Abstract
Over the last several decades, a wealth of information has become available regarding various sources of stem cells and their potential use for regenerative purposes. Given the intense debate regarding embryonic stem cells, much of the focus has centered around application of adult stem cells for regenerative engineering along with other relevant aspects including use of growth factors and scaffolding materials. The more recent discovery of tooth-derived stem cells has sparked much interest in their application to regenerative dentistry to treat and alleviate the most prevalent oral diseases—i.e., dental caries and periodontal diseases. Also exciting is the advent of induced pluripotent stem cells, which provides the means of using patient-derived somatic cells for their creation, and their eventual application for generation of the dental complex. Thus, evolving developments in the field of regenerative dentistry indicate the prospect of constructing “custom-made” tooth and supporting structures thereby fostering the realization of “personalized dentistry.” On the other hand, others have explored the possibility of augmenting endogenous regenerative capacity through utilization of small molecules to regulate molecular signaling mechanisms that mediate regeneration of tooth structure. This review is focused on these aspects of regenerative dentistry in view of their relevance to personalized dentistry.
Keywords: Stem cells, Molecular mechanisms, Dentin/pulp complex, Tissue repair, Tooth regeneration, Personalized dentistry, Predictive preventive personalized medicine (PPPM)
Introduction
Prevalence of common oral diseases
Common oral diseases include dental caries and periodontal diseases which, left untreated, can lead to eventual tooth loss. Worldwide, dental caries, which often leads to pain and discomfort, are very prevalent in upward of 90% of school-aged children and nearly 100% of adults. Also, severe periodontal disease is common (e.g., in upward of 20% of adults), which can lead to loss of tooth as reflected by data indicating that about 30% of people aged 65–74 have no natural teeth. Indeed, complete loss of natural teeth is widespread and particularly affects older people [1, 2].
In Europe, dental caries remains a major problem, especially in Eastern Europe and in socio-economically deprived groups in Europe as exemplified by data indicating that almost all adults have experienced dental caries and that only 41% of Europeans retain all their natural teeth. The total expenditure on dental health care each year is 40 billion euros and around 66% of these costs relate to treating dental caries and its consequences [3].
In the USA, the Center for Disease Control (CDC) report, for 2011–2012, indicates dental caries remains a major public health concern [4]. Collectively, the data indicate that dental caries is common among various age groups of children and adults and that disparities exist among various socioeconomic groups both in dental caries prevalence and access and barrier to dental care.
Similar to dental caries, periodontal diseases are also very prevalent in both developed and developing countries and estimated to affect about 20–50% of people worldwide. In Europe, severe periodontal disease affects 5–20% of middle-aged (35–44%) adults and up to 40% of older individuals, 65–74 years old [5]. In the USA, a recent CDC report indicates that about 47% of adults aged 30 years or older have some form of periodontal diseases. Periodontal disease increases with age affecting 70% of adults 65 years or older [6]. Indeed, high prevalence of periodontal diseases makes it a major public health concern because aside from adversely impacting oral health and eventually leading to tooth loss, periodontitis is believed to increase the risk for systemic disorders such as cardiovascular diseases [7, 8].
Predictive, preventive, and personalized medicine/dentistry
Thus far, clinical dentistry has relied heavily on a variety of dental biomaterials and restorative options/protocols to prevent and/or treat dental caries and lost tooth structure; these include dental amalgam, composite materials, and fixed and removal prosthesis. Similarly, a variety of protocols are used for prevention and treatment of periodontal diseases including basic treatment (e.g., scaling and root planning/curettage) and guided tissue regeneration. More recently, dental implants have provided a very attractive option for replacement of teeth lost to dental caries and/or periodontal diseases. Although dental implants are considered as a “gold standard” for replacement of missing teeth, they do not exhibit many of the properties of natural teeth and can be associated with complications leading to their failure. However, developments in tissue engineering and the recognition of the usefulness of stem cells in tissue repair and regeneration have sparked much interest in application of tissue engineering principles and protocols to regenerate the dental complex or its associated structures. Also exciting is the prospect of use of small molecules to modulate signaling mechanisms that can promote repair and regeneration of the tooth structure. Clearly, such advances pave the way for personalized dentistry which is at the core of the paradigm shift from “reactive medicine/dentistry” to predictive, preventive, and personalized medicine/dentistry [9, 10]. These aspects are the focus of this review. However, at the outset, an overview of normal tooth development would be helpful for better understanding of current research aimed at repair and regeneration of diseased tooth and the periodontium.
Tooth development
Tooth development is the culmination of reciprocal epithelial-mesenchymal interactions occurring in sequential stepwise fashion [11–13]. The ability to generate teeth initially resides in the dental epithelium which causes induction of tooth formation in mesenchyme of cranial neural crest origin (Fig. 1). Initially, dental lamina forms at the site of future tooth development via thickening of dental epithelium. The formation of tooth placode heralds the initiation of tooth development and the invagination of the epithelial component into the underlying mesenchyme at specific locations. Hallmark features of subsequent stages include proliferation of the epithelial cells and transition of the epithelium through various shapes ranging from bud-, cap-, to bell-like shapes thereby determining the 3-dimensional form and shape of the future crown (Fig. 1). During tooth development, the placodal stage coincides with the shift of odontogenic capacity from the epithelium to the dental mesenchyme; thereafter, dental mesenchyme can induce tooth formation when combined with epithelium.
Fig. 1.
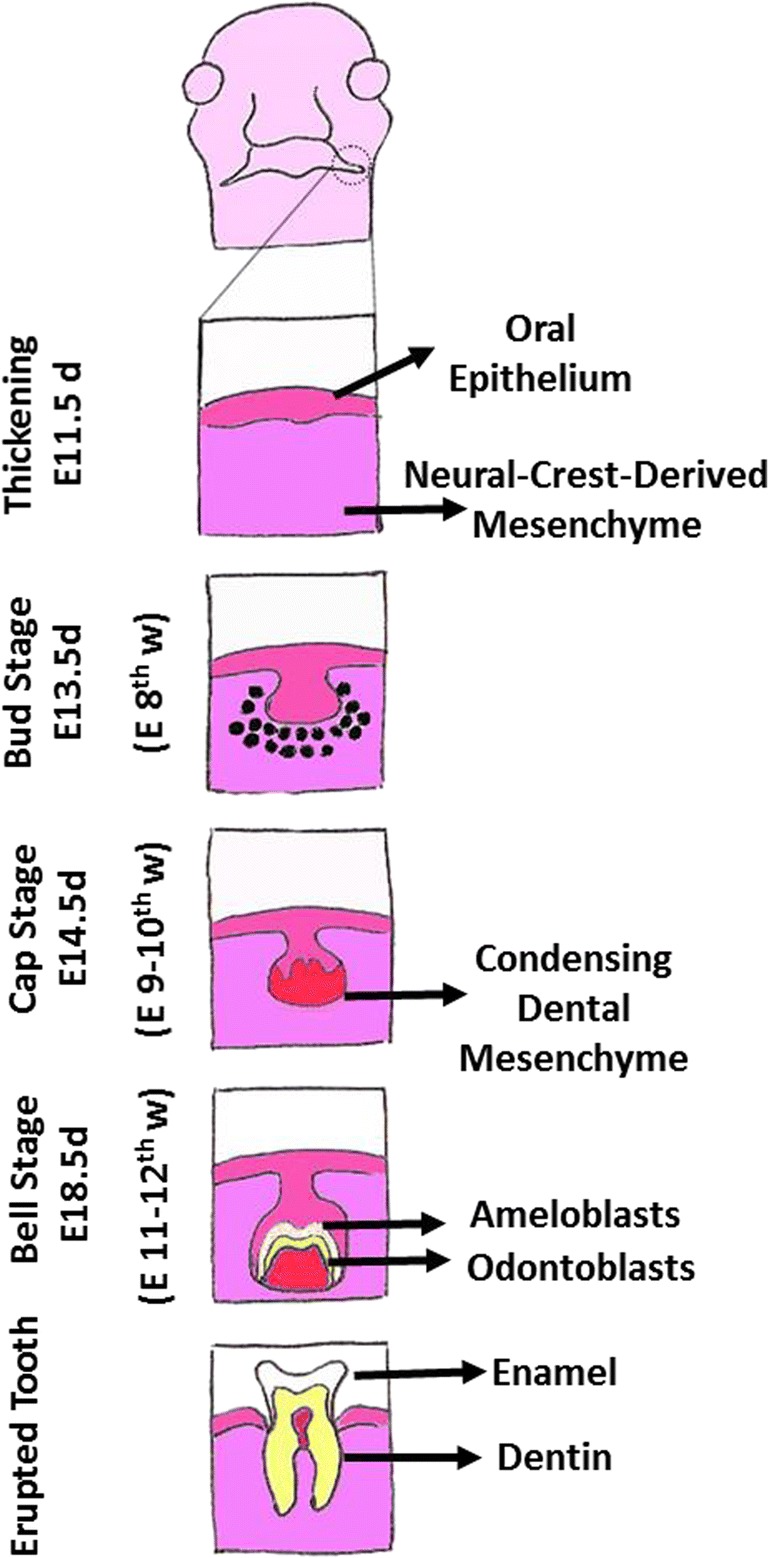
Diagram depicts developmental stages of the tooth. E = embryonic; d = days (for mouse); w = weeks (for human)
The condensation of dental mesenchyme is believed to be accompanied with expression of odontogenic markers such as Pax9, Msx1, and BMP4 [11–13]. The condensed dental mesenchyme initially surrounds the invaginating epithelium, but later on it becomes surrounded by the epithelium (i.e., dental epithelium). Hallmark features of the late bell stage include cellular differentiation and the production of mineralized matrices, namely enamel and dentin and subsequent development of crown of the tooth. The formation of enamel and dentin occurs at the interface between inner dental epithelium (or inner enamel epithelium) and dental mesenchyme (or dental papilla); cells of the inner dental epithelium differentiate into ameloblasts while those of dental papilla differentiate into odontoblasts with the ultimate production of enamel and dentin, respectively.
Completion of development of the crown of the tooth marks the beginning of root formation which occurs as a result of joining of inner and outer enamel cells and their proliferation along with their downward movement to progressively encircle dental papilla and assuming 3-dimensional shape, form, and size of the root of the developing tooth. The epithelial cuff encircling the dental papilla is known as the epithelial root sheath or the Hertwig root sheath. The inner enamel epithelium of the root sheath no longer differentiates into ameloblasts but continues to induce differentiation of the dental papilla cells to become odontoblasts which produce root dentin. This is followed by fragmentation of the Hertwig’s root sheath and consequent access of the ectomesenchymal cells of the dental follicle to the root surface and their differentiation to cementoblasts which ultimately produce cementum. The final stages of root development occur during and after tooth eruption and are characterized by further elongation of the root and formation of periodontal tissues that provide anchorage of the tooth to underlying bone; the source of periodontal tissues is the ectomesenchymal cells of the dental follicle, which produce the alveolar socket and the periodontal ligament thereby connecting the tooth with its socket. It is noteworthy that remnants of Hertwig’s root sheath become a component of the periodontal ligament (i.e., rests of Malassez) which can be the source of some cystic jaw lesions [11–13].
Stem cells
Embryonic and adult stem cells—potential applications
Stem cells are non-specialized cells which possess two important characteristics (Fig. 2). First, they are capable of self-renewal, via cell division, even after long periods of inactivity. Second, under certain physiological or experimental conditions, they can give rise to functional cells of a specific tissue or organ [14, 15]. Consequently, stem cells are recognized as possessing the remarkable potential to develop into many different cell types during early life and growth. Importantly, in many tissues, stem cells serve as an endogenous reservoir system to replenish other cells during the lifetime of a person or an animal. For example, in the gastrointestinal system and bone marrow, stem cells regularly undergo cell division in order to repair and replace worn out or injured tissues. On the other hand, in some organs (e.g., heart and pancreas), stem cells undergo cell division only under special conditions [14–16].
Fig. 2.
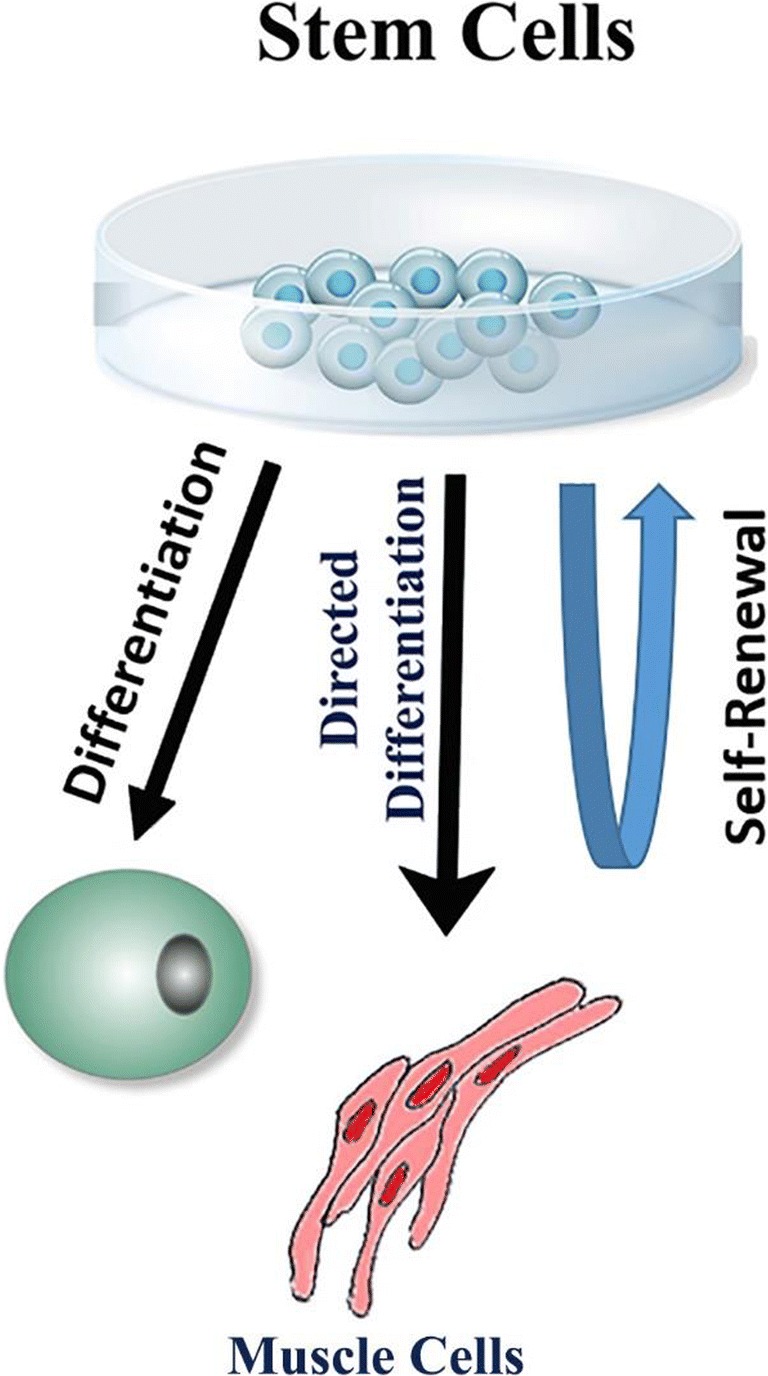
Diagram shows hallmark features of stem cells
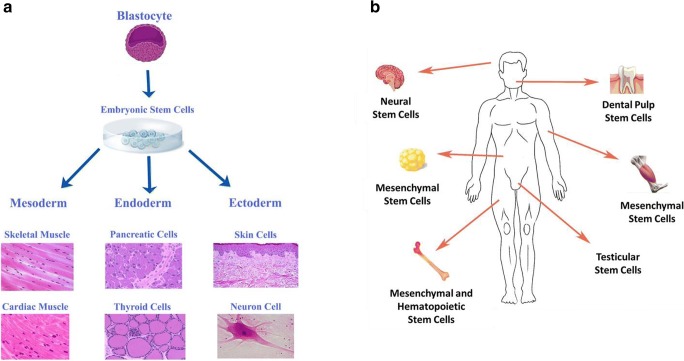
The types of stem cells, in humans and animals, are the embryonic stem cells and the somatic (i.e., cells of the body) or adult stem cells (Fig. 3). The initial discovery of mouse embryonic stem cells, in 1981, and subsequent detailed investigation of their biology paved the way for development of the methods and techniques to derive stem cells from human embryos (1998) and grow them under laboratory conditions [14, 15]. It is noteworthy that human embryos were created through in vitro fertilization for reproductive purposes; when no longer needed for the intended purpose, they were donated for research purpose with the informed consent of the donor. Importantly, in early embryonic stage (i.e., blastocyst), the inner cells give rise to the entire body of the organism which requires specialized cells for all germ layers (Fig. 3). Thus, embryonic stem cells are pluripotent cells capable of producing specialized cells for all tissues and organs [14, 15].
Fig. 3.
Diagrams show source and differentiation potential of embryonic stem cells (a) and tissue sources of adult stem cells (b)
Adult stem cells, on the other hand, are undifferentiated cells which reside among differentiated cells in a tissue or organ; unlike embryonic stem cells which originate from blastocyst, the origin of adult stem cells in some mature tissues remains unclear and is under investigation (i.e., endogenous to the tissue/organ and/or mobilized from other parts of the body, e.g., bone marrow, to another tissue/organ). The adult stem cell can renew itself and can differentiate to yield some, but not all, specialized cell types of the tissue or organ; thus, adult stem cells are considered as multipotent cells [14, 15] (Fig. 3). Investigation of adult stem cells dates back to the 1950s when it was discovered that the bone marrow contains at least two types of stem cells, namely hematopoietic stem cells and mesenchymal (or bone marrow stromal) stem cells [14, 15]. While hematopoietic stem cells give rise to all blood cell types, mesenchymal stem cells can generate a variety of specialized types including bone, cartilage, and fat cells. Further, endothelial progenitor cells play a major role in bone marrow angiogenesis due to their relevant clonogenic potential; these cells are also mobilized into the peripheral blood, giving rise to mature endothelial cells in newly formed blood vessels after injury (e.g., myocardial infarction) or during tumor development. Thus, bone marrow-derived endothelial cells likely represent a reservoir for the entire body angiogenesis and vasculogenesis. Adult stem cells have been found in many more tissues than once thought possible (e.g., in the heart and brain) leading to the prospect of their use for transplants in order to repair or regenerate cells/tissues that have sustained injury. Indeed, hematopoietic stem cells (e.g., from bone marrow) have been used for over 40 years as transplants for several conditions including leukemia and lymphoma [14, 15]. Thus, transplantation-based therapies are being explored even for repair and regeneration of tissues/organs which once were thought not to harbor stem cells. For example, while initial evidence for dividing cells that ultimately became nerve cells was provided from studies in the 1960s, it was not until the 1990s that the preponderance of evidence convinced the scientific community that adult brain does contain stem cells capable of generating astrocytes, oligodendrocytes, and neurons [14, 15, 17, 18]. Similarly, adult myocardium is now considered to possess resident (progenitor) stem cells which can be identified by the use of surface markers (e.g., c-Kit, Sca1, or platelet-derived growth factor receptor a) [16]. Nonetheless, the challenge still remains how to effectively mobilize such endogenous repair/regenerative capacity of the heart to treat cardiac diseases (e.g., myocardial infarction).
Induced-pluripotent stem cells—potential applications
The more novel introduction in the field of stem cells is the advent of induced pluripotent stem cells (iPSCs) (Fig. 4). The scientific basis for this breakthrough actually dates back to the early 1960s with the landmark discovery of Sir John B. Gurdon that specialization of cells can be reversible [19, 20]. This conclusion was based on Gurdon’s elegant studies whereby the nucleus of a fertilized egg cell from a frog was removed and replaced with the nucleus of a cell taken from a tadpole’s intestine. This modified egg cell grew into a new frog, proving that the mature cell still contained the genetic information needed to form all types of cells [19, 20]. Relying on this information, Yamanaka and colleagues [21] identified conditions that would allow some specialized adult cells to be reprogrammed to assume a stem cell-like state—iPSCs. These cells were derived from mouse (embryonic or adult) fibroblasts infected with viruses encoding the transcription factors Oct3/4, Sox2, c-Myc, Klf4, and Sox2. Accordingly, mouse iPSCs exhibit the morphology and growth properties of embryonic stem cells and express their marker genes. Authors also showed that subcutaneous transplantation of iPSCs into nude mice-produced tumors containing tissues from all three germ layers thereby establishing pluripotency of mouse iPSCs [21]. Soon, thereafter, human iPSCs were introduced which express cell markers and are capable of generating cells characteristics of all three germ layers [22]. Accordingly, it was shown that only four factors, namely, Oct4, Sox2, Nanog, and Lin28, are sufficient to program human somatic cells to pluripotent stem cells that display the essential features of embryonic stem cells. The human iPSCs possess normal karyotypes, display telomerase activity, and express cell surface markers and genes that are characteristic of human embryonic stem cells. Importantly, the advent of iPSCs provides the unique opportunity for a number of applications including regenerative dentistry as described below. Indeed, recognition of the revolutionary impact of iPSCs led to the award of Nobel Prize in Medicine and Physiology, in 2012, to Gurdon and Yamanaka.
Fig. 4.
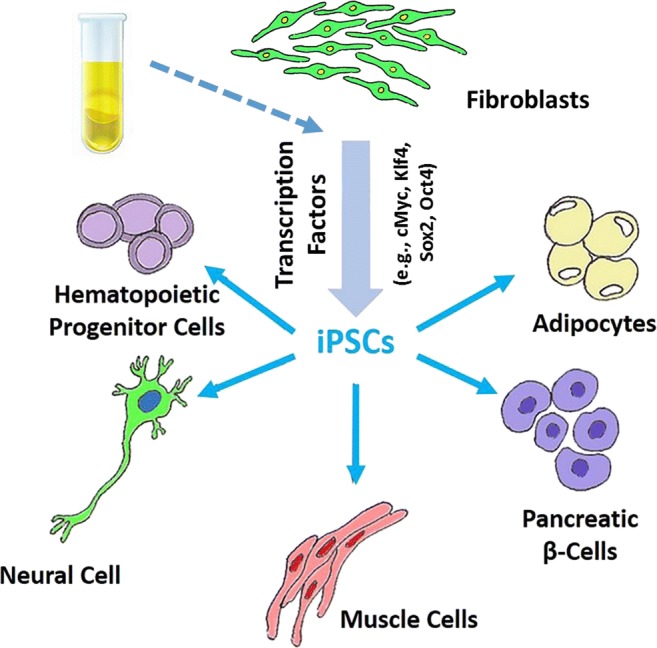
Diagram depicts potential source (e.g., fibroblasts or epithelial cells in urine) for development if iPSCs and their differentiation potentials
More recently, research has focused on generation of integration-free iPSCs [23–25]. This was prompted by the fact that the original method for generation of iPSCs uses retrovirus or lentivirus vectors that require integration of viral DNA into target cells. The integration of exogenous genes encoding required transcription factors for generation of iPSCs (e.g., Oct4, Sox2, C-Myc, and Klf4) raises concern about the risk of mutagenesis and tumor formation [26]. Thus, non-integration gene delivery systems (e.g., Sendai virus, recombinant proteins, synthetic mRNA and episomal vectors) are increasingly utilized for generation of iPSCs—i.e., generation of integration-free iPSCs [27]. In this context, it is important to note that generation of integration-free iPSCs from human urine-derived cells has further raised the prospect for personalized medicine and dentistry as described below [28].
Dental stem cells
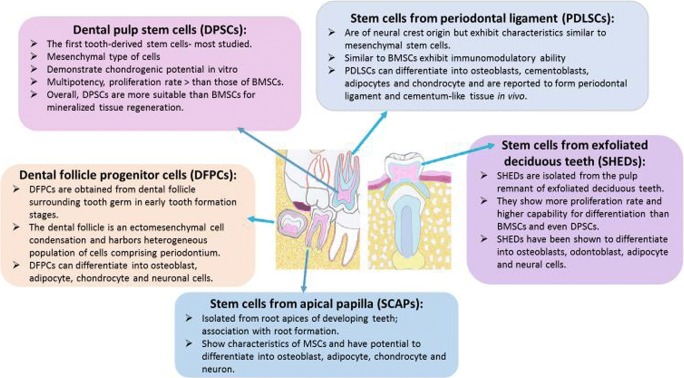
The dental pulp has long been known to respond to an injurious stimulus by mobilizing its endogenous defenses culminating in generation of reparative dentin. This innate ability to repair damaged tissue has been suggestive of existence of odontogenic progenitor cells or stem cells that participate in the regenerative process. This recognition ultimately led to the seminal work of Gronthos and colleagues [29] in 2000 who reported identification and isolation of dental pulp stem cells (DPSCs) from third molars; DPSCs manifest high clonogenic capacity and great multilineage potential (e.g., osteogenic, dentinogenic, adipogenic, chondrogenic, myogenic, neurogenic; [30]). Identification of DPSCs laid the foundation for subsequent studies to explore existence of stem cells in other parts of the oral cavity (Fig. 5). Accordingly, stem/progenitor cells have been identified and characterized in the apical papilla (SCAPs), in human exfoliated deciduous teeth (SHED), periodontal ligament (PDLSCs), and dental follicle (DFPCs); others have reported existence of alveolar bone marrow-derived mesenchymal stem cells (ABMSCs), gingival-derived mesenchymal stem cells (GMSCs), and tooth-germ progenitor cells (TGPCs). Stem/progenitor cells derived from oral cavity express several mesenchymal markers including CD29, CD73, CD90, and CD105 as well as embryonic markers such as Sox2, Nanog and Oct4 and can differentiate into multiple cell lineages [11, 13, 30–33]. Importantly, some dental stem cells exhibit more embryonic-like features than bone marrow stem cells and umbilical cord [31–34]. Therefore, mesenchymal stem cells derived from the oral cavity are believed to be very important and valuable resource for eventual development of cells for clinical/therapeutic applications not only in dentistry but also in medicine. Indeed, tooth-derived stem cells have been used to regenerate cells of a variety of organs including bone, liver, pancreas, salivary gland, vascular system, skeletal muscle, nerve, and cornea [33]. Some features of major tooth-derived stem cells are summarized in Fig. 6.
Fig. 5.
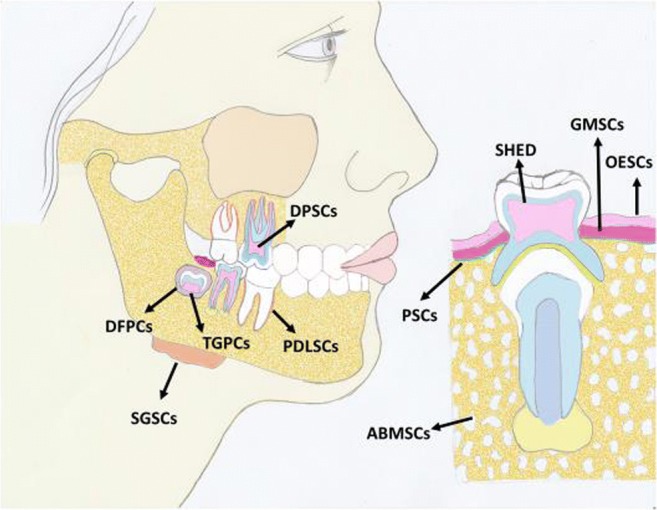
Diagram shows sources of stem cells from the oral cavity
Fig. 6.
Summary of major features of several types of stem cells derived from the dental complex. BMSCs = bone marrow-derived stem cells
Aside from identification of mesenchymal stem cells in the oral cavity, the existence of tooth epithelial stem cells niche was initially identified in continuously growing mouse incisors [11, 35–37]. Dental epithelial progenitor cells differentiate into four cell types as follow: inner enamel epithelium (ameloblast cell-lineage), stratum intermedium, stellate reticulum, and outer enamel epithelium. The cervical loop which develops after the bud stage, at the apical end of the inner and outer epithelium, is composed of the inner and outer dental epithelium. The cervical loop encircles the loosely arranged stellate reticulum cells (which are lost after first layer of enamel is formed). Tooth epithelial stem cells originate from Sox2+ cells of the component of dental epithelium and maintain competence to generate teeth; this is best observed during the formation of succedaneous (or replacement) teeth; these cells were later shown to be capable of generating all epithelial components of the tooth. Thus, tooth-derived epithelial stem cells could potentially be used to generate new teeth. Importantly, however, in humans, this is not feasible because epithelial stem cell niche remains active until the onset of root formation which coincides with the loss of stellate reticulum from the cervical loop and consequent loss of epithelial stem cells. Indeed, during tooth eruption, the entire epithelial compartment, including ameloblasts, is lost through apoptosis. Thus, the inability of human teeth to regenerate enamel relates to the loss of the epithelial compartment along with loss of epithelial stem cells and their niche; this recognition has invigorated research to explore potential options for enamel regeneration as described below. This aspect of normal human tooth development is critically relevant to stem cell-based tooth regeneration given that both epithelial and mesenchymal interactions are required for tooth formation as described below.
Stem cells and tooth regeneration
The dental complex is a multistructure organ composed of highly mineralized tissues (i.e., enamel, dentin, and cementum) and soft tissues of the dental pulp and the periodontium. These structures are the target of most common dental diseases—dental caries and periodontal diseases. Thus, regenerative dentistry is focused on exploring the potential of stem cells derived from the dental complex or iPSCs for regeneration of dentin/pulp complex, whole tooth, bioroot, and periodontal tissue following similar general principles and protocols [11–13, 29–36].
As alluded earlier, existing interventional and therapeutic options utilize restorative and periodontal techniques/protocols coupled with use of biocompatible materials to address these prevalent oral diseases. While such approaches have markedly improved oral health of humans across the globe, long-term clinical success is unpredictable and limited. Given the great strides that have been made in tissue regenerative sciences, attention is increasingly shifted to empowering endogenous healing, repair, and regenerative capacities of the human body with the ultimate objective of repair and regeneration of oral tissues lost to disease and injury. Thus, regeneration of dental complex and its associated structures (i.e., whole tooth, dentin-pulp, tooth root, or periodontal tissues) is the focus of intense research and development.
As described earlier, hallmark characteristic of tooth development is the epithelial-mesenchymal interactions; this aspect is critically relevant to any attempt at regeneration of tooth or its components. This is evident from the seminal studies of Kollar and Baird [38, 39], who established that the interaction of lip-furrow epithelium with dental mesoderm produces teeth. Shortly thereafter, the group further established the development of tooth germs, in vitro, when recombinants of embryonic dental epithelium and mesenchyme were placed into the anterior chamber of mouse eye which produced properly patterned tooth constructs with enamel and dentin. Later studies used cell lines of the dental epithelium and ectomesenchyme for tooth generation. Accordingly, dental epithelium cell line was established from a p53-deficient fetal mouse and shown to express ameloblastin and amelogenin. Thereafter, reconstructed tooth germs, using cell lines and fetal mesenchyme, were implanted under the renal capsule which resulted in tooth generation with calcified structures resembling natural tooth; germs without cell lines developed bone [40]. Nakao and colleagues [41] developed a protocol whereby epithelial and mesenchymal tissues were initially isolated from incisor tooth germ of embryonic day 14.5 (ED14.5) followed by dissociation into single cells. Thereafter, these single cells were reconstituted, which manifested compartmentalization, within a collagen gel drop, at high cell density which were, in turn, used for generation of bioengineered incisor tooth germ. The explants were transplanted beneath the kidney capsule. After a 10-day period, the bioengineered tooth germ, placed under the renal capsule, generated incisors with proper orientation of tissue components (e.g., odontoblasts, dentin, dental tubules, ameloblasts, enamel, dental pulp, root) in comparison to natural tooth. Authors also examined the potential of either the bioengineered tooth germ or the developing tooth (under the renal capsule) to be successfully transplanted thereby developing the tooth in the oral cavity after extraction of a mandibular incisor in the mouse. Thus, they used bioengineered tooth which developed under the kidney capsule (for 2 weeks) or the bioengineered tooth germ which was developed in organ culture for 2 days. Accordingly, individual bioengineered teeth or primordia were implanted into the tooth cavities in a cusp-to-root direction. Two weeks after transplantation, both the single primordia isolated from cultured tooth germ and the single teeth isolated from explants in the subrenal capsules developed in extraction sites and formed correct tooth structure comprising of enamel, dentin, root, dental pulp, blood vessels, and bone by histological observations at high frequencies (77% and 85%, respectively). Utilizing similar protocols, Ikeda and colleagues [42] achieved transplantation of a bioengineered tooth germ into the socket of a lost tooth in an adult mouse. The bioengineered tooth erupted into the oral cavity and achieved occlusion. Further, the bioengineered tooth possessed hardness of mineralized tissues for mastication and responded to orthodontic treatment and noxious stimulation. Nonetheless, the bioengineered tooth did not achieve the 3-dimensional geometric shape and form of the intended tooth—the maxillary first molar. Thus, while significant strides have been made in developing the methods and technology for tooth generation, major hurdles remain in regeneration of tooth that replicates collective biological, functional, and morphological features of natural tooth. More recently, utilizing a murine transplantation model system, successful transplantation of a bioengineered tooth unit was reported, which was comprised of the mature tooth, periodontal ligament, and alveolar bone into a poorly sized bony socket achieving bone integration by recipient bone remodeling. The bioengineered tooth manifested physiological tooth functions such as mastication, periodontal ligament function for bone remodeling, and responsiveness to noxious stimulations. The bioengineered tooth unit restored enough of the alveolar bone in a vertical direction into an extensive bone defect of murine lower jaw [43].
An outcome of trauma to developing tooth is the ensuing pulpal necrosis accompanied with impaired root development. A recent study has explored the ability of autologous tooth stem cells to regenerate dental pulp following implantation into injured teeth [44]. Utilizing animal models, authors initially showed that implantation of autologous tooth stem cells from deciduous teeth was capable of regenerating the dental pulp as manifested with development of an odontoblast layer, blood vessels, and nerves. This led authors to explore the relevance of these findings to human subjects. Thus, authors compared the efficacy of transplantation of autologous DPSCs from deciduous teeth to that of apexification. The results indicated that DPSCs implantation, compared to apexification treatment, resulted in regeneration of three-dimensional pulp tissue with blood vessels and sensory nerves, a year later, accompanied with longer root but reduced width of the apical foramen. Further, no adverse effects were noted 2 years after DPSC transplantation. Based on their findings, authors suggest that autologous human DPSCs can regenerate whole dental pulp and may be useful for treating tooth following traumatic injury [44].
As alluded earlier, loss of the epithelial compartment along with loss of epithelial stem cells and their niche occur during normal human tooth development thereby accounting for the inability of human tooth to regenerate enamel. Thus, while great strides have been made to regenerate stem cell-based dentin/pulp complex, regeneration of enamel structure has lagged behind. Consequently, enamel regeneration remains a major challenge given its distinctive apatite composition, hierarchical architecture, and corresponding properties [45, 46]. Nonetheless, initial attempts at enamel generation involved development of inorganic methods for growth of aligned enamel-like apatite nanocrystals on dental enamel [47, 48]. More recently, attention has turned to use of organic matrices for guided mineralization via a biomimetic approach based on regulated organic-inorganic interaction. Accordingly, amelogenin and gelatin have been used to grow aligned apatite nanocrystals directly on enamel surface [49, 50]. More recently, a protein-mediated mineralization process was reported that capitalizes on disorder-order interplay using elastin-like recombinamers to program organic–inorganic interactions into hierarchically ordered mineralized structures. The materials comprise elongated apatite nanocrystals that are aligned and organized into microscopic prisms, which grow together into spherulite-like structures, hundreds of micrometers in diameter, that come together to fill macroscopic areas. This approach could offer a potential strategy for hard tissue repair and regeneration such as the enamel [51].
Scaffolds and growth factors for regenerative dentistry
While this review has primarily focused on potential cellular sources for tooth regeneration, it is important to emphasize that this aspect is just one component of many requirements of tissue engineering. Other components may include scaffolds and growth factors which have been the focus of other reviews and will be briefly alluded to here.
Several criteria are described for an ideal scaffold including chemical stability, mechanical strength, biocompatibility, controlled degradation, and being permissive of cell adhesion and proliferation. Scaffold materials with potential for regenerative dentistry include natural polymers (e.g., collagen, chitosan, alginate and hyaluronic acid); synthetic materials (e.g., polyglycolic acid, polylactic acid, polylactic polyglycolic acid); and bioactive ceramic (e.g., hydroxyapatite and bioglass) [13, 31, 52]. While these scaffolding materials have shown effectiveness and usefulness in tooth repair/regeneration attempts, a potential problem associated with the use of scaffolding material relates to risk of infection and inflammation thereby prompting exploration of scaffold-independent regeneration techniques and protocols [31]. In this context, a novel approach has been described whereby scaffold-free 3-dimensional cell constructs, using thermoresponsive hydrogel, have been fabricated using DPSCs and their viability have been assessed for in vitro and in vivo dental pulp regeneration [53]. The results indicate formation of pulp-like tissues with rich blood vessels within the human root canal (of tooth devoid of pulp) 6 weeks after implantation. Further, histologic analyses indicated that transplanted DPSCs differentiated into odontoblast-like mineralizing cells at sites in contact with dentin and that human CD31-positive endothelial cells were found at the center of regenerated tissue. These observations indicate the self-organizing ability of 3-dimensional DPSCs constructs within the pulpless root canal in vivo and that pulp-like tissue, rich in blood vessel, can be formed with DPSCs without the use of scaffolds or growth factors [53]. More recently, Smith et al. [54] reported on their work to create highly cellularized bioengineered tooth bud constructs that would develop hallmark features of natural tooth buds such as the dental epithelial stem cell niche, enamel knot signaling centers, transient amplifying cells, and mineralized dental tissues. These constructs consisted of postnatal dental cells which were encapsulated within gelatin methacryloyl hydrogel and were implanted under the skin of immunocompromised rats which demonstrated evidence of natural tooth development. On the other hand, another study used decellularized tooth buds which were reseeded with postnatal dental epithelium, dental mesenchymal, and endothelial cells; the protocol supported the formation of mineralized whole teeth in an in vivo large animal mini-pig jaw thereby establishing the potential use of decellularized tooth bud as scaffold for bioengineered tooth replacement therapy in humans [55].
With respect to growth factors, a number of them have been used to regulate proliferation of and induction of differentiation of stem cells into desired cells; these molecules bind to specific cell membrane-associated receptors thereby mobilizing a cascade of pathways and processes culminating in tissue generation [56, 57]. Indeed, the relevance and importance of several growth factors in generation or repair of dentin and pulp (e.g., barrier formation at site of pulp exposure) have been demonstrated. For example, bone morphogenetic protein (BMP)-2 mediates dentin-induced odontoblastic differentiation of dental pulp stem cells. On the other hand, transforming growth factor- β (TGF-β) can stimulate odontoblast-like cell differentiation and DPSCs-mediated mineralization [56]. Thus, establishing critical growth factors, or combinations thereof, that would be conducive to regeneration of dental complex or associated structures is a major focus of ongoing research.
Finally, an emerging field in tooth regeneration relates to epigenetics and stem cells. As indicated earlier, various sources of stem cells in the oral cavity have been identified including the dental pulp—i.e., DPSCs; sources of these cells from humans include the pulp of primary teeth or third molars. While these cells have multi-lineage potential, cellular, and molecular mechanisms that determine their lineage specification remain to be better established. Emerging evidence indicates that conformation of chromatin structure can impact cell fate. Thus, reversible chemical modifications at the DNA and/or histone levels can influence gene sequences thereby impacting cell fate. Further, miRNAs are emerging as potential players in determining somatic lineage. These aspects are the focus of a recent review [58].
Signaling mechanisms and use of small molecules in reparative and regenerative dentistry
Recent studies have explored responses of resident dental pulp cells to infection and inflammation due to dental caries which encroaches upon the dental pulp accompanied with apoptosis of odontoblasts [59]. It is believed that the ensuing damage can/may be partially repaired due to migration, to the injured site, and activity of DPSCs which are present in niches around the blood vessels of the pulp. Consequently, DPSCs can differentiate to odontoblasts and generate reparative, or tertiary, dentin. Thus, chemotaxis of DPSCs to pulpal site of injury is a critical event for the endogenous capacity of the dental complex to repair/regenerate itself. Consequently, unraveling the signaling mechanism(s) culminating in migration of DPSCs to the site of injury within the tooth has been a focus of research in the field. Accordingly, a recent study explored the role of stromal cell-derived factor-1 (SDF-1) and its interaction with its G protein-coupled receptor, C-X-C chemokine receptor type 4 (CXCR4) and associated signaling pathway in migration/recruitment of DPSCs [60]. The results indicate that SDF-1/CXCR4 axis induces migration of human DPSCs via signaling mechanisms involving focal adhesion kinase (FAK)/phosphatidylinositol-3 kinase (PI3K)/Akt and glycogen synthase kinase 3-β (GSK-3β)/β-catenin pathways. Authors concluded that these signaling pathways are involved in repair of the dental pulp and that SDF-1 maybe a novel therapeutic option for treatment of pulpitis [60]. A subsequent study, utilizing a murine model of dental pulp exposure in molar teeth, explored the impact of pharmacologic inhibition of GSK-3β in formation of dentin; inhibition of GSK-3β is known to upregulate Wnt/β-catenin signaling immediate response in the dental pulp. For these studies, tideglusib (a potent and selective inhibitor of GSK-3β) was used in order to activate the aforementioned signaling pathway and to mobilize resident stem cells in the pulp thereby promoting reparative dentin formation; tideglusib is known for its neuroprotective, anti-inflammatory and neurogenesis-inducing effects with potential usefulness in neurodegenerative diseases such as Alzheimer’s disease [61, 62]. Thus, tideglusib was delivered to tooth preparations, with intentional mechanical pulp exposure, using biodegradable collagen sponges, in order to promote dentin formation as collagen sponge progressively degrades. This protocol was able to promote natural repair at the site of pulp exposure by depositing reactionary dentin 6 weeks after treatment thereby filling the whole injury site from occlusal to pulp chamber roof. Importantly, the dental pulp remained vital in comparison to control teeth with exposed pulp without capping and those with glass ionomer not revealing evidence of reparative dentin formation with pulp becoming severely hypoplastic [61]. A more recent study from this group explored potential role of Wnt/β-catenin pathway, TGF-β and BMP in reparative dentin formation; it is noteworthy that damage to dentin causes the release of fossilized TGF-β and BMP within dentin which, in turn, stimulate odontoblasts to generate reactionary dentin which is formed on the pulpal surface of exiting dentin resulting in its thickening [62]. This study utilized a nonexposed pulp injury model in mouse coupled with use of the pharmacologic agents to modulate aforementioned pathways. The results indicate that Wnt activation increases reactionary dentin formation while inhibition of Wnt, TGF-β, or BMP pathways do not impair reactionary dentin formation. Interestingly, however, inhibition of BMP and/or TGF-β signaling produces a more disorganized, nontubular reactionary dentin. Authors conclude that Wnt/β-catenin signaling pathway does not play a major role in the formation of reactionary dentin; however, in concert with reparative dentin generation, exogenous activation of Wnt/β-catenin pathway can enhance tertiary dentin formation. These observations are encouraging as they raise the prospect of empowering endogenous capability of the dental complex for repair and regeneration.
Conclusion
The discovery of stem cells in various components of the dental complex along with the advent of iPSCs have sparked a flurry of research activity around the world with the noble and ultimate objective of repair and regeneration of the dental complex or its associated structures. These extensive preclinical studies have paved the way for several ongoing clinical studies to determine the feasibility of utilization of dental complex-derived stem cells to promote dental pulp regeneration or their efficacy in treatment of chronic periodontitis, among others [31]. However, the outcome of these clinical trials largely remains to be established. Nonetheless, the potential feasibility of generation of various components of the dental complex utilizing iPSCs raises the prospects of use of urine-derived cells in order to prepare if-iPSCs to be further utilized for production of the tooth germ and its subsequent implantation with the hope of generation of biologically and functionally competent dental complex in humans (Fig. 7). Clearly, major obstacles must be overcome (e.g., generation of tooth of appropriate 3-dimensional form and shape that would function normally) before full realization of regenerative dentistry. This is not unusual given the fact that stem cell-based therapies for other human diseases (e.g., myocardial infarction) still remain a dream despite several decades of intense research and development in related fields [16]. In this context and as alluded earlier, while the reversibility of cell differentiation/specialization was established in the early 1960s [17, 18], the advent of iPSCs which relied on that knowledge occurred in 2006 with the first report of generation of murine iPSCs [18]. Also exciting is the progress in the area of tooth repair via modulation of signaling mechanisms which regulate migration and function of resident stem cells in the dental pulp and their ultimate differentiation to odontoblasts and dentin generation. Thus, while the ability of the tooth to generate reactionary and reparative dentin has been known for a long time, emerging studies are unraveling relevant signaling mechanisms as potential novel target(s) of therapies via use of small molecules for their modulation. Thus, harnessing the ability of the dental complex to repair and regenerate could ultimately lead to providing alternatives to existing protocols/biomaterials for treatment of the most common oral diseases—i.e., dental caries and periodontal diseases.
Fig. 7.
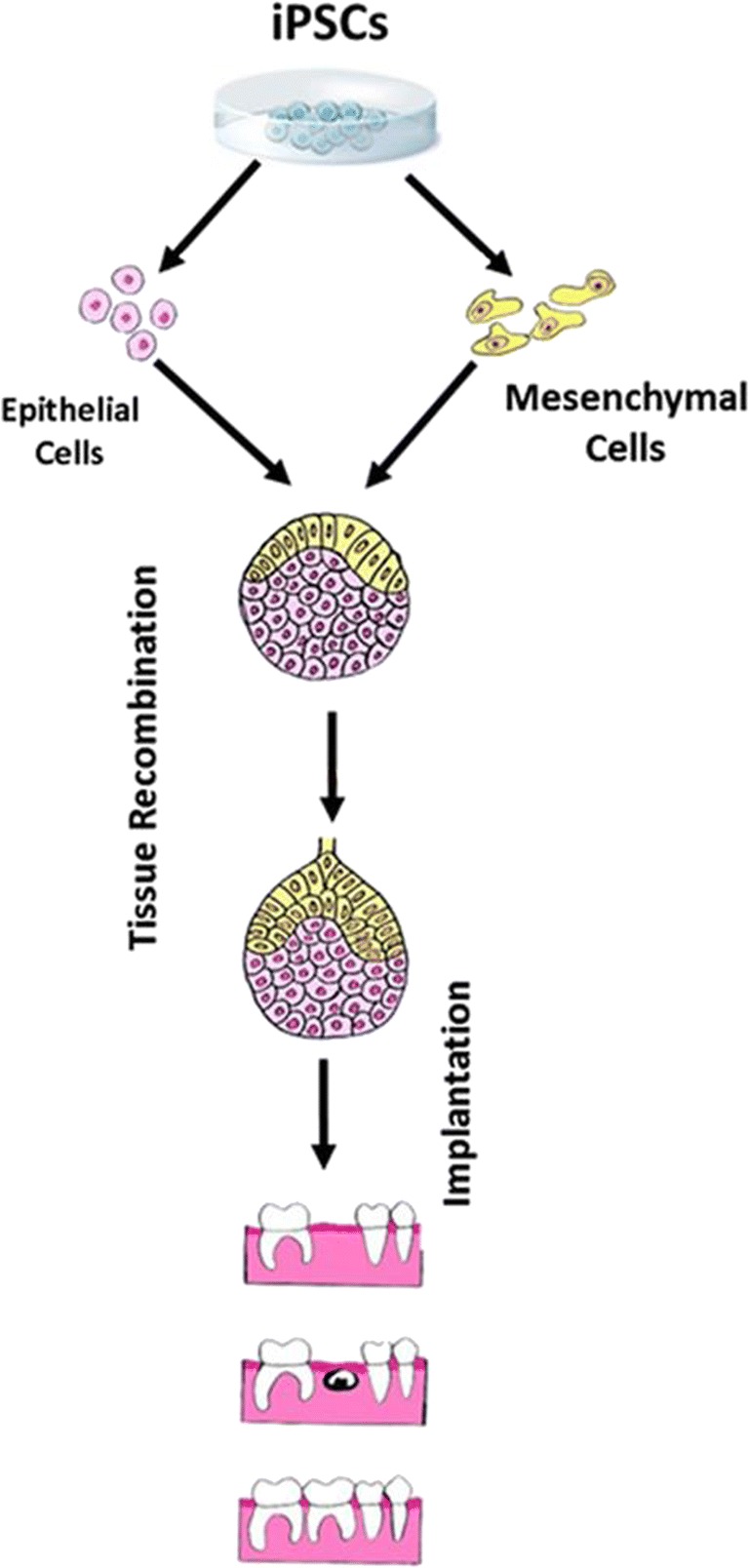
Diagram shows the potential for use of iPSCs to generate mesenchymal and epithelial stem cells followed by in vitro tissue recombination prior to implantation of the tooth germ into extraction socket for regeneration of the dental complex
Expert recommendations
As described above, major advances have been made towards regeneration of dentin/pulp, bioroot, whole tooth and periodontal support in preclinical studies with clinical studies ongoing. Nonetheless, it is important to note that much of our knowledge regarding regenerative dentistry emanates from studies which have utilized otherwise “normal” conditions and/or healthy animals. Since the ultimate objective of regenerative dentistry is applicability to diverse patient populations, it is important to explore how various disease conditions (e.g., metabolic diseases such as diabetes mellitus) may affect the ability to regenerate the dental complex or its associated structures. Also, if patient-derived iPSCs are to be employed for such purposes, it is important to establish how disease conditions affect their utility for regenerative purposes. Thus, realization of use of stem cells for personalized dentistry is very complex and multifaceted albeit a noble objective.
Compliance and ethical standards
Conflict of interest
Authors declare that they have no competing interest.
Consent for publication
Not applicable.
Ethical approval
This submission does not involve the use of human subjects.
Human and animal rights
No experiments have been performed using patients and/or animals.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Oral disease burdens and common risk factors. 2018. http://www.who.int/oral_health/disease_burden/global/en/. Accessed 25 Dec 2018.
- 2.Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol. 2012;60(1):15–39. doi: 10.1111/j.1600-0757.2011.00425.x. [DOI] [PubMed] [Google Scholar]
- 3.Patel R. The state of oral health in Europe. Report Commissioned by the Platform for Better Oral Health in Europe. 2012. http://www.oralhealthplatform.eu/wp-content/uploads/2015/09/Report-the-State-of-Oral-Health-in-Europe.pdf. Accessed 25 Dec 2018.
- 4.Centers for Disease Control and Prevention. Health, United States, 2016. https://www.cdc.gov/nchs/data/hus/hus16.pdf#060. Accessed 25 Dec 2018.
- 5.World Health Organization. Oral health: data and statics. http://www.euro.who.int/en/health-topics/disease-prevention/oral-health/data-and-statistics. Accessed 25 Dec 2018.
- 6.Centers for Disease Control and Prevention. Periodontal disease. https://www.cdc.gov/oralhealth/periodontal_disease/index.htm. Accessed 25 Dec 2018.
- 7.Bartold MP, Mariotti A. The future of periodontal-systemic associations: raising the standards. Curr Oral Health Rep. 2017;4(3):258–262. doi: 10.1007/s40496-017-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschfeld J, Kawai T. Oral inflammation and bacteremia: implications for chronic and acute systemic diseases involving major organs. Cardiovasc Hematol Disord Drug Targets. 2015;15(1):70–84. doi: 10.2174/1871529x15666150108115241. [DOI] [PubMed] [Google Scholar]
- 9.Golubnitschaja O, Costigliola V, EPMA. General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14. 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed]
- 10.Golubnitschaja O, Costigliola V, Grech G. EPMA World Congress: Traditional forum in predictive, preventive and personalised medicine for multi-professional consideration and consolidation. EPMA J. 2017;8(Suppl 1):1–54. [Google Scholar]
- 11.Balic A. Biology explaining tooth repair and regeneration: a mini-review. Gerontology. 2018;64(4):382–388. doi: 10.1159/000486592. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Parada C, Chai Y. Cellular and molecular mechanisms of tooth root development. Development. 2017;144(3):374–384. doi: 10.1242/dev.137216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amrollahi P, Shah B, Seifi A, Tayebi L. Recent advancements in regenerative dentistry: a review. Mater Sci Eng C Mater Biol Appl. 2016;69:1383–1390. doi: 10.1016/j.msec.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health. Stem cells information. Stem Cell Basics IV. https://stemcells.nih.gov/info/basics/4.htm. Accessed 25 Dec 2018.
- 15.National Institutes of Health. Stem cells information. Stem Cell Basics I. https://stemcells.nih.gov/info/basics/1.htm. Accessed 25 Dec 2018.
- 16.Tzahor E, Poss KD. Cardiac regeneration strategies: staying young at heart. Science. 2017;356(6342):1035–1039. doi: 10.1126/science.aam5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egawa N, Takase H, Josephine L, Takahashi R, Arai K. Clinical application of oligodendrocyte precursor cells for cell-based therapy. Brain Circ. 2016;2(3):121–125. doi: 10.4103/2394-8108.192515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog Neurobiol. 2018. 10.1016/j.pneurobio.2018.05.004. [DOI] [PMC free article] [PubMed]
- 19.Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- 20.Gurdon JB. Nuclear transplantation, the conservation of the genome, and prospects for cell replacement. FEBS J. 2017;284(2):211–217. doi: 10.1111/febs.13988. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Vodyanik MA, Smuga Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Routti V, Stewart R, Slukvin II, Thompson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 23.Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, Li A, Huang K, Luo R, Wang L, Liu Y, Zhou T, Wei S, Pan G, Pei D. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen (Lond) 2013;2(1):6. doi: 10.1186/2045-9769-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010;19(4):469–480. doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen W, Zhang JP, Chen W, Arakaki C, Li X, Baylink D, et al. Generation of integration-free induced pluripotent stem cells from human peripheral blood mononuclear cells using episomal vectors. J Vis Exp. 2017;119. 10.3791/55091. [DOI] [PMC free article] [PubMed]
- 26.Durcova-Hills G. Induced reprogramming of human somatic cells into pluripotency: a new way how to generate pluripotent stem cells. Differentiation. 2008;76(4):323–325. doi: 10.1111/j.1432-0436.2008.00266.x. [DOI] [PubMed] [Google Scholar]
- 27.Bang JS, Choi NY, Lee M, Ko K, Lee HJ, Park YS, Jeong D, Chung H-M, Ko K. Optimization of episomal reprogramming for generation of human induced pluripotent stem cells from fibroblasts. Anim Cells Syst. 2018;22(2):132–139. doi: 10.1080/19768354.2018.1451367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YM, Zampieri BL, Scott-McKean JJ, Johnson MW, Costa ACS. Generation of integration-free induced pluripotent stem cells from urine-derived cells isolated from individuals with down syndrome. Stem Cells Transl Med. 2017;6(6):1465–1476. doi: 10.1002/sctm.16-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2015;9(11):1205–1216. doi: 10.1002/term.1899. [DOI] [PubMed] [Google Scholar]
- 31.Hu L, Liu Y, Wang S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018;24(5):696–705. doi: 10.1111/odi.12703. [DOI] [PubMed] [Google Scholar]
- 32.Miran S, Mitsiadis TA, Pagella P. Innovative dental stem cell-based research approaches: the future of dentistry. Stem Cells Int. 2016;2016:7231038. doi: 10.1155/2016/7231038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park YJ, Cha S, Park YS. Regenerative applications using tooth derived stem cells in other than tooth regeneration: a literature review. Stem Cells Int. 2016;2016:9305986. doi: 10.1155/2016/9305986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhai Q, Dong Z, Wang W, Li B, Jin Y. Dental stem cell and dental tissue regeneration. Front Med. 2018. 10.1007/s11684-018-0628-x. [DOI] [PubMed]
- 35.Chavez MG, Hu J, Seidel K, Li C, Jheon A, Naveau A, et al. Isolation and culture of dental epithelial stem cells from the adult mouse incisor. J Vis Exp. 2014;87. 10.3791/51266. [DOI] [PMC free article] [PubMed]
- 36.Yu T, Volponi AA, Babb R, An Z, Sharpe PT. Stem cells in tooth development, growth, repair, and regeneration. Curr Top Dev Biol. 2015;115:187–212. doi: 10.1016/bs.ctdb.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Li Y, Shi R, Zhang S, Liu H, Zheng Y, Li Y, Cai J, Pei D, Wei S. Generation of tooth-periodontium complex structures using high-odontogenic potential dental epithelium derived from mouse embryonic stem cells. Stem Cell Res Ther. 2017;8(1):141. doi: 10.1186/s13287-017-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kollar EJ, Baird GR. Tissue interactions in embryonic tooth germs. I. Reorganization of the dental epithelium during tooth germ reconstruction. J Embryol Exp Morphol. 1970;24(1):173–186. [PubMed] [Google Scholar]
- 39.Kollar EJ, Baird GR. Tissue interactions in embryonic mouse tooth germs. II. The inductive role of dental papilla. J Embryol Exp Morphol. 1970;24(1):173–186. [PubMed] [Google Scholar]
- 40.Komine A, Suenaga M, Nakao K, Tsuji T, Tomooka Y. Tooth regeneration from newly established cell lines from a molar tooth germ epithelium. Biochem Biophys Res Commun. 2007;355(3):758–763. doi: 10.1016/j.bbrc.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 41.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T. The development of a bioengineered organ germ method. Nat Methods. 2007;4(3):227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, Ogawa M, Mizuno M, Kasugai S, Tsuji T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A. 2009;106(32):13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oshima M, Mizuno M, Imamura A, Ogawa M, Yasukawa M, Yamazaki H, Morita R, Ikeda E, Nakao K, Takano-Yamamoto T, Kasugai S, Saito M, Tsuji T. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS One. 2011;6(7):e21531. doi: 10.1371/journal.pone.0021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xuan K, Li B, Guo H, Sun W, Kou X, He X, Zhang Y, Sun J, Liu A, Liao L, Liu S, Liu W, Hu C, Shi S, Jin Y. Decidous autologous tooth stem cell regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10(455):eaaf3227. doi: 10.1126/scitranslmed.aaf3227. [DOI] [PubMed] [Google Scholar]
- 45.Habelitz S, Marshall SJ, Marshall GWJ, Balooch M. Mechanical properties of human dental enamel on the nanometre scale. Arch Oral Biol. 2001;46(2):173–183. doi: 10.1016/s0003-9969(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 46.Jayasudha, Baswaraj, Navin HK, Prasanna KB. Enamel regeneration—current progress and challenges. J Clin Diagn Res. 2014;8(9):ZE06–ZE09. doi: 10.7860/JCDR/2014/10231.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamagishi K, Onuma K, Suzuki T, Okada F, Tagami J, Otsuki M, Senawangse P. A synthetic enamel for rapid tooth repair. Nature. 2005;433(7028):819. doi: 10.1038/433819a. [DOI] [PubMed] [Google Scholar]
- 48.Yin Y, Yun S, Fang J, Chen H. Chemical regeneration of human tooth enamel under near-physiological conditions. Chem Commun (Camb). 2009;(39):5892–4. 10.1039/b911407f. [DOI] [PubMed]
- 49.Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev. 2008;108(11):4754–4783. doi: 10.1021/cr8004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruan Q, Zhang Y, Yang X, Nutt S, Moradian-Oldak J. An amelogenin chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013;9(7):7289–7297. doi: 10.1016/j.actbio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elsharkawy S, Al-Jawad M, Pantano MF, Tejeda-Montes E, Mehta K, Jamal H, Agarwal S, Shuturminska K, Rice A, Tartullo Makina NV, Wilson RM, Bushby AJ, Alonso M, Rodriguez-Cabello JC, Barbieri E, del Río Hernández A, Stevens MM, Pugno NM, Anderson P, Mata A. Protein disorder–order interplay to guide the growth of hierarchical mineralized structures. Nat Commun. 2018;9(1):2145. doi: 10.1038/s41467-018-04319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashemi-Beni B, Khoroushi M, Foroughi MR, Karbasi S, Khademi AA. Tissue engineering: dentin–pulp complex regeneration approaches (a review) Tissue Cell. 2017;49(5):552–564. doi: 10.1016/j.tice.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Itoh Y, Sasaki JI, Hashimoto M, Katata C, Hayashi M, Imazato S. Pulp regeneration by 3-dimensional dental pulp stem cell constructs. J Dent Res. 2018;97(10):1137–1143. doi: 10.1177/0022034518772260. [DOI] [PubMed] [Google Scholar]
- 54.Smith EE, Angstadt S, Monteiro N, Zhang W, Khademhosseini A, Yelick PC. Bioengineered tooth buds exhibit features of natural tooth buds. J Dent Res. 2018;97(10):1144–1151. doi: 10.1177/0022034518779075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang W, Vazquez B, Oreadi D, Yelick PC. Decellularized tooth bud scaffolds for tooth regeneration. J Dent Res. 2017;96(5):516–523. doi: 10.1177/0022034516689082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oshima M, Tsuji T. Functional tooth regenerative therapy: tooth tissue regeneration and whole-tooth replacement. Odontology. 2014;102(2):123–136. doi: 10.1007/s10266-014-0168-z. [DOI] [PubMed] [Google Scholar]
- 57.Kitamura C, Nishihara T, Terashita M, Tabata Y, Washio A. Local regeneration of dentin-pulp complex using controlled release of fgf-2 and naturally derived sponge-like scaffolds. Int J Dent. 2012;2012:190561. doi: 10.1155/2012/190561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodas-Junco BA, Canul-Chan M, Rojas-Herrera RA, De-la-Peña C, Nic-Can GI. Stem cells from dental pulp: what epigenetics can do with your tooth. Front Physiol. 2017;8:999. doi: 10.3389/fphys.2017.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang HS, Pei F, Chen Z, Zhang L. Increased apoptosis of inflamed odontoblasts is associated with CD47 loss. J Dent Res. 2016;95(6):697–703. doi: 10.1177/0022034516633639. [DOI] [PubMed] [Google Scholar]
- 60.Li M, Sun X, Ma L, Jin L, Zhang W, Xiao M, Yu Q. SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3β/β-catenin pathways. Sci Rep. 2017;7:40161. doi: 10.1038/srep40161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neves VCM, Babb R, Chandrasekaran D, Sharpe PT. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci Rep. 2017;7:39654. doi: 10.1038/srep39654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neves VCM, Sharpe PT. Regulation of reactionary dentine formation. J Dent Res. 2018;97(4):416–422. doi: 10.1177/0022034517743431. [DOI] [PubMed] [Google Scholar]