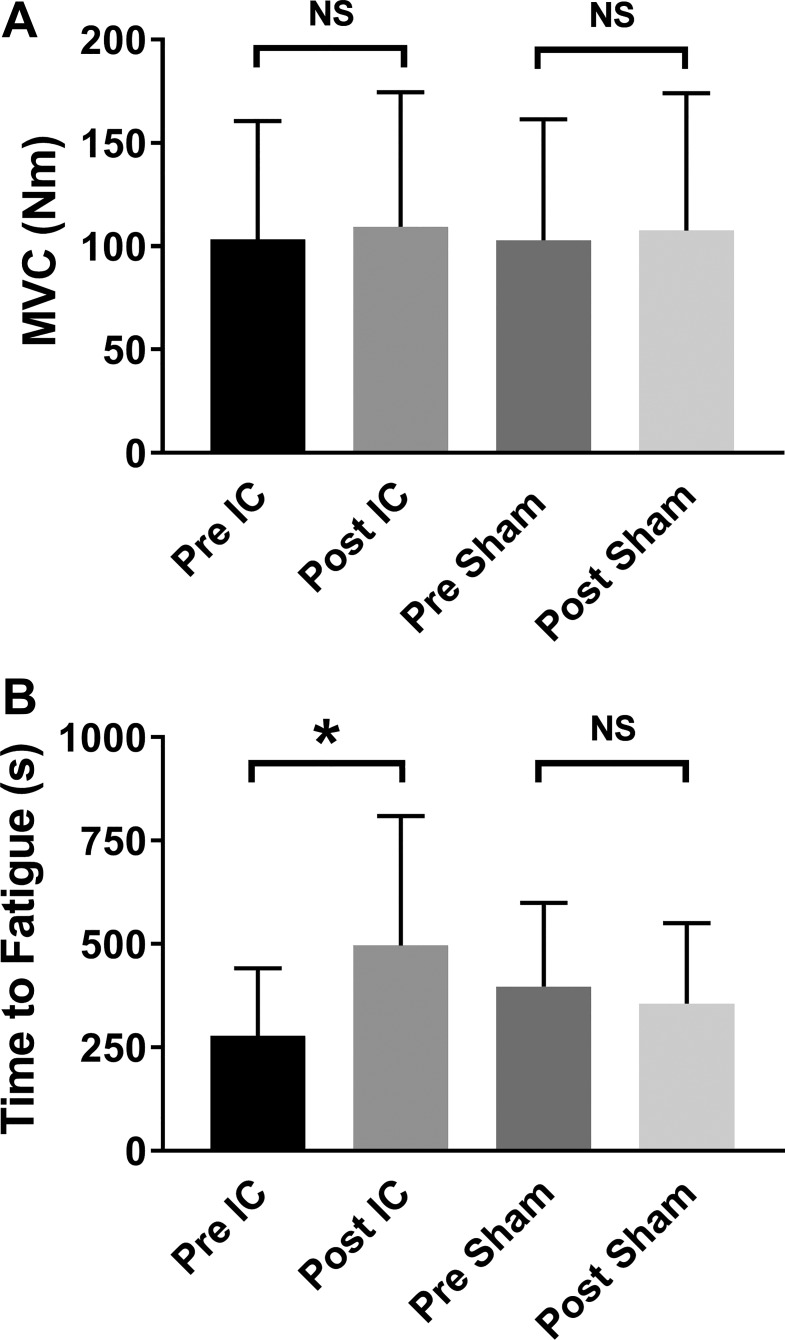
For MVC, there was no interaction effect of time × group (P = 0.82; Fig. 3A), nor was there a difference in pre-post mean changes between the groups (IC mean change = 6 ± 11 N·m and IC Sham mean change = 8 ± 12 N·m; P = 0.76, unpaired t-test). As shown in Fig. 3B, there was an interaction effect of time × group (P = 0.008) for fatigue task duration (n = 8 IC, n = 10 IC Sham). Pairwise multiple comparisons of group means indicated that IC significantly improved task duration from pretest values (278 ± 163 to 496 ± 313 s; P = 0.004), whereas there was no difference observed in participants in the IC Sham group (397 ± 203 s pretest vs. 355 ± 195 s posttest; P = 0.46). There was also a difference in pre-post mean changes between the groups (IC mean change = 218 ± 246 s and IC Sham mean change = −42 ± 114 s; P = 0.01, unpaired t-test). Of the eight individuals in the IC group who completed the fatigue task, seven had improved task duration after 2 wk of IC.
Fig. 3.
Changes in paretic knee extensor muscle strength and fatigability, as assessed by measuring maximum voluntary contractions (MVCs) of the knee extensors and the time study participants could sustain an isometric contraction equal to 30% of their MVC, in chronic stroke survivors after 2 wk of either ischemic conditioning (IC) or IC Sham training on the paretic leg. A: neither IC nor IC Sham had any effect on knee extensor MVC (P > 0.05). B: time to muscle fatigue increased from 278 ± 163 to 496 ± 313 s in the IC group (P < 0.05), whereas no change was observed in the IC Sham group (397 ± 202 s pre-IC Sham vs. 355 ± 195 s post-IC Sham; P = 0.46). Data are shown as means ± SD. NS, not significant. *P < 0.05 pre-IC vs. post-IC.
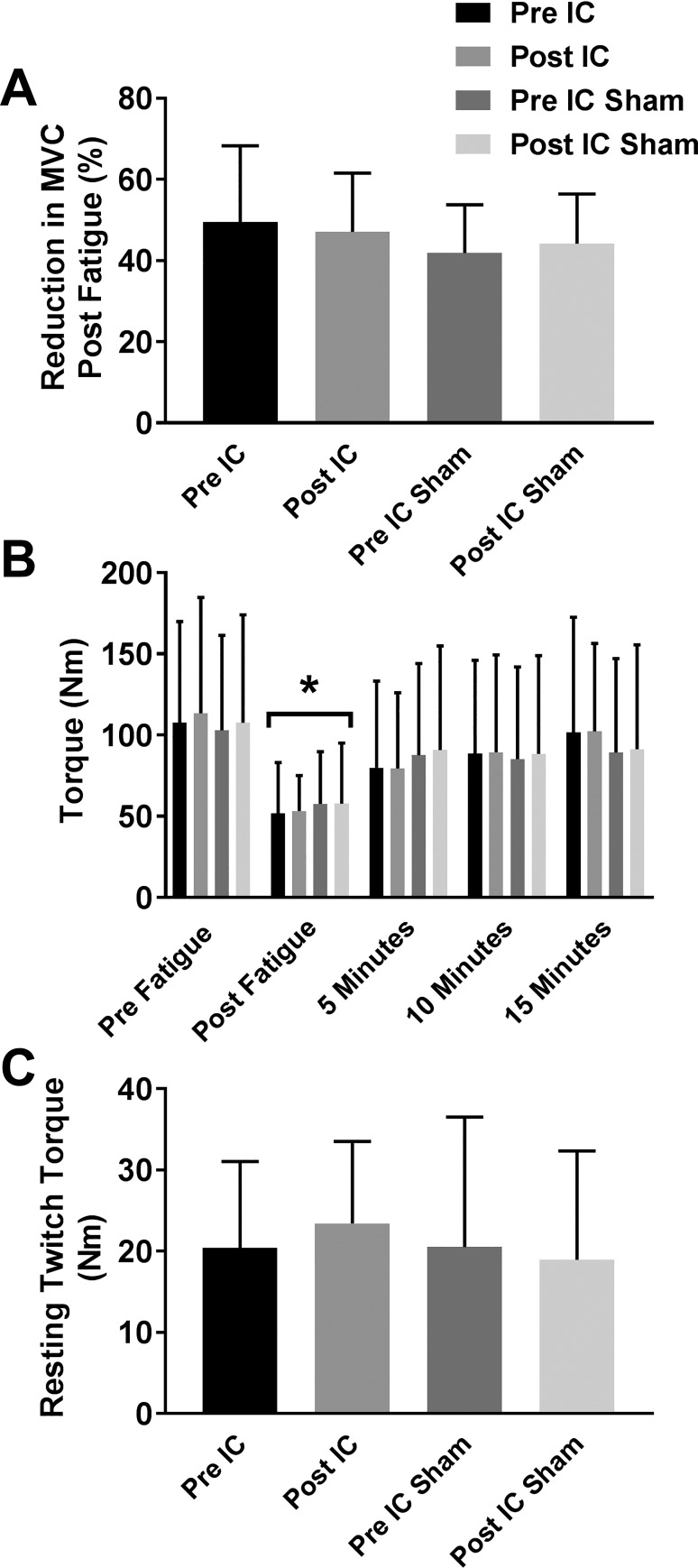
As shown in Fig. 4A, participants in both groups were equally fatigued after completing the fatigue test sessions as there was no interaction effect of time × group (P = 0.33) on the drop in MVC following fatigue. Fatigue recovery, as measured by the recovery of MVC torque at 5, 10, and 15 min postfatigue, was also not different between the groups (Fig. 4B; P = 0.67). As shown in Fig. 4C, there was also no interaction effect of time × group (P = 0.28) on muscle contractile properties following either IC or IC Sham as the torque amplitude elicited by a maximal electrical stimulation of the muscle was similar in both groups pretest versus posttest.
Fig. 4.
Reductions in maximum voluntary contraction (MVC) following fatigue and resting twitch responses of paretic muscle. All participants had a marked reduction in MVC immediately following termination of the fatiguing muscle contraction, and there were no differences in the percent reduction in MVC between groups (A; P = 0.42, 2-way repeated-measures ANOVA). As shown in B, MVC torque increased equally among all groups 5, 10, and 15 min postfatigue. There were also no differences in muscle contractile properties among the four treatment groups as evidenced by the amount of torque generated by maximal electrical stimulation of the paretic knee extensor muscles at rest (C; P = 0.72, 2-way repeated-measures ANOVA). All data are shown as means ± SD; n = 8 pre- and post-ischemic conditioning (IC); n = 10 pre- and post-IC Sham. *P < 0.05 vs. prefatigue.
The effect sizes (Cohen’s d) of IC or IC Sham on self-selected walking speed, fast walking speed, MVC, and muscle fatigability are shown in Table 2. IC had a large effect on both self-selected walking speed and the time to fatigue, a small-to-medium effect on fast walking speed, and little to no effect on paretic leg MVC. IC Sham had no effect on any of the study outcome measures.
Table 2.
Effect of IC or IC Sham on study outcome measures
| Outcome Measure | IC | IC Sham |
|---|---|---|
| Self-selected walking speed | 0.84 | 0.09 |
| Fast walking speed | 0.41 | 0.02 |
| Time to fatigue | 0.91 | −0.21 |
| Maximum voluntary contraction | 0.10 | 0.08 |
Values are Cohen’s d values. Effect size threshold: d = 0.20, small effect; d = 0.50, medium effect; d = 0.80, large effect; d = 1.30, very large effect. IC, ischemic conditioning.
DISCUSSION
There are three novel findings from this pilot study. First, we show that repeated IC training results in a clinically significant improvement in self-selected walking speed in chronic stroke survivors. Second, 2 wk of IC training reduced neuromuscular fatigability of the paretic leg knee extensors. Finally, repetitive IC training on the paretic leg was well tolerated and feasible in chronic stroke survivors as no study participants withdrew from the study because of pain or discomfort from the procedure and all participants who completed the study performed the prescribed number of cuff inflation sessions.
This study is the first to apply IC training to chronic stroke survivors and examine the effects of IC on a functional outcome such as walking. When compared with cardioprotection, the investigation of applying IC to promote neural plasticity and stroke recovery is still in its relative infancy. In addition to our group, to date, there have been only a few small clinical trials that have examined the effects of IC on stroke recovery and neural protection or on motor function in the elderly. The first studies demonstrated that IC on the leg was safe and well tolerated in patients with acute subarachnoid hemorrhage (<96 h from onset; 25, 32). Since 2016, pilot studies have shown that IC has the potential to reduce National Institutes of Health stroke score when performed within 24 h of cerebrovascular accident (20). When performed twice daily on the arms for 1 yr, IC reduces white matter hyperintensity, slows cognitive decline, and increases middle cerebral artery blood flow in patients with cerebral small vessel disease (37, 57). Ongoing trials are currently examining the efficacy of IC to reduce infarct size during acute cerebral infarction (NCT02169739) or within 6 mo of stroke (NCT02189928). Additionally, Sutter et al. showed in the elderly that IC performed on the upper extremity has the potential in enhance motor learning, but the degree to which subjects benefit may be affected by age and comorbidities (53). To our knowledge, there have been no studies that have examined whether IC improves walking function or promotes motor recovery in chronic stroke survivors. As the results from this pilot study show, IC has the potential to be used as a novel therapy (or as an adjunct to standard therapy) to promote motor recovery poststroke.
The previous work by Sutter et al. (53) showed that the effect size of IC on motor learning may decline with age and an increased number of comorbid conditions: important considerations for applying IC to stroke rehabilitation strategies. Nonetheless, we report positive effects of IC on motor function, specifically self-selected walking speed and skeletal muscle fatigue resistance, in stroke survivors in this pilot study. However, our study participants were relatively young (average age 58 ± 18 yr; range 20–80 yr) and did not have comorbidities. Furthermore, there was no association with age and the improvements in either walking speed or fatigue resistance (data not shown) in our study participants. Finally, there was also no correlation between the effect size of IC on walking speed or fatigue resistance and each individual’s number of years poststroke (data also not shown). Larger cohort studies will ultimately be necessary to determine which individuals will derive the most benefit from IC poststroke.
Broadly, our study is also one of the first to apply IC as an intervention to improve physical function in a population with reduced functional capacity or motor deficits. Numerous studies have examined the ability of IC to improve strength and endurance in young, healthy individuals. The results of these studies have very recently been summarized in two separate meta-analyses by Salvador et al. (48) and by Incognito et al. (30). Both groups similarly concluded that although IC does appear to have beneficial effects on exercise performance in most cases, a ceiling effect likely exists, and the training status of the test subjects must be carefully considered. Both groups of authors also concluded that an important and logical future direction for this field is the application of IC to clinical populations. Although our study specifically examined the effects of IC on stroke survivors, other clinical populations with reduced physical function such as individuals with spinal cord injury, frail, elderly patients, and individuals undergoing cardiac rehabilitation may benefit from IC.
The mechanisms by which IC improves gait speed in stroke survivors remain to be elucidated, but there are numerous possible mechanisms that warrant consideration. Unexpectedly, knee extensor strength did not significantly increase as we saw in our previous work, which examined the effects of a single session of IC on leg strength (28). However, we only measured strength in one muscle group (the paretic knee extensors), and it is possible that changes in strength of muscles known to improve propulsion forces, such as the ankle plantar flexors, may have contributed to the improved gait speed. We did show that IC dramatically improved the ability to maintain activation of the paretic knee extensors at a submaximal level (Fig. 3), which may be potentially more relevant than strength increases for self-selected walking speed, where maximal force generation is not required. All 20 study participants were able to walk faster during the fast walking test than during the self-selected walking test (individual data not shown), reflecting their ability to volitionally increase walking speed if needed. We did not, however, observe a significant improvement in fast walking speed following IC. As fast walking is more reliant on leg strength than self-selected walking speed (8, 38, 46), this lack of improvement in fast walking speed is consistent with our findings that paretic leg knee extensor MVC did not increase following 2 wk of IC.
Also, it is well known in the cardioprotection literature that IC has both a short and a long phase of protection whereby different mechanisms mediate the response [reviewed by Koch et al. (31a)]. In our previous study (28) we only examined the changes in knee extensor strength immediately after the IC session (i.e., in the short phase). In this study, knee extensor strength, muscle fatigue, and walking speed were examined 24–48 h after the final of ~7 IC sessions (i.e., in the long phase). It could also be the case with IC-induced changes in motor function that different “windows” of effect size and mechanism exist, and future studies should be designed to examine the differential effects of short- and long-phase IC on strength, muscle fatigue, and walking speed, as well as the effects of single-session versus multisession IC.
Study Limitations and Future Directions
We recognize several limitations and suggest numerous future study directions. Here we show that in chronic stroke survivors 2 wk of IC alone resulted in an increase in self-selected walking speed of 0.17 m/s, which exceeds the MCID for this population (43, 55) and is larger than what has been reported in many gait- and strength-training rehabilitation programs (51). This is a remarkable improvement given that study participants were many years poststroke, study participants were not undergoing physical therapy, and IC was done without concurrent strength or gait training. It is therefore conceivable that when used as an adjunct with mainstream therapies, changes in gait speed could exceed effects seen with traditional training therapy alone. Future studies will be necessary to determine whether IC, when performed concurrently with therapy or exercise, has an additive effect on motor outcomes.
One limitation we recognize is that this pilot study was only conducted in a single-blinded manner, with only the study participants being blinded to the purpose of the different cuff inflation pressures. However, the assessor of the 10-m walk test read from a script, and equal encouragement was given to all study participants. The assessors of paretic leg MVCs and fatigue task duration were also not blinded to the group assignments. Assessor bias for these measures is highly unlikely as MVC was determined strictly by the force output from the Biodex dynamometer and fatigue was determined using the abovementioned criteria as determined by the algorithm in the custom-written LabVIEW program. All study participants were given equal verbal encouragement.
Another limitation is that we did not examine the duration of the beneficial effects of IC following the final testing session. As we show, IC improves gait speed, so it will be important to determine whether this improvement in walking function is sustained in the long term as gait speed dramatically affects both functional indoor and outdoor ambulation and strongly correlates with quality of life after stroke (31, 33, 45, 49). Sustained improvements in walking speed with a concordant reduction in neuromuscular fatigability would also likely increase physical fitness, which is dramatically reduced in stroke survivors (3, 4), and reduce the incidence of recurrent stroke. We also recognize that we did not have a maximum cutoff for self-selected walking speed, and the self-selected walking speed of 3/22 study participants was equal to that of age- and sex-matched norms (5). However, all study participants had a lower extremity Fugl-Meyer score <34, all study participants had weakness in their paretic leg compared with their nonparetic leg, and there was no difference in baseline walking speed between the IC and IC Sham groups. Initial walking speed also had no correlation with the degree of improvement in the IC group (data not shown).
The relatively small number of participants in this pilot study is also a limitation; however, gait speed was improved in 9/10 participants in the IC group, and neuromuscular fatigue resistance was improved in 7/8 tested participants. We also did not examine the effects of IC on the nonparetic leg or the upper extremities (remote conditioning) and recognize that some of the improvement in self-selected walking speed could have been due to an unmeasured improvement in nonparetic leg function. Finally, our study cohort consisted only of individuals >1 yr poststroke who had reached the “plateau” phase of their recovery and were no longer undergoing neurorehabilitation. Given the ease of translating IC to the clinic or hospital setting, future studies that examine the effect of IC on stroke recovery during the subacute stroke phase where neuroplasticity is greatest should be prioritized.
GRANTS
The project was supported by National Center for Advancing Translational Sciences Grants UL1-TR-001436 (to A. S. Hyngstrom and M. J. Durand) and KL2-TR-001438 (to M. J. Durand, A. S. Hyngstrom, and D. D. Gutterman) and by National Institute of Neurological Disorders and Stroke Grants 1R21-NS-088818 (to A. S. Hyngstrom, M. J. Durand, and D. D. Gutterman) and R15-NS-084130 (to A. S. Hyngstrom).
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.D., D.D.G., and A.S.H. conceived and designed research; M.J.D., T.F.B., J.N.N., S.Z.A., and M.T.W. performed experiments; M.J.D., T.F.B., J.N.N., and A.S.H. analyzed data; M.J.D., B.D.S., D.D.G., and A.S.H. interpreted results of experiments; M.J.D. prepared figures; M.J.D. and A.S.H. drafted manuscript; M.J.D., T.F.B., J.N.N., S.Z.A., M.T.W., B.D.S., D.D.G., and A.S.H. edited and revised manuscript; M.J.D., T.F.B., J.N.N., S.Z.A., M.T.W., B.D.S., D.D.G., and A.S.H. approved final version of manuscript.
REFERENCES
- 1.Andreas M, Schmid AI, Keilani M, Doberer D, Bartko J, Crevenna R, Moser E, Wolzt M. Effect of ischemic preconditioning in skeletal muscle measured by functional magnetic resonance imaging and spectroscopy: a randomized crossover trial. J Cardiovasc Magn Reson 13: 32, 2011. doi: 10.1186/1532-429X-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Bailey TG, Jones H, Gregson W, Atkinson G, Cable NT, Thijssen DH. Effect of ischemic preconditioning on lactate accumulation and running performance. Med Sci Sports Exerc 44: 2084–2089, 2012. doi: 10.1249/MSS.0b013e318262cb17. [DOI] [PubMed] [Google Scholar]
- 2.Barbosa TC, Machado AC, Braz ID, Fernandes IA, Vianna LC, Nobrega AC, Silva BM. Remote ischemic preconditioning delays fatigue development during handgrip exercise. Scand J Med Sci Sports 25: 356–364, 2015. doi: 10.1111/sms.12229. [DOI] [PubMed] [Google Scholar]
- 3.Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, MacKay-Lyons M, Macko RF, Mead GE, Roth EJ, Shaughnessy M, Tang A; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Clinical Cardiology . Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45: 2532–2553, 2014. doi: 10.1161/STR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 4.Billinger SA, Coughenour E, Mackay-Lyons MJ, Ivey FM. Reduced cardiorespiratory fitness after stroke: biological consequences and exercise-induced adaptations. Stroke Res Treat 2012: 959120, 2012. doi: 10.1155/2012/959120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy 97: 182–189, 2011. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Cherry-Allen KM, Gidday JM, Lee JM, Hershey T, Lang CE. Remote limb ischemic conditioning at two cuff inflation pressures yields learning enhancements in healthy adults. J Mot Behav 49: 337–348, 2017. doi: 10.1080/00222895.2016.1204268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherry-Allen KM, Gidday JM, Lee JM, Hershey T, Lang CE. Remote limb ischemic conditioning enhances motor learning in healthy humans. J Neurophysiol 113: 3708–3719, 2015. doi: 10.1152/jn.01028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark DJ, Manini TM, Fielding RA, Patten C. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp Gerontol 48: 358–363, 2013. doi: 10.1016/j.exger.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clevidence MW, Mowery RE, Kushnick MR. The effects of ischemic preconditioning on aerobic and anaerobic variables associated with submaximal cycling performance. Eur J Appl Physiol 112: 3649–3654, 2012. doi: 10.1007/s00421-012-2345-5. [DOI] [PubMed] [Google Scholar]
- 10.Cruz RS, de Aguiar RA, Turnes T, Pereira KL, Caputo F. Effects of ischemic preconditioning on maximal constant-load cycling performance. J Appl Physiol (1985) 119: 961–967, 2015. doi: 10.1152/japplphysiol.00498.2015. [DOI] [PubMed] [Google Scholar]
- 11.Cruz RS, de Aguiar RA, Turnes T, Salvador AF, Caputo F. Effects of ischemic preconditioning on short-duration cycling performance. Appl Physiol Nutr Metab 41: 825–831, 2016. doi: 10.1139/apnm-2015-0646. [DOI] [PubMed] [Google Scholar]
- 12.da Cunha IT Jr, Lim PA, Qureshy H, Henson H, Monga T, Protas EJ. Gait outcomes after acute stroke rehabilitation with supported treadmill ambulation training: a randomized controlled pilot study. Arch Phys Med Rehabil 83: 1258–1265, 2002. doi: 10.1053/apmr.2002.34267. [DOI] [PubMed] [Google Scholar]
- 13.de Groot PC, Thijssen DH, Sanchez M, Ellenkamp R, Hopman MT. Ischemic preconditioning improves maximal performance in humans. Eur J Appl Physiol 108: 141–146, 2010. doi: 10.1007/s00421-009-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickstein R. Rehabilitation of gait speed after stroke: a critical review of intervention approaches. Neurorehabil Neural Repair 22: 649–660, 2008. doi: 10.1177/1545968308315997. [DOI] [PubMed] [Google Scholar]
- 16.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol 8: 398–412, 2009. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobkin BH, Plummer-D’Amato P, Elashoff R, Lee J; SIRROWS Group . International randomized clinical trial, Stroke Inpatient Rehabilitation with Reinforcement of Walking Speed (SIRROWS), improves outcomes. Neurorehabil Neural Repair 24: 235–242, 2010. doi: 10.1177/1545968309357558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, Dobkin BH, Rose DK, Tilson JK, Cen S, Hayden SK; LEAPS Investigative Team . Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med 364: 2026–2036, 2011. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durand MJ, Murphy SA, Schaefer KK, Hunter SK, Schmit BD, Gutterman DD, Hyngstrom AS. Impaired hyperemic response to exercise post stroke. PLoS One 10: e0144023, 2015. doi: 10.1371/journal.pone.0144023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.England TJ, Hedstrom A, O’Sullivan S, Donnelly R, Barrett DA, Sarmad S, Sprigg N, Bath PM. RECAST (Remote Ischemic Conditioning After Stroke Trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke 48: 1412–1415, 2017. doi: 10.1161/STROKEAHA.116.016429. [DOI] [PubMed] [Google Scholar]
- 21.Enoka RM, Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol 586: 11–23, 2008. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther 32: 46–49, 2009. doi: 10.1519/00139143-200932020-00002. [DOI] [PubMed] [Google Scholar]
- 23.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 24.Gibson N, White J, Neish M, Murray A. Effect of ischemic preconditioning on land-based sprinting in team-sport athletes. Int J Sports Physiol Perform 8: 671–676, 2013. doi: 10.1123/ijspp.8.6.671. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez NR, Connolly M, Dusick JR, Bhakta H, Vespa P. Phase I clinical trial for the feasibility and safety of remote ischemic conditioning for aneurysmal subarachnoid hemorrhage. Neurosurgery 75: 590–598, 2014. doi: 10.1227/NEU.0000000000000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil 83: 1629–1637, 2002. doi: 10.1053/apmr.2002.35473. [DOI] [PubMed] [Google Scholar]
- 27.Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol 65: 177–195, 2015. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hyngstrom AS, Murphy SA, Nguyen J, Schmit BD, Negro F, Gutterman DD, Durand MJ. Ischemic conditioning increases strength and volitional activation of paretic muscle in chronic stroke: a pilot study. J Appl Physiol (1985) 124: 1140–1147, 2018. doi: 10.1152/japplphysiol.01072.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Incognito AV, Burr JF, Millar PJ. The effects of ischemic preconditioning on human exercise performance. Sports Med 46: 531–544, 2016. doi: 10.1007/s40279-015-0433-5. [DOI] [PubMed] [Google Scholar]
- 31.Khanittanuphong P, Tipchatyotin S. Correlation of the gait speed with the quality of life and the quality of life classified according to speed-based community ambulation in Thai stroke survivors. NeuroRehabilitation 41: 135–141, 2017. doi: 10.3233/NRE-171465. [DOI] [PubMed] [Google Scholar]
- 31a.Koch S, Della-Morte D, Dave KR, Sacco RL, Perez-Pinzon MA. Biomarkers for ischemic preconditioning: finding the responders. J Cereb Blood Flow Metab 34: 933–941, 2014. doi: 10.1038/jcbfm.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke 42: 1387–1391, 2011. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil 85: 234–239, 2004. doi: 10.1016/j.apmr.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, Yellon DM, Deanfield JE, MacAllister RJ. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a KATP-channel dependent mechanism. Circulation 116: 1386–1395, 2007. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- 36.Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst Rev 1: CD002840, 2014. doi: 10.1002/14651858.CD002840.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mi T, Yu F, Ji X, Sun Y, Qu D. The interventional effect of remote ischemic preconditioning on cerebral small vessel disease: a pilot randomized clinical trial. Eur Neurol 76: 28–34, 2016. doi: 10.1159/000447536. [DOI] [PubMed] [Google Scholar]
- 38.Middleton A, Fulk GD, Herter TM, Beets MW, Donley J, Fritz SL. Self-selected and maximal walking speeds provide greater insight into fall status than walking speed reserve among community-dwelling older adults. Am J Phys Med Rehabil 95: 475–482, 2016. doi: 10.1097/PHM.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris SL, Dodd KJ, Morris ME. Outcomes of progressive resistance strength training following stroke: a systematic review. Clin Rehabil 18: 27–39, 2004. doi: 10.1191/0269215504cr699oa. [DOI] [PubMed] [Google Scholar]
- 40.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 41.Paradis-Deschênes P, Joanisse DR, Billaut F. Ischemic preconditioning increases muscle perfusion, oxygen uptake, and force in strength-trained athletes. Appl Physiol Nutr Metab 41: 938–944, 2016. doi: 10.1139/apnm-2015-0561. [DOI] [PubMed] [Google Scholar]
- 43.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 54: 743–749, 2006. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 44.Pérez-Pinzón MA. Neuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemia. J Bioenerg Biomembr 36: 323–327, 2004. doi: 10.1023/B:JOBB.0000041762.47544.ff. [DOI] [PubMed] [Google Scholar]
- 45.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke 26: 982–989, 1995. doi: 10.1161/01.STR.26.6.982. [DOI] [PubMed] [Google Scholar]
- 46.Peterson CL, Kautz SA, Neptune RR. Braking and propulsive impulses increase with speed during accelerated and decelerated walking. Gait Posture 33: 562–567, 2011. doi: 10.1016/j.gaitpost.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pohl M, Werner C, Holzgraefe M, Kroczek G, Mehrholz J, Wingendorf I, Hoölig G, Koch R, Hesse S. Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living after stroke: a single-blind, randomized multicentre trial (Deutsche GangtrainerStudie, DEGAS). Clin Rehabil 21: 17–27, 2007. doi: 10.1177/0269215506071281. [DOI] [PubMed] [Google Scholar]
- 48.Salvador AF, De Aguiar RA, Lisbôa FD, Pereira KL, Cruz RS, Caputo F. Ischemic preconditioning and exercise performance: a systematic review and meta-analysis. Int J Sports Physiol Perform 11: 4–14, 2016. doi: 10.1123/ijspp.2015-0204. [DOI] [PubMed] [Google Scholar]
- 49.Schmid A, Duncan PW, Studenski S, Lai SM, Richards L, Perera S, Wu SS. Improvements in speed-based gait classifications are meaningful. Stroke 38: 2096–2100, 2007. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 51.States RA, Salem Y, Pappas E. Overground gait training for individuals with chronic stroke: a Cochrane systematic review. J Neurol Phys Ther 33: 179–186, 2009. doi: 10.1097/NPT.0b013e3181c29a8c. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan KJ, Brown DA, Klassen T, Mulroy S, Ge T, Azen SP, Winstein CJ; Physical Therapy Clinical Research Network (PTClinResNet) . Effects of task-specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Phys Ther 87: 1580–1602, 2007. doi: 10.2522/ptj.20060310. [DOI] [PubMed] [Google Scholar]
- 53.Sutter EN, Mattlage AE, Bland MD, Cherry-Allen KM, Harrison E, Surkar SM, Gidday JM, Chen L, Hershey T, Lee JM, Lang CE. Remote limb ischemic conditioning and motor learning: evaluation of factors influencing response in older adults. Transl Stroke Res, 2018. doi: 10.1007/s12975-018-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka D, Suga T, Tanaka T, Kido K, Honjo T, Fujita S, Hamaoka T, Isaka T. Ischemic preconditioning enhances muscle endurance during sustained isometric exercise. Int J Sports Med 37: 614–618, 2016. doi: 10.1055/s-0035-1565141. [DOI] [PubMed] [Google Scholar]
- 55.Tilson JK, Sullivan KJ, Cen SY, Rose DK, Koradia CH, Azen SP, Duncan PW; Locomotor Experience Applied Post Stroke (LEAPS) Investigative Team . Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther 90: 196–208, 2010. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Meng R, Song H, Liu G, Hua Y, Cui D, Zheng L, Feng W, Liebeskind DS, Fisher M, Ji X. Remote ischemic conditioning may improve outcomes of patients with cerebral small-vessel disease. Stroke 48: 3064–3072, 2017. doi: 10.1161/STROKEAHA.117.017691. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Reis C, Applegate R II, Stier G, Martin R, Zhang JH. Ischemic conditioning-induced endogenous brain protection: Applications pre-, per- or post-stroke. Exp Neurol 272: 26–40, 2015. doi: 10.1016/j.expneurol.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, Kramer RS. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int 80: 861–867, 2011. doi: 10.1038/ki.2011.156. [DOI] [PubMed] [Google Scholar]