Abstract
Objective
The need for orthodontic treatment continues to increase. Strategies that shorten the treatment course and reduce discomfort are most welcome in clinic. Circadian rhythm plays important role in various physiological processes, including bone formation. This study intended to depict a possible circadian releasing property of the osteogenic factors within the periodontal tissue during orthodontic treatment, which may direct a more efficient and satisfactory orthodontic treatment to the patient.
Methods
Primary periodontal ligament cells (PDLCs) were obtained from the Sprague-Dawley (SD) rats. An equibiaxial strain value of 12% was applied on rat PDLCs (rPDLCs). After 2 h stimuli of 10−7 M dexamethasone (DX), the osteogenic genes’ expressions were detected by real-time polymerase chain reaction (RT-PCR) at Zeitgeber times 0, 4, 8, 12, 16, 20, and 24. An orthodontic appliance was placed on 45 SD rats. Animals were maintained under 12-h light/dark periods and euthanized at 9 time points over the diurnal cycle. The orthodontic sensitive tissues of the mesial root of the maxillary first molar were collected for RT-PCR and immunohistological assay.
Results
The rPDLCs displayed typical fibroblastic spindle shape, and subcultured steadily in vitro. Induced by DX, the mRNA expression of Col-1, OPN, and IBSP within the loaded/unloaded rPDLCs oscillated as that of the main clock gene Per-1. The osteogenic genes’ expressions as well as the protein releases sustained a circadian oscillation trend in vivo.
Conclusions
This study indicates the existence of a circadian rhythm of the osteogenic factors within the orthodontic sensitive tissues, which highlights the importance of precise timing of force loading in further orthodontic treatment. Thus, a periodicity pattern of orthodontic traction at night may prove a more efficient tooth movement while minimizing the treatment window and discomfort complains.
Keywords: Circadian rhythm, Orthodontic tooth movement, Osteogenesis, Animal study, Mechanical force, Predictive preventive personalised medicine, Prognosis, Biomarker panel, Expression pattern, Animal model, Personalized orthodonic care
Introduction
Orthodontic treatment deals with the study and treatment of malocclusions. Nowadays, the need for efficient orthodontic treatment continues to increase across the world [1]. Historically, orthodontic tooth movement (OTM) was described as a site-specific bone remodeling through the histological change of periodontal ligament (PDL) under mechanical loading [2]. PDL is a layer of soft tissue between the tooth and the alveolar bone that provides the necessary microenvironment for cells involved in the alveolar bone remodeling, and is now recognized as the cellular basis for OTM [3]. Orthodontic mechanical force acts on PDL cells (PDLCs) and induces the secretion of a series of genes relating inflammation, osteogenesis, and osteoclastogenesis, which finally leads to the reestablishment of the involved periodontal apparatus and completes the realignment of the tooth [4].
Circadian rhythms are self-sustained endogenous oscillations occurring over a 24-h period [5]. The master circadian clock, locating in the suprachiasmatic nuclei (SCN), mainly synchronizes by light and coordinates the peripheral circadian clocks in nearly every part of the human body. The genes of the molecular clocks, such as period (Per), cryptochrome (Cry), brain and muscle Arnt-like protein (BMAL), and circadian locomotor output cycles kaput (CLOCK), exist in the SCN and peripheral tissue clocks [6]. These genes regulate many physiological processes, including organ development, metabolic functions, and tissue remodeling/repair in mammals, and are believed to exert important functions [7]. Lately, studies unveiled a relationship between circadian rhythm and mineralized tissue development, including osteogenesis and osteoclastogenesis [6, 8, 9]. The findings elicited a periodic expression mode of both osteogenesis and bone resorption related genes under the manipulation of peripheral nerve system. More recent studies also indicated that Cry2 and Per2 affected distinct pathways in the regulation of bone volume [5, 10, 11]. Meanwhile, researchers have observed that rat maxillary first molar receiving orthodontic force only at night moved faster than those loading only at daytime as well as those loading all day long [12]. This result strongly suggested the effect of circadian rhythm on bone metabolism during OTM. However, direct evidence of the periodicity expression of relative genes remains unavailable.
Hormonal signaling is one of the two routes through which the central circadian clock coordinates the peripheral circadian clocks [13]. In current study, we adopted dexamethasone (DX) to mimic the dictation of hormonal signaling from SCN to arouse the circadian variation of rat PDLCs (rPDLCs). Real-time polymerase chain reaction (RT-PCR) was applied to evaluate Q1, whether osteogenic gene expression of rPDLCs exhibit periodicity; Q2, whether the addition of mechanical force changes the periodicity; Q3, whether the addition of mechanical force influences the gene expression level. Furthermore, the in vivo expression of the osteogenic factors was also tested by RT-PCR and immunohistological staining on an orthodontic model of rats. This study detected the existence of circadian rhythm in orthodontic sensitive tissue and laid foundation for chromobiological design of force timing design in a more effective personalized orthodontic care.
Materials and methods
Rat PDLCs isolation and culture
Eight-week-old male Sprague-Dawley (SD) rats were obtained from the Tongji Medical College Animal Center. (Wuhan, China). All procedures concerning animal use were conformed to the guidelines of the Animal Ethics Committee of Huazhong University of Science and Technology (Wuhan, China) [14, 15]. rPDLCs of the maxillary first molars were isolated and cultured according to a modified way of the previous study [16]. Briefly, maxillary first molars were thoroughly washed with phosphate buffered saline (PBS) after careful extraction, and the periodontal ligament within the mid-third portion of the mesial root of the maxillary upper molar was scraped by a surgical steel scalpel (no. 11 blade, Junbei Ltd., Shanghai, China). All the explants were then placed onto the bottom of a six-well culture plates. Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Gibco), 100 U/ml penicillin, and 100 mg/L streptomycin (Invitrogen, Carlsbad, CA, USA), was applied to sustain the primary rPDLCs. Cells were cultured in a humidified 37 °C, 5% CO2 incubator. In the present study, cells after passage 3 were used.
Application of tensile strain to PDL cells in vitro
Primary rPDLCs (5 × 104 per ml) were seeding into six-well, 35-mm flexible-bottomed Uniflex culture plates coated with type-I collagen. One day after seeding, the supplied DMEM containing 2% FBS were afforded for a further 24 h. After that, the seeded cells were subjected to an intermittent deformation of 12% for 6 s every 90 s with a FX-5000™ Tension System (Flexcell Corporation, Hillsborough, NC, USA), as described previously [17].
Treatment with dexamethasone on rPDLCs
After 4 h loading of PDLCs, 10−7 M DX (Sigma-Aldrich) was added to the culture medium to mimic the dictation of hormonal signaling from SCN. The culture medium was replaced 2 h later with fresh supplied DMEM containing 2% FBS, and cells were collected every 3 h. All time interval calculations are based at the indicated Zeitgeber (ZT—is an event that provides the sets of a biological clock) and ZT-0 is considered 2 h after medium changing. Cells are first harvested at ZT-0, and then are collected every 3 h for a total of 9 time points. RNAs were isolated and analyzed by RT-PCR [18].
Animals for OTM
Forty-five 12-week-old male SD rats with a weight of 250 g ± 15 g were obtained from the Tongji Medical College Animal Center. Five rats were kept in one cage under identical condition: room temperature at 24 °C ± 1 °C, humidity at 55~65%, standardized laboratory rat diet, free access to water, and a controlled light condition (12 h:12-h light: dark, 330 lx).
In vivo orthodontic treatment
After a 2-week-adapting period, all animals received an orthodontic appliance that was installed before (Fig. 1) [19, 20]. Generally, the maxillary right first molar was connected with both maxillary central incisors with a nickel-titanium closed-coil spring (3M Unitek, Monrovia, California, USA) to perform the mesial movement. The orthodontic force was continuous and maintained about 30 g for 2 weeks.
Fig. 1.
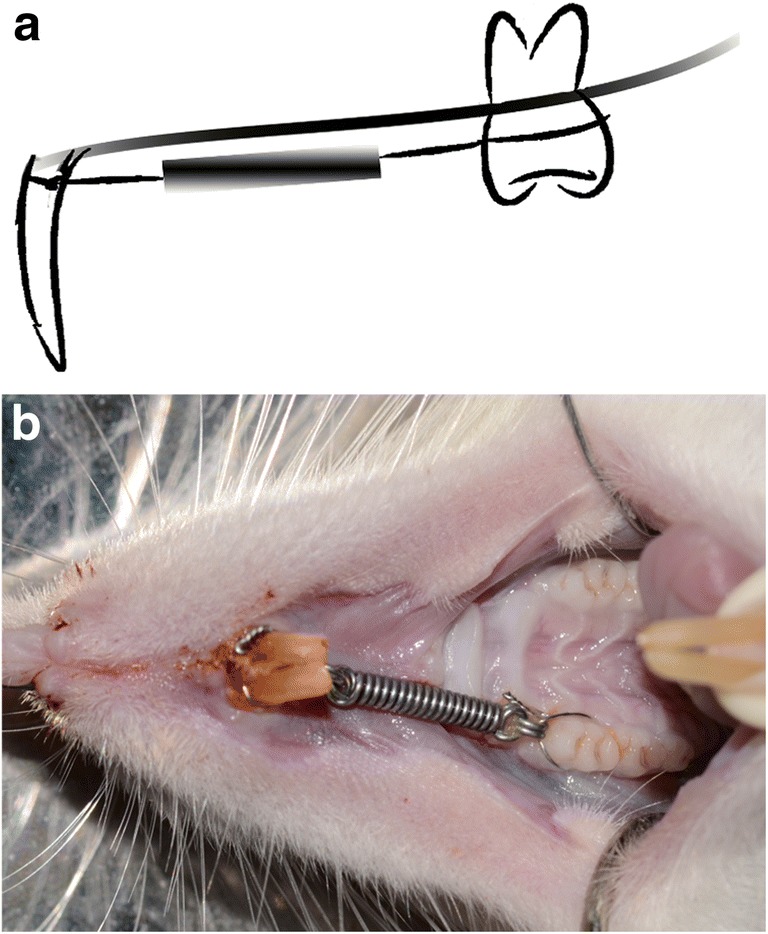
Established orthodontic tooth movement (OTM) model on Sprague-Dawley (SD) rats. a The schematic illustration of the animal model. b Photograph of the orthodontic device in the mouth of SD rats
Sample harvest
Two weeks after orthodontic appliance placement, animals were euthanized. All time interval calculations are based at the indicated Zeitgeber. At the designed time point, orthodontic sensitive tissue of each animal, including the cervical half of the mesial root of the moved molar, its adjacent alveolar and the periodontal ligament, was immediately dissected and bisected into the buccal and palatal halves along the maximal sagittal plane. One half was directly cryoconserved in liquid nitrogen for RT-PCR test (n = 5 for each group); the other half was quickly immersed into 4% paraformaldehyde for histomorphometric analysis (n = 5 for each group). The cryoconserved samples were stored at − 80 °C before tissue homogenizations (mixer mill MM 200, 30 s/sample at 25 Hz, Retsch® GmbH, Haan, Germany). The total RNAs were then extracted by Trizol reagent (Invitrogen, USA).
Immunohistological evaluation
The fixed pieces were immersed in 4% paraformaldehyde solution for 24 h at 4 °C followed by decalcification in EDTA for 15 days at room temperature. After embedding in paraffin, 5 μm sections were prepared for immunohistochemistry (IHC) [21, 22]. Briefly, sections were deparaffinized, rehydrated, and incubated with primary antibodies against OPN (Abcam, USA, 1:200 dilution) overnight at 4 °C. The slides were then incubated with HRP-conjugated secondary antibody before staining in diaminobenzidine (DAB) kit. Finally, all sections were counterstained with hematoxylin. The average optical density (AOD) of the immunohistochemistry assay was measured using Image-Pro Plus 6.0 software.
Real-time quantitative PCR
cDNA was synthesized using a Prime-Script™ RT reagent kit (Takara Bio, Shiga, Japan) according to the manufacturer’s instructions. The related genes, including Period 1 (Per-1), collegen-1 (Col-1), osteopontin (OPN), and integrin-binding sialoprotein (IBSP) were measured, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene for the normalization of RNA expression levels. The PCR primer sequences are displayed in Table 1.
Table 1.
Nucleotide sequences for real-time polymerase chain reaction primers (rat)
| Genes | Forward primer | Reverse primer | Accession no. |
|---|---|---|---|
| PER-1 | ACATCTGAATACACTCTCCGCAAC | GCAGGCGAGATGGTGTAGTAGAG | NM_001034125.1 |
| COL1-1 | CAGATTGAGAACATCCGCAGC | CGGAACCTTCGCTTCCATACTC | NM_053304.1 |
| OPN | GATGAACAGTATCCCGATGCC | CCCTCTGCTTATACTCCTTGGAC | NM_012881.2 |
| IBSP | GAAAGAGCAGCACGGTTGAGTAT | CGTCATAGGTTTCATACGCAGTG | NM_012587.2 |
| β-actin | TGCTATGTTGCCCTAGACTTCG | GTTGGCATAGAGGTCTTTACGG | NM_031144.3 |
Statistical analysis
To determine the periodicity, the data of RT-PCR was tested with Time Series Analysis Single Cosinor v.6.0. The period was determined using the chronobiometric ellipse test. The mean ± standard deviation of the gene expression levels were compared to ZT-0 within a group using the Student’s t test. The gene expression levels at per time point were compared between the static and the dynamic group using the ANOVA at the 0.950 probability level by an SAS 6.12 software package (SAS, Cary, NC, USA). [9]
Results
Primary rPDLCs culture
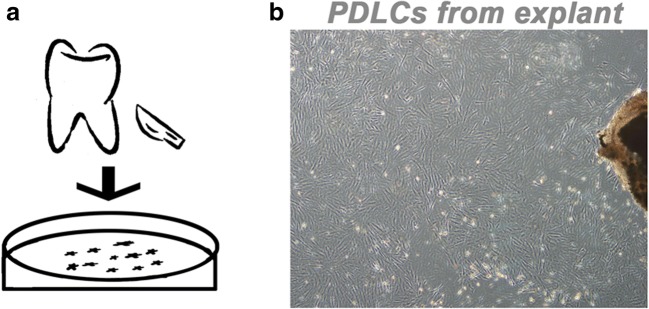
Five days after tissue explant placed on the bottom of the dish, sporadic long-shuttle rPDLCs were seen around the edge of it. After 2 weeks, 80~90% confluence was reached as shown in Fig. 2.
Fig. 2.
Primary PDLCs culture. a The schematic illustration of the harvestment of PDLCs from the upper first molar of SD rat. b Spindle-shaped PDLCs migrated from explant 14 days after implantation
Per-1 and osteogenesis-related genes expression of loaded/unloaded PDLCs
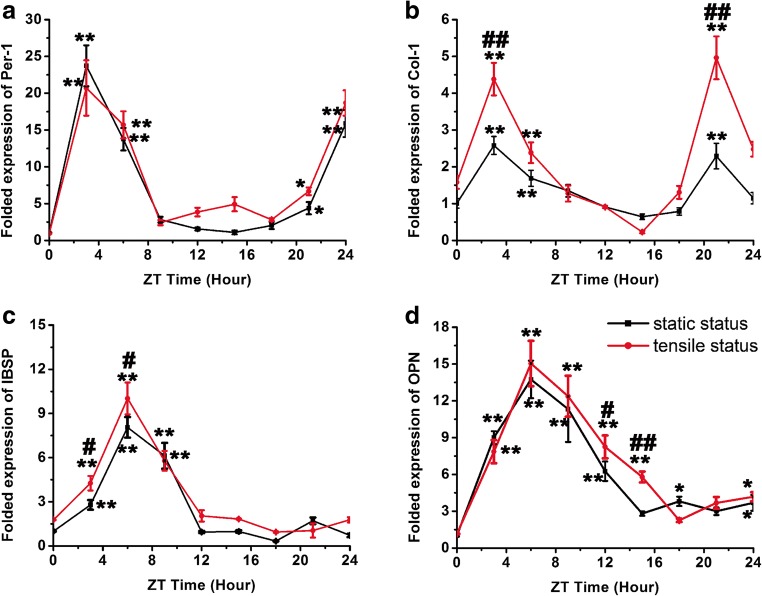
The gene expression levels of Col-1, OPN, and IBSP were assessed every 3 h over a 24-h period to determine whether these osteogenic genes expression of rPDLCs coincided with the circadian cycle-related gene under loaded/unloaded condition. The results showed that, in the static condition, the osteogenic genes’ levels fluctuated in a manner largely coincident with the light/dark cycle indicated by Per-1. Specifically, the levels of osteogenic related genes initially began to rise at 0:00 h, peaking between 03:00 and 06:00 h, and then declined starting at 06:00 h (Col-1) and 09:00 h (OPN and IBSP). To be noticed, levels of Col-1 have two peaks as that of Per-1, and they (03:00 h and 21:00 h) were significantly higher than levels at 00:00 h (p < 0.01). Otherwise, levels of OPN and IBSP at 06:00 h~09:00 h were statistically higher as that of the 00:00 h (p < 0.01). On the other hand, dexamethasone also induced the oscillation of the osteogenic-related gene expressions of rPDLCs under loaded situation. Interestingly, the fluctuation mode kept identically when compared to unloaded situation. The differences of Col-1, OPN, and IBSP expression between static/tensile situations mainly appears in the elevated levels instead of oscillating pattern, while the expression level of Per-1 remains steady under both culture conditions (Fig. 3). A synergistic effect was also noticed to increase the level of Col-1 (03:00 h and 21:00 h) and IBSP (03:00 h and 06:00 h) at the highest expression points.
Fig. 3.
Osteogenic genes’ expression of loaded/unloaded rPDLCs stimulated by dexamethasone. a Per-1 expression of rPDLCs. b Col-1 expression of rPDLCs. c OPN expression of rPDLCs. d IBSP expression of rPDLCs. (*p < 0.05 and **p < 0.01 as compared to 00:00 h gene expression in identical culture environment; #p < 0.05 and ##p < 0.01 as compared to the static culture environment group at identical time point)
Per-1 and osteogenesis-related genes expression during OTM
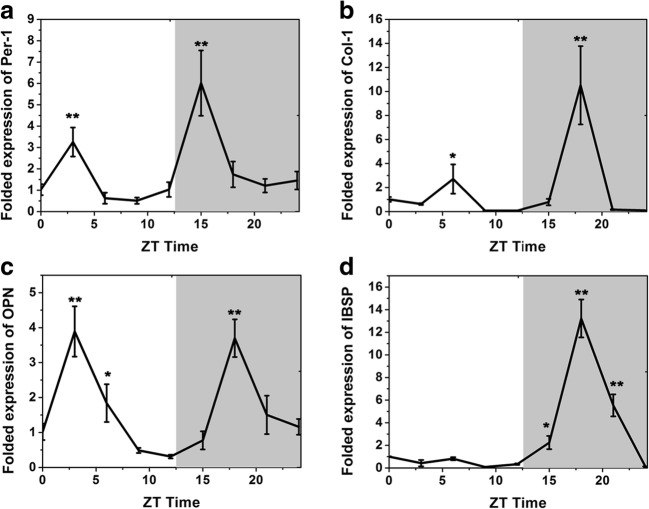
To determine whether or not the expression of osteogenic genes fluctuated over a 24-h time period during orthodontic treatment, real-time RT-PCR analyses were conducted on total RNA isolated from the periodontal tissue of the moving teeth in the orthodontic model on rats. As shown in Fig. 4, Per-1, Col-1, and OPN mRNA levels showed two troughs within a 24-h observation window. For Per-1, the highest expressions appeared at 03:00 h and 15:00 h, while Col-1 and OPN appeared at 03:00 h~06:00 h and 18:00 h. The trough of IBSP delayed 12 h as compared to in vitro test and increased to (13.22 ± 1.68) folds to IBSP at 00:00 h in vivo.
Fig. 4.
Osteogenic genes’ expression of periodontal tissue during OTM. a Per-1 expression. b Col-1 expression. c OPN expression. d IBSP expression. (*p < 0.05 and **p < 0.01 as compared to 00:00 h gene expression)
OPN expression during OTM
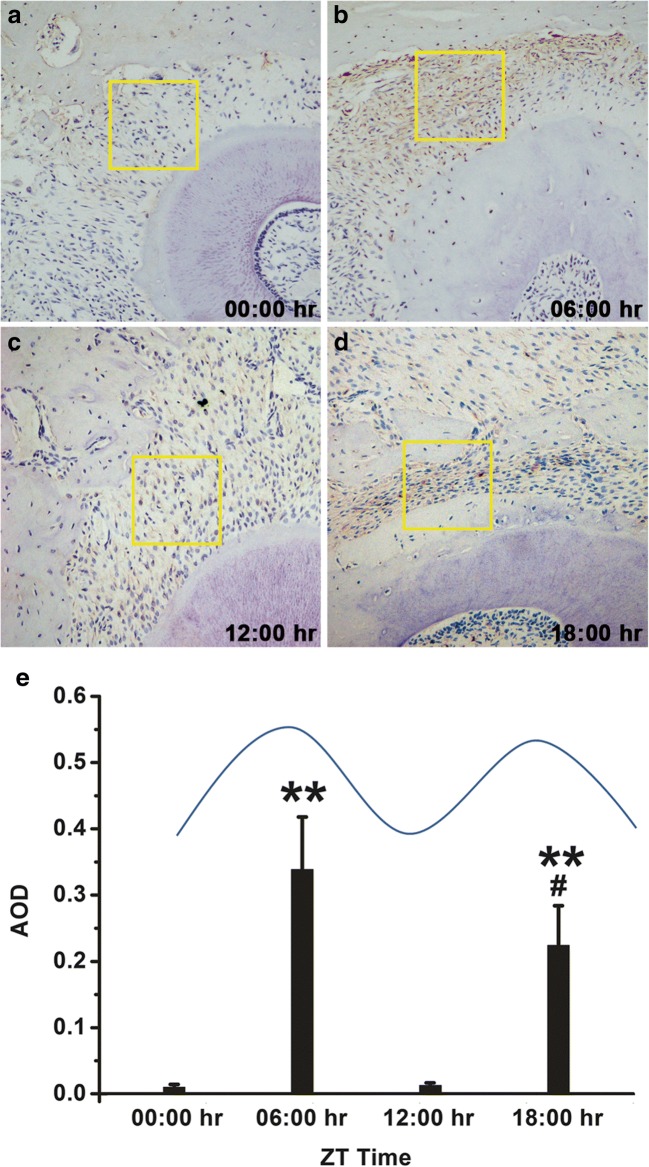
Immunohistochemical analyses were conducted to detect the expression of OPN in periodontal tissue during OTM [22]. OPN was released in cells of the periodontal ligament region between alveolar bone and root surface. Expression of protein was upregulated in the 06:00 h and 18:00 h time points compared with the 00:00 h and 12:00 h (Fig. 5). The AOD was also measured. We noted that the 06:00 h and 18:00 h points presented an increase in the content of OPN. Compared to 00:00 h, 12:00 h, and 18:00 h time points, the 06:00 h showed significantly enhanced levels of OPN (Fig. 5e).
Fig. 5.
Immunohistological staining of OPN in the periodontal tissue during OTM. a OPN staining at the 00:00 h time point. b OPN staining at the 06:00 h time point. c OPN staining in the 12:00 h time point. d OPN staining in the 18:00 h time point. e Average optical density (AOD) of OPN immunohistological staining at 00:00 h, 06:00 h, 12:00 h, and 18:00 h (*p < 0.05 and **p < 0.01 as compared to 00:00 h AOD; #p < 0.05 as compared to 06:00 h AOD)
Discussion
Biological clocks have evolved as an organic evolution and an adaptation to life on a rhythmic planet, synchronizing physiological processes to the environmental light-dark [23]. Although accumulating evidences implied the significance of circadian rhythm in bone metabolism, there are few studies focusing on that within the periodontal tissue. The current study showed that the expression of osteogenic-related genes oscillated along with that of main clock gene in rPDLCs. Also, the in vivo expressions of circadian gene and osteogenic genes/proteins in periodontal tissue were detected on the OTM model for the first time to demonstrate the potential function of circadian cycle in orthodontic moving tooth.
PDLCs play essential role in periodontal metabolism. Previously, researchers found that cyclic tension promotes osteogenic differentiation in human PDLCs, which indicated the main role of PDLCs during OTM on the molecular level [24]. Similarly, we chose PDLCs to reflect the status of periodontal tissue during OTM. On the other hand, an equibiaxial strain value of 12% was adopted on rPDLCs to represent the in vivo deformation of the cells during occlusal loading and OTM [17, 25].
To mimic the hormonal signal, one of the main routes from SCN to transmit central circadian time to peripheral tissue, we introduce dexamethasone to induce the clock gene Per-1 expression. Consistent with previous reports, 10−7 M DX stimulation was effective to initiate the oscillatory expression of Per1 in rPDLCs as shown in the human osteoblast line by Komoto et al. [6]. The maximum expression emerged at 03:00 h and 24:00 h, and exhibited a sinusoidal wave-like pattern. This indicated the existence of a molecular expression periodicity in periodontal apparatus. The oscillatory expressions of osteogenic genes Col-1, OPN, and IBSP in rPDLCs were also stimulated, with the Col-1 expression pattern being closely similar to that of Per1. As Col-1, OPN, and IBSP are important markers of osteoblastogenesis and cementogenesis [26], their circadian expression specifically indicated an oscillatory property of the periodontal formation. We also noticed that the intervention of mechanical force did not interfere with the circadian expression of these osteogenic genes. Interestingly, a synergistic effect between hormonal signal (DX) and orthodontic force (mechanical loading) was first noted in the expression of rPDLCs’ osteogenic genes on the mRNA level, which coincided with previous findings that there are considerable variations in tooth movement to orthodontic force when the force is applied at different times of the day in rats [12, 27].
To further clarify the circadian rhythm of bone metabolism during OTM, the in vivo study was conducted on rats. A nickel-titanium closed-coil spring appliance was applied to establish an orthodontic model on the right maxillary first molar. The force applied was 30 g, which moderately affects the normal tooth of rats and has been shown to be comparable to the force used in human tooth movement [28–30]. The maxillary first molar of rat has five roots. The compressed zone appeared on the cervical half of the middle and mesial roots (mesial sides) [31, 32]. Since the mesial root is the biggest and relatively easy to expose, we took it and its neighboring alveolar as the orthodontic sensitive sample for the histological analysis, including RT-PCR and IHC. Also, the observation level was taken within the cervical half of the mesial root. Meanwhile, to avoid the interference of tension or compression side differences, we divided the orthodontic sensitive tissue along the mesio-distal direction. Thus, the images were taken at the buccal/palatal side of the mesial root of the moved molar. The stress distribution could be considered identically based on previous report [32]. The RT-PCR results showed that the expression of osteogenic genes as Col-1, OPN, and IBSP all demonstrated a circadian cycle pattern. A synchronizing change could also be noticed in these genes, as they simultaneously reached their highest expression level at 18:00 h. This time point was 3 h later compared to Per1, which suggested the controlling role of clock gene in osteogenesis [33, 34]. The immunohistological staining of OPN further proved the fluctuated secretion pattern on the protein expression level. This trend was basically consistent with the circadian rhythm of osteocalcin in the maxillomandibular complex [33], which strongly implied the circadian oscillation state of osteogenic-related factors in periodontal tissue during OTM. This finding first unveiled the relationship between OMT and circadian rhythm on a protein level. It is worth mentioning that, our previous clinical observation found 21:00 h the acrophage of the rapid palate expansion [35]. In this study, we further proved that the acrophase of the osteogenic genes were mainly appeared around 17:00 h~18:00 h (2 or 3 h earlier), which may explained the human change from a mRNA level and indicate new strategy of activation time for a more efficient orthodontic treatment.
Outlook and expert recommendations
This research is especially relevant to the objectives of predictive, preventive, and personalized medicine (PPPM) given that, circadian rhythm affects bone formation, in turn, contribute to a periodicity-regulated OTM [36]. The results presented herein are used to guide further research, like accelerating the tooth movement rate by controlling peripheral oscillation. Also, depending on individual genetic and environmental conditions, different activation time should be recognized to give personized care. In a broader interest for maxillofacial surgery, rehabilitation, and plastic surgery, the role played by circadian rhythm and its underlying potential in provoking more efficient soft/hard tissue reconstruction worth further consideration [37].
In conclusion, our research highlights the circadian rhythm of osteogenic factors within the periodontal tissue during OTM. This study may benefit the prognosis and personalized treatment design of the current orthodontic therapy through the manipulation of circadian rhythm genes. Furthermore, the understanding of the physiological adjustment in OMT from the perspective of light-dark cycle would be more comprehensive and may suggest revolutionary change to its current concept and therapy.
Acknowledgements
The authors would like to thank the Tongji Medical College Animal Center, Anyi Li and the Stomatology faculty of Tongji Medical College Zuojiao Yin for technical support.
Funding
The study was funded by the National Science Foundation of China (No. 81170986 and No.81800891).
Compliance with ethical standards
All procedures concerning animal use were conformed to the guidelines of the Animal Ethics Committee of Huazhong University of Science and Technology (Wuhan, China)
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, Krapfenbauer K, Mozaffari MS, Costigliola V. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. doi: 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloul SS, Gerstenfeld LC, Morgan EF, Carvalho RS, Van Dyke TE, Kantarci A. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofac Orthop. 2011;139(4 Suppl):S83–101. doi: 10.1016/j.ajodo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Cao H, Kou X, Yang R, Liu D, Wang X, Song Y, Feng L, He D, Gan Y, Zhou Y. Force-induced Adrb2 in periodontal ligament cells promotes tooth movement. J Dent Res. 2014;93(11):1163–1169. doi: 10.1177/0022034514551769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Li W, Swain MV, Ali Darendeliler M, Li Q. A periodontal ligament driven remodeling algorithm for orthodontic tooth movement. J Biomech. 2014;47(7):1689–1695. doi: 10.1016/j.jbiomech.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Zheng L, Seon YJ, Mourao MA, Schnell S, Kim D, Harada H, et al. Circadian rhythms regulate amelogenesis. Bone. 2013;55(1):158–165. doi: 10.1016/j.bone.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komoto S, Kondo H, Fukuta O, Togari A. Comparison of beta-adrenergic and glucocorticoid signaling on clock gene and osteoblast-related gene expressions in human osteoblast. Chronobiol Int. 2012;29(1):66–74. doi: 10.3109/07420528.2011.636496. [DOI] [PubMed] [Google Scholar]
- 7.Cavalcanti P, Campos T, Araujo J. Actigraphic analysis of the sleep-wake cycle and physical activity level in patients with stroke: implications for clinical practice. Chronobiol Int. 2012;29(9):1267–1272. doi: 10.3109/07420528.2012.719960. [DOI] [PubMed] [Google Scholar]
- 8.Fujihara Y, Kondo H, Noguchi T, Togari A. Glucocorticoids mediate circadian timing in peripheral osteoclasts resulting in the circadian expression rhythm of osteoclast-related genes. Bone. 2014;61:1–9. doi: 10.1016/j.bone.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Wu X, Yu G, Parks H, Hebert T, Goh BC, Dietrich MA, Pelled G, Izadpanah R, Gazit D, Bunnell BA, Gimble JM. Circadian mechanisms in murine and human bone marrow mesenchymal stem cells following dexamethasone exposure. Bone. 2008;42(5):861–870. doi: 10.1016/j.bone.2007.12.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, Rothman PB, Takahashi JS, Hooper LV. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342(6159):727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janich P, Toufighi K, Solanas G, Luis NM, Minkwitz S, Serrano L, Lehner B, Benitah SA. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell. 2013;13(6):745–753. doi: 10.1016/j.stem.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Miyoshi K, Igarashi K, Saeki S, Shinoda H, Mitani H. Tooth movement and changes in periodontal tissue in response to orthodontic force in rats vary depending on the time of day the force is applied. Eur J Orthod. 2001;23(4):329–338. doi: 10.1093/ejo/23.4.329. [DOI] [PubMed] [Google Scholar]
- 13.Gan EH, Quinton R. Physiological significance of the rhythmic secretion of hypothalamic and pituitary hormones. Prog Brain Res. 2010;181:111–126. doi: 10.1016/S0079-6123(08)81007-2. [DOI] [PubMed] [Google Scholar]
- 14.Qin X, Raj RM, Liao XF, Shi W, Ma B, Gong SQ, Chen WM, Zhou B. Using rigidly fixed autogenous tooth graft to repair bone defect: an animal model. Dent Traumatol. 2014;30(5):380–384. doi: 10.1111/edt.12101. [DOI] [PubMed] [Google Scholar]
- 15.Qin X, Zou F, Chen W, Xu Y, Ma B, Huang Z, Zhu G, Zhou B. Demineralized dentin as a semi-rigid barrier for guiding periodontal tissue regeneration. J Periodontol. 2015;86(12):1370–1379. doi: 10.1902/jop.2015.150271. [DOI] [PubMed] [Google Scholar]
- 16.Kato T, Hattori K, Deguchi T, Katsube Y, Matsumoto T, Ohgushi H, Numabe Y. Osteogenic potential of rat stromal cells derived from periodontal ligament. J Tissue Eng Regen Med. 2011;5(10):798–805. doi: 10.1002/term.379. [DOI] [PubMed] [Google Scholar]
- 17.Wescott DC, Pinkerton MN, Gaffey BJ, Beggs KT, Milne TJ, Meikle MC. Osteogenic gene expression by human periodontal ligament cells under cyclic tension. J Dent Res. 2007;86(12):1212–1216. doi: 10.1177/154405910708601214. [DOI] [PubMed] [Google Scholar]
- 18.Qin X, Hoda MN, Susin C, Wheeler JN, Marshall B, Perry L, Saad N, Yin L, Elsayed R, Elsalanty M, Abdelsayed R, Yu JC, Dhandapani KM, Akbari O, Mozaffari MS, Baban B. Increased innate lymphoid cells in periodontal tissue of the murine model of periodontitis: the role of AMP-activated protein kinase and relevance for the human condition. Front Immunol. 2017;8:922. doi: 10.3389/fimmu.2017.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usumi-Fujita R, Hosomichi J, Ono N, Shibutani N, Kaneko S, Shimizu Y, Ono T. Occlusal hypofunction causes periodontal atrophy and VEGF/VEGFR inhibition in tooth movement. Angle Orthod. 2013;83(1):48–56. doi: 10.2319/011712-45.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzen TJ, Zahra SE, El-Kadi A, Vandevska-Radunovic V. The influence of low-level laser on orthodontic relapse in rats. Eur J Orthod. 2015;37(1):111–117. doi: 10.1093/ejo/cju053. [DOI] [PubMed] [Google Scholar]
- 21.Qin X, Liu JY, Wang T, Pashley DH, Al-Hashim AH, Abdelsayed R, et al. Role of indoleamine 2,3-dioxygenase in an inflammatory model of murine gingiva. J Periodontal Res. 2017;52(1):107–113. doi: 10.1111/jre.12374. [DOI] [PubMed] [Google Scholar]
- 22.Qin X, Liu JY, Abdelsayed R, Shi X, Yu JC, Mozaffari MS, Baban B. The status of glucocorticoid-induced leucine zipper protein in the salivary glands in Sjogren’s syndrome: predictive and prognostic potentials. EPMA J. 2015;7:3. doi: 10.1186/s13167-016-0052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang ZC, Wang YG, Li L, Yin HD, Li DY, Wang Y, Zhao XL, Liu YP, Zhu Q. Circadian clock genes are rhythmically expressed in specific segments of the hen oviduct. Poult Sci. 2016;95(7):1653–1659. doi: 10.3382/ps/pew051. [DOI] [PubMed] [Google Scholar]
- 24.Shen T, Qiu L, Chang H, Yang Y, Jian C, Xiong J, Zhou J, Dong S. Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells. Int J Clin Exp Pathol. 2014;7(11):7872–7880. [PMC free article] [PubMed] [Google Scholar]
- 25.Witt-Enderby PA, Slater JP, Johnson NA, Bondi CD, Dodda BR, Kotlarczyk MP, Clafshenkel WP, Sethi S, Higginbotham S, Rutkowski JL, Gallagher KM, Davis VL. Effects on bone by the light/dark cycle and chronic treatment with melatonin and/or hormone replacement therapy in intact female mice. J Pineal Res. 2012;53(4):374–384. doi: 10.1111/j.1600-079X.2012.01007.x. [DOI] [PubMed] [Google Scholar]
- 26.Ratajczak J, Hilkens P, Gervois P, Wolfs E, Jacobs R, Lambrichts I, Bronckaers A. Angiogenic capacity of periodontal ligament stem cells pretreated with deferoxamine and/or fibroblast growth factor-2. PLoS One. 2016;11(12):e0167807. doi: 10.1371/journal.pone.0167807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada S, Saeki S, Takahashi I, Igarashi K, Shinoda H, Mitani H. Diurnal variation in the response of the mandible to orthopedic force. J Dent Res. 2002;81(10):711–715. doi: 10.1177/154405910208101011. [DOI] [PubMed] [Google Scholar]
- 28.Boas Nogueira AV, Chaves de Souza JA, Kim YJ, Damiao de Sousa-Neto M, Chan Cirelli C, Cirelli JA. Orthodontic force increases interleukin-1beta and tumor necrosis factor-alpha expression and alveolar bone loss in periodontitis. J Periodontol. 2013;84(9):1319–1326. doi: 10.1902/jop.2012.120510. [DOI] [PubMed] [Google Scholar]
- 29.Leiker BJ, Nanda RS, Currier GF, Howes RI, Sinha PK. The effects of exogenous prostaglandins on orthodontic tooth movement in rats. Am J Orthod Dentofac Orthop. 1995;108(4):380–388. doi: 10.1016/S0889-5406(95)70035-8. [DOI] [PubMed] [Google Scholar]
- 30.King GJ, Keeling SD, McCoy EA, Ward TH. Measuring dental drift and orthodontic tooth movement in response to various initial forces in adult rats. Am J Orthod Dentofac Orthop. 1991;99(5):456–465. doi: 10.1016/S0889-5406(05)81579-3. [DOI] [PubMed] [Google Scholar]
- 31.Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian JH, Darendeliler MA, Yoshida N. Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod. 2008;78(3):502–509. doi: 10.2319/052007-240.1. [DOI] [PubMed] [Google Scholar]
- 32.Gonzales C, Hotokezaka H, Arai Y, Ninomiya T, Tominaga J, Jang I, Hotokezaka Y, Tanaka M, Yoshida N. An in vivo 3D micro-CT evaluation of tooth movement after the application of different force magnitudes in rat molar. Angle Orthod. 2009;79(4):703–714. doi: 10.2319/071308-366.1. [DOI] [PubMed] [Google Scholar]
- 33.Gafni Y, Ptitsyn AA, Zilberman Y, Pelled G, Gimble JM, Gazit D. Circadian rhythm of osteocalcin in the maxillomandibular complex. J Dent Res. 2009;88(1):45–50. doi: 10.1177/0022034508328012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McElderry JD, Zhao G, Khmaladze A, Wilson CG, Franceschi RT, Morris MD. Tracking circadian rhythms of bone mineral deposition in murine calvarial organ cultures. J Bone Miner Res. 2013;28(8):1846–1854. doi: 10.1002/jbmr.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai YM, Mao J. Correlation between circadian rhythm and rapid palatal expansion. Zhonghua Kou Qiang Yi Xue Za Zhi. 2010;45(11):655–658. [PubMed] [Google Scholar]
- 36.Kunin A, Polivka J, Jr, Moiseeva N, Golubnitschaja O. “Dry mouth” and “Flammer” syndromes-neglected risks in adolescents and new concepts by predictive, preventive and personalised approach. EPMA J. 2018;9(3):307–317. doi: 10.1007/s13167-018-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lechner J, Noumbissi S, von Baehr V. Titanium implants and silent inflammation in jawbone-a critical interplay of dissolved titanium particles and cytokines TNF-alpha and RANTES/CCL5 on overall health? EPMA J. 2018;9(3):331–343. doi: 10.1007/s13167-018-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]