Abstract
In addition to its role as an environmental stressor, scientists have recently demonstrated the potential for heat to be a therapy for improving or mitigating declines in arterial health. Many studies at both ends of the scientific controls spectrum (tightly controlled, experimental vs. practical) have demonstrated the beneficial effects of heating on microvascular function (e.g., reactive hyperemia, cutaneous vascular conductance); endothelial function (e.g., flow-mediated dilation); and arterial stiffness (e.g., pulse-wave velocity, compliance, β-stiffness index). It is important to note that findings of beneficial effects are not unanimous, likely owing to the varied methodology in both heating protocols and assessments of outcome measures. Mechanisms of action for the effects of both acute and chronic heating are also understudied. Heat science is a very promising area of human physiology research, as it has the potential to contribute to approaches addressing the global cardiovascular disease burden, particularly in aging and at risk populations, and those for whom exercise is not feasible or recommended.
Keywords: arterial stiffness, endothelial function, heat stress, heat therapy, vascular physiology
INTRODUCTION
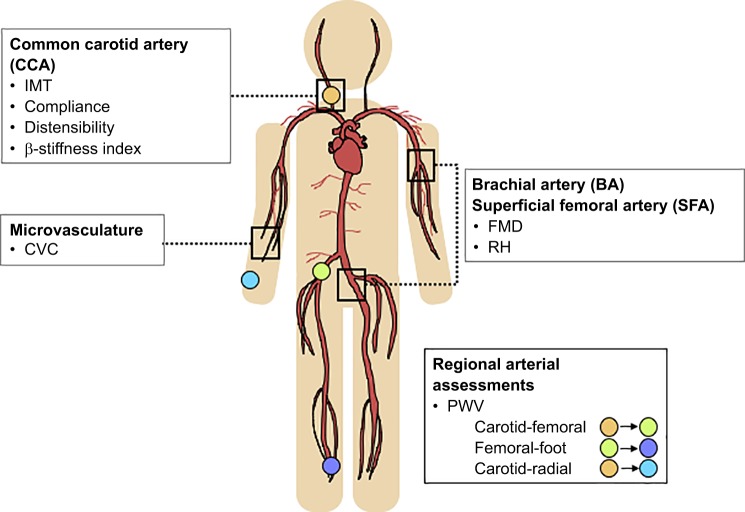
The search for innovative approaches to the management and prevention of cardiovascular disease (CVD) has created a potential niche for the use of heat as a therapeutic tool for improving vascular health outcomes. On a global scale, CVDs, ischemic heart disease and stroke in particular, are still the leading causes of death (World Health Organization, January 2017), and it is now known that endothelial dysfunction precedes the development of atherosclerotic plaques along the arterial wall that are characteristically associated with these diseases (22). The arterial wall is made up of three layers that are distinct in their composition and function. The tunica adventitia contains elastin, collagen, nerves, and blood vessels and provides the artery structural integrity; the tunica media contains smooth muscle cells and allows the artery to control blood flow; and the tunica intima is lined with endothelial cells that secrete substances that promote an antiatherogenic environment (80). Various vascular properties have emerged as indicators of CVD risk, including microvascular function (e.g., reactive hyperemia, cutaneous vascular conductance); endothelial function (e.g., flow-mediated dilation); and arterial stiffness (e.g., pulse-wave velocity, compliance, β-stiffness index) (Fig. 1).
Fig. 1.
Assessment methods for common vascular structure and function outcomes. Structural and functional characteristics are typically assessed at the common carotid artery (CCA), brachial artery (BA), superficial femoral artery (SFA), and the microvasculature. Microvascular function can be assessed through cutaneous vascular conductance (CVC) or reactive hyperemia (RH) response. Endothelial function can be assessed through flow-mediated dilation (FMD). Arterial stiffness can be assessed locally through compliance, distensibility, and the β-stiffness index, or regionally through pulse-wave velocity (PWV) at a variety of arterial sites. Arterial intima-media thickness (IMT) describes the thickness of the arterial wall, most typically at the CCA, and is a precursor to atherosclerosis.
Heat stress is emerging as a potential alternative to exercise for improving cardiovascular health, because it too increases vascular shear stress, a critical stimulus for changing vascular function (94). Indeed, many studies have shown that heat, applied acutely or chronically, has the ability to alter a number of indices of vascular structure and function, which may be promising for the aging population, as well as at risk groups that may not be physically able to partake of the recommended amount of exercise. Activities such as Waon therapy, Japanese onsen bathing, Finnish sauna bathing, and Bikram yoga have been promoted in some venues for their heat-related benefits for many years (27, 31, 63, 81), but recent studies have also demonstrated that simpler alternatives, such as a hot foot spa or hot tub bath, may elicit similar positive effects on vascular health with regular use (8, 9, 89). Accordingly, the objectives of this paper are as follows: 1) to describe currently used practical models of heat therapy and their effects on vascular structure and function; 2) to outline the physiological changes in vascular structure and function previously observed in studies of chronic and acute heat exposure; and 3) to propose potential avenues of future research on heat science as it relates to vascular health.
COMMON HEAT THERAPIES AND THEIR EFFECTS ON VASCULAR STRUCTURE AND FUNCTION
Commonly used heat therapies have been previously demonstrated to be successful at creating a general sense of well-being, whether it be through symptom alleviation, cultural inclusion, or physical fitness (27, 28, 63, 81, 87). These effects have contributed to the worldwide adoption of these heat therapies. Recent studies have highlighted the potential role of improved vascular health in contributing to the efficacy of these heat therapies (27, 31, 32, 37, 47, 67).
Waon Therapy
Waon therapy, which translated means “soothing warm therapy,” was developed in Japan by Dr. Chuwa Tei in 1989 as a treatment for chronic heart failure (CHF) (63). It involves being seated for 15 min in a far infrared dry sauna set to 60°C, with the goal of increasing core temperature (Tc) by 1–1.5°C, followed by 30 min outside the sauna in supine rest while wrapped in blankets to retain heat. Water is provided ad libitum to prevent dehydration from sweating (63). This therapy has almost exclusively been used in CHF patients and individuals with risk factors for CVD, and its effect has not been explored in young, healthy individuals (63). Dr. Tei’s research group has successfully shown that Waon therapy is able to improve cardiac hemodynamics and function, clinical symptoms, and prognosis of individuals with CHF (46, 48, 62, 88).
Improvements in cardiovascular health with Waon therapy may be attributable, in part, to improvements in vascular function with exposure to frequent therapeutic sessions (range: 5–7 times/wk for 2–5 wk). Using a modified Waon therapy protocol in Syrian gold hamsters and TO-2 cardiomyopathic hamsters, researchers showed that treatment upregulated endothelial nitric oxide synthase (eNOS) mRNA and protein content in endothelial cells (35, 36). In apolipoprotein E-deficient mice, Waon therapy increased limb perfusion, capillary density, and eNOS expression (1). Furthermore, angiogenesis (i.e., increased capillary density) was abolished with administration of NG-nitro-l-arginine methyl ester (l-NAME) and in eNOS knockout mice, suggesting that these beneficial adaptations are heavily nitric oxide (NO) mediated (1). Reduced oxidative stress may also play a role in improved vascular function with Waon therapy, as the marker urinary 8-epi-PGF2α decreased with 2 wk of daily treatment in humans with at least one coronary risk factor (57). Although most previous experiments examining the impact of Waon therapy were performed in animal models, the mechanisms of action in the animal experiments may provide an explanation for the observed improvements in endothelial function assessed by brachial artery (BA) flow-mediated dilation (FMD) in human intervention studies of Waon therapy in individuals with either CHF or at least one coronary risk factor (37, 47, 67). It should be noted that in these studies that have measured BA FMD in response to Waon therapy, methods used do not align perfectly with the established guidelines, which should be taken into consideration when their findings are interpreted (90).
Japanese Onsen Bathing
Onsen bathing is widely used in Japan regardless of age and disease status. Onsens are natural hot springs, which are now typically developed to include nearby bathing facilities and traditional inns (81). The temperature of water in an onsen, although not typically recorded, can vary between 25 and 45°C (81). The effects of onsen bathing on cardiovascular outcomes have scarcely been explored. Very recently, data have been published from the Shimanami Health Promoting Program, a longitudinal study evaluating the factors that contribute to cardiovascular disease, dementia, and death in an elderly Japanese population (49). In total, 873 participants filled out a questionnaire regarding their onsen bathing habits posted to them 1 yr after study cessation in December 2014. Participants bathed, on average, 5.8 ± 1.9 times/wk for 12.4 ± 9.9 min/session. Researchers observed that individuals who bathed more than or equal to five times per week had lower brachial-to-ankle pulse wave velocity (PWV), central pulse pressure, and plasma B-type natriuretic peptide (BNP). They also showed, in a subset of 166 participants with a mean follow-up duration of 4.9 yr, that maintaining these bathing habits (≥5 times/wk) eventually lowered resting levels of plasma BNP, which suggests improved blood pressure regulation (49). In another study conducted in the city of Beppu, Japan, a questionnaire regarding hot spa-bathing habits and disease history was administered to a random selection of 20,000 citizens aged 65 yr of age and older. Of the 9,252 valid responses received, analysis revealed that habitual onsen bathing may translate into the prevention of hypertension in women and cardiovascular disease in men, among a variety of other diseases surveyed (56). Some mechanisms of action have been demonstrated in a study in Sprague-Dawley rats (68) in which thickening of the intimal wall, a known precursor for atherosclerosis, was induced by surrounding right femoral arteries of rats with a polyethylene cuff. Following cuff treatment, rats underwent thermal therapy involving 15 min of hot water bathing (40.5–41.5°C) daily for 28 days. Rectal temperature was reported to have increased from 35 to 38°C from beginning to end of a hot water bathing session. Remarkably, thermal therapy decreased neointimal thickening and markers of atherosclerotic progression (i.e., ED-1, -2, -3-positive cells, MCP-1, and p22-phox); and increased the expression of heat shock protein (HSP) 72, which is thought to be a key component of the adaptive response to heat stress (68).
Finnish Sauna Bathing
In Finland, sauna bathing typically involves sitting in a room with 80–100°C dry, circulating air for anywhere between 5 and 20 min, interspersed with brief periods in a cold environment (27). Although common worldwide, sauna bathing is so pervasive in Finnish culture that when the Kuopio Ischemic Heart Disease risk factor study recruited middle-aged men between 42 and 61 yr old, sauna bathing habits were included in the baseline characteristics of participants. In recent publications using this data set, with a follow-up duration of at least 20 yr in >2,000 participants, researchers observed that increased sauna bathing frequency (4–7 times/wk) and duration (>19 min/session) were associated with a decreased risk of sudden cardiac death, coronary heart disease, cardiovascular disease, and all-cause mortality, for which the effect was amplified when combined with high cardiorespiratory fitness (50, 52). Frequent sauna bathing was also found to be associated with a lower risk of incident hypertension (104). Of note, sauna bathing habits were only assessed at baseline using a self-reported questionnaire; therefore, interpretation of the data relies on the assumption that these habits remained the same over the course of the succeeding 20+ yr. Currently, a mechanistic perspective on the risk-lowering effects of sauna bathing is lacking. One study has examined the acute changes in arterial stiffness with sauna bathing in 102 asymptomatic participants with at least one cardiovascular risk factor (54). Participants underwent a single session of sauna bathing at 73°C and 10–20% humidity for 30 min. Carotid-femoral (cf) PWV decreased immediately following sauna exposure, but returned to baseline levels after 30 min of recovery (54). The significance of this transient decrease in arterial stiffness remains to be determined, although it is likely more representative of a decrease in vascular tone.
Bikram Yoga
Bikram yoga was created and popularized by Bikram Choudhury in the early 1970s, and involves a set sequence of pranayama (deep breathing), asanas (poses), and kapalabhati (quick, strong exhalations) performed in a heated environment (35–42°C and 40% humidity) (28). Despite widespread adoption in Western cultures, there is a surprising lack of research examining the physiological impact of the practice of Bikram yoga. Previous studies on hatha (non-heated) yoga revealed no change in arterial stiffness [i.e., common carotid artery (CCA) compliance and β-stiffness index] or endothelial function (i.e., BA FMD) with regular practice (2–3 sessions/wk for 6–12 wk) (34, 85). The addition of the heated component in Bikram yoga yielded different results, but showed that the arterial response might differ based on factors such as age and training duration (31, 32). When groups of young and middle-aged to older adults practiced Bikram yoga for three times per week for 8 wk, CCA compliance and the β-stiffness index improved only in the younger group, while BA FMD improved only in the middle-aged to older group (31, 32). Interestingly, when a subsequent experiment was conducted by the same research group to directly compare heated and non-heated yoga in middle-aged to older adults only, BA FMD increased in the non-heated yoga group, but only trended toward an increase in the heated yoga group (P = 0.056) (33). Based on the available data, it appears that there is much greater variability around the BA FMD responses after heated vs. non-heated yoga, which may explain the lack of statistical significance observed in some of the previous studies. There is no clear consensus on the impact of Bikram (heated) yoga on arterial function, and it is possible that training duration (8 vs. 12 wk), which was different between the two studies in middle-aged to older adults, plays a role in the response.
While there is considerable evidence to suggest a general benefit of these commonly used heat therapies on vascular health and CVD risk, limited fundamental mechanistic work prompted researchers to expand on this area of research using controlled experiments to isolate the role of heat on the vasculature in the absence of other confounding elements such as muscular contraction.
PHYSIOLOGICAL RESPONSE TO HEATING
The hallmark physiological response to heat stress is the cutaneous vasodilation that allows for the necessary redirection of blood flow to the surface of the skin for heat dissipation (16) (Fig. 2). The role of vasodilators and vasoconstrictors changes as heat stress progresses in both duration and magnitude. Mild heat levels (Tc increase of ~0.5°C), characterized by isolated elevated skin temperature, generate minor adjustments in skin blood flow due to slight changes in both vasoconstrictor (decreased) and vasodilator (increased) input. Moderate heat levels (Tc increase of ~1.0–1.5°C), characterized by increases in Tc in conjunction with elevations in skin temperature, cause further increases in skin blood flow through exclusive vasodilator mechanisms in the absence of vasoconstrictor action. Severe heat levels (Tc >40°C) are characterized by significant increases in Tc that trigger active vasodilator pathways to further increase skin blood flow (16, 42). All modes of heat therapy aim to elicit a Tc increase between ~1.0 and 1.5°C; as such, the mechanisms and studies described throughout will reflect those of moderate heating. Complex neural and local mechanisms are involved in the cutaneous vasodilatory responses with heating, which are not necessarily the same as the mechanisms that regulate conduit and peripheral arteries. Neural mechanisms of action remain unclear but are believed to exert their effects through a cotransmitter system, which suggests the release of multiple neurotransmitters to stimulate vasodilation (5, 45). Substances that are potentially involved in this mechanism include acetylcholine (45, 100), NO (43, 82, 84), vasoactive intestinal peptide (5, 99), substance P (neurokinin-1 receptors) (101), histamine (H1 receptors) (102), and prostaglandins (59). Local mechanisms create a two-tiered pattern of vasodilation in response to heating. Initial vasodilation occurs when temperature-sensitive vanilloid type 1 receptors in afferent cutaneous sensory nerves detect heat and prompt the reflex, antidromic release of vasodilatory neurotransmitter(s), the identity and action of which are currently unknown (60, 69, 86). Prolonged vasodilation occurs when HSP90 binds to, and subsequently activates, eNOS to generate NO, which diffuses to the smooth muscle layer of the arteriolar wall to cause it to relax (44, 60, 83). Well-controlled experiments using topical capsaicin (anesthetic) and l-NAME locally at the site of heating provide supporting evidence for the mechanisms of initial and prolonged vasodilation, respectively (44, 60, 86). Further information on this topic is detailed in other reviews (15, 40, 100).
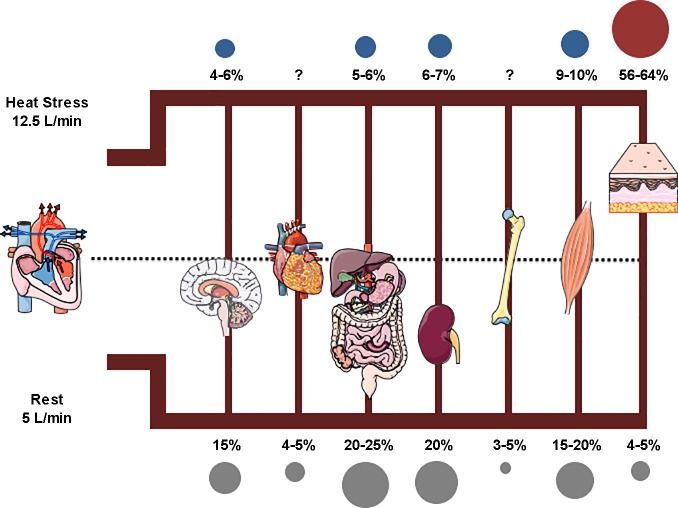
Fig. 2.
Blood flow distribution at rest and during heat stress. During whole-body heat stress, cardiac output (CO) increases from ~5 to 12.5 l/min, a 2.5-fold increase. Blood flow is redirected to the skin (↑ 7–8 l/min) (39) from the splanchnic (↓ 40%) (61) and renal (↓ 15–30%) (72) regions, such that the organs comprise ~60, 5–6, and 6–7% of cardiac output with heating, respectively. Gray circles indicate the percent contribution to CO at rest, blue circles indicate a decrease in percent contribution to CO during heat stress, and red circles indicate an increase in percent contribution to CO during heat stress. Illustrations of human body organs produced by Servier Medical Art.
With heating, the capacity for 7–8 l/min of increased skin blood flow with heating is facilitated predominantly by increased cardiac output (up to ~12.5 l/min) and decreased blood flow to the splanchnic (by ~40%) and renal (by ~15–30%) circulations (74) (Fig. 2). As a consequence, in the conduit (i.e., carotid) and peripheral (i.e., brachial, femoral) arteries, which are the focus of this review, heating results in increased blood flow and shear stress, particularly in the anterograde direction (forward, away from heart) (73, 91, 92, 94). The elevation in heart rate, concurrent with negligible changes in stroke volume, has been suggested to be mainly responsible for the increases in cardiac output of up to 2.5 times resting values. Changes in temperature and autonomic nervous system (ANS) activity with heat stress alter cardiac nodal cells such that action potentials are triggered more frequently (i.e., ↓ time to reach threshold for depolarization) and travel more quickly (i.e., ↑ conduction velocity) through cardiac myocytes (18, 23, 41, 98). Heating alters ANS balance, boosting sympathetic nervous system (SNS) activity and withdrawing parasympathetic nervous system (PNS) activity, to place the body in a global hyperadrenergic state that facilitates more frequent sinoatrial node firing. Although the goal of this mechanism is to satisfy the need for blood flow to the skin, it is very likely that these changes also influence the conduit and peripheral vascular environments, although it is currently unknown exactly how this happens. The reduction in splanchnic and renal blood flow has been attributed to decreased perfusion pressure and increased vasoconstriction in these vascular beds (20, 76–78). Overall, the redistribution of blood flow during heating allows for blood pressure to be maintained despite the substantial drop in vascular resistance to the skin blood vessels.
The SNS is a key component of the physiological response to heating in humans, controlling both the sweating and skin blood flow responses needed to dissipate heat (24). Additionally, the SNS is responsible for the compensatory increases in cardiac output and vascular resistance in noncutaneous beds necessary for the maintenance of blood pressure (75, 78). Approximately 80–95% of the increase in cutaneous blood flow with passive heat stress occurs through active vasodilation, a process that is mediated by cholinergic nerves evoking the release and action of acetylcholine and other neurotransmitters (42). In support of this idea, many studies have shown that whole-body heat stress sufficient to increase Tc by ~0.7 up to 1.3°C increases skin sympathetic nerve activity (SSNA) to trigger active vasodilation (24, 55). Muscle sympathetic nerve activity (MSNA), a more common index of sympathetic activation that is strongly associated with vasoconstriction, also increases by 40–90% to drive the increase in cardiac output and decrease in blood flow to the renal and splanchnic regions with whole-body heating (24, 55, 61). Changes in both SSNA and MSNA with heating are thought to occur through direct temperature-related mechanisms in an intensity-dependent manner (24).
IN VIVO HUMAN EXPERIMENTAL HEATING MODALITIES
Since practical heat therapies have amassed interest in recent years, researchers have begun to explore research questions on its effects in more controlled, experimental settings. Several methods have been used, thus far, to apply heat stress to the human body. Water immersion of various body parts is the most commonly used method as it is inexpensive and offers even heating of the immersed areas. Studies have employed this method to heat forearms (25, 65, 94), feet (30, 89), and lower legs (73), from the waist down (13, 14, 91, 92) and shoulders down (8–10). A limitation of this method may include the inability to collect any data involving electrical equipment (e.g., ECG), although protective seals can be applied to overcome this issue. Water-perfused tube-lined heating suits are also used, typically for whole-body heating (21, 64). There is also the option to leave an arm exposed to remove possible local skin-heating effects on measurements of arterial function. This option requires technical expertise for the construction of the suit, but has the benefit of leaving the skin surface dry for obtaining measurements. Our laboratory has previously used a heating pad/blanket to apply heat to the forearm, which, although inexpensive, is not ideal because of the uneven heating owing to difficulty wrapping fabric around irregularly shaped body parts as well as the fixed and subjective heat settings (e.g., low, medium, high). Other options include heat chambers and/or portable saunas, which would most closely replicate the conditions experienced in practical models of heat therapy.
The upcoming sections will summarize the acute and chronic effects of experimentally applied heat stress on the vasculature. Unless otherwise stated, findings highlighted are from cohorts of younger, healthy participants (both men and women) to describe the general observed response, rather than the influence of age and/or disease status.
ACUTE EFFECTS OF HEAT ON THE VASCULATURE
Few studies have examined the acute effect of a single exposure to heat on indices of vascular structure and function. Microvascular function has been shown to be improved in the lower limb following 45 min of lower leg immersion in water at 42°C (73), and unchanged in the upper limb following 60 min of combined whole-body and waist-down immersion in water at 40.5°C (10). In both studies, microvascular function was assessed indirectly using the reactive hyperemia peak and/or area under the curve response to a period of brief ischemia. More robust methods of microvascular function assessment, such as perfusion via laser Doppler, microdialysis, or contrast-enhanced ultrasound, should be used in this context for a more comprehensive evaluation. It might also be worth noting that Romero et al. (73) examined the superficial femoral artery (SFA) 30 min postheating while Brunt et al. (10) examined the BA 60 min postheating; either factor could explain the divergent findings.
Acute conduit artery endothelial function responses to a bout of passive heat stress have also been examined via FMD. An important consideration of findings is that the measurement of FMD is sensitive to baseline arterial diameter, and the appropriate statistical steps must be taken to account for acute diameter changes when responses pre- and postheating protocols are compared (2). Tinken et al. (94) demonstrated improvements in BA FMD without a change in baseline arterial diameter following 30 min of bilateral forearm heating in a water bath set to 40°C, but the lack of core and skin temperature data limits the characterization of the magnitude of heating stimulus (94). Brunt et al. (10) showed no change in BA FMD after 60 min of combination whole-body and waist-down hot water immersion at 40.5°C; but the postintervention FMD test was conducted 1 h after heating, and resting arterial diameter was observed to be increased at this time point compared with the preintervention FMD test (3.24 ± 0.24 to 3.46 ± 0.25 mm); therefore, it is possible that the expected change was missed (10). Romero et al. (73) and Thomas et al. (91) assessed acute responses to heat exposure via SFA FMD, which although relevant due to the atherosclerosis-prone nature of the SFA, has not been validated as a surrogate for coronary endothelial function or as an indicator of cardiovascular disease risk. Nevertheless, neither 45 min of waist-down water immersion nor 30 min of lower limb (up to 33 cm) water immersion, both at a temperature of 42°C, were sufficient to change SFA FMD, despite using allometric scaling statistical procedures (73, 92). In addition to the inconsistencies in statistically accounting for changes in baseline arterial diameter across studies, disparities in heating protocols and location of an artery examined preclude any overarching conclusions with respect to the acute impacts of local limb heating on conduit artery endothelial function.
Arterial stiffness responses to acute heat stress have previously been assessed via PWV and cardio-ankle vascular index (CAVI) and are likely reflective of transient changes in blood pressure and vascular tone with heating. There is no consensus with respect to the current literature, with findings ranging from increased (64), decreased (30), and no change (21, 64) in stiffness metrics with whole-body or lower limb heating. Examining special populations seemed to follow the same lack of trend, with the increased cfPWV in Moyen et al. (64) also observed in smokers and the decreased CAVI in Hu et al. (30) also observed in older patients (64). Additionally, Thomas et al. (92) observed decreases in both cfPWV and carotid-radial PWV in older individuals and those with peripheral arterial disease (91). All protocols except for that used in Hu et al. (30), which saw a Tc change of ~0.3–0.4°C, generated increases in Tc in the range of 1.5–1.8°C (21, 30, 64, 91). Discrepancies in the results of these four studies may be attributable to the various populations examined and assessment techniques used. The lack of change in Ganio et al. (21) may have been due to an underpowered sample size (n = 8) and the inclusion of both men and women (without control of menstrual phase). For comparison, Moyen et al. (64) had 26 men, with 13 in each of the smoking and nonsmoking groups, and Hu et al. (30) had 32 women, with 16 in each of the younger and older age groups. Timing of arterial stiffness assessment may have also contributed to the divergent findings in these studies. Moyen et al. (64) measured PWV while the body was still under heat stress, in which case it is plausible that the SNS resulted in increased vascular tone and arterial stiffness. On the other hand, Hu et al. (30) measured CAVI in the absence of heat stress, albeit immediately after water immersion, and found transiently decreased arterial stiffness that returned to approximate resting levels after 30 min. However, to further complicate the issue, Thomas et al. (92) observed decreased PWV despite assessment 30 min after the heating intervention. It is worth mentioning that only Thomas et al. (92) followed the guidelines on the recommended method of arterial stiffness assessment (7), which should be considered when findings between studies are compared.
Overall, regardless of the vascular outcome examined, the literature with respect to acute vascular responses to heating is sparse, and results are equivocal at best. Understanding the acute vascular responses to heating are important for informing the mechanisms by which heating may influence the vasculature with repeated use and should be studied further.
CHRONIC EFFECTS OF HEAT ON THE VASCULATURE
Recent research conducted in controlled experimental settings has begun to focus on the effects of chronic application of heat on measures of micro- and macrovascular function in cohorts of young, healthy men and women. In previous studies, microvascular function has been commonly assessed as the cutaneous vascular conductance (CVC) response to a heat stimulus using laser Doppler array probes. A series of two studies employed 8-wk heat training protocols where participants came in for 30-min sessions three times a week for hot water immersion (40–42°C). Green et al. (25) used a bilateral forearm heating model intended to increase local temperature and found increased forearm CVC after 4 wk, and Carter et al. (13) used a waist-down heating model intended to increase Tc and found increased forearm CVC after 8 wk (13, 25). Restricting the normal blood flow and shear stress elevations to the BA, where assessment was conducted, negated the improvements in CVC. In the study by Carter et al. (13), skin temperature at the nonheated forearms also appeared to play a key role in modulating the microvascular function response to lower limb heat training, as CVC was found to decrease when the natural rise in skin temperature was restricted and instead clamped at resting levels. Taking it a step further, Brunt et al. (8) used the same laser Doppler microvascular function assessment technique but layered on a microdialysis approach to determine the mechanisms underlying the observed changes in microvascular function with 8 wk of a combination of whole-body and waist-down heat training. Before and after the training period, a heat challenge was applied to several sites on the ventral forearm where microdialysis fibers had been inserted. Microdialysis fibers received either lactated Ringer (control), the NOS inhibitor Nω-nitro-l-arginine (l-NNA) to reduce NO production, or the superoxide dismutase mimetic tempol to reduce oxidative stress (8). Administration of l-NNA decreased CVC, while tempol increased CVC but not above that of the control condition, demonstrating that improved NO bioavailability is the key heat training-induced change responsible for improved microvascular function (8). Most recently, Francisco et al. (19) tested whether microvascular adaptations could be elicited by 10 days of 60 min/day forearm heating using a cylindrical water-spray device. Throughout the heating sessions, the contralateral arm was submerged in a thermoneutral (32°C) water bath to serve as a within-subject control. The Tc remained unchanged throughout forearm heating in a subset of two subjects in whom this outcome was assessed (19). When comparing microvascular function before and after short-term acclimation, researchers found that 10 days of repeated heating was insufficient to elicit changes in the cutaneous vascular responses to local heating, acetylcholine administration, or prolonged forearm heating, suggesting that either a longer acclimation period or less localized stimulus is needed (19).
In previous examinations of vascular responses to heat training, macrovascular function was most typically assessed as endothelial function using a FMD test. In separate studies from the Green research group, BA FMD was examined before, during, and after 8 wk of 3 times/wk 30-min limb hot water (40–42°C) immersion sessions. Naylor et al. (65) heated both forearms and observed an increase in BA FMD after 2 wk of heat training (65), while Carter et al. (14) heated from the waist down and observed an increase in BA FMD after 4 wk. In both cases, BA endothelial function improved early on, but reverted to baseline levels by the end of heat training, which is consistent with the proposed time course of vascular adaptations to exercise training (93). Accompanying structural outcomes would have given a more complete picture, although the observed lack of change in resting arterial diameter in either study at any time point during the 8 wk of heat training suggests no structural remodeling in the form of increased arterial dimension occurs (14, 65). It also seems that the location of heating may impact the time course of change in FMD (i.e., increase after 2 vs. 4 wk). Because these earlier studies were designed for the purpose of investigating the effect of shear stress alterations rather than heating, skin and Tc measurements were absent or limited, making it difficult to characterize the magnitude of the heating stimulus. The most recent study by Brunt et al. (9) is the most comprehensive and robust study on passive heat stress training to date, including measures of arterial wall thickness and stiffness in addition to endothelial function. A combination whole-body and waist-down water immersion protocol was used to heat young, sedentary but otherwise healthy men and women, with the ultimate goal of maintaining a Tc between 38.5 and 39°C (~1.5°C above resting) for up to 90 min (9). For comparison, the heating protocol used by Carter et al. (14) increased Tc by only ~0.5°C by the end of the 30 min. Heat training in the Brunt et al. (9) study was also more rigorous with 4–5 sessions per week of heating for a total of 36 sessions throughout the 8-wk period. Improvements were observed in BA FMD, SFA compliance, SFA β-stiffness index, cfPWV, CCA intima-media thickness (IMT), and blood pressure (BP) at various time points throughout heat training (9). Increases in BA FMD (after 2 wk), SFA compliance (after 4 wk), and mean BP (after 2 wk) persisted until the end of the 8-wk training period. Decreases in diastolic BP were observed at weeks 4 and 8 only. Decreases in the SFA β-stiffness index, cfPWV, and CCA IMT were observed only at the end of the 8 wk (9). The IMT findings in this study should be interpreted with caution, as calipers were used for analysis (96). Otherwise, gold standard methodologies were used for all other vascular outcome measures (7, 53, 90). The differential adaptations in different arterial locations (e.g., CCA vs. SFA) is very interesting and suggests that different mechanisms may be involved in the response to chronic heating. Furthermore, the maintenance of improved endothelial function up to 8 wk of training surpasses the time course of adaptations suggested by Tinken et al. (93), which may point to alternate signaling pathways being activated with chronic combined local and core temperature elevation compared with other interventions, such as local heating alone and exercise (14, 65, 93). Recently, Bailey et al. (3) demonstrated that 8 wk (30-min sessions, 3 times/wk) of whole-body hot water immersion improved BA FMD to the same extent as moderate-intensity cycling training, providing support for the eventual use of heat stress as an alternative or adjunct therapy to exercise (3). Certainly, more research is warranted as this study was conducted in a group of young recreationally active women, which represents only a fraction of the world population.
POTENTIAL MECHANISMS AND FUTURE DIRECTIONS
Shear stress is a critical physiological stimulus thought to be imperative for eliciting vascular adaptation in response to stressors through its ability to trigger the production of substances (e.g., NO) that are vasodilatory and thought to be atheroprotective (Fig. 3) (17, 26, 51). Endothelial cells detect shear stress through the alteration and deformation of the morphology of their stress fibers, which subsequently trigger the production of vasodilators or vasoconstrictors. The effects of shear stress on vascular function have been demonstrated in cells, animals, and humans (12, 26, 51, 66, 97). Seminal work by Pohl et al. (70) demonstrated, through their removal, that endothelial cells are essential to the FMD response. In this study conducted in vivo in canine femoral arteries, the responses to nitroglycerine and norepinephrine, both vasoactive agonists, were preserved, while FMD was lost after intimal denudation (70). In experiments that followed by Laughlin and colleagues (38), researchers observed tight coupling between shear stress induced by increased flow and FMD ex vivo in rat superficial femoral arteries. Additionally, they demonstrated that higher vs. lower or no flow resulted in greater mRNA expression of eNOS, an enzyme needed to produce NO, and Cu/Zn superoxide dismutase, an enzyme that scavenges superoxide (O2−), thereby slowing down the rate of degradation of NO (103). With regard to heat stress, it is known that vascular shear stress is increased during heating to satisfy the demand for blood flow at the skin surface; and it has been postulated that these repeated increases in shear stress drive the improvements in micro- and macrovascular artery function that arise with chronic heat exposure (94). Recent work in humans in vivo has recapitulated some of the early shear stress work conducted in cells and animals, with a series of experiments repeatedly demonstrating that the improvement in FMD and CVC in response to heating, as well as a number of other interventions, is abolished when a cuff is inflated to 80–100 mmHg throughout the intervention to prevent the naturally occurring increase in shear stress (6, 25, 94, 95). The obligatory role of increased shear stress on the heat-induced improvements in vascular function has become accepted in the literature, as it is one of the most well-studied and reproduced phenomenon in this research area.
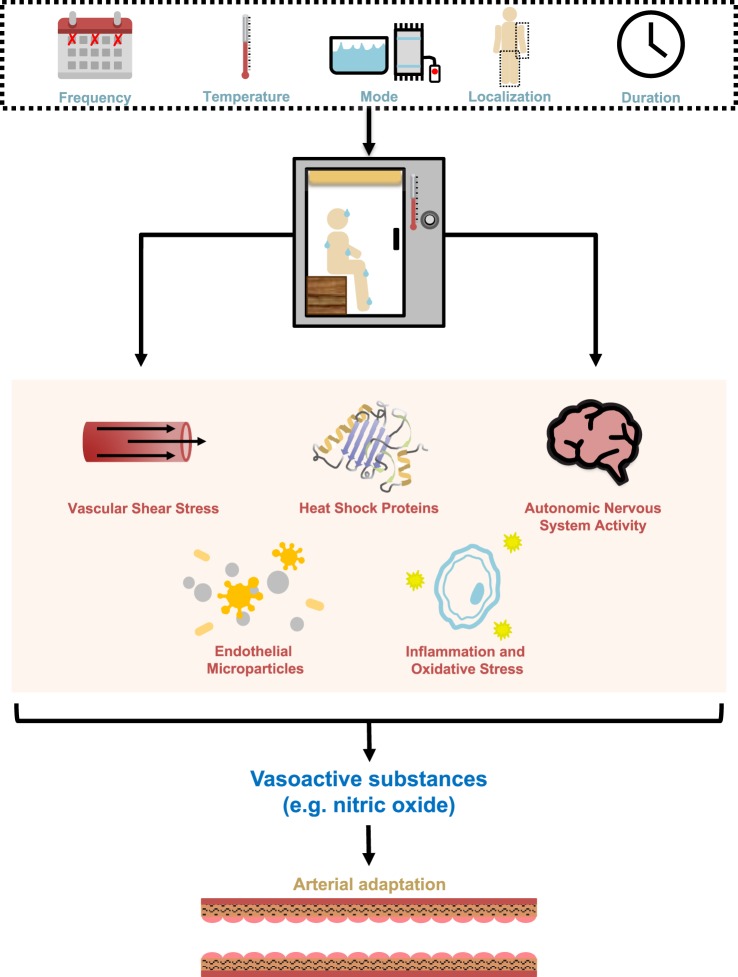
Fig. 3.
Potential control mechanisms for the effect of heat stress on the vasculature. Heat stress can be characterized by frequency, temperature, mode, localization, and duration, to determine the magnitude and intensity of the stimulus. Heat alters vascular shear stress, heat shock protein content, autonomic nervous system activity, endothelial cell damage pathways, and inflammation and oxidative stress, all of which are associated with the regulation of secreted vasoactive substances to mediate arterial adaptation.
Changes in arterial stiffness with acute and chronic heating are more challenging to explain, as both passive (i.e., deposition of elastin and collagen in arterial wall) and active (i.e., vascular tone) components of the arterial wall can contribute to measured values (79). Currently available assessment methods and outcome measures, such as PWV, compliance, and the β-stiffness index, are not capable of discerning which of the two contributors is responsible for observed changes in the stiffness profile, although duration of intervention or training can help to inform this question. Modifications to passive stiffness are likely restricted to chronic intervention durations owing to the time course for deposition of new elastin or collagen protein, while active stiffness can reasonably fluctuate over a shorter period of time and may be transient, since neural drive is a primary effector of this type of change (Fig. 3) (79). It may be worthwhile to assess sympathetic tone through muscle or skin sympathetic nerve activity in future studies to provide additional insight on arterial stiffness changes, particularly with chronic heat stress.
Much of the recent work alludes to the potential roles of increased HSPs and decreased inflammation and oxidative stress in mediating changes in vascular function (Fig. 3). HSPs play an important role in activating eNOS, as supported by data that show that the HSP90 inhibitor geldanamycin attenuates the NO-mediated vasodilation in skin (83). In vitro experiments in bovine coronary endothelial cells have demonstrated that acute heat activates HSP90, yielding increases in circulating NO through elevated eNOS activity (71), whereas ex vivo experiments in peripheral blood mononuclear cells from humans have shown that chronic heat increases the amount of HSP72 and HSP90 protein (58). HSPs also work to stabilize and activate other proteins, and those involved in inflammatory and oxidative stress responses are likely relevant to vascular function. Although the main purpose was to examine responses to hypoxia-reoxygenation, recent work by Brunt et al. (11) showed that in human umbilical vein endothelial cells incubated for 24 h in “mild heating” (39°C) media or with serum from participants that have undergone an 8-wk heat therapy intervention, there was reduced production of O2− and elevated production of manganese superoxide dismutase (O2− scavenger) at rest compared with sham conditions (37°C media and thermoneutral water immersion therapy, respectively). These differences coincided with increased HSP70 protein levels in mild heating-pretreated cells and peripheral blood mononuclear cells isolated from venous blood collected from human participants (11). Additionally, Bain et al. (4) show in humans that arterial concentrations of activation- and apoptosis-derived endothelial microparticles and platelet microparticles are reduced after ~1 h of whole-body heat stress (4). These outcome measurements indicate endothelial cell damage and activation of inflammatory and thrombotic pathways, respectively, which provide further information on mechanisms by which heating improves vascular function (Fig. 3). Further work in this area should concurrently explore the changes in HSPs, inflammation, and oxidative stress, and indices of cardiovascular disease risk in response to heating interventions.
As some of the literature in both practical and experimental models of heat therapy suggests, age and disease risk status may be moderators of the effect of heat on the vasculature. It would be advantageous to extend the acute and chronic heating findings in experimental studies to groups of individuals that are unable to perform traditional physical activities, or undergo periods of reduced physical activity, such as older adults and clinical populations. Very few tightly controlled, experimental studies have been conducted in these populations for whom heat therapy may be most transformative in terms of health outcomes (73, 91). In a recent study, Teixeira et al. (89) showed, albeit in a young healthy population, that immersing one foot in a hot bath for 30 min, three times a day, can mitigate the impairment in SFA endothelial function that occurs with just 5 days of reduced physical activity (89). This study should be replicated in clinical groups, since it is reasonable to expect even greater positive results considering they are likely beginning with reduced endothelial function and have more to gain from the heating intervention.
CONCLUSIONS
Based on a wealth of evidence in Waon therapy, Japanese onsen bathing, Finnish Sauna bathing, Bikram yoga, and other heat-related activities, it seems that chronic exposure to heat in a therapeutic paradigm has a beneficial effect on vascular health (27, 31, 63). Recent efforts to characterize the vascular responses to acute and chronic heating using experimental models have generally demonstrated improvements across all aspects of vascular health, including IMT, compliance, β-stiffness index, FMD, and CVC with chronic heat exposure. In contrast, there is less consensus regarding the responses to acute heat exposure due to a variety of factors, but most notably suboptimal and inconsistent assessment methods and protocols. For instance, a major challenge of acute investigations is the wide range in the definition of the acute phase, anywhere between 0 min to 24 h postintervention. With most research groups assessing outcomes at just one or two time points, this makes it extremely difficult to compare findings and determine which responses are scientifically relevant. A study aiming to establish a time course of change in vascular outcomes in response to acute heat stress is needed to further progress in this area. Another major limitation of the current literature is the extensive array of heating protocols and assessment methodologies, making it difficult to arrive at a consensus for any given effect (e.g., acute vs. training, degree of heating, location of heating, duration of heating). The future of heat stress therapies may benefit from utilizing the F.I.T.T. (frequency, intensity, type, time) principle more commonly used in exercise, as studies will need to consider these aspects of the stimulus as moderating factors of the effect (Fig. 3). The literature on Finnish sauna bathing is likely the most advanced in this regard, with studies having demonstrated negative dose-dependent relationships between frequency and/or duration of sauna use and CVD and all-cause mortality risk (50, 52). It is reasonable to believe that indicators of vascular dysfunction that precede many CVDs would be even more sensitive to detect the effects of nuances in these heating parameters. Although not much has been done to explore the impact of heating intensity (i.e., temperature), many heating mechanisms are thought to only be activated beyond certain threshold temperatures, which suggests that milder heating may not be as effective as moderate heating at triggering the atheroprotective signaling pathways known to be beneficial for vascular function. Overall, important caveats to many of the observations made, as well as the limited number of studies in both acute and chronic heating models, necessitate additional studies be conducted that are comprehensive and well controlled.
GRANTS
M. J. MacDonald is funded by the Natural Sciences and Engineering Research Council (DG 238819-13).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.C. and M.J.M. conceived and designed research; J.L.C. and M.J.M. prepared figures; J.L.C. and M.J.M. drafted manuscript; J.L.C. and M.J.M. edited and revised manuscript; J.L.C. and M.J.M. approved final version of manuscript.
REFERENCES
- 1.Akasaki Y, Miyata M, Eto H, Shirasawa T, Hamada N, Ikeda Y, Biro S, Otsuji Y, Tei C. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ J 70: 463–470, 2006. doi: 10.1253/circj.70.463. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson G, Batterham AM, Thijssen DHJ, Green DJ. A new approach to improve the specificity of flow-mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens 31: 287–291, 2013. doi: 10.1097/HJH.0b013e32835b8164. [DOI] [PubMed] [Google Scholar]
- 3.Bailey TG, Cable NT, Miller GD, Sprung VS, Low DA, Jones H. Repeated warm water immersion induces similar cerebrovascular adaptations to 8 weeks of moderate-intensity exercise training in females. Int J Sports Med 37: 757–765, 2016. doi: 10.1055/s-0042-106899. [DOI] [PubMed] [Google Scholar]
- 4.Bain AR, Ainslie PN, Bammert TD, Hijmans JG, Sekhon M, Hoiland RL, Flück D, Donnelly J, DeSouza CA. Passive heat stress reduces circulating endothelial and platelet microparticles. Exp Physiol 102: 663–669, 2017. doi: 10.1113/EP086336. [DOI] [PubMed] [Google Scholar]
- 5.Bennett LAT, Johnson JM, Stephens DP, Saad AR, Kellogg DL Jr. Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol 552: 223–232, 2003. doi: 10.1113/jphysiol.2003.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH, Green DJ. Brachial artery adaptation to lower limb exercise training: role of shear stress. J Appl Physiol (1985) 112: 1653–1658, 2012. doi: 10.1152/japplphysiol.01489.2011. [DOI] [PubMed] [Google Scholar]
- 7.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FUS, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries . Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30: 445–448, 2012. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 8.Brunt VE, Eymann TM, Francisco MA, Howard MJ, Minson CT. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J Appl Physiol (1985) 121: 716–723, 2016. doi: 10.1152/japplphysiol.00424.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol 594: 5329–5342, 2016. doi: 10.1113/JP272453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunt VE, Jeckell AT, Ely BR, Howard MJ, Thijssen DHJ, Minson CT. Acute hot water immersion is protective against impaired vascular function following forearm ischemia-reperfusion in young healthy humans. Am J Physiol Regul Integr Comp Physiol 311: R1060–R1067, 2016. doi: 10.1152/ajpregu.00301.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunt VE, Wiedenfeld-Needham K, Comrada LN, Minson CT. Passive heat therapy protects against endothelial cell hypoxia-reoxygenation via effects of elevations in temperature and circulating factors. J Physiol 596: 4831–4845, 2018. doi: 10.1113/JP276559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension 17: 187–193, 1991. doi: 10.1161/01.HYP.17.2.187. [DOI] [PubMed] [Google Scholar]
- 13.Carter HH, Spence AL, Atkinson CL, Pugh CJA, Cable NT, Thijssen DHJ, Naylor LH, Green DJ. Distinct effects of blood flow and temperature on cutaneous microvascular adaptation. Med Sci Sports Exerc 46: 2113–2121, 2014. doi: 10.1249/MSS.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 14.Carter HH, Spence AL, Atkinson CL, Pugh CJA, Naylor LH, Green DJ. Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol 114: 859–865, 2014. doi: 10.1007/s00421-013-2817-2. [DOI] [PubMed] [Google Scholar]
- 15.Charkoudian N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol (1985) 109: 1221–1228, 2010. doi: 10.1152/japplphysiol.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crandall CG, Wilson TE. Human cardiovascular responses to passive heat stress. Compr Physiol 5: 17–43, 2015. doi: 10.1002/cphy.c140015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 115: 1285–1295, 2007. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 18.DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res 106: 434–446, 2010. doi: 10.1161/CIRCRESAHA.109.208041. [DOI] [PubMed] [Google Scholar]
- 19.Francisco MA, Brunt VE, Jensen KN, Lorenzo S, Minson CT. Ten days of repeated local forearm heating does not affect cutaneous vascular function. J Appl Physiol (1985) 123: 310–316, 2017. doi: 10.1152/japplphysiol.00966.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganio MS, Brothers RM, Lucas RA, Hastings JL, Crandall CG. Validity of auscultatory and Penaz blood pressure measurements during profound heat stress alone and with an orthostatic challenge. Am J Physiol Regul Integr Comp Physiol 301: R1510–R1516, 2011. doi: 10.1152/ajpregu.00247.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganio MS, Brothers RM, Shibata S, Hastings JL, Crandall CG. Effect of passive heat stress on arterial stiffness. Exp Physiol 96: 919–926, 2011. doi: 10.1113/expphysiol.2011.057091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118: 620–636, 2016. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorman AJ, Proppe DW. Mechanisms producing tachycardia in conscious baboons during environmental heat stress. J Appl Physiol 56: 441–446, 1984. doi: 10.1152/jappl.1984.56.2.441. [DOI] [PubMed] [Google Scholar]
- 24.Greaney JL, Kenney WL, Alexander LM. Sympathetic regulation during thermal stress in human aging and disease. Auton Neurosci 196: 81–90, 2016. doi: 10.1016/j.autneu.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DHJ, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 588: 1571–1577, 2010. doi: 10.1113/jphysiol.2010.186965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Green DJ, Hopman MTE, Padilla J, Laughlin MH, Thijssen DHJ. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol Rev 97: 495–528, 2017. doi: 10.1152/physrev.00014.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinonen I, Laukkanen JA. Effects of heat and cold on health, with special reference to Finnish sauna bathing. Am J Physiol Regul Integr Comp Physiol 314: R629–R638, 2018. doi: 10.1152/ajpregu.00115.2017. [DOI] [PubMed] [Google Scholar]
- 28.Hewett ZL, Cheema BS, Pumpa KL, Smith CA. The effects of Bikram yoga on health: critical review and clinical trial recommendations. Evid Based Complement Alternat Med 2015: 428427, 2015. doi: 10.1155/2015/428427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Q, Zhu W, Zhu Y, Zheng L, Hughson RL. Acute effects of warm footbath on arterial stiffness in healthy young and older women. Eur J Appl Physiol 112: 1261–1268, 2012. doi: 10.1007/s00421-011-2066-1. [DOI] [PubMed] [Google Scholar]
- 31.Hunter SD, Dhindsa MS, Cunningham E, Tarumi T, Alkatan M, Nualnim N, Elmenshawy A, Tanaka H. The effect of Bikram yoga on endothelial function in young and middle-aged and older adults. J Bodyw Mov Ther 21: 30–34, 2017. doi: 10.1016/j.jbmt.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Hunter SD, Dhindsa MS, Cunningham E, Tarumi T, Alkatan M, Nualnim N, Tanaka H. The effect of Bikram yoga on arterial stiffness in young and older adults. J Altern Complement Med 19: 930–934, 2013. doi: 10.1089/acm.2012.0709. [DOI] [PubMed] [Google Scholar]
- 33.Hunter SD, Laosiripisan J, Elmenshawy A, Tanaka H. Effects of yoga interventions practised in heated and thermoneutral conditions on endothelium-dependent vasodilatation: the Bikram yoga heart study. Exp Physiol 103: 391–396, 2018. doi: 10.1113/EP086725. [DOI] [PubMed] [Google Scholar]
- 34.Hunter SD, Tarumi T, Dhindsa MS, Nualnim N, Tanaka H. Hatha yoga and vascular function: results from cross-sectional and interventional studies. J Bodyw Mov Ther 17: 322–327, 2013. doi: 10.1016/j.jbmt.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda Y, Biro S, Kamogawa Y, Yoshifuku S, Eto H, Orihara K, Kihara T, Tei C. Repeated thermal therapy upregulates arterial endothelial nitric oxide synthase expression in Syrian golden hamsters. Jpn Circ J 65: 434–438, 2001. doi: 10.1253/jcj.65.434. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda Y, Biro S, Kamogawa Y, Yoshifuku S, Eto H, Orihara K, Yu B, Kihara T, Miyata M, Hamasaki S, Otsuji Y, Minagoe S, Tei C. Repeated sauna therapy increases arterial endothelial nitric oxide synthase expression and nitric oxide production in cardiomyopathic hamsters. Circ J 69: 722–729, 2005. doi: 10.1253/circj.69.722. [DOI] [PubMed] [Google Scholar]
- 37.Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, Otsuji Y, Minagoe S, Toyama Y, Tei C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol 38: 1083–1088, 2001. doi: 10.1016/S0735-1097(01)01467-X. [DOI] [PubMed] [Google Scholar]
- 38.Jasperse JL, Laughlin MH. Flow-induced dilation of rat soleus feed arteries. Am J Physiol 273: H2423–H2427, 1997. doi: 10.1152/ajpheart.1997.273.5.H2423. [DOI] [PubMed] [Google Scholar]
- 39.Johnson JM, Brengelmann GL, Hales JR, Vanhoutte PM, Wenger CB. Regulation of the cutaneous circulation. Fed Proc 45: 2841–2850, 1986. [PubMed] [Google Scholar]
- 40.Johnson JM, Minson CT, Kellogg DLJ Jr. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol 4: 33–89, 2014. doi: 10.1002/cphy.c130015. [DOI] [PubMed] [Google Scholar]
- 41.Jose AD, Stitt F, Collison D. The effects of exercise and changes in body temperature on the intrinsic heart rate in man. Am Heart J 79: 488–498, 1970. doi: 10.1016/0002-8703(70)90254-1. [DOI] [PubMed] [Google Scholar]
- 42.Kellogg DL., Jr In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol (1985) 100: 1709–1718, 2006. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- 43.Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol (1985) 85: 824–829, 1998. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 44.Kellogg DL Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol (1985) 86: 1185–1190, 1999. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 45.Kellogg DL Jr, Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995. doi: 10.1161/01.RES.77.6.1222. [DOI] [PubMed] [Google Scholar]
- 46.Kihara T, Biro S, Ikeda Y, Fukudome T, Shinsato T, Masuda A, Miyata M, Hamasaki S, Otsuji Y, Minagoe S, Akiba S, Tei C. Effects of repeated sauna treatment on ventricular arrhythmias in patients with chronic heart failure. Circ J 68: 1146–1151, 2004. doi: 10.1253/circj.68.1146. [DOI] [PubMed] [Google Scholar]
- 47.Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 39: 754–759, 2002. doi: 10.1016/S0735-1097(01)01824-1. [DOI] [PubMed] [Google Scholar]
- 48.Kihara T, Miyata M, Fukudome T, Ikeda Y, Shinsato T, Kubozono T, Fujita S, Kuwahata S, Hamasaki S, Torii H, Lee S, Toda H, Tei C. Waon therapy improves the prognosis of patients with chronic heart failure. J Cardiol 53: 214–218, 2009. doi: 10.1016/j.jjcc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Kohara K, Tabara Y, Ochi M, Okada Y, Ohara M, Nagai T, Ohyagi Y, Igase M. Habitual hot water bathing protects cardiovascular function in middle-aged to elderly Japanese subjects. Sci Rep 8: 8687, 2018. doi: 10.1038/s41598-018-26908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunutsor SK, Khan H, Laukkanen T, Laukkanen JA. Joint associations of sauna bathing and cardiorespiratory fitness on cardiovascular and all-cause mortality risk: a long-term prospective cohort study. Ann Med 50: 139–146, 2018. doi: 10.1080/07853890.2017.1387927. [DOI] [PubMed] [Google Scholar]
- 51.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol (1985) 104: 588–600, 2008. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med 175: 542–548, 2015. doi: 10.1001/jamainternmed.2014.8187. [DOI] [PubMed] [Google Scholar]
- 53.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 54.Lee E, Laukkanen T, Kunutsor SK, Khan H, Willeit P, Zaccardi F, Laukkanen JA. Sauna exposure leads to improved arterial compliance: findings from a non-randomised experimental study. Eur J Prev Cardiol 25: 130–138, 2018. doi: 10.1177/2047487317737629. [DOI] [PubMed] [Google Scholar]
- 55.Low DA, Keller DM, Wingo JE, Brothers RM, Crandall CG. Sympathetic nerve activity and whole body heat stress in humans. J Appl Physiol (1985) 111: 1329–1334, 2011. doi: 10.1152/japplphysiol.00498.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maeda T, Mimori K, Suzuki S, Horiuchi T, Makino N. Preventive and promotive effects of habitual hot spa-bathing on the elderly in Japan. Sci Rep 8: 133, 2018. doi: 10.1038/s41598-017-18488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masuda A, Miyata M, Kihara T, Minagoe S, Tei C. Repeated sauna therapy reduces urinary 8-epi-prostaglandin F(2α). Jpn Heart J 45: 297–303, 2004. doi: 10.1536/jhj.45.297. [DOI] [PubMed] [Google Scholar]
- 58.McClung JP, Hasday JD, He J-R, Montain SJ, Cheuvront SN, Sawka MN, Singh IS. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol 294: R185–R191, 2008. doi: 10.1152/ajpregu.00532.2007. [DOI] [PubMed] [Google Scholar]
- 59.McCord GR, Cracowski J-L, Minson CT. Prostanoids contribute to cutaneous active vasodilation in humans. Am J Physiol Regul Integr Comp Physiol 291: R596–R602, 2006. doi: 10.1152/ajpregu.00710.2005. [DOI] [PubMed] [Google Scholar]
- 60.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 61.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol (1985) 84: 1323–1332, 1998. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- 62.Miyata M, Kihara T, Kubozono T, Ikeda Y, Shinsato T, Izumi T, Matsuzaki M, Yamaguchi T, Kasanuki H, Daida H, Nagayama M, Nishigami K, Hirata K, Kihara K, Tei C. Beneficial effects of Waon therapy on patients with chronic heart failure: results of a prospective multicenter study. J Cardiol 52: 79–85, 2008. doi: 10.1016/j.jjcc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Miyata M, Tei C. Waon therapy for cardiovascular disease: innovative therapy for the 21st century. Circ J 74: 617–621, 2010. doi: 10.1253/circj.CJ-09-0939. [DOI] [PubMed] [Google Scholar]
- 64.Moyen NE, Ganio MS, Burchfield JM, Tucker MA, Gonzalez MA, Dougherty EK, Robinson FB, Ridings CB, Veilleux JC. Effect of passive heat stress on arterial stiffness in smokers versus non-smokers. Int J Biometeorol 60: 499–506, 2016. doi: 10.1007/s00484-015-1046-2. [DOI] [PubMed] [Google Scholar]
- 65.Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DHJ, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol 300: H664–H669, 2011. doi: 10.1152/ajpheart.00985.2010. [DOI] [PubMed] [Google Scholar]
- 66.Noris M, Morigi M, Donadelli R, Aiello S, Foppolo M, Todeschini M, Orisio S, Remuzzi G, Remuzzi A. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ Res 76: 536–543, 1995. doi: 10.1161/01.RES.76.4.536. [DOI] [PubMed] [Google Scholar]
- 67.Ohori T, Nozawa T, Ihori H, Shida T, Sobajima M, Matsuki A, Yasumura S, Inoue H. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol 109: 100–104, 2012. doi: 10.1016/j.amjcard.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 68.Okada M, Hasebe N, Aizawa Y, Izawa K, Kawabe J, Kikuchi K. Thermal treatment attenuates neointimal thickening with enhanced expression of heat-shock protein 72 and suppression of oxidative stress. Circulation 109: 1763–1768, 2004. doi: 10.1161/01.CIR.0000124226.88860.55. [DOI] [PubMed] [Google Scholar]
- 69.Pérgola PE, Kellogg DL Jr, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol 265: H785–H792, 1993. doi: 10.1152/ajpheart.1993.265.3.H785. [DOI] [PubMed] [Google Scholar]
- 70.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986. doi: 10.1161/01.HYP.8.1.37. [DOI] [PubMed] [Google Scholar]
- 71.Pritchard KA Jr, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem 276: 17621–17624, 2001. doi: 10.1074/jbc.C100084200. [DOI] [PubMed] [Google Scholar]
- 72.Radigan LR, Robinson S. Effects of environmental heat stress and exercise on renal blood flow and filtration rate. J Appl Physiol 2: 185–191, 1949. doi: 10.1152/jappl.1949.2.4.185. [DOI] [PubMed] [Google Scholar]
- 73.Romero SA, Gagnon D, Adams AN, Cramer MN, Kouda K, Crandall CG. Acute limb heating improves macro- and microvascular dilator function in the leg of aged humans. Am J Physiol Heart Circ Physiol 312: H89–H97, 2017. doi: 10.1152/ajpheart.00519.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 75.Rowell LB. Human Circulation Regulation During Physical Stress. New York: Oxford University Press, 1986. [Google Scholar]
- 76.Rowell LB, Brengelmann GL, Blackmon JR, Murray JA. Redistribution of blood flow during sustained high skin temperature in resting man. J Appl Physiol 28: 415–420, 1970. doi: 10.1152/jappl.1970.28.4.415. [DOI] [PubMed] [Google Scholar]
- 77.Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol 27: 673–680, 1969. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- 78.Rowell LB, Detry JR, Profant GR, Wyss C. Splanchnic vasoconstriction in hyperthermic man—role of falling blood pressure. J Appl Physiol 31: 864–869, 1971. doi: 10.1152/jappl.1971.31.6.864. [DOI] [PubMed] [Google Scholar]
- 79.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107: 2864–2869, 2003. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 80.Seeley R, VanPutte C, Regan J, Russo A. Seeley’s Anatomy & Physiology. New York: McGraw-Hill, 2011. [Google Scholar]
- 81.Serbulea M, Payyappallimana U. Onsen (hot springs) in Japan—transforming terrain into healing landscapes. Health Place 18: 1366–1373, 2012. doi: 10.1016/j.healthplace.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 82.Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol (1985) 85: 830–834, 1998. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- 83.Shastry S, Joyner MJ. Geldanamycin attenuates NO-mediated dilation in human skin. Am J Physiol Heart Circ Physiol 282: H232–H236, 2002. doi: 10.1152/ajpheart.2002.282.1.H232. [DOI] [PubMed] [Google Scholar]
- 84.Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and l-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol (1985) 88: 467–472, 2000. doi: 10.1152/jappl.2000.88.2.467. [DOI] [PubMed] [Google Scholar]
- 85.Sivasankaran S, Pollard-Quintner S, Sachdeva R, Pugeda J, Hoq SM, Zarich SW. The effect of a six-week program of yoga and meditation on brachial artery reactivity: do psychosocial interventions affect vascular tone? Clin Cardiol 29: 393–398, 2006. doi: 10.1002/clc.4960290905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stephens DP, Charkoudian N, Benevento JM, Johnson JM, Saumet JL. The influence of topical capsaicin on the local thermal control of skin blood flow in humans. Am J Physiol Regul Integr Comp Physiol 281: R894–R901, 2001. doi: 10.1152/ajpregu.2001.281.3.R894. [DOI] [PubMed] [Google Scholar]
- 87.Strandberg TE, Strandberg A, Pitkälä K, Benetos A. Sauna bathing, health, and quality of life among octogenarian men: the Helsinki Businessmen Study. Aging Clin Exp Res 30: 1053–1057, 2018. doi: 10.1007/s40520-017-0855-z. [DOI] [PubMed] [Google Scholar]
- 88.Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, Tanaka N. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation 91: 2582–2590, 1995. doi: 10.1161/01.CIR.91.10.2582. [DOI] [PubMed] [Google Scholar]
- 89.Teixeira AL, Padilla J, Vianna LC. Impaired popliteal artery flow-mediated dilation caused by reduced daily physical activity is prevented by increased shear stress. J Appl Physiol (1985) 123: 49–54, 2017. doi: 10.1152/japplphysiol.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas KN, van Rij AM, Lucas SJE, Cotter JD. Lower-limb hot-water immersion acutely induces beneficial hemodynamic and cardiovascular responses in peripheral arterial disease and healthy, elderly controls. Am J Physiol Regul Integr Comp Physiol 312: R281–R291, 2017. doi: 10.1152/ajpregu.00404.2016. [DOI] [PubMed] [Google Scholar]
- 92.Thomas KN, van Rij AM, Lucas SJE, Gray AR, Cotter JD. Substantive hemodynamic and thermal strain upon completing lower-limb hot-water immersion; comparisons with treadmill running. Temperature (Austin) 3: 286–297, 2016. doi: 10.1080/23328940.2016.1156215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586: 5003–5012, 2008. doi: 10.1113/jphysiol.2008.158014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tinken TM, Thijssen DHJ, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension 54: 278–285, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tinken TM, Thijssen DHJ, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 96.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Fatar M, Hernandez Hernandez R, Jaff M, Kownator S, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS, Zannad F, Zureik M. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 23: 75–80, 2007. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 97.Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol 269: C1371–C1378, 1995. doi: 10.1152/ajpcell.1995.269.6.C1371. [DOI] [PubMed] [Google Scholar]
- 98.Verkerk AO, van Ginneken ACG, Wilders R. Pacemaker activity of the human sinoatrial node: role of the hyperpolarization-activated current, I(f). Int J Cardiol 132: 318–336, 2009. doi: 10.1016/j.ijcard.2008.12.196. [DOI] [PubMed] [Google Scholar]
- 99.Wilkins BW, Wong BJ, Tublitz NJ, McCord GR, Minson CT. Vasoactive intestinal peptide fragment VIP10-28 and active vasodilation in human skin. J Appl Physiol (1985) 99: 2294–2301, 2005. doi: 10.1152/japplphysiol.00500.2005. [DOI] [PubMed] [Google Scholar]
- 100.Wong BJ, Hollowed CG. Current concepts of active vasodilation in human skin. Temperature (Austin) 4: 41–59, 2016. doi: 10.1080/23328940.2016.1200203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilatation in humans. J Physiol 577: 1043–1051, 2006. doi: 10.1113/jphysiol.2006.112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol 560: 941–948, 2004. doi: 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woodman CR, Muller JM, Rush JW, Laughlin MH, Price EM. Flow regulation of ecNOS and Cu/Zn SOD mRNA expression in porcine coronary arterioles. Am J Physiol 276: H1058–H1063, 1999. doi: 10.1152/ajpheart.1999.276.3.H1058. [DOI] [PubMed] [Google Scholar]
- 104.Zaccardi F, Laukkanen T, Willeit P, Kunutsor SK, Kauhanen J, Laukkanen JA. Sauna bathing and incident hypertension: a prospective cohort study. Am J Hypertens 30: 1120–1125, 2017. doi: 10.1093/ajh/hpx102. [DOI] [PubMed] [Google Scholar]