Abstract
Cardiac myocytes are the cells responsible for the robust ability of the heart to pump blood throughout the circulatory system. Cardiac myocytes grow in response to a variety of physiological and pathological conditions; this growth challenges endoplasmic reticulum-protein quality control (ER-PQC), a major feature of which includes the unfolded protein response (UPR). ER-PQC and the UPR in cardiac myocytes growing under physiological conditions, including normal development, exercise and pregnancy, are sufficient to support hypertrophic growth of each cardiac myocyte. However, the ER-PQC and UPR are insufficient to respond to the challenge of cardiac myocyte growth under pathological conditions, including myocardial infarction and heart failure. In part, this insufficiency is due to a continual decline in the expression levels of important adaptive UPR components as a function of age and during myocardial pathology. This chapter will discuss the physiological and pathological conditions unique to the heart that involve ER-PQC, and whether the UPR is adaptive or maladaptive under these circumstances.
1 Introduction
In most cells, including those comprising the heart, the rough endoplasmic reticulum (ER) serves as the location for the synthesis of most secreted and membrane proteins(Palade and Siekevitz 1956; Reid and Nicchitta 2012). This accounts for at least 35% of all proteins, emphasizing the importance of ensuring the quality of these proteins. In the heart, a number of pathological conditions including ischemia, ischemia/reperfusion and hypertrophic myocyte growth can place high demands on the ER protein-folding machinery and, in some cases can cause ER stress (Glembotski 2007; Minamino and Kitakaze 2010) (Fig. 1A), which impairs ER protein folding and leads to activation of the unfolded protein response (UPR). As in other tissue types, the UPR in the heart, which comprises the ER protein quality control (ER-PQC) system, consists of three major branches that are mediated be the ER-transmembrane sensors of ER-misfolded proteins, PERK, IRE-1 and ATF6 (Fig. 1B–D), each of which transduces the signal initiated by SR/ER protein misfolding to downstream events(Glembotski 2007, 2008; Doroudgar and Glembotski 2013b). The ER-PQC is responsible for ensuring that proteins made in the ER are properly folded and correctly modified post-translationally, for example by glycosylation or disulfide bond formation, before they are permitted to the leave the ER on the way to their eventual target destinations(Walter and Ron 2011; Gardner et al. 2013). Proteins that do not pass ER-PQC are not permitted to move on, and instead are translocated back out of the ER lumen and subjected to proteasome-mediated degradation in a process called ER associated degradation, or ERAD (Plemper and Wolf 1999; Pisoni and Molinari 2016) (Fig. 1E). Acute activation of PERK, IRE-1 and ATF6 leads to restoration of ER protein folding and cell survival. However, in the event that ER protein folding is not restored, the UPR remains activated, and this chronic activation leads to initiation of cell death. While the precise mechanisms responsible for switching the UPR from adaptive to maladaptive are not well understood, it is apparent that, if activated chronically, each branch of the UPR can lead to initiation of death pathways. Temporal changes in the UPR gene program are likely to be at least partly responsible for the transition of the UPR from adaptive to maladaptive(Walter and Ron 2011; Hetz et al. 2015; Ryoo 2016). In the heart it is apparent that prolonged activation of the UPR leads to decreased expression of adaptive UPR gene products, such as ER chaperones and protein disulfide isomerases, and increased expression of pro-death UPR gene products, such as C/EBP homologous protein (CHOP), which can contribute to the switch of the UPR from adaptive to maladaptive (Sano and Reed 2013; Li et al. 2014; Ryoo 2016). Targeted gene deletion studies, which are discussed in the last section of this review, have shown that PERK is adaptive in the heart (Fig. 1B), and is required for optimal cardiac function in models of myocardial disease, while CHOP, which lies downstream of PERK, is maladaptive (Fig. 1F). Additional studies have shown that the UPR-regulated transcription factors XBP1s and ATF6 serve novel adaptive roles in the heart by inducing genes required for cytosolic protein O-GlcNAcylation (Fig. 1G) and an array of about 400 UPR gene products (Fig. 1H), respectively.
Figure 1. Effects of Cardiac Pathology on ER Stress and the Unfolded Protein Response in Cardiac Myocytes.
Shown is a diagram of the rough ER with attached ribosomes translating mRNAs that encode ER luminal proteins. Conditions that impair the folding of nascent ER proteins, which include ischemia, ischemia/reperfusion and hypertrophy can result in ER stress (A). Under non-stressed conditions, the ER-luminal chaperone, glucose-regulated protein 78 kDa (GRP78) associates with the luminal domains of the 3 proximal effectors of ER stress, PERK (B), IRE-1 (C) and ATF6 (D). Upon ER stress, GRP78 relocates from the luminal domains of these proteins to misfolded proteins and either facilitates their folding, or escorts them to the degradation machinery (E). The disassociation of GRP78 from PERK, IRE-1, as well as the binding of misfolded proteins to IRE-1 and, possibly to PERK, allows their oligomerization, which fosters trans-phosphorylation and activation of these effectors. In the case of ATF6, dissociation of GRP78 and the association of escorts, such as thrombospondin 4, facilitate the movement of ATF6 to the Golgi, where it is cleaved by site 1 and site 2 proteases that reside in the Golgi. The resulting N-terminal fragment is liberated from the Golgi, translocates to the nucleus, and binds to ER stress response elements in ER stress response (ERSR) genes, and regulates their transcription (D). Activated PERK (B) phosphorylates eIF2α, which fosters transient global translational repression and the translation of the ATF4 mRNA from an alternate start site to generate active ATF4 using an alternate open reading frame (ORF). While PERK appears to be cardioprotective, the ATF4-mediated induction of CHOP is pro-apoptotic in the heart (F). Activated IRE-1 splices the unspliced form of XBP1 mRNA (XBP1u mRNA) to generate a splice variant form (XBP1s mRNA) that encodes the active transcription factor, XBP1s (C). Like ATF6, XBP1s and ATF6 translocate to the nucleus, and bind to various types of regulator elements in ERSRs to regulate their expression. One important group of XBP1s genes in the heart are those that increase cytosolic protein O-GlcNAcylation, which is cardioprotective (G). Depending on whether ER stress and UPR gene induction is acute or chronic, as with other tissues, in the heart the results are either adaptive (acute), oriented toward resolution of ER stress and cell survival (G,H), or maladaptive (chronic), activating death pathways (F).
2 ER-protein Quality Control in Cardiac Myocytes
Heart disease includes both vascular and cardiac diseases. Vascular diseases, such as atherosclerosis, affect blood vessels and blood, while cardiac diseases affect the heart muscle, or myocardium and impair its ability to efficiently propel blood to organs and tissues. While ER-PQC, ER stress and the UPR have been studied extensively in the setting of vascular diseases(Zhou and Tabas 2013; Ivanova and Orekhov 2016), until relatively recently, these topics have attracted less attention in studies of the myocardium. Among the possible reasons for this are the lack of appreciation of the importance of the ER as a site of protein synthesis in cardiac myocytes, as well as the relatively ill-defined location and function of the ER protein synthetic machinery in cardiac myocytes. Contributing to these reasons is the complicated structure of the ER in cardiac myocytes, most of which comprises the sarco/endoplasmic reticulum (SR), a specialized form of the ER that surrounds the contractile elements of myocytes, and is recognized primarily for its roles in the regulated release and storage of the calcium that underlies contraction and relaxation, respectively, in a process called contractile calcium handling (Fig. 2A) (Bers 2002, 2008; Sobie and Lederer 2012). In addition to the SR, there is a peri-nuclear ER that is structurally contiguous with the SR and as in most cells(Franke 1977), in cardiac myocytes appears to have the molecular machinery necessary for the synthesis of secreted and membrane proteins (Fig. 2B)(Nakayama et al. 2010). What remains unknown is t the extent of overlap between the SR and ER in terms of contractile calcium handling, and secreted and membrane protein synthesis(Glembotski 2012; Millott et al. 2012; Groenendyk et al. 2013). Although there have been few studies that directly address these unknowns, it appears as though both the ER and SR of cardiac myocytes have some of the molecular machinery usually identified with secreted and membrane protein synthesis (Fig. 2C) and quality control, such as the ER chaperone GRP78, consistent with a possible role for the SR in the synthesis of secreted and membrane proteins. It also appears as though the calcium stored within the peri-nuclear ER of cardiac myocytes may be released into the nucleus (Fig. 2D), perhaps in coordination with contraction and each depolarization event(Ljubojevic and Bers 2015). Moreover, in contrast to SR calcium, ER calcium in cardiac myocytes can also be released in an IP3-dependent manner, somewhat like other cells types; this IP3-sensitive calcium pool may facilitate cell surface receptor regulated events in cardiac myocytes that rely on the local release of calcium that does not drive contraction (Fig. 2E)(Nakayama et al. 2010). Thus, there seems to be some, but not complete overlap in ER and SR function.
Figure 2. Sarco/endoplasmic Reticulum Network in a Cardiac Myocyte.
Shown is a diagram of a cardiac myocyte depicting the relationships between the sarcoplasmic reticulum (SR) involved in contractile calcium handling (A), the perinuclear ER involved in secreted and membrane protein synthesis (B), the SR that may be involved in secreted and membrane protein synthesis (C), the perinuclear ER involved in nuclear calcium signaling (D) and the perinuclear ER involved in local cytosolic calcium signaling (E). Also shown are the actin and myosin myofilaments that comprise the contractile apparatus of cardiac myocytes.
Cardiac myocytes comprise the major mass of the myocardium(Zak 1974; Banerjee et al. 2007), where they are responsible for contraction and the movement of blood throughout the body. Therefore, conditions that impair cardiac myocyte contraction, such as those that affect contractile calcium handling, or the ability of sarcomeres to generate force can be life threatening. The left ventricle (LV) is the largest chamber of the heart and is the main pump that supplies all organs and tissues with oxygenated blood. Because of its dominant role as a pumping chamber, diseases that affect LV function have widespread impact and can be life threatening. Therefore, most studies of the heart focus on the structure and function of the myocytes that comprise the LV, with a particular emphasis on sarcomeric proteins and SR calcium handling, and less focus on the synthesis of secreted and membrane proteins in the ER. This is surprising, considering that many membrane and secreted proteins required for proper cardiac contractility and endocrine/paracrine function are made in the ER(Glembotski 2012). It is interesting to note that atrial myocytes, which are less critical for propelling blood than ventricular myocytes, are distinct from ventricular myocytes, since they are professional secretory cells, responsible for the production of the hemodynamic hormone, atrial natriuretic peptide(de Bold 2011). Relatively recent studies demonstrating that, in addition to ER-targeted proteins, proteins bound for other locations are also translated on ER ribosomes, coupled with the finding that ER ribosome translation is more efficient that free ribosome translation(Reid and Nicchitta 2012, 2015) have spawned new interest in determining roles for ER-PQC and the UPR in cardiac mycoytes under physiological and pathological conditions. A number of conditions can arise in cardiac myocytes that can affect ER-PQC, including decreased ER/SR calcium and the generation of reactive oxygen species (ROS), both of which occur during a number of cardiac pathologies(Kranias and Bers 2007; Ward et al. 2014). However, when it comes to the UPR, one often-overlooked condition that may challenge the ER-PQC in health and disease is cardiac myocyte growth(Glembotski 2014; Doroudgar et al. 2015b).
3 Physiological Conditions that Challenge the ER-PQC in Cardiac Myocytes
Because of the increased protein synthesis, and thus, protein folding demands during growth, PQC in the ER and elsewhere are of great importance in cardiac myocytes during physiological and pathological growth of the heart(Shimizu and Minamino 2016). The heart is a very plastic organ(Hill and Olson 2008), and there are numerous conditions under which cardiac myocytes exhibit dramatic changes in number and size, beginning with development(Heineke and Molkentin 2006). The embryonic myocardium grows mostly by the rapid division of relatively small cardiac myocytes, or hyperplasia. This process is depicted for the LV in Figure 3A. However, soon after birth, within 7–14 days in mice, the replicative capacity of cardiac myocytes decreases dramatically; thus, during most of post-natal development, when the heart increases in size to meet the demand for circulating greater volumes of blood, myocardial growth is primarily the result of increased cardiac myocyte size, or hypertrophy (Fig. 3B). During both of these growth phases it is essential that the protein-folding machinery in cardiac myocytes, including the ER-PQC system, have the capacity to meet the demands placed on it by increased protein synthesis. Thus, we posited that the UPR contributes significantly to the PQC machinery that supports hyperplasic and hypertrophic cardiac myocyte growth. Consistent with this hypothesis is the finding that expression of numerous UPR genes is relatively high in the neonatal and juvenile rodent heart, compared to the adult heart, when cardiac myocyte growth has ceased(Doroudgar et al. 2015a; Taylor 2016). Moreover, neonatal and juvenile mouse hearts exhibit a robust adaptive UPR, which is much weaker in the adult mouse heart. It is precisely this decrease of UPR gene expression in the adult heart that poses a potential problem in the event that cardiac myocyte growth is reinitiated, depending on the stimulus for that growth.
Figure 3. Growth of the Left Ventricle during Pre- and Post-natal Development.
Cross sections of the mammalian left ventricle (LV) of the heart are shown diagrammatically, with the relative size depicting the changes in LV mass at different stages of development in the newborn (A) or the adult (B). The red areas are representations of the myocardium. The relative sizes of the arrows and blood represent the relative amounts of blood pumped, or ejected by the LV at different stages of development. The cardiac myocyte diagrams below each LV represent the relative changes in number and size during development. Since increases in LV muscle mass by hyperplasic or hypertrophic growth require increases in protein-folding machinery, and since there is not ER stress or cell death associated with LV myocyte growth during pre- and post-natal development, endoplasmic reticulum-protein quality control (ER-PQC) and unfolded protein response (UPR) machinery are sufficient to support myocardial growth.
There are also physiological conditions under which cardiac myocytes resume growth in the adult, including exercise and pregnancy(Hill and Olson 2008; Shimizu and Minamino 2016). Under these conditions myocardial growth is adaptive, resulting in increases in the ability of the LV to pump blood to meet the new demands for increased blood flow. During both exercise and pregnancy, myocardial growth is hypertrophic (Fig. 4). In this case, as with myocardial growth during development, myocytes grow in diameter more than in length, a process that is called concentric hypertrophy(van Berlo et al. 2013). Myocardial growth during exercise and pregnancy is reversible and does not lead to a pathological growth of the myocardium(Wasfy and Weiner 2015; Roh et al. 2016). Finally, while it is not well studied, the increased myocyte size during exercise and pregnancy is associated with increased protein synthesis(Catalucci et al. 2008; Li et al. 2012; Chung and Leinwand 2014) and thus would be expected to increase the demands on protein folding machinery in the ER, as well as in the cytosol; however, it is evident that the UPR in the adult heart is sufficient to meet the demands of increased protein synthesis, because under these conditions, there does not appear to be any myocyte death. Therefore, since this growth is limited in extent and time, and since the signal transduction initiated during physiological growth differs significantly from that during pathological growth(Maillet et al. 2013; van Berlo et al. 2013; Lerchenmuller and Rosenzweig 2014), it appears as though physiological growth of the adult myocardium does not pose a challenge to the ER-PQC machinery. However, the impact on the ER-PQC may be different under conditions of growth during myocardial pathology(Glembotski 2014).
Figure 4. Growth of the Left Ventricle during Exercise and Pregnancy.
Cross sections of the LV and cardiomyocytes are depicted, as in Figure 3. During exercise and pregnancy the LV grows adaptively by concentric hypertrophy of each cardiomyocyte, i.e. increased diameter but not increased length, while the number of myocytes remains unchanged. Since this growth is adaptive, and is not associated with ER stress or myocyte death, the ER-PQC and UPR are sufficient to support the growth.
4 Pathological Conditions that Challenge the ER-PQC in Cardiac Myocytes
Diseases that affect the myocardium can be genetic or environmental. For example, mutations(Burke et al. 2016; Wilsbacher and McNally 2016), certain drugs, such as anti-neoplastics(Lee 2015; Molinaro et al. 2015), and pathogens(Fung et al. 2016; Yajima and Knowlton 2009) can affect sarcomere structure and impact contractile function to the point where LV pump action is severely impaired, leading eventually to heart failure and death (Fig. 5A). Although less common than mutations in sarcomeric proteins, mutations in calcium handling proteins and ion channels can lead to arrhythmias as well as contraction deficits that can contribute to sudden death, cardiac arrest and, in some cases heart failure(Bezzina et al. 2015; Wagner et al. 2015; Curran and Mohler 2015; Vatta and Ackerman 2010). In many of these cases, expression of UPR components is upregulated, although it is currently unclear as to whether this upregulation is a cause or a consequence of the myocardial defect(Liu and Dudley 2015).
Figure 5. Effect of Ischemia, Myocardia infarction, Mutations, Drugs and Pathogens on Cardiac Contractility.
(A) Myocardial infarction (MI) results in irreparable damage to the myocardium, which decreases LV contractility. Ischemic cardiomyocytes that eventually die from ischemia have insufficient ER-PQC and UPR machinery. (B) Mutations, drugs, mostly anti-neoplastic agents, and pathogens, such as viruses, also impair cardiomyocyte contractility. These insults are associated with an insufficient ER-PQC and UPR, leading to maladaptive ER stress, which contributes to myocyte dropout by apoptosis.
Diseases that affect the myocardium can also develop as a result of injuries, such as myocardial infarction (MI) (Fig. 5B). In many cases, MI is the result of insufficient coronary blood flow to the myocardium, which is sometimes a consequence of atherosclerosis. This insufficient flood flow causes a lack of oxygen and nutrients, i.e. ischemia, that can eventually lead to death of the affected myocardium(Frangogiannis 2015). Surrounding the infarcted myocardium is the peri-infarct region, or border zone, in which the myocytes remain viable, but are stressed, due to the near lethal ischemia. In border zone myocytes, markers of the UPR are increased, and it is possible that these genes encode proteins that increase the survival of cardiac myocytes in the border zone.
Since, the damaged myocardium in adults cannot be regenerated, MI damage is essentially permanent, and can set in motion a pathological remodeling of the heart that can lead to serious functional impairment, culminating eventually in life threatening heart failure(Rosenzweig 2012). Heart failure afflicts about 6 million people(Roger et al. 2012) and is the single most common discharge diagnosis in those over 65(DeFrances and Podgornik 2006). Moreover, the disease is especially sobering because the limited regenerative capacity of the myocardium, coupled with the current absence of an effective therapy for the disease, leave heart transplant as the only cure. In the early stages of heart failure the myocardium grows by concentric hypertrophy by a processes called hypertrophic cardiomyopathy, or HCM (Fig. 6A). In addition to MI, hypertension can also initiate HCM(Hoenig et al. 2008). In contrast to physiological hypertrophy, which stops when optimal benefit is obtained, this pathological hypertrophy, which is caused by a concentric growth of cardiac myocytes, does not resolve(van Berlo et al. 2013; Hill and Olson 2008; Frey et al. 2004). Initially, HCM is thought by some to be compensatory, providing for sustained, perhaps even improved LV function. However, as the myocardium remodels, this HCM often transforms into dilated cardiomyopathy (DCM), a condition associated with a decrease in myocyte numbers and an eccentric hypertrophy associated with the elongation of cardiomyocytes, in the absence of a compensating increase in diameter (Fig. 6B). This chamber dilation, associated with a severe weakening of the LV muscle, leads to decreased LV blood ejection and ultimately, to life-threatening heart failure (HF)(Lazzeroni et al. 2016).
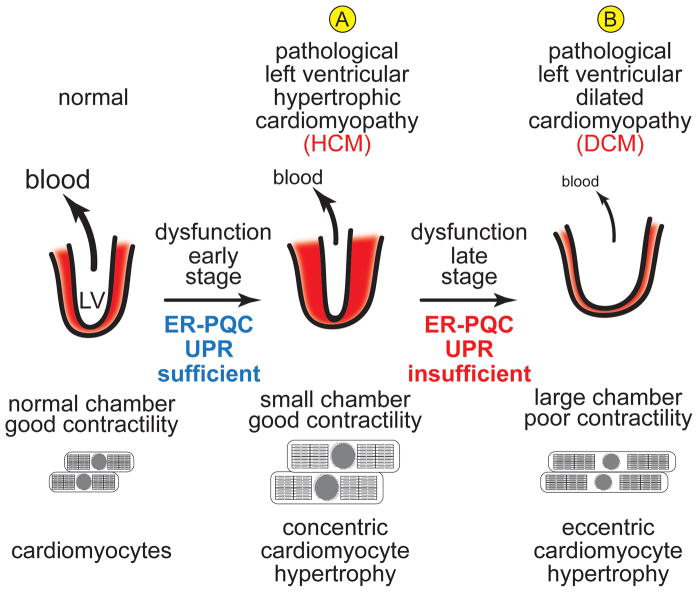
Figure 6. Development of Cardiomyopathy in the Diseased Heart.
Ischemia, MI, mutations, drugs and pathogens often lead to (A) hypertrophic cardiomyopathy (HCM), a condition in which each LV myocyte grows concentrically, i.e. increased diameter. This hypertrophic growth of the LV does not lead to increased pump function, and in many cases LV pump function is decreased, as the LV wall growth is so extensive that the chamber size is decreased. This hypertrophy is considered pathological because it usually leads to further remodeling and deterioration of LV structure and LV dysfunction in the late stage of pathology, associated with cardiomyocyte apoptosis, as well as an eccentric growth of remaining cardiomyocyte, i.e. increased length, which does not enhance cardiomyocyte contractility. This results in a dilation of the LV so chamber size increases, but since LV muscle mass is decreased, the ability of the LV to pump blood is severely impaired, leading to dilated hypertrophic cardiomyopathy (DCM) and eventually to heart failure (B). In the early stage of the disease, the ER-PQC and UPR are sufficient to handle cardiomyocyte growth; however, in the late stage of the disease, maladaptive ER stress ensues, and the ER-PQC and UPR are not sufficient to maintain LV structure and function.
While ER-PQC is compromised in ischemic heart disease and hypertrophic cardiomyopathy, where misfolded proteins accumulate within cardiac myocytes, it is underappreciated that cardiac pathology can also stem from accumulation of misfolded proteins in the extracellular space(Shi et al. 2010). In cancer patients diagnosed with light chain amyloidosis, clonally expanded plasma cells secrete amyloidogenic light chains that misfold in the circulation(Cooley et al. 2014). These circulating amyloids aggregate into soluble proteotoxic oligomers, forming amyloid fibril deposits predominantly in the heart. This leads to impaired myocyte contractility, myocyte oxidative stress, and ultimately, myocyte death. Patients diagnosed with this disease typically cannot tolerate standard treatments meant to preserve cardiac function during heart failure, including β-adrenergic receptor blockers, angiotensin converting enzyme inhibitors, and digoxin(Meier-Ewert et al. 2011). Another form of cardiac amyloidosis is familial atrial fibrillation, in which the electrical conduction system of the heart, specifically in the atria, is disrupted by deposition of mutant amyloidogenic forms of transthyretin and atrial natriuretic peptide, secreted by the liver and the atria, respectively(Hodgson-Zingman et al. 2008). Patients with dilated cardiomyopathy stemming from cardiac amyloidosis and atrial fibrillation patients do not tolerate standard arrhythmia treatments. In heart failure patients diagnosed with light chain cardiac amyloidosis, cancer remission coincides with improved cardiac function(Meier-Ewert et al. 2011), suggesting that stemming the production of amyloidogenic proteins may be a therapeutic approach to treating cardiac pathologies resulting from compromised protein quality control in the extracellular space. Interestingly, it has been shown that activation of the UPR increases expression and secretion of a subset of chaperones that prevent aggregation of misfolded proteins in the extracellular space(Genereux et al. 2015). Additionally, it has been shown that stress-independent activation of the UPR can prevent secretion of amyloidogenic light chains while not affecting secretion of non-amyloidogenic light chains(Cooley et al. 2014). Given that the activation of the UPR can also attenuate oxidative stress-mediated myocyte death, the UPR can be therapeutically targeted to treat forms of heart disease where proteotoxicity occurs from outside of the cell.
5 Experiment Methods and Models for Studying ER-PQC in Cardiac Pathology
For the most part, the relationship between cardiac pathologies and the UPR has been examined in several models, including primary cultures of cardiac myocytes and hearts of genetically modified mice. In these model systems there are several maneuvers that are accepted as mimics of heart disease in humans. For example, cultured cardiac myocytes can be subjected to conditions that simulate those in the ischemic heart, i.e. oxygen and glucose deprivation. But even more clinically relevant is subjecting cultured cardiac myocytes to simulated ischemia followed by reperfusion (I/R), which is meant to mimic the clinically relevant scenario the heart of an MI patient might experience upon reopening of a blocked coronary vessel by angioplasty(Webster et al. 1995). Cardiac myocyte damage and viability are impacted during both ischemia and reperfusion, with reperfusion damage being caused primarily by reactive oxygen species (ROS) (Chen and Zweier 2014). Finally, in some cultured cardiac myocyte models, hypertrophic growth of myocytes can be induced with growth promoters, such as α-adrenergic receptor agonists, which mimic some aspects of pathological growth(O’Connell et al. 2014). However, each of these cultured cell models have limitations. For example, due to technical reasons, hypertrophic myocyte growth is studied in cultured neonatal rat cardiac myocytes, which has limitations because, at this stage of development cardiac myocytes are much more plastic and able to exhibit growth in ways that do not replicate the growth observed in the adult rodent heart(Louch et al. 2011).
As important as cultured cell models are for examining molecular mechanisms, more relevant to human pathology are mouse models in which heart disease can be studied in vivo. Three surgical mouse models of myocardial heart disease are commonly used to mimic ischemia, ischemia/reperfusion and pathological hypertrophy; they are myocardial infarction (MI), ischemia/reperfusion (I/R) and transaortic constriction (TAC), respectively(Abarbanell et al. 2010). MI surgery involves permanent ligation of the left anterior descending coronary artery (LAD), which is the artery most commonly blocked in patients suffering from an MI. I/R surgery involves ligating the LAD the same manner as MI surgery, but for only about 30 min, followed by release and subsequent reperfusion. TAC surgery involves a partial restriction of the aorta, which increases the pressure against which the ventricle must work to expel blood, thus mimicking hypertension. Following these surgical procedures, cardiac function can be examined several ways, but the most widely used non-invasive approach is echocardiography. The effect of each of these surgeries on cardiac structure is generally examined in postmortem histological examination of hearts for myocyte size and number, as well as apoptosis and gene expression.
6 Studies of ER-PQC in Cardiac Pathology
While there have been numerous reports of how various mouse models of heart disease effect changes in UPR gene expression, such reports are of limited impact to our understanding of whether and how the UPR contributes to, or is a result of cardiac pathology. Instead, more revealing are mouse models in which specific genes of interest have been overexpressed, knocked down or out in the heart, which allows a more reliable interrogation of the effects of gain- or loss-of-function of known master regulators of the UPR on cardiac structure and function in response to models of heart disease. Accordingly, instead of compiling many studies that demonstrate a correlation between heart disease and the UPR, in the following section, a few of the highest impact studies that more clearly establish cause and effect between the UPR and heart disease are summarized. These studies have been categorized by which major branch of the UPR has been examined.
6.1 PERK/CHOP
The major downstream effect of activating the PERK branch of the UPR is eIF2α phosphorylation on serine 51, which attenuates global translation but increases translation of the transcription factor, ATF4 (Fig. 1B). Upon prolonged activation of the UPR, ATF4 increases expression of the pro-apoptotic transcription factor, C/EBP homologous protein (CHOP)(Malhotra and Kaufman 2007; Ron and Walter 2007). Since apoptosis is a major contributor to the decline in cardiac function observed during heart failure and other cardiac pathologies(Foo et al. 2005), and since CHOP expression is increased in experimental models of heart disease(Okada et al. 2004), several studies have focused on the effects of CHOP gene deletion in the mouse heart.
In genetically modified mice in which CHOP has been deleted in all tissues, i.e. global knockout, in the setting of MI it was shown that compared to WT mice, CHOP KO mice exhibited increased death; however, the mice that survived had increased pathological hypertrophy of the LV, but no significant change in cardiac function or fibrosis(Luo et al. 2015). These results suggested that CHOP deletion does not negatively effect cardiac structure and function during permanent occlusion of the LAD, which is not usually associated with significant increases in apoptosis, leaving unanswered the question of whether CHOP contributes to apoptosis in the diseased heart.
The effect of CHOP on apoptosis in the diseased myocardium was examined in a different study in which the same line of CHOP KO mice was subjected to I/R surgery(Miyazaki et al. 2011), a maneuver known to increase apoptosis in the heart. In that study, it was shown that CHOP deletion decreased infarct size by decreasing apoptosis, as well as decreasing myocardial inflammation. In the same study, a mechanistic examination in mouse hearts and in cultured cardiac myocytes showed that in WT cardiac myocytes, the ROS generated upon simulated reperfusion activated CHOP, which induced apoptosis and inflammation gene expression.
Since CHOP is increased in heart failure, a study designed to examine the effects of CHOP in the hearts of mice subjected to TAC, which induces heart failure, also used the same line of CHOP KO mice(Fu et al. 2010). In that study it was found that the CHOP KO mice exhibited reduced signs of heart failure after TAC surgery, including reduced hypertrophy, myocyte size, myocyte apoptosis, lung weight and cardiac fibrosis, compared to WT mice. These results suggested that CHOP contributes to UPR-mediated apoptotic myocyte death and, therefore, is responsible for at least a portion of heart failure in this model of hypertension.
The effects of PERK deletion have also been examined in the mouse heart(Liu et al. 2014). However, since global deletion of PERK causes mouse growth retardation, this study examined the effects of deleting PERK specifically in cardiac myocytes using a conditional gene targeting approach. In this study it was found that under non-stress conditions, PERK deletion had no effect on mouse heart structure and function. However, in a TAC model of heart failure, compared to WT mice, PERK KO mice exhibited decreased cardiac function, as well as exacerbated LV fibrosis and myocyte apoptosis. These findings indicate that PERK is required to protect the heart from pressure-overload induced heart failure. Taken together, these studies indicate that the in the heart, the PERK branch of the UPR moderates the damaging effects of TAC-induced heart failure, so in that regard PERK is in some way protective. While the effects of PERK deletion on MI and I/R have not yet been studied, it is apparent that the apoptotic effector of the PERK branch of the UPR, CHOP, exacerbates the TAC-induced heart failure, as well as I/R damage in the mouse heart.
6.2 IRE-1/XBP1
One of the major targets of IRE-1 is the transcription factor, XBP1. When activated by ER stress, the endonuclease activity of IRE1 promotes the splicing or the mRNA encoding XBP1 from a form encoding an inactive protein, to one encoding an active transcription factor, spliced XBP1, XBP1s(Groenendyk et al. 2013). In mouse hearts ischemia activates the UPR and increases the formation of XBP1s, which protects from I/R damage of cardiac myocytes(Thuerauf et al. 2006). Moreover, I/R increases protein O-GlcNAcylation in the hearts of mice subjected to I/R, in vivo, and O-GlcNAcylation decreases I/R damage in mouse hearts(Ngoh et al. 2011). In a study of the effects of XBP1 gain- and loss-of-function in cardiac myocytes of mice, it was shown that XBP1s protects mouse heart from I/R damage by transcriptionally inducing key genes in the pathway responsible for the biosynthesis of hexoses required for protein O-GlcNAcylation(Wang et al. 2014). Interestingly, O-GlcNAcylation in the heart is not always protective. For example, in models of Type II diabetes, O-GlcNAcylation has been shown to increase cardiac, dysfunction(Dassanayaka and Jones 2014), partly through the O-GlcNAcylation and hyperactivation of CaMKII, which deleteriously affects myocardial contractility(Erickson et al. 2013).
6.3 ATF6
ATF6 is activated, and many of its downstream target genes are induced in a variety of cardiac pathologies, including those induced in mice by MI, I/R and TAC(Glembotski 2014). Moreover, acute conditional activation of ATF6 in mouse hearts protects the heart from I/R damage(Martindale et al. 2006). It has been known for some time that in the absence of ER stress, the ER-resident chaperone, GRP78 can bind to, and anchor ATF6 in the lumen of the ER(Shen et al. 2002). Upon ER stress, GRP78 releases its stronghold on ATF6, allowing it to leave the ER and translocate to the Golgi, where it is cleaved by S1 and S2 proteases to generate its transcription active form(Ye et al. 2000). However, a recent study showed that thrombospondin 4, a component of the extracellular matrix that is made and folded in the ER lumen, serves an escort role by binding to, and facilitating the movement of ATF6 from the ER to the Golgi(Lynch et al. 2012). In this study it was shown that even in the absence of ER stress, ATF6 translocates from ER to Golgi upon overexpression of thromspondin 4 in the heart, while thrombospondin 4 deletion decreases this translocation. Moreover, thrombospondin 4 gain- and loss-of-function in the heart decreased or increased, respectively, the damage in mouse hearts subjected to MI surgery. Thus, during secretion thrombospondin 4 functions as an escort for ATF6 relocation from the ER to the Golgi, and then after it is secreted (Fig. 1D escort), thrombospondin 4 plays a second role as an extracellular matrix protein. As a result of this study, it has been hypothesized that thrombospondin 4 competes with GRP78 for binding to ATF6, and that while GRP78 cloaks the Golgi localization sequence on ATF6, by displacing GRP78, thrombospondin 4 reveals this sequence, thus facilitating ATF6 translocation(Doroudgar and Glembotski 2013a). In other studies it was shown that, in the mouse heart acute activation of ATF6 induces about 400 genes in cardiac myocytes, some of which were not previously known to be ATF6 ER stress-inducible genes, and have subsequently been shown to participate in the adaptive gene program regulated by ATF6 in the mouse heart upon MI, I/R or TAC(Belmont et al. 2008). Recently, it was shown that, compared to WT mice, ATF6 KO mouse hearts exhibit greater I/R damage, decreased function, and increased apoptosis and necrosis compared(Jin et al. 2016). In this study it was shown that the expression levels of numerous oxidative stress response genes were decreased in ATF6 KO mouse hearts, identifying many of them for the first time as ATF6-inducible, ER stress response genes. Interestingly, the ATF6-dependent oxidative stress genes in the mouse heart encode antioxidant proteins that reside in outside of the ER, demonstrating, for the first time that ATF6 links ER stress and oxidative stress response pathways, thus explaining a mechanism by which acute activation of ATF6 can mitigate I/R damage in the heart, which is caused mainly by ROS generation in mitochondria.
7 Conclusions
Secreted and membrane protein synthesis in the ER is of critical importance to normal cardiac myocyte contractile function; however, the processes that oversee and govern the quality of secreted and membrane protein synthesis in cardiac myocytes have been unappreciated and, therefore, unstudied until relatively recently. The ER-PQC and UPR in cardiac myocytes appear to be robust and able to meet the challenges of ER protein synthesis and folding during physiological growth of the heart. However, during pathologies that can also initiate myocardial growth, especially in the adult, the adaptive ER-PQC and UPR in cardiac myocytes is insufficient, leading to accumulation of proteotoxic, terminally misfolded proteins, aggregate formation and eventual impairment of cardiac myocyte contractile function and heart failure. Emerging areas of interest in the field include determining what is different about physiological and pathological cardiac myocyte growth in terms of ER-PQC and the UPR, in hopes of designing therapeutic approaches to decrease myocyte death and loss of LV function during pathology. One clear area of interest has been spawned by the finding that the expression levels of essential components of the adaptive UPR machinery decrease as a function of age, while the propensity for developing cardiac pathology increases as a function of age. This finding has led to the exploration of therapeutic approaches aimed at enhancing the adaptive UPR in the aged, pathologic heart in hopes of improving the synthesis, folding and trafficking of secreted and membrane proteins in ways that are anticipated to enhance cardiac myocyte contractility and reduce the progression to heart failure.
References
- Abarbanell AM, Herrmann JL, Weil BR, Wang Y, Tan J, Moberly SP, Fiege JW, Meldrum DR. Animal models of myocardial and vascular injury. J Surg Res. 2010;162(2):239–249. doi: 10.1016/j.jss.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293(3):H1883–1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- Belmont PJ, Tadimalla A, Chen WJ, Martindale JJ, Thuerauf DJ, Marcinko M, Gude N, Sussman MA, Glembotski CC. Coordination of growth and endoplasmic reticulum stress signaling by regulator of calcineurin 1 (RCAN1), a novel ATF6-inducible gene. J Biol Chem. 2008;283(20):14012–14021. doi: 10.1074/jbc.M709776200. M709776200 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- Bezzina CR, Lahrouchi N, Priori SG. Genetics of sudden cardiac death. Circ Res. 2015;116(12):1919–1936. doi: 10.1161/CIRCRESAHA.116.304030. [DOI] [PubMed] [Google Scholar]
- Burke MA, Cook SA, Seidman JG, Seidman CE. Clinical and Mechanistic Insights Into the Genetics of Cardiomyopathy. J Am Coll Cardiol. 2016;68(25):2871–2886. doi: 10.1016/j.jacc.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalucci D, Latronico MV, Ellingsen O, Condorelli G. Physiological myocardial hypertrophy: how and why? Front Biosci. 2008;13:312–324. doi: 10.2741/2681. [DOI] [PubMed] [Google Scholar]
- Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114(3):524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Leinwand LA. Pregnancy as a cardiac stress model. Cardiovasc Res. 2014;101(4):561–570. doi: 10.1093/cvr/cvu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley CB, Ryno LM, Plate L, Morgan GJ, Hulleman JD, Kelly JW, Wiseman RL. Unfolded protein response activation reduces secretion and extracellular aggregation of amyloidogenic immunoglobulin light chain. Proc Natl Acad Sci U S A. 2014;111(36):13046–13051. doi: 10.1073/pnas.1406050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J, Mohler PJ. Alternative paradigms for ion channelopathies: disorders of ion channel membrane trafficking and posttranslational modification. Annu Rev Physiol. 2015;77:505–524. doi: 10.1146/annurev-physiol-021014-071838. [DOI] [PubMed] [Google Scholar]
- Dassanayaka S, Jones SP. O-GlcNAc and the cardiovascular system. Pharmacol Ther. 2014;142(1):62–71. doi: 10.1016/j.pharmthera.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bold AJ. Thirty years of research on atrial natriuretic factor: historical background and emerging concepts. Canadian Journal of Physiology and Pharmacology. 2011;89(8):527–531. doi: 10.1139/Y11-019. [DOI] [PubMed] [Google Scholar]
- DeFrances CJ, Podgornik MN. 2004 National Hospital Discharge Survey. Adv Data. 2006;(371):1–19. [PubMed] [Google Scholar]
- Doroudgar S, Glembotski CC. ATF6 and thrombospondin 4: the dynamic duo of the adaptive endoplasmic reticulum stress response. Circ Res. 2013a;112(1):9–12. doi: 10.1161/CIRCRESAHA.112.280560. 112/1/9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudgar S, Glembotski CC. New concepts of endoplasmic reticulum function in the heart: Programmed to conserve. J Mol Cell Cardiol. 2013b doi: 10.1016/j.yjmcc.2012.10.006. S0022-2828(12)00374-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudgar S, Volkers M, Thuerauf DJ, Khan M, Mohsin S, Respress JL, Wang W, Gude N, Muller OJ, Wehrens XH, Sussman MA, Glembotski CC. Hrd1 and ER-Associated Protein Degradation, ERAD, are Critical Elements of the Adaptive ER Stress Response in Cardiac Myocytes. Circ Res. 2015a;117(6):536–546. doi: 10.1161/CIRCRESAHA.115.306993. CIRCRESAHA.115.306993 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroudgar S, Volkers M, Thuerauf DJ, Khan M, Mohsin S, Respress JL, Wang W, Gude N, Muller OJ, Wehrens XHT, Sussman MA, Glembotski CC. Hrd1 and ER-Associated Protein Degradation, ERAD, Are Critical Elements of the Adaptive ER Stress Response in Cardiac Myocytes. Circulation Research. 2015b;117(6):536–546. doi: 10.1161/CIRCRESAHA.115.306993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502(7471):372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115(3):565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG. Pathophysiology of Myocardial Infarction. Compr Physiol. 2015;5(4):1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- Franke WW. Structure and function of nuclear membranes. Biochem Soc Symp. 1977;(42):125–135. [PubMed] [Google Scholar]
- Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109(13):1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB109/13/1580. [pii] [DOI] [PubMed] [Google Scholar]
- Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, Asanuma H, Asakura M, Takashima S, Komuro I, Kitakaze M, Minamino T. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122(4):361–369. doi: 10.1161/CIRCULATIONAHA.109.917914. [DOI] [PubMed] [Google Scholar]
- Fung G, Luo H, Qiu Y, Yang D, McManus B. Myocarditis. Circ Res. 2016;118(3):496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5(3):a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genereux JC, Qu S, Zhou M, Ryno LM, Wang S, Shoulders MD, Kaufman RJ, Lasmezas CI, Kelly JW, Wiseman RL. Unfolded protein response-induced ERdj3 secretion links ER stress to extracellular proteostasis. EMBO J. 2015;34(1):4–19. doi: 10.15252/embj.201488896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glembotski CC. Endoplasmic reticulum stress in the heart. Circ Res. 2007;101(10):975–984. doi: 10.1161/CIRCRESAHA.107.161273. 101/10/975 [pii] [DOI] [PubMed] [Google Scholar]
- Glembotski CC. The role of the unfolded protein response in the heart. J Mol Cell Cardiol. 2008;44(3):453–459. doi: 10.1016/j.yjmcc.2007.10.017. S0022-2828(07)01283-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glembotski CC. Roles for the sarco-/endoplasmic reticulum in cardiac myocyte contraction, protein synthesis, and protein quality control. Physiology (Bethesda) 2012;27(6):343–350. doi: 10.1152/physiol.00034.2012. 27/6/343 [pii] [DOI] [PubMed] [Google Scholar]
- Glembotski CC. Roles for ATF6 and the sarco/endoplasmic reticulum protein quality control system in the heart. J Mol Cell Cardiol. 2014;71:11–15. doi: 10.1016/j.yjmcc.2013.09.018. S0022-2828(13)00291-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenendyk J, Agellon LB, Michalak M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol. 2013;75:49–67. doi: 10.1146/annurev-physiol-030212-183707. [DOI] [PubMed] [Google Scholar]
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. nrm1983 [pii] [DOI] [PubMed] [Google Scholar]
- Hetz C, Chevet E, Oakes SA. Proteostasis control by the unfolded protein response. Nat Cell Biol. 2015;17(7):829–838. doi: 10.1038/ncb3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358(13):1370–1380. doi: 10.1056/NEJMra072139. 358/13/1370 [pii] [DOI] [PubMed] [Google Scholar]
- Hodgson-Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, Ballew JD, de Andrade M, Burnett JC, Jr, Olson TM. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359(2):158–165. doi: 10.1056/NEJMoa0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. The cardiac microvasculature in hypertension, cardiac hypertrophy and diastolic heart failure. Curr Vasc Pharmacol. 2008;6(4):292–300. doi: 10.2174/157016108785909779. [DOI] [PubMed] [Google Scholar]
- Ivanova EA, Orekhov AN. The Role of Endoplasmic Reticulum Stress and Unfolded Protein Response in Atherosclerosis. Int J Mol Sci. 2016;17(2) doi: 10.3390/ijms17020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JK, Blackwood EA, Azizi KM, Thuerauf DJ, Fahem AG, Hofmann C, Kaufman RJ, Doroudgar S, Glembotski CC. ATF6 Decreases Myocardial Ischemia/Reperfusion Damage and Links ER Stress and Oxidative Stress Signaling Pathways in the Heart. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.310266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranias EG, Bers DM. Calcium and cardiomyopathies. Subcell Biochem. 2007;45:523–537. doi: 10.1007/978-1-4020-6191-2_20. [DOI] [PubMed] [Google Scholar]
- Lazzeroni D, Rimoldi O, Camici PG. From Left Ventricular Hypertrophy to Dysfunction and Failure. Circ J. 2016;80(3):555–564. doi: 10.1253/circj.CJ-16-0062. [DOI] [PubMed] [Google Scholar]
- Lee CS. Mechanisms of Cardiotoxicity and the Development of Heart Failure. Crit Care Nurs Clin North Am. 2015;27(4):469–481. doi: 10.1016/j.cnc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Lerchenmuller C, Rosenzweig A. Mechanisms of exercise-induced cardiac growth. Drug Discov Today. 2014;19(7):1003–1009. doi: 10.1016/j.drudis.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Li J, Umar S, Amjedi M, Iorga A, Sharma S, Nadadur RD, Regitz-Zagrosek V, Eghbali M. New frontiers in heart hypertrophy during pregnancy. Am J Cardiovasc Dis. 2012;2(3):192–207. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 2014;46(8):629–640. doi: 10.1093/abbs/gmu048. [DOI] [PubMed] [Google Scholar]
- Liu M, Dudley SC., Jr Role for the Unfolded Protein Response in Heart Disease and Cardiac Arrhythmias. Int J Mol Sci. 2015;17(1) doi: 10.3390/ijms17010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kwak D, Lu Z, Xu X, Fassett J, Wang H, Wei Y, Cavener DR, Hu X, Hall J, Bache RJ, Chen Y. Endoplasmic reticulum stress sensor protein kinase R-like endoplasmic reticulum kinase (PERK) protects against pressure overload-induced heart failure and lung remodeling. Hypertension. 2014;64(4):738–744. doi: 10.1161/HYPERTENSIONAHA.114.03811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubojevic S, Bers DM. Nuclear calcium in cardiac myocytes. J Cardiovasc Pharmacol. 2015;65(3):211–217. doi: 10.1097/FJC.0000000000000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louch WE, Sheehan KA, Wolska BM. Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol. 2011;51(3):288–298. doi: 10.1016/j.yjmcc.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Li Q, Zhang X, Shen L, Xie J, Zhang J, Kitakaze M, Huang X, Liao Y. Ablation of C/EBP homologous protein increases the acute phase mortality and doesn’t attenuate cardiac remodeling in mice with myocardial infarction. Biochem Biophys Res Commun. 2015;464(1):201–207. doi: 10.1016/j.bbrc.2015.06.117. [DOI] [PubMed] [Google Scholar]
- Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, Lynch KA, Okada T, Aronow BJ, Osinska H, Prywes R, Lorenz JN, Mori K, Lawler J, Robbins J, Molkentin JD. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149(6):1257–1268. doi: 10.1016/j.cell.2012.03.050. S0092-8674(12)00572-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14(1):38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18(6):716–731. doi: 10.1016/j.semcdb.2007.09.003. S1084-9521(07)00149-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98(9):1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. 01.RES.0000220643.65941.8d [pii] [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Sanchorawala V, Berk JL, Ruberg FL. Cardiac amyloidosis: evolving approach to diagnosis and management. Curr Treat Options Cardiovasc Med. 2011;13(6):528–542. doi: 10.1007/s11936-011-0147-4. [DOI] [PubMed] [Google Scholar]
- Millott R, Dudek E, Michalak M. The endoplasmic reticulum in cardiovascular health and disease. Can J Physiol Pharmacol. 2012;90(9):1209–1217. doi: 10.1139/y2012-058. [DOI] [PubMed] [Google Scholar]
- Minamino T, Kitakaze M. ER stress in cardiovascular disease. J Mol Cell Cardiol. 2010;48(6):1105–1110. doi: 10.1016/j.yjmcc.2009.10.026. S0022-2828(09)00469-6 [pii] [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, Ogawa H, Oike Y. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):1124–1132. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- Molinaro M, Ameri P, Marone G, Petretta M, Abete P, Di Lisa F, De Placido S, Bonaduce D, Tocchetti CG. Recent Advances on Pathophysiology, Diagnostic and Therapeutic Insights in Cardiac Dysfunction Induced by Antineoplastic Drugs. Biomed Res Int. 2015;2015:138148. doi: 10.1155/2015/138148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Bodi I, Maillet M, DeSantiago J, Domeier TL, Mikoshiba K, Lorenz JN, Blatter LA, Bers DM, Molkentin JD. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ Res. 2010;107(5):659–666. doi: 10.1161/CIRCRESAHA.110.220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40(3):895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell TD, Jensen BC, Baker AJ, Simpson PC. Cardiac alpha1-adrenergic receptors: novel aspects of expression, signaling mechanisms, physiologic function, and clinical importance. Pharmacol Rev. 2014;66(1):308–333. doi: 10.1124/pr.112.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;1106:705–712. doi: 10.1161/01.CIR.0000137836.95625.D401.CIR.0000137836.95625.D4. [pii] [DOI] [PubMed] [Google Scholar]
- Palade GE, Siekevitz P. Liver microsomes; an integrated morphological and biochemical study. J Biophys Biochem Cytol. 1956;2(2):171–200. doi: 10.1083/jcb.2.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni GB, Molinari M. Five Questions (with their Answers) on ER-Associated Degradation. Traffic. 2016;17(4):341–350. doi: 10.1111/tra.12373. [DOI] [PubMed] [Google Scholar]
- Plemper RK, Wolf DH. Endoplasmic reticulum degradation. Reverse protein transport and its end in the proteasome. Mol Biol Rep. 1999;26(1–2):125–130. doi: 10.1023/a:1006913215484. [DOI] [PubMed] [Google Scholar]
- Reid DW, Nicchitta CV. Primary role for endoplasmic reticulum-bound ribosomes in cellular translation identified by ribosome profiling. J Biol Chem. 2012;287(8):5518–5527. doi: 10.1074/jbc.M111.312280. M111.312280 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DW, Nicchitta CV. Diversity and selectivity in mRNA translation on the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2015;16(4):221–231. doi: 10.1038/nrm3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J, Rhee J, Chaudhari V, Rosenzweig A. The Role of Exercise in Cardiac Aging: From Physiology to Molecular Mechanisms. Circ Res. 2016;118(2):279–295. doi: 10.1161/CIRCRESAHA.115.305250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. nrm2199 [pii] [DOI] [PubMed] [Google Scholar]
- Rosenzweig A. Medicine. Cardiac regeneration. Science. 2012;338(6114):1549–1550. doi: 10.1126/science.1228951. 338/6114/1549 [pii] [DOI] [PubMed] [Google Scholar]
- Ryoo HD. Long and short (timeframe) of endoplasmic reticulum stress-induced cell death. FEBS J. 2016;283(20):3718–3722. doi: 10.1111/febs.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833(12):3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. S0167-4889(13)00251-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3(1):99–111. doi: 10.1016/s1534-5807(02)00203-4. S1534580702002034 [pii] [DOI] [PubMed] [Google Scholar]
- Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, Ward JE, Connors LH, Sawyer DB, Semigran MJ, Macgillivray TE, Seldin DC, Falk R, Liao R. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci U S A. 2010;107(9):4188–4193. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. 2016;97:245–262. doi: 10.1016/j.yjmcc.2016.06.001. [DOI] [PubMed] [Google Scholar]
- Sobie EA, Lederer WJ. Dynamic local changes in sarcoplasmic reticulum calcium: physiological and pathophysiological roles. J Mol Cell Cardiol. 2012;52(2):304–311. doi: 10.1016/j.yjmcc.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC. Aging and the UPR(ER) Brain Res. 2016;1648(Pt B):588–593. doi: 10.1016/j.brainres.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99(3):275–282. doi: 10.1161/01.RES.0000233317.70421.03. 01.RES.0000233317.70421.03 [pii] [DOI] [PubMed] [Google Scholar]
- van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest. 2013;123(1):37–45. doi: 10.1172/JCI62839. 62839 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatta M, Ackerman MJ. Genetics of heart failure and sudden death. Heart Fail Clin. 2010;6(4):507–514. ix. doi: 10.1016/j.hfc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Wagner S, Maier LS, Bers DM. Role of sodium and calcium dysregulation in tachyarrhythmias in sudden cardiac death. Circ Res. 2015;116(12):1956–1970. doi: 10.1161/CIRCRESAHA.116.304678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, Criollo A, Luo X, Tan W, Jiang N, Lehrman MA, Rothermel BA, Lee AH, Lavandero S, Mammen PP, Ferdous A, Gillette TG, Scherer PE, Hill JA. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 2014;156(6):1179–1192. doi: 10.1016/j.cell.2014.01.014. S0092-8674(14)00025-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward CW, Prosser BL, Lederer WJ. Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle. Antioxid Redox Signal. 2014;20(6):929–936. doi: 10.1089/ars.2013.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasfy MM, Weiner RB. Differentiating the athlete’s heartPath6-from hypertrophic cardiomyopathy. Curr Opin Cardiol. 2015;30(5):500–505. doi: 10.1097/HCO.0000000000000203. [DOI] [PubMed] [Google Scholar]
- Webster KA, Discher DJ, Bishopric NH. Cardioprotection in an in vitro model of hypoxic preconditioning. J Mol Cell Cardiol. 1995;27(1):453–458. doi: 10.1016/s0022-2828(08)80041-7. [DOI] [PubMed] [Google Scholar]
- Wilsbacher L, McNally EM. Genetics of Cardiac Developmental Disorders: Cardiomyocyte Proliferation and Growth and Relevance to Heart Failure. Annu Rev Pathol. 2016;11:395–419. doi: 10.1146/annurev-pathol-012615-044336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T, Knowlton KU. Viral myocarditis: from the perspective of the virus. Circulation. 2009;119(19):2615–2624. doi: 10.1161/CIRCULATIONAHA.108.766022. [DOI] [PubMed] [Google Scholar]
- Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6(6):1355–1364. doi: 10.1016/s1097-2765(00)00133-7. S1097-2765(00)00133-7 [pii] [DOI] [PubMed] [Google Scholar]
- Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res. 1974;35(2 suppl II):17–26. [PubMed] [Google Scholar]
- Zhou AX, Tabas I. The UPR in atherosclerosis. Semin Immunopathol. 2013;35(3):321–332. doi: 10.1007/s00281-013-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]