Abstract
A new determination of the 229Th half-life was made based on measurements of the 229Th massic activity of a high-purity solution for which the 229Th molality had previously been measured. The 229Th massic activity was measured by direct comparison with SRM 4328C using 4παβ liquid scintillation counting, NaI counting, and standard addition liquid scintillation counting. The massic activity was confirmed by isotope dilution alpha spectrometry measurements. The calculated 229Th half-life is (7825 ± 87) years (k = 2), which is shorter than the three most recent half-life determinations but is consistent with these values within uncertainties.
Keywords: 229Th, Half-life, Liquid scintillation counting, NaI counting, Isotope dilution alpha spectrometry
Introduction
229Th is a relatively long-lived nuclide in the 237Np decay series that occurs, primarily, as a daughter product of 233U [1]. The nuclide decays by a emission to 225Ra which, in turn, decays to 225Ac and then, through a series of short-lived nuclides, to 209Bi (Fig. 1). The 229Th nuclide does not occur in nature, making it useful to the environmental and geological sciences where it is used as a yield tracer for thorium α spectrometry [2] and as an isotope dilution mass spectrometry (IDMS) spike for thorium amount measurements [3]. In addition, 229Th is a source of high-purity 225Ac for research in nuclear medicine [4] and is being evaluated for use as a high accuracy nuclear clock [5]. The nuclide is particularly important for nuclear forensics where it is used as an IDMS spike for 230Th “dating” of uranium materials associated with the nuclear fuel cycle, e.g. [6, 7].
Fig. 1.
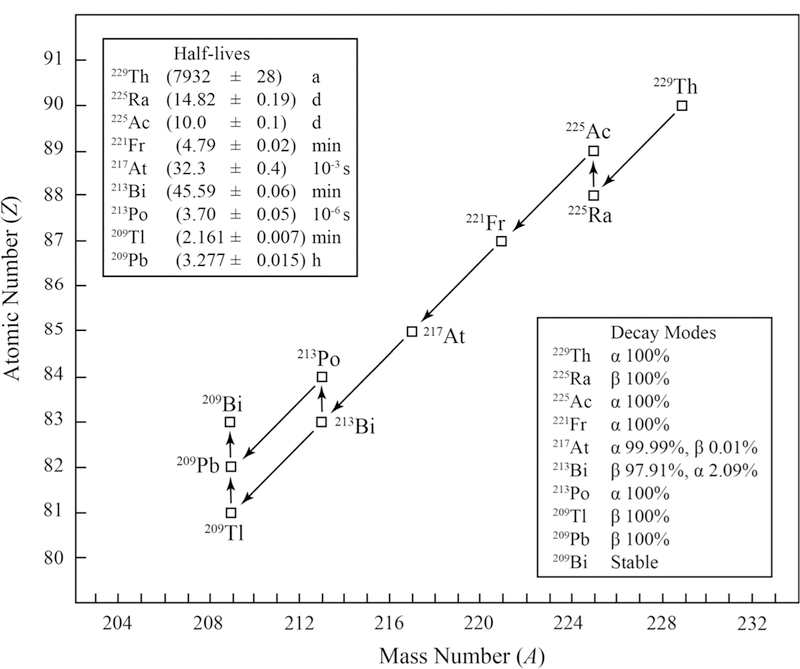
Simplified decay scheme for 229Th. The uncertainties for the half-lives are standard uncertainties. Half-life and decay mode data for 229Th are from [8]. Half-life and decay mode data for all other isotopes are from [9]
Due to ongoing interest in 229Th, the National Institute of Standards and Technology (NIST) has produced four issues of Standard Reference Material (SRM) certified for 229Th massic activity (Bq g−1). These standards are SRM 4328 issued in 1984, SRM 4328A issued in 1995, SRM 4328B issued in 1996, and SRM 4328C issued in 2008 [10–13]. Fitzgerald et al. [14] describe the massic activity calibration for the 229Th Master (M) solution used to prepare SRM 4328C. To produce the stock solution for the SRM, an aliquot of the M solution was quantitively diluted by a factor (DS) of 308.85 ± 0.09. The 229Th massic activity of the M solution was then measured by live-timed 4παβ-γ anticoincidence counting (LTAC). The massic activity of the SRM solution was calculated using the measured massic activity of the M solution and the gravimetrically determined dilution factor. Relative liquid scintillation (LS) counting of the M and SRM 4328C solutions was used to verify the gravimetric dilution factor for the SRM and the calculated activity value for the SRM was also confirmed by four additional radioactivity measurement methods, including LS counting with 3H CIEMAT/NIST efficiency tracing,1 triple-to-double coincidence counting (LS TDCR), 2πα proportional counting, and α spectrometry.
In a separate project, two ampoules of the 229Th M solution were combined and diluted by a factor (DX) of approximately 56.02 to produce a new reference material that was characterized for 229Th molality (229Th mol g−1) and Th isotopic composition [15]. The molality of the new 229Th reference material (referred to here as 229Th RM) was measured in two characterization campaigns by IDMS using 232Th from a high purity metal as a spike and verified using the SRM 4342A 230Th massic activity standard as an independent IDMS spike. The resulting 229Th molality value for the 229Th RM solution is (0.11498 ± 0.00008) nmol g−1 for a reference date of 15 September 2012.
Considering the variety of uses for 229Th, an accurate half-life value for the nuclide is important for decay corrections, converting from activity to amount, and estimating the production rate of decay-chain nuclides. To date, four half-life measurements have been published for 229Th, with the three most recent determinations being relatively consistent. Hagemann et al. [1] published an initial 229Th half-life of (7340 ± 160)3 years as part of a study of the 233U decay chain. This value is significantly shorter than subsequent measurements (≈ 7%) with the discrepancy attributed to a combination of factors including a biased 233U half-life value used to calculate the quantity of 229 Th in the analysis samples, a possible underestimate of 228Th contributions to 229Th activity measurements, or 233U interference in 229Th activity measurements [16–18]. Goldstein et al. [16] performed a study to refine the 229Th half-life value by measuring the molality of 229Th in a solution with a well-characterized massic activity, SRM 4328. The resulting measurement indicated a half-life of (7880 ± 120) years (k = 2). More recently, Kikunaga et al. [17] calculated a half-life of (7932 ± 55) years (k = 2) based on measured A(233U)/A(232U) and A(229Th)/A(228Th) activity ratios. Finally, Varga et al. [18] measured the molality of the SRM 4328C massic activity standard by IDMS using two different Th spikes. One spike was a commercially prepared natural Th solution [Method A: (7889 ± 32) years] and the second spike was a high purity U material with a calculated quantity of ingrown 230Th [Method B: (7952 ± 36) years]. The half-lives calculated for each set of IDMS measurements were combined for a reported half-life value of (7917 ± 48) years (k = 2). It was noted in [15], however, that the estimated massic activity and the measured molality of the 229Th RM indicated a half-life value that was not consistent with the two most recent half-life determinations published for the nuclide.
The following study was performed to investigate the apparent discrepancy between recently published 229Th half-lives and a half-life based on the estimated massic activity of the newly calibrated 229Th RM. The massic activity of the 229Th RM was quantitively determined by direct comparison with the SRM 4328C massic activity standard and was confirmed by isotope dilution alpha spectrometry (IDAS). A new value for the 229Th half-life is calculated from the measured 229Th massic activity and the previously measured 229Th molality.
Experimental
Direct activity comparison
A massic activity value for 229Th in the 229Th RM was determined by measuring the ratio of relative activities (i.e. activity per gram solution) for the 229Th RM solution and the SRM 4328C activity standard. Three counting methods were used to make the relative activity measurements. The first method compared the relative LS counting rates for carefully prepared sources of the two solutions. Then, the relative integral photon emission rates of the LS counting sources were directly compared using a NaI(Tl) well counter. Finally, a standard addition LS counting method was used to determine the activity ratio from a series of mixtures of the two reference materials.
Relative LS counting
The 229Th decay series consists of a seven-nuclide-long decay chain (Fig. 1) with an LS efficiency of > 6.9 s−1 - Bq−1. To achieve accurate results for the comparative LS counting measurements, it is imperative that the two 229Th solutions are in identical states of radioactive equilibrium and that the solutions were counted with identical detection efficiency. Nearly identical counting efficiency was achieved by gravimetrically determining the components in the counting source cocktails to assure similar quenching. Identical radioactive equilibrium is also a reasonable assumption when the history and handling of the solutions are considered. Both 229Th solutions were prepared from quantitative dilutions of aliquots from the same M solution; both solutions were diluted with 1 mol L−1 HNO3; and no additional separations or processing were performed on either solution prior to sealing in glass ampoules. Ampoules of both reference materials were opened specifically for the activity measurements performed in April 2017, however, the SRM 4328C solution was prepared, dispensed into ampoules, and sealed in October 2007 while the 229Th RM was prepared and sealed in March 2012. Although it is not possible to categorically rule out chemical fractionation of the sealed M solution aliquots over the 4.5-year period between preparation of the 229Th solutions (e.g., preferential adsorption or precipitation of an element in the 229Th decay chain), there is no a priori reason to suspect a difference in the radioactive equilibrium of the two solutions.
Counting sources for high precision LS were prepared from the 229Th RM and SRM 4328C solutions (Table 1). Two different scintillation fluids were used to create liquid scintillation sources for both 229Th solutions, Ultima Gold AB4 (PerkinElmer, Waltham, MA) and Ecoscint (National Diagnostics, Tampa, FL). Three sources were gravimetrically prepared in 20 mL glass LS vials for each scintillant-229Th solution combination, resulting in a total of 12 sources. To prepare the sources, aliquots from ampoules of the SRM and 229Th RM were dispensed using polyethylene aspirating pycnometers with masses measured on an electronic microbalance (AT20, Mettler Toledo, USA). Other cocktail components were weighed with a mechanical analytical balance (Mettler B5, Mettler Toledo, USA). Close matching of the aqueous mass fraction in the sets of cocktails was achieved by the addition of 1 mol L−1 HNO3 to some of the sources. In addition, each cocktail contained approximately 60 mg of di-(2-ethylhexyl) phosphoric acid (DEHPA or HDEHP), (C8H17O)2PO2H, which is a complexing agent. Matched blank sources of similar composition over the same quench range were also prepared for background subtraction.
Table 1.
229Th RM and SRM 4328C LS source preparation details and characteristics
| LS source series |
Mass of scintillant (g) |
Mass of 229Th solution aliquot (g) |
Mass of blank HNO3 (g) |
Total mass cocktail (g) |
faq (%) | H# |
|---|---|---|---|---|---|---|
|
229Th RM UGAB |
9.850 (3) | 0.2039 (9) | 0.798 (23) | 10.912 (19) | 9.18 (19) | 89.0 (3) |
| 4328Cs UGAB | 9.852 (1) | 0.9909 (10) | – | 10.906 (11) | 9.09 (8) | 91.2 (5) |
|
229Th RM ECOS |
8.916 (17) | 0.1993 (40) | 0.790 (26) | 9.969 (42) | 9.92 (2) | 118.4 (3) |
| 4328Cs ECOS | 8.910 (7) | 1.0103 (26) | – | 9.987 (8) | 10.12 (2) | 121.1 (3) |
Each LS Source Series consisted of 3 separately prepared counting sources. UGAB and ECOS refer to the Ultima Gold AB and Ecoscint scintillation solutions, respectively. The values for the masses (g) and aqueous mass fraction faq (in %) are averages for the three sources in the series with the values in parentheses representing the standard deviation of the means. H# is the average quench indicating parameter (as measured in Counter B) which is an additional indicator of the close composition matching between the two 229Th solutions for each scintillant cocktail
Each source was counted in triplicate on a LS 6500 counter (Beckman Coulter, Fullerton, CA, USA) and on an Accuflex LSC-8000 (Hitachi Aloka Medical Ltd., Tokyo, Japan). Two LS counters were used for this set of experiments to demonstrate that results are independent of the operating characteristics of a particular instrument (detection threshold, photomultiplier efficiency, deadtime, amplification, signal conversion, etc.). Individual LS measurements consisted of integrating the entire LS spectrum, and were of sufficient duration to accumulate over 106 counts (≈ 1 h), such that the relative Poisson counting statistics on each was < 0.1%. In addition to closely matching quenching conditions and cocktail compositions in counting sources, both instruments use quench indicating parameters (QIP) to monitor quenching. The Accuflex LSC-8000 (Counter H) uses the External Standard Channel Ratio (ESCR) quench parameter, which relates quenching to the ratio of two counting windows in the Compton spectrum of 133Ba. The Beckman LS 6500 (Counter B) uses a Horrocks Number (H#) to monitor the quench level, which is based on the downward spectrum shift of the Compton edge of an external 137Cs source with increasing quenching in the cocktail.
Relative photon emission
As an independent measurement of the 229Th RM and SRM 4328C activity ratio, relative photon emission rates were measured on the LS sources described above. Six replicate measurements of each source were made on a NaI (Tl) well-type counter (2480 WIZARD2, PerkinElmer, Waltham, MA). The detector crystal is 75 mm in diameter with a height of 80 mm and is shielded with a minimum of 50 mm of lead. A total of 6 trials for each source were measured sequentially, alternating between SRM and 229Th RM vials and the spectrum for each measurement was accumulated for an interval of 10800 s (live time). Stability between trials was monitored with a 1.97 kBq 129I check source (Half-life = 1.6 × 107 years). The region from, nominally, 20 to 2000 keV was integrated to obtain an integral counting rate that was corrected for background. The Poisson counting statistics for any single measurement were approximately 0.13%.
Results for LS and photon emission counting rates
The measurement results are provided in terms of grandmeans5 and great-grand-means of the massic count ratios (Table 2). The measured count rates from the LS and NaI(Tl) experiments are proportional to the massic activity of the respective solutions. If the 229Th decay chain equilibrium is the same for both solutions, then the ratio of the massic count rates is equivalent to the 229Th relative activity ratio (AX/AS) which, in turn is equivalent of the ratio of M solution dilution factors (DS/DX) for the two reference materials. The uncertainty values for the grandmean massic count ratios in Table 2 are computed precision estimators, equivalent to standard deviations of the mean (S¯x), that consider both the within-source and between-source components of variance. The within-source component is a “typical” (i.e., average) value for the standard deviation of the replicate measurements and the between-source component is the standard deviation of the mean values. The two variance components are combined in quadrature to calculate the standard deviation of the mean for each counter-scintillant combination. Separate great grand-means are calculated for the LS and Na(Tl) measurements with a relative precision estimator calculated as described above.
Table 2.
Count ratios of 229Th RM—SRM 4328C as measured by LS and NaI(Tl) counters
| LS measured count ratios |
NaI(Tl) measured count ratios |
|||||
|---|---|---|---|---|---|---|
| LS counter | Scintillant | Grand mean massic count ratio | Sx | Scintillant | Grand mean massic count ratio | Sx |
| B | UGAB | 5.5186 | 0.0121 | UGAB | 5.5148 | 0.0143 |
| B | ECOS | 5.5199 | 0.0155 | |||
| H | UGAB | 5.5216 | 0.0088 | ECOS | 5.5303 | 0.0166 |
| H | ECOS | 5.5301 | 0.0166 | |||
| Great grand mean | 5.5226 | 0.0109 | 5.5226 | 0.0170 | ||
Counters H and B are as described in the text. UGAB and ECOS are as previously defined. S¯x is a relative precision estimator equivalent to the standard deviation of the mean
Activity ratio by LS standard addition
A LS standard addition experiment was performed using a series of counting sources prepared as scintillation cocktails that contain mixtures of SRM 4328C and the 229Th RM in varying proportions (Table 3). A total of five standard addition LS sources were prepared using the Ecosint scintillation solution. Aliquots of both SRM 4328C and the 229Th RM were added to each source but source preparation was, otherwise, as previously described. The LS counting rate (Rc) for the mixtures of the SRM 4328C, with massic activity (AS), and the 229Th RM, with massic activity (AX), can be given by
| (1) |
where ms and mx are the respective aliquot masses of the two solutions in the cocktail and where ε is the overall 229Th decay chain detection efficiency for the mixture in matched cocktails. Simple rearrangement, by normalization of each term in Eq. (1) by mS, yields a linear function
| (2) |
with intercept ε AS and slope ε AX . From a series of matched counting sources with rates Rc for varying mS and mX, a linear fit of Rc/mS as a function of mX/mS will determine slope and intercept parameters whose ratio gives AX/AS. Additionally, the linear equation, in two independent variables of Eq. (1), can be fit by multiple regression to yield ε AS and ε AX directly. The two calculational approaches give identical results, though extraction of the covariance between ε AS and ε AX from the covariance matrix is easier with multiple regression.
Table 3.
Standard addition source preparation details and characteristics
| Source ID |
Mass of scintillant (g) |
Mass of 229Th RM aliquot (g) |
Mass of SRM 4328C aliquot (g) |
Mass of blank HNO3 (g) |
Total mass cocktail (g) |
faq (%) |
H#2 |
|---|---|---|---|---|---|---|---|
| SA1 | 13.5365 | 0.076775 | 1.697757 | 0 | 15.3669 | 11.54 | 123.5 (5) |
| SA2 | 13.5657 | 0.201698 | 1.304430 | 0.2647 | 15.3962 | 11.50 | 123.2 (4) |
| SA3 | 13.5808 | 0.348458 | 0.995254 | 0.4289 | 15.416 | 11.50 | 123.6 (4) |
| SA4 | 13.5832 | 0.476746 | 0.600704 | 0.6948 | 15.4124 | 11.50 | 122.9 (4) |
| SA5 | 13.5711 | 0.632714 | 0.327851 | 0.8073 | 15.4002 | 11.48 | 123.1 (3) |
Mass values have a standard uncertainty of < 0.01%. H# is a quench indicating parameter (as measured in Counter B for n = 10 replications) which is an additional indicator of the close composition matching. The values in parentheses represent the numerical values of the standard deviation of the mean for the quoted H#
Ten (10) replicate measurements of the five sources were made in both Counter B and Counter H. The counting rates for the sources integrated over the entire LS spectrum ranged from about 520 s−1 to 950 s−1. So, a duration of 3600 s was used for the measurements in order to accumulate approximately 1.9 106 to 3.4 106 integral counts in each spectrum (corresponding to relative Poisson counting statistics of about 0.07 to 0.05% for each of the 10 measurements).
Results for LS standard addition measurement
The standard deviation of the mean for the average of the 10 replicate measurements of a source on Counter B ranged from 0.25 to 0.37%, and from 0.060 to 0.083% on Counter H. Multiple regressions of the Counter B and Counter H data sets for the Rc (mX, mS) two-independent-variable function of Eq. (3) were performed with the LAB Fit code [19]. Table 4 summarizes the results of these regressions. The ratio AX/AS was obtained from the fitted parameters ε AX and ε AS and its relative standard deviation was computed from
| (3) |
where SX and SS are the standard deviations on AX and AS, respectively, and where SXS is the covariance between AX and AS (refer to Table 4). The reduced χ2 values for the two regressions for ν = 3 degrees of freedom were 3.1 and 0.61, with corresponding percentile points of p = 0.024 and p = 0.61 for Counters B and H data, respectively.
Table 4.
Standard addition activity ratio measurement results
| Quantities | Counter B | Counter H |
|---|---|---|
| 1347.9 | 1369.4 | |
| 245.57 | 249.98 | |
| 0.08892 | 0.6583 | |
| 0.008953 | 0.03474 | |
| − 0.01646 | − 0.07668 | |
| 5.4888 | 5.4780 | |
| S | 0.0030 | 0.0067 |
| Grand mean | Massic activity ratio 5.4834 | SD 0.0087 |
The fitted parameters and are for the 229Th RM and SRM 4328C solutions, respectively, with corresponding variances and and covariance . The estimator S is the propagated standard deviation of the massic activity ratio
Isotope dilution alpha spectrometry
The massic activity of the 229Th RM was measured on five subsamples that were prepared for IDAS from a single unit of the reference material. This measurement method is independent of the three activity ratio methods and is not reliant upon comparison with SRM 4328C. The IDAS analysis is a nuclide-specific measurement of 229Th activity so any perturbation of the radioactive equilibrium of the decay chain for the isotope will not bias the measurement results. Accordingly, the analyses represent a robust verification of massic activity calculated from the previously described relative activity measurements.
The IDAS analysis samples were prepared by transferring aliquots of the SRM 4342A 230Th activity standard to 5 separate beakers as an isotope dilution spike, followed by aliquots of the 229Th RM (Table 5). The masses of the SRM 4342A and 229Th RM aliquots were measured by difference on XP 205 balance (Mettler, Toledo USA). Approximately 2 mL of 0.4 mol L−1 NaHSO4 (Fisher Scientific, Hampton, NH) and 2 mL 2 mol L−1 HNO3 were also added to sample beakers which were then refluxed on a hotplate for roughly 1 h. Additional 2 mol L−1 HNO3 was added to each beaker, as necessary, to yield 10 mL of solution in preparation for ion exchange separation using UTEVA resin (Eichrom Technologies, Chicago, USA). Prior to loading, approximately 1 mg of Zr in a 4 mol L−1 nitrate solution (SPEX Industries, Metuchen, NJ) was added to each sample to assist in the recovery of Th from the UTEVA columns. The Th was eluted from the columns into a beaker using 20 mL of 6 mol L−1 HCl. Approximately 1 mL concentrated H2SO4 was added to the samples to break down any organic compounds that may have washed through the UTEVA column during elution and the solution was heated until the sulfuric acid was completely fumed off. Once dry, the samples were cooled and then re-dissolved in 10 mL of 2 mol L−1 HNO3. Preparation of the samples for counting on the alpha spectrometer entailed co-precipitation of the Th with ≈ 50 µg of NdF3, (Lindsay Rare Earth Chemicals, West Chicago, IL) in 1 mol L−1 HCL and collection of the sample on a 0.1 µm Metricel filter (Pall Corp. Baltimore, USA). The α-decays from 230Th and 229Th were counted for approximately 106 s on an Ortec Octete alpha spectrometer (Ortec, Oak Ridge, USA) with silicon detectors. The measured α activities for 230Th and 229Th were corrected for peak tailing, background, and decay probability. The ratio of the corrected activities in conjunction with the known aliquot weights were then used to calculate the 229Th massic activity using a standard isotope dilution algorithm.
Table 5.
229Th IDAS sample data. Mass values have a relative standard uncertainty of 0.3%
| Source ID | 229Th Aliquot (g) | 4342A aliquot (g) | 229Th corrected counts | 230Th corrected counts | Massic activity (Bq g−1) |
|---|---|---|---|---|---|
| 1A | 0.04681 | 0.23224 | 201134 | 208015 | 195.7 (0.9) |
| 2A | 0.03359 | 0.23442 | 146521 | 214837 | 194.2 (1.0) |
| 3A | 0.04935 | 0.22853 | 193851 | 187416 | 195.5 (0.9) |
| 4A | 0.03323 | 0.21738 | 121015 | 160688 | 201.0 (1.0) |
| 5A | 0.03362 | 0.23496 | 138180 | 203920 | 193.2 (1.0) |
The values in parentheses are combined standard uncertainties estimated for the individual massic activity measurements
Results from IDAS
The combined IDAS measurement data indicate a 229Th massic activity of (195.9 ± 3.2) Bq g−1 (k = 2) for a measurement date of March 5, 2012. The uncertainty of the measured value is dominated by the variability of the replicate massic activity measurements (Table 6) with the uncertainty for solution masses, the SRM 4342A tracer, and peak tail corrections representing minor contributions. Uncertainty contributions from Th blank and α branching ratios were insignificant.
Table 6.
Uncertainty Budget for 229Th massic activity by IDAS
| Component | Comment | Type | ui% | |
|---|---|---|---|---|
| Massic activity | Standard uncertainty of replicate IDAS measurements of 229Th massic activity | A | 0.69 | |
| Weighing | Estimated relative uncertainty for potential bias in mass of IDAS solution aliquots | B | 0.30 | |
| Peak tail | Estimated uncertainty of 229Th peak tail correction applied to the α count data | B | 0.21 | |
| 230Th spike | Combined standard uncertainty of the massic activity for SRM 4342A | B | 0.20 | |
| Background | Estimated uncertainty of 229Th and 230Th background corrections | B | 0.04 | |
| Relative combined standard uncertainty (uc) | 0.81% | |||
| 229Th massic activity (Bq g−1) | Expanded uncertainty (Bq g−1) | |||
| 195.9 | 3.2 (Relative: 1.6%) | |||
The uncertainty estimate for the massic activity was calculated in accordance with Taylor and Kuyatt, [20] and GUM protocols [21] using the GUM Workbench software [22]. ui% represents the relative standard uncertainty for the listed components. The expanded uncertainty (U = k × uc) for the massic activity value is the product of the combined standard uncertainty (uc) and a coverage factor (k), with k = 2 to achieve an approximately 95% level of confidence
Discussion
The physical and analytical linkages between the M solution, SRM 4328C, and the 229Th RM allow for the measurement of the 229Th RM massic activity through a direct comparison to SRM 4328C (Fig. 2). The three counting methods used to measure the activity ratios of the 229Th RM and SRM 4328C should yield the same values, within uncertainties. Results from the LS and NaI(Tl) relative counting methods are consistent among all three counters and both scintillation cocktails used to prepare the sources (Table 2). The LS standard additions results are also consistent for both LS counters (Table 4) but the relative difference between the standard addition and the relative counting methods (δ) is 0.7%, while the relative standard deviation for the results of the two standard addition measurements are both approximately 0.16%. No clear explanation for the apparent discrepancy between the AX/AS ratio for the methods has been identified. The composition matching of the five standard addition sources dictated nearly identical detection efficiencies across the series. Secondly, the quality of the fits, as reflected in the adjusted R2, reduced χ2, and F-values (for ANOVA ratio of variances), suggests that there should have been better agreement. The 95.4% coverage factor on the standard deviation SX and SS for AX and AS is k = 3.31, such that the expanded relative uncertainties kS on AX/AS of 0.18 and 0.40% are roughly a factor of 2 to 3 smaller than the δ from the AX/AS ratio. In the absence of identifiable analytical problems for the three activity ratio measurement methods, the overall mean values for each method were averaged to yield a combined activity ratio (AX/AS)c of 5.510 with a standard uncertainty of 0.026. This standard uncertainty is calculated by adding, in quadrature, two variance components. These components are the within-method variability, represented by the typical standard deviation of the mean for the great-grand-means multiplied by the Student’s T distribution for 2 degrees of freedom (n = 3 measurement methods), and the between-method variability represented by the standard deviation of the 3 activity ratio values.
Fig. 2.
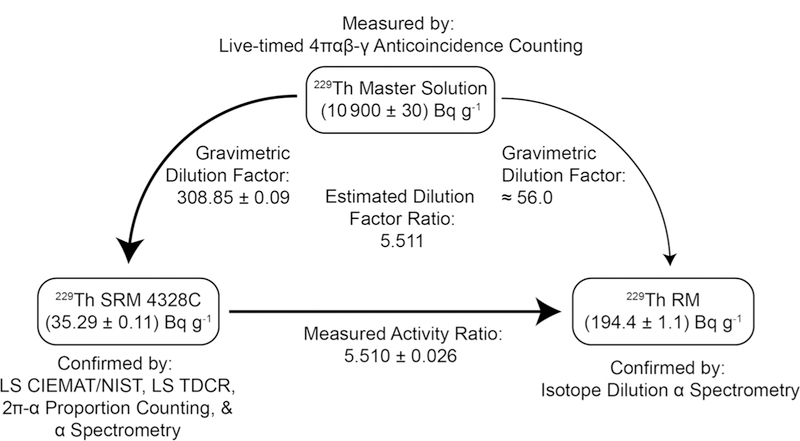
Schematic of material and analytical linkages between the M solution, SRM 4328C, and the 229Th RM. Bold arrows indicate the activity measurement linkages. The lighter arrow indicates direct production of the 229Th RM from the M solution. Activity values shown in the figure are for the December 31, 2007 reference date of the massic activity value of the M solution. All uncertainties are combined standard uncertainties
As previously described, the ratio of dilution factors (DS/DX) for the two reference materials should be identical to the 229Th activity ratio, representing an independent confirmation of the measured ratio. Although the dilution factor for the 229Th RM is an approximation, a calculated DS/DX of 5.511 is essentially indistinguishable from the measured activity ratio value indicating the measured ratio is consistent with the values expected based on preparation of the reference material from the M solution.
The massic activity of the 229Th RM solution (AX) can be calculated (Eq. 4) from the combined activity ratio (AX/AS)c, as described above, and the measured activity of the M solution (Am) and the dilution factor for production of SRM 4328C stock solution (DS), as reported in [14].
| (4) |
The resulting massic activity value for the 229Th RM is (194.4 ± 1.1) Bq g−1. For comparison, the 229Th massic activity measured by IDAS, corrected to the reference date for SRM 4328C (December 31, 2007), is 196.0 Bq g−1 with an expanded uncertainty of 3.2 Bq g−1 (k = 2). The relative difference between the activity measurements is 0.8% which is well within the 1.6% relative uncertainty of the IDAS measurement (Fig. 3).
Fig. 3.
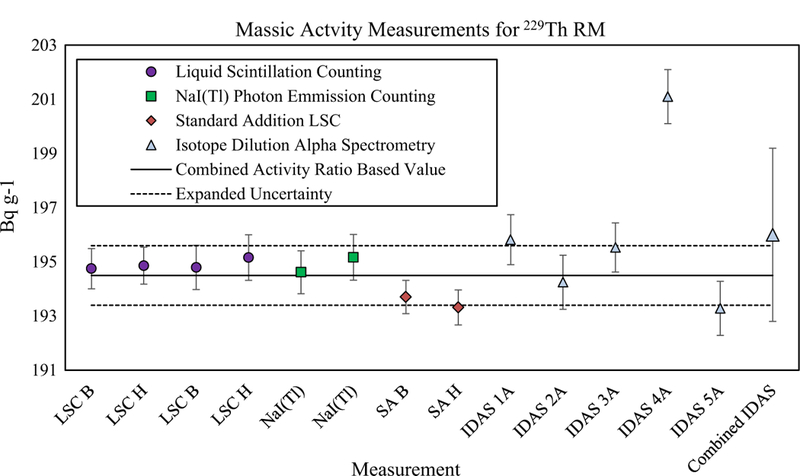
Massic activity values calculated from measured 229Th RM—SRM 4328C activity ratios and direct measurements by IDAS. Error bars for data points are combined standard uncertainties with the exception of the “Combined IDAS” values which is shown with an expanded uncertainty (k = 2). Note that only the LSC, Standard Addition LSC, and NaI(Tl) data were used to determine the “Combined Activity Ratio Based Value” and “Expanded Uncertainty” interval shown in the figure. All data are presented for a reference date of 31 December 2007
The massic activity from Eq. 4 (AX) and the 229Th molality (bX) of (0.11498 ± 0.00008) nmol g−1, as measured for the 229Th RM in [15], can then be used to calculate a decay constant (λ) for 229Th but a decay correction for the (4.7 ± 0.25) year interval between the reference dates for the massic activity and the molality measurements (t) must also be incorporated into the calculation (Eq. 5).
| (5) |
where L is the Avogadro’s Constant. This function is solved for the decay constant by numerical iteration, which converges rapidly because of the small magnitude of the exponential term. A half-life (T) is then calculated from the decay constant (Eq. 6).
| (6) |
The best-fit decay constant for the 229Th data is 8.858 10−5 a−1 with a combined standard uncertainty of 0.049 10−5 a−1 which corresponds to a half-life and expanded uncertainty of (7825 ± 87) years (k = 2). The uncertainty for the half-life determination is dominated by components from the M solution activity measurement and the measured activity ratio for the 229Th RM (see Table 7). The uncertainties for the measured molality of the 229Th RM solution, the SRM 4328C dilution factor, and the decay interval between the SRM reference date and the 229Th molality measurements represent minor contributions to the overall uncertainty for the half-life.
Table 7.
Uncertainty budget for 229Th half-life determination
| Component | Comment | Type | ui%2 | |
|---|---|---|---|---|
| Activity ratio | Standard uncertainty of combined activity ratio measurements from this study | A | 0.48 | |
| LTAC 229Th activity | Standard uncertainty of the M solution massic activity | B | 0.28 | |
| 229Th molality | Combined standard uncertainty of the measured 229Th molality | B | 0.07 | |
| Dilution factor | Standard uncertainty of the dilution factor for production of SRM 4328C from the M solution | B | 0.03 | |
| Decay interval | Relative uncertainty associated with the interval between the M solution reference date and molality measurements |
B | 0.00 | |
| Relative combined standard uncertainty (uc) | 0.56% | |||
| 229Th half-life (a) | Expanded uncertainty3 (a) | |||
| 7825 | 87 (Relative: 1.1%) | |||
The uncertainty estimate for the half-life was calculated in accordance with Taylor and Kuyatt, [20] and GUM protocols [21] using the GUM Workbench software [22]. ui% represents the relative standard uncertainty for the listed components. The expanded uncertainty (U = k × uc) for the half-life value is the product of the combined standard uncertainty (uc) and a coverage factor (k), with k = 2 to achieve an approximately 95% level of confidence
The 229Th half-life measured in this study is largely consistent with previous determinations (Fig. 4) with the exception of the first published half-life for the nuclide presented in Hagemann et al. [1]. As previously discussed, the value from Hagemann et al. is significantly shorter than subsequent half-life measurement. The present half-life measurement is consistent with the published half-life reported by Goldstein et al. [16] but is only marginally consistent with the values of Varga, et al. [18] and Kiku-naga et al. [17] as indicated by overlapping expanded uncertainty envelopes. Pommé [23] noted that discrepancies between half-life measurements for the same nuclide are common and speculated that a major cause for the apparent differences is underestimated measurement uncertainty. While it is not possible to assess the uncertainty cited by Hagemann et al. [1] and the uncertainty cited by Goldstein et al. appears to be reasonably conservative, we speculate that the uncertainty of the half-lives from Kikunaga et al. and Varga et al. may be underestimated.
Fig. 4.
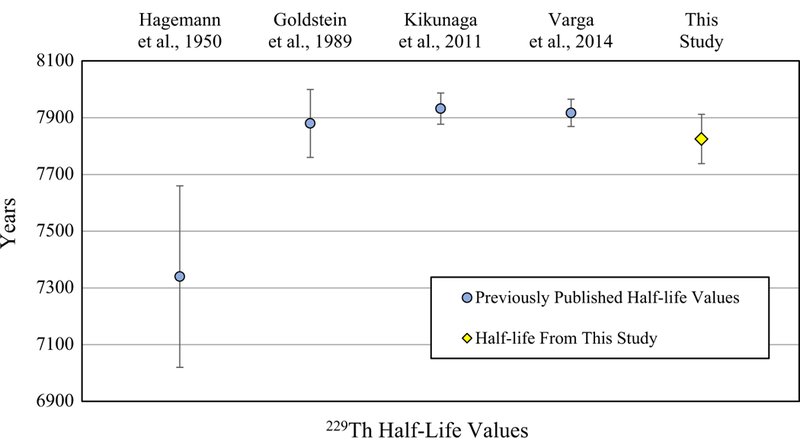
Published half-lives for 229Th. Error bars for data points are expanded uncertainties (k = 2)
Kikunaga et al. [17] measured the half-life by an indirect method based on known ingrowth periods for Th in a high purity 233U material that also contains a small proportion of 232U. In their study, the measured α count ratios of A(233U)/A(232U) and A(229Th)/A(228Th) and the half-lives of 232U and 228Th were used to calculate a 229Th half-life without the necessity of measuring the absolute amount or activity for the nuclide. Accordingly, their calculations are highly sensitive to the 228Th half-life and the measured 229Th to 228Th count ratio, for which they indicate an average uncertainty of 0.2%. The 228Th half-life appears to be well constrained [9], and Kikunaga, et al. addressed potential sources for significant bias in their data such as corrections for background and interferences in the α spectra. However, the combined alpha spectra for 229Th and 228Th are complex and there is potential that their choice of region-of-interest for the nuclides, the magnitude of applied corrections, and/or uncertainties in decay data for the nuclides could result in a bias that is not covered by the 0.15% relative “systematic” uncertainty cited for their measurements.
Varga et al. [18] provided detailed uncertainty budgets for each of the measurement methods used for their half-life determination (Methods A and B). The uncertainties for each method is dominated by the Type B uncertainties for SRM 4328C massic activity. Three replicates of the 229Th molality measurement were performed for each method but no variability component associated with the replicate IDMS measurements was specified. Varga et al. then combined the two measured half-lives to obtain a weighted mean value of 7917 years with an expanded uncertainty of only 48 years (k = 2), despite a 63-year difference in the half-lives for the two methods. It does not appear that the combined measurement uncertainty incorporated a component for the variability evident between measurement methods and it is unclear whether the type B uncertainty components shared by the methods were appropriately propagated.
Conclusion
A new measured half-life of (7825 ± 87) years (k = 2) is reported for 229Th. This value is based on the previously determined 229Th molality of a new thorium IDMS reference material and the detailed characterization of the massic activity value for the reference material, presented here. The measured massic activity is tied directly to the SRM 4328C activity standard through multiple replicate activity ratio measurements using three independent methods and was also independently confirmed by IDAS measurements. This new 229Th half-life measurement is between 0.7 and 1.4% shorter than other modern half-life determinations but is otherwise consistent with these values (overlapping uncertainties at the 95% level of confidence).
Acknowledgements
Project activities at NIST were supported by the United States Department of Homeland Security.
Footnotes
The acronym CIEMAT/NIST refers to the two laboratories that collaborated in developing the protocol for this LS tracing methodology; viz., the Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas (CIEMAT) and the National Institute of Standards and Technology (NIST).
The dilution factor for the 229Th RM (DX) is only used for comparison purposes. During the dilution of the M solution to create the 229Th RM stock solution, the masses measured on a large-capacity Jupiter 3000 balance appeared to be inconsistent with normal operation of the balance. Accordingly, the calculated dilution factor value is an approximation.
Uncertainties for values in text are combined standard uncertainties (uc) unless otherwise indicated with a coverage factor value (k) of 2 or greater for the expanded uncertainties (U = k × uc).
Certain commercial equipment, instruments, software, or materials are identified in this paper to foster understanding. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Multiple counting sources were made for each sample-scintillant combination and each source was measured multiple times on two the LS counters and the NaI(Tl) counter. Mean count ratio values were calculated for the replicate measurements of sample-scintillant combinations for each of the counters. A “Grand-Mean” represent the mean value of the “Mean” count ratios for a specified counter-scintillant combination. “Great Grand Means” represent the mean values of the Grand Means for the LSC and NaI(Tl) analysis methods.
References
- 1.Hagemann F, Katzin LI, Studier MH, Seaborg GT, Ghiorso A (1950) The 4n + 1 radioactive series: the decay products of U233. Phys Rev 79(3):435–443 [Google Scholar]
- 2.de Lavison P, Husband LJ, Jerome SM, Keightley JD, Woodman AP, Woods DH, Woods SA (2000) The standardization of the 229Th for an environmental yield tracer. Appl Rad Iso 53:243–249 [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal SK (2016) A review on the mass spectrometric analysis of thorium. Radiochim Acta 104(7):445–456 [Google Scholar]
- 4.Apostolidis C, Molinet R, Rasmussen G, Morgenstern A (2005) Production of Ac-225 from Th-229 for targeted alpha therapy. Anal Chem 77:6288–6291 [DOI] [PubMed] [Google Scholar]
- 5.Campbell CJ, Radnaev AG, Kuzmich A, Dzuba VA, Flambaum VV, Derevianko A (2012) A single-ion nuclear clock for metrology at the 19th decimal place. Phys Rev Lett 108(120802):1–5 [DOI] [PubMed] [Google Scholar]
- 6.Lamont SP, Hall GJ (2005) Uranium age determination by measuring the 230Th/234U ratio. Radioanal Nuc Chem 264:423–427 [Google Scholar]
- 7.Williams RW, Gaffney AM (2011) 230Th-234U model ages of some uranium standard reference materials. Proc Radiochim Acta 1:31–35 [Google Scholar]
- 8.Tuli J K (2011) Nuclear Wallet Cards, Nation Nuclear Data Center, https://www.nndc.bnl.gov. Accessed 1 Jan 2018
- 9.BIPM (2013) Monograph 5: Table of Radionuclides, 7-A:14–245 [Google Scholar]
- 10.NBS (1984) Standard Reference Material 4328 Radioactivity Standard, National Bureau of Standards, Gaithersburg, MD, certificates available at https://www.nist.gov/srm. Accessed 16 May 2017 [Google Scholar]
- 11.NIST (1995) Standard Reference Material 4328A Thorium-229 Radioactivity Standard, National Institute of Standards and Technology, Gaithersburg, MD, certificate available at https://www.nist.gov/srm. Accessed 16 May 2017 [Google Scholar]
- 12.NIST (1996) Standard Reference Material 4328B Thorium-229 Radioactivity Standard, National Institute of Standards and Technology, Gaithersburg, MD, certificate available at https://www.nist.gov/srm. Accessed 16 May 2017 [Google Scholar]
- 13.NIST (2008) Standard Reference Material 4328C Thorium-229 Radioactivity Standard, National Institute of Standards and Technology, Gaithersburg, MD, certificate available at https://www.nist.gov/srm. Accessed 16 May 2017 [Google Scholar]
- 14.Fitzgerald R, Collé R, Laureano-Pérez L, Pibida L, Hammond MM, Nour S, Zimmerman BE (2010) A new primary standardization of 229Th. Appl Radiat Iso 68:1303–1308 [DOI] [PubMed] [Google Scholar]
- 15.Essex RM, Mann JL, Williams RW, Kinman WS, Hubert A, Bennett ME, Alkiviadias G (2018) A new thorium-229 reference material. Appl Rad Iso 134:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein SJ, Murrell MT, Williams RW (1989) Half-life of 229Th. Phys Rev C 40(6):2793–2795 [DOI] [PubMed] [Google Scholar]
- 17.Kikunaga H, Suzuki T, Nomura M, Mitsugashira T, Shinohara A (2011) Determination of the half-life of the ground state of 229Th by using 232U and 233U decay series. Phys Rev C 84(014316):1–6 [Google Scholar]
- 18.Varga Z, Nicholl A, Mayer K (2014) Determination of the 229Th half-life. Phys Rev C 89(064310):1–6 [Google Scholar]
- 19.da Silva W, da Silva C P (2011) Lab Fit Curve Fitting Software. Universidade Federal de Campina Grande, Campina Grande, Brazil, https://www.zeus.df.ufcg.edu.br/labfit/. Accessed 21 June 2017 [Google Scholar]
- 20.Taylor BN, Kuyatt CE (1994) Guidelines for Evaluating and Expressing the Uncertainty of NIST Measurement Results: NIST Technical Note 1297. National Institute of Standards and Technology, Gaithersburg [Google Scholar]
- 21.JCGM (2008) Evaluation of measurement data—Guide to the expression of uncertainty in measurement. JCGM 100, (E/F)
- 22.Metrodata GmbH (2009) GUM Workbench. Weil am Rhein, Germany [Google Scholar]
- 23.Pomme S (2015) The uncertainty of the half-life. Metrologia 52:S51–S65 [Google Scholar]


