Abstract
Soil DNA extraction encounters numerous challenges that can affect both yield and purity of the recovered DNA. Clay particles lead to reduced DNA extraction efficiency, and PCR inhibitors from the soil matrix can negatively affect downstream analyses when applying DNA sequencing. Further, these effects impede molecular analysis of bacterial community compositions in lower biomass samples, as often observed in deeper soil layers. Many studies avoid these complications by using indirect DNA extraction with prior separation of the cells from the matrix, but such methods introduce other biases that influence the resulting microbial community composition. To address these issues, a direct DNA extraction method was applied in combination with the use of a commercial product, the G2 DNA/RNA Enhancer, marketed as being capable of improving the amount of DNA recovered after the lysis step. The results showed that application of G2 increased DNA yields from the studied clayey soils from layers from 1.00 to 2.20 m. Importantly, the use of G2 did not introduce bias, as it did not result in any significant differences in the biodiversity of the bacterial community measured in terms of alpha and beta diversity and taxonomical composition. Finally, this study considered a set of customised lysing tubes for evaluating possible influences on the DNA yield. Tubes customization included different bead sizes and amounts, along with lysing tubes coming from two suppliers. Results showed that the lysing tubes with mixed beads allowed greater DNA recovery compared to the use of either 0.1 or 1.4 mm beads, irrespective of the tube supplier. These outcomes may help to improve commercial products in DNA/RNA extraction kits, besides raising awareness about the optimal choice of additives, offering opportunities for acquiring a better understanding of topics such as vertical microbial characterisation and environmental DNA recovery in low biomass samples.
Introduction
The complex chemical and physical structure of soil greatly influences the binding strength of DNA to its particles. Soil factors, such as clay type and content, concentration and valence of cations, the amount of humic substances and pH, dictate the adsorption of DNA into the soil matrix [1, 2]. Clay minerals effectively bind DNA and other charged molecules, such as Ca2+, Mg2+ and Al3+. The binding effect processes between clay and DNA was reviewed in previous works such as Nielsen et al., Greaves and Wilson, and Paget et al. [2–4]. The increased binding of DNA to soil particles significantly reduces degradation of this biological material by extracellular microbial DNases and nucleases [5–7]. However, the same binding force that protects DNA from degradation reduces the amount of DNA recovered during DNA extraction. Appropriate DNA recovery from the soil matrix is also affected when the soil layers, especially those located below the topsoil, contain lower biomass and hence smaller DNA quantities. Indeed, deeper soil layers generally have a lower carbon content, lower nutrient concentrations and consequently lower microbial biomass [8, 9]. Despite the decrease in microbial biomass with soil depth, the hitherto poorly characterised communities dwelling in the deeper layers of the soil perform important roles in carbon sequestration [10], nutrient cycling [11, 12], mineral weathering and soil formation [13, 14], contaminant degradation [13] and groundwater quality [11].
Depending on the methodological approach applied, DNA extraction methods can be divided into direct and indirect methods. In direct extraction protocols, lysis is the first step and the microorganisms are treated with the matrix. During indirect extraction, however, the first step involves the detachment of the microbes from the soil matrix, generally conducted in a liquid media or supplemented by the use of density centrifugations such as in Nycodenz extractions [15]. Both these methods introduce a different extraction bias [16]. During direct extractions from inorganic soils that are particularly rich in clay, the DNA liberated from the microbial cells is quickly adsorbed onto clay particles, preventing complete recovery. During indirect extractions, regardless of which sample or soil type, the bias is due to the different efficacy of the separation treatment on specific microbes, which may enrich one particular microbial fraction over another [17]. These complications may impede the extractions from soil and sediment layers, particularly from lower depths, and thus hamper the study of topics such as vertical microbial characterisation and environmental ancient DNA. The importance of obtaining high DNA yields is also related to greater representativeness of the soil gene pool, reducing the bias introduced into the successive analyses [18, 19].
Although many authors working with soil or sediments have opted for indirect DNA extraction methods to overcome these problems [20–23], the present study focused on a direct approach. Direct extraction methods have been shown to recover the greatest diversity in terms of the number of OTUs, especially if based on mechanical lysis such as bead beating when compared with other lysis methods [24]. With the aim of reducing the retention capacity of clay particles, the commercial product G2 DNA/RNA Enhancer (Ampliqon A/S, Odense, Denmark), hereafter referred to as G2, has been introduced into lysing matrix tubes. The G2 product is marketed as being able to improve the amount of DNA recovered after the lysis step [25]. G2 is a product made from freeze-dried highly mutagenised salmon sperm DNA [25] that adsorbs, like environmental DNA, to the clay particle before cell lysis [26]. However, unlike salmon sperm DNA, the G2 enhancer is not amplifiable in downstream polymerase chain reaction (PCR) applications [25].
The primary objective of the present study was to test the effects of the commercial product G2 on DNA yield and the diversity of the bacterial community from silty clay soil samples from layers between 1.00 and 2.20 m, thus aiming to develop an improved and bias-reduced direct DNA extraction protocol for this type of challenging sample. In addition to the effect of G2 on DNA yield, tests were conducted using both customised and commercially available DNA extraction kits. Customised tubes for evaluating possible influences on the DNA yield were prepared using different bead sizes and amounts, along with different plastic lysing tubes from different suppliers.
Material and methods
Soil core sampling
Soil sampling was performed in September 2016 at a vineyard located in the municipality of La Horra in the Ribera del Duero region in Spain (41°43'46.82"N, 3°53'29.85"W). The sampling approach was designed to obtain two undisturbed soil cores, allowing later sub-sampling for DNA analysis at specific soil core depths. To do so, a Fraste Multidrill model PL was applied for intact soil core sampling using a hydraulic hammer. This model is typically used for standard penetration test (SPT) analysis, but in this case it was fitted with a PVC tube adapted internally to the metal probe rod to allow undisturbed soil core recovery. The soil cores were recovered in the PVC tubes with a 2.5” diameter in lengths of 0.60 m. The cores were then sealed at both ends, labelled and stored in a cold room (4–6 °C) until further sub-sampling.
Soil sub-sampling and homogenisation
Sub-sampling was performed by opening the cores and recovering soil from specific depths and putting the samples into sterile 15 ml plastic tubes. Sub-samples were taken from the central, untouched part of the cores collected using a sterile spatula and tweezers. After sub-sampling, the 15 mL plastic tubes were promptly frozen at -18 °C, shipped to Denmark and kept frozen until DNA extraction.
To obtain a homogenous soil sample for distribution between the DNA extraction tubes, a composite soil sample was produced by mixing 22.5 g of soil. This pool was composed by mixing 1.5 g of soil from 15 selected soil sub-samples representing soil depths ranging from 1.00 to 2.20 m, a depth range chosen on the basis of previous pilot studies. The pilot studies showed that below the depth of approximately 2.20 m, no measurable DNA could be recovered without using G2, which would make a comparison of microbial communities between the extractions with and without G2 unviable. To proceed with the DNA extraction, 0.4 g of the homogenised soil pool was placed in 51 lysing tubes. The DNA extraction followed the protocol of the FastDNA Spin Kit for Soil (MP Biomedicals, LLC, Solon, OH, USA), but was modified with regard to the lysing tubes. For this study, different types of lysing tubes were prepared using commercial products made by MP Biomedicals and Ampliqon in order to test the effect of plastics, beads and G2. All the preparations are summarised in Table 1. With these lysing tube combinations we aimed to compare the microbial communities for samples extracted with: 1) tubes containing different bead sizes (series compared: “g” with “h” and “i” and “a with “c” and “d”), 2) tubes containing G2 (series compared: “a” with “b”; “e” with “g”; and “f” with “h”), and 3) different tubes supplier (series compared: “a” with “i”; “c” with “g”; and “d” with “h”).
Table 1. All the lysing tubes used in the experiments.
| Series | n | Tube | Beads | G2 |
|---|---|---|---|---|
| a* | 5 | MP-Bio | Mixed | No |
| b | 5 | MP-Bio | Mixed | Yes |
| c | 5 | MP-Bio | 1.4 mm | No |
| d | 5 | MP-Bio | 0.1 mm | No |
| e* | 5 | Ampliqon | 1.4 mm | Yes |
| f* | 5 | Ampliqon | 0.1 mm | Yes |
| g | 5 | Ampliqon | 1.4 mm | No |
| h | 5 | Ampliqon | 0.1 mm | No |
| i | 5 | Ampliqon | Mixed | No |
| A-NEG+G2 | 3 | MP-Bio | Mixed | Yes |
| A-NEG | 3 | MP-Bio | Mixed | No |
*Products already commercialised, while the remainder are customised preparations. A-NEG+G2 represents a negative control of the extraction with G2 added; A-NEG represents a negative control of the extraction using the kit’s lysing tube without a sample. With mixed beads, the intention was to have an exact proportion of 0.1 and 1.4 mm beads plus a large 4 mm glass bead. When mixed beads are used, the final weight of the beads is greater than the single type beads. It was decided not to modify this parameter since the test was intended to compare already existing commercial products. The customised preparations were made to evaluate the statistical effect of the different variables involved. Ampliqon and MP-Bio define the different tube supplier: Ampliqon A/S (Denmark) and MP-Biochemicals (Germany).
DNA quantification qPCR, library preparation and sequencing
Following the extractions, the DNA yields were measured using Qubit 2.0 fluorometer (Thermo Scientific). Qubit measurements were taken from all the tube preparations in triplicates and the average results reported in S1 Table. All PCR reactions were prepared using UV sterilised equipment and negative controls were run alongside the samples.
The qPCR with primers targeting the 16S rRNA gene was carried out on a CFX Connect Real-Time PCR Detection System (Bio-Rad). The primers used were 341F (TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-CCTAYGGGRBGCASCAG) and 806R (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-GGACTACNNGGGTATCTAAT) [27], complete with adapters for Illumina MiSeq sequencing.
Single qPCR reactions contained 4 μL of 5x HOT FIREPol EvaGreen qPCR Supermix (Solis BioDyne, Tartu, Estonia), 0.4 μL of forward and reverse primers (10 μM), 2 μL of bovine serum albumin (BSA) to a final concentration of 0.1 mg/mL, 12.2 μL of PCR grade sterile water and 1 ng of template DNA. A standard curve consisting of dilution series of 16S standard was prepared from DNA extracts of Escherichia coli K-12, with seven 16S rRNA gene copies per genome [28]. The quantity of 10−1 16S standard was 8.45 × 107 16S genes/μL. Quantification parameters showed an efficiency of E = 87.7% and R2 of 0.997. The qPCR cycling conditions included initial denaturation at 95 °C for 12 min, followed by 40 cycles of denaturation at 95 °C for 15 sec, annealing at 56 °C for 30 sec, and an extension at 72 °C for 30 sec, with a final extension performed at 72 °C for 3 min [29].
Amplicon library preparation was performed by a two-step PCR, as described by Feld et al. [30] and Albers et al. [31] with slight modifications. Sample concentration was approximately 5 ng of DNA, and both PCRs were carried out using a Veriti Thermal Cycler (Applied Biosystems). In each reaction of the first PCR, the mix contained 12 μL of AccuPrime SuperMix II (Thermo Scientific), 0.5 μL of forward and reverse primer from a 10 μM stock, 0.5 μL of bovine serum albumin (BSA) to a final concentration of 0.025 mg/mL, 1.5 μL of sterile water and 5 μL of template. The reaction mixture was pre-incubated at 95 °C for 2 min, followed by 33 cycles of 95 °C for 15 sec, 55 °C for 15 sec, 68 °C for 40 sec, with a final extension performed at 68 °C for 4 min.
Samples were subsequently indexed by a second PCR using the following PCR protocol. Amplification was performed in 28 μL reactions with 12 μL of AccuPrime SuperMix II (Thermo Scientific), 2 μL of primers complete with indexes and P7/P5 ends, 7 μL of sterile water and 5 μL of PCR1 product. The cycling conditions included initial denaturation at 98 °C for 1 min, followed by 13 cycles of denaturation at 98 °C for 10 sec, annealing at 55 °C for 20 sec, and extension at 68 °C for 40 sec, with a final extension performed at 68 °C for 5 min. The primer dimers formed in the PCR were removed, along with PCR components, using a clean-up step. In this step, HighPrep PCR reagent (MAGBIO) was used to selectively bind the DNA fragments to sequence according to the manufacturer’s protocol. PCR products were finally checked by electrophoresis on a 1.5% agarose gel. Samples were then pooled in an equimolar amount of 10 ng and sequenced at Aarhus University (Roskilde, Denmark) on Illumina MiSeq instrument using 2x250 paired-end reads with V2 Chemistry.
Bioinformatics
Sequencing data were analysed and visualised using QIIME 2 v. 2017.9 [32]. Demultiplexed reads from the Illumina MiSeq were quality filtered using the plugin quality filter with default parameters of QIIME2 [33]. Reads were then denoised, chimera checked and dereplicated using a DADA2 denoise-paired plugin [34]. The output was rarefied to the lowest sample at 16047 reads using qiime feature-table rarefy [35]. Thereafter a multiple-sequence alignment was performed using MAFFT [36] and subsequently a phylogenetic tree generated using FastTree [37]. Alpha and beta diversity analyses were performed through a q2-diversity plugin [38] with the core-metrics-phylogenetic method on the rarefied sequence-variant table. For the alpha diversity, two different parameters were measured: richness and evenness. Richness was measured based on Faith-pd [39], while evenness [40] was reported through the Pielou score [41]. This produced box plots and PCoA plots visualised through Emperor [42]. Taxonomic assignments were performed using qiime feature-classifier classify-sklearn in which a pre-trained Naïve-Bayes classifier with Greengenes v_13.8 [43] was used. Taxa bar plots were built using the plugin qiime taxa bar plot with different filtered, unfiltered and grouped rarefied tables. All the data are available in the Sequence Read Archive (SRA) with the accession number PRJEB24676.
Statistics
A statistical evaluation of the results was performed separately for DNA quantification and sequencing dataset. For DNA quantification, assessments based on one-way ANOVA followed by Scheffe’s test were performed. The ANOVA test indicates whether there is at least one statistically significant (p-value below 0.05) difference in the whole dataset. After this we performed a t-test, on Qubit and qPCR results, to evaluate the impact of G2 on the whole dataset. Scheffe’s method, instead, indicates which group comparison is statistically significant (comparison score > critical Scheffe’s score). The critical value of Scheffe’s method is calculated starting from the F-critic of ANOVA test multiplied by (N-1), where N is the number of comparisons performed with Scheffe’s method. Scheffe’s test was used here because it is less sensitive to an unequal number of samples representing the different variables analysed. Furthermore Scheffe’s methods have been used to reduce false positive results due to type I errors when multiple comparisons are performed on the same dataset [44]. Both tests were applied to the dataset of the DNA quantification obtained using Qubit and qPCR for the gene copy number. Since both results were consistent and G2 is mainly used to obtain DNA for PCR-based downstream applications, only the results of the qPCR are discussed. All statistical evaluations and visualisations regarding DNA amount and gene copy number quantification were performed in Microsoft Office Excel 2010.
Sequencing data after QIIME 2 pipeline processing (2.4) were statistically evaluated using the Kruskal-Wallis test for alpha and beta diversity, a non-parametric method substitute of ANOVA when the normal distribution of data cannot be assumed. The resulting p-value of the alpha diversity comparison was based on the medians of different parameters (richness and evenness) calculated between the different series analysed. Beta diversity analyses were performed using both Kruskal Wallis and PERMANOVA with 999 permutations. Finally a statistical evaluation was performed of differentially abundant features based on an analysis of composition of microbiomes (ANCOM). ANCOM computes Aitchinson’s [45] log-ratio of relative abundance for each taxon, controlling the false discovery rate (FDR) using the Benjamini-Hochberg procedure. This test is based on the assumption that few features change in a statistical way between the samples, and hence it is very conservative [46]. All these statistical tests were applied using QIIME2 v2017.9.
Results
The main aim of the present study was to evaluate the impact of the commercial product G2 on DNA extraction from a silty clay soil layer between 1.00 and 2.20 m. Soil samples description, defined as silty clay, was performed together with an experienced geologist. G2, freeze-dried inside the lysing tubes, was applied with the purpose of preventing or reducing the adsorption of environmental DNA onto the clay particles in the soil, thus improving the recovery of nucleic acids during cellular lysis. This paper presents the results of the impact of G2 on final DNA extraction efficiency and its possible impact on the composition of soil microbial communities. Furthermore the G2 effect was compared with other variables such as bead size and tube supplier in different commercialised and customised lysing tubes, as summarised in Table 1.
G2 enhanced DNA recovery from deep soil layers
In order to test the influence of the presence of G2 on DNA yield, a series of extractions were set up using different bead size and tube combinations. Following DNA quantification via Qubit, the results were analysed. A first evaluation, using t-test to compare the series that differ for the presence of G2, resulted in a p-value of 9.29*10−16, indicating a higher yield when G2 is used. As seen in Fig 1A, the G2 component consistently improved the DNA yield (Scheffe’s score > 27.612 –S2 Table). Fig 1A shows that there were differences between the different tube preparations, irrespective of the presence of G2. Different bead combinations and tubes without the addition of G2 were therefore tested. These variations could potentially be attributed to the different beads and the plastic of the tubes (Fig 1B). The mixed beads allowed greater DNA recovery compared to the use of either 1.4 mm or 0.1 mm beads, regardless of the type of plastic tubes used (Scheffe’s score > 27.612 –S2 Table). The differences in the DNA yield between the 1.4 mm and 0.1 mm beads were less pronounced, including for the differences due to the plastic composition of the tubes. Mixed beads always allowed recovery of the highest DNA yield in all the tube preparations in which they were used.
Fig 1. Results based on Qubit quantification after DNA extraction expressed as ng/μl.
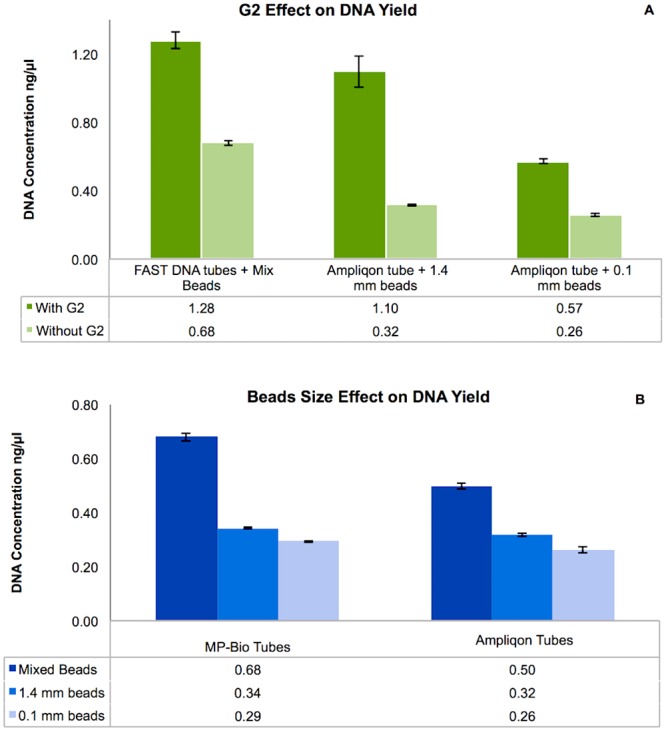
(A) Comparison of three commercial products with and without G2. (B) Comparison of three different bead sizes and the two tube types, without added G2. Values shown are averages of n = 3 independent measurements on n = 5 biological replicates. Error bars are calculated from the standard deviation for each series. There was a statistical significance (Scheffe’s score > 27.612) for the three comparisons with and without G2 in test A, while for test B, it occurred in the comparisons between mixed vs. 1.4 mm beads and mixed vs. 0.1 mm beads, but not between 1.4 mm vs. 0.1 mm beads or between the two tube suppliers (S2 Table).
Greater DNA yield corresponded to a higher number of 16S genes
Considering that G2 is a DNA-based product, this could potentially affect the Qubit measurements since the signal recorded using a fluorescent dye is emitted when it binds dsDNA. In such a situation there might be an overestimation of soil DNA from the Qubit result. To examine whether this was the case, the copy number variation of a genetic marker, such as the 16S rRNA gene for bacteria, was tested via qPCR.
The results of the qPCR (Fig 2 and S3 Table) confirmed the trend observed with the DNA yield provided by the Qubit analysis. The ANOVA based on the qPCR results described the dataset with a p-value of 1.0147*E-36 (S4 Table). This meant that in all the data produced by qPCR, taking into account the variance within and between groups, there was at least one difference that was statistically significant. We, therefore, performed a t-test comparing the sample with and without G2, resulting in a p-value of 4.3*10−9, which indicate the statistical relevance of the usage of G2. However, to identify all the other differences statistically relevant in our database such as beads and tube suppliers, Scheffe’s method was applied (S5 Table). Based on these results, it was evident that G2 always allowed the recovery of the highest number of genes, while mixed beads also improved gene recovery (Scheffe’s score > 26.117 –category a in S5 Table). These results were also evident and consistent when looking at the DNA yield measured via Qubit, as reported in S1 Table. The comparison of different plastic tubes (category e in S5 Table) showed a non-significant difference. Instead, the beads used in lysis were shown to have a significant effect on DNA yield (category b in S5 Table) and, as discussed below, also on microbial composition. From the Scheffe’s statistics, it was possible to observe that the mixed beads from the original lysing tube of the FastDNA Spin Kit for Soil always recovered more DNA than the other bead preparations containing either 0.1 mm or 1.4 mm beads. Furthermore it was noted that although there was no statistically relevant difference between the use of 1.4 mm or 0.1 mm beads, the results changed, showing a statistical significance, when G2 was added (category d in S5 Table).
Fig 2. 16S rRNA gene copy number variation after qPCR expressed as genes/μl.
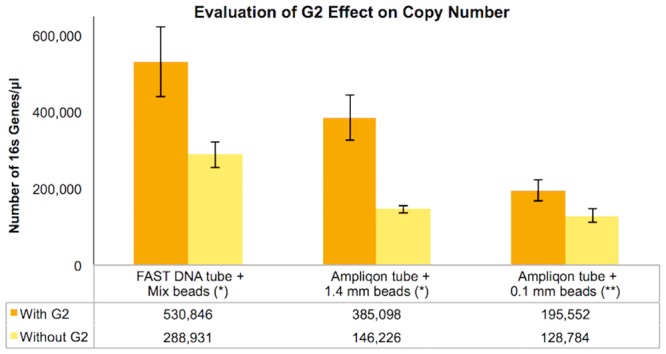
A comparison between three commercial products with and without G2. Values shown are averages of n = 3 technical replicates for n = 3 biological replicates for each series. Error bars are calculated from the standard deviation for each series. (*) Significantly relevant comparison (Scheffe’s score > 26.117). (**) Not statistically relevant (Scheffe’s score < 26.117) (S5 Table).
Furthermore, the results reported in S5 Table (category c) Table considered the possibility of basal contamination of the kit that may be relevant during the analyses or alternatively the possibility of a source of contamination introduced during the G2 freeze-drying process. In both cases, the control samples showed that basal contamination of the kit did not affect the results, and furthermore that the freeze-drying process did not introduce any more detectable DNA than the negative control.
To visualise this statistical evaluation based on all the different comparisons using Scheffe’s method, the following procedure was applied: Scheffe’s score (S5 Table) was averaged by grouping different comparisons based on the variables examined, i.e. G2, bead size and tube plastic. The average score was then plotted and the results shown in Fig 3. Calculations are reported in S6 Table. The results illustrated in Fig 3 show that the main influence to DNA yield came from the presence or absence of G2 in the lysing tubes, while the bead effect showed a smaller, but still relevant, significance. The results related to the two plastic tubes tested were non-significant.
Fig 3. Visualisation of the average score of the Scheffe’s test.
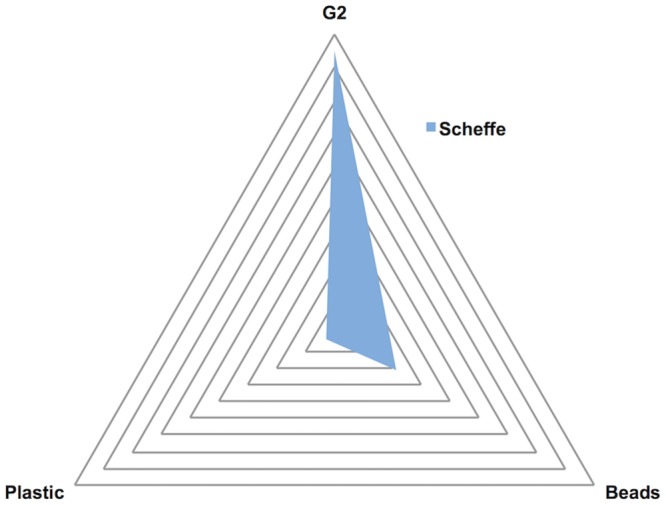
Visualisation of the average score of the Scheffe’s test of categories a, b and e, according to S5 Table, visualising the impact of G2 compared with the effect of plastics and beads. The values on which this visualisation is based are reported in S6 Table.
Influence of G2 on the bacterial community structure
We also tested whether the use of G2 influenced microbial community composition, for example by enriching particular taxa or skewing the relative taxa abundance between samples. Hence, a subset of the DNA samples was sequenced for the V3-V4 region of the 16S rRNA gene. A statistical evaluation of the differences in alpha and beta diversity allowed an assessment of the impact of G2 on the microbial community DNA from this sample. Similarly, the impact of other variables, such as tubes and beads, on microbial community DNA composition was also evaluated.
After data pre-processing through the QIIME2 pipeline, denoised and rarefied exact sequence variants were obtained. From these, the effects of G2 and bead size on alpha diversity were examined, focusing on two parameters: richness and evenness. These results are summarised in Fig 4.
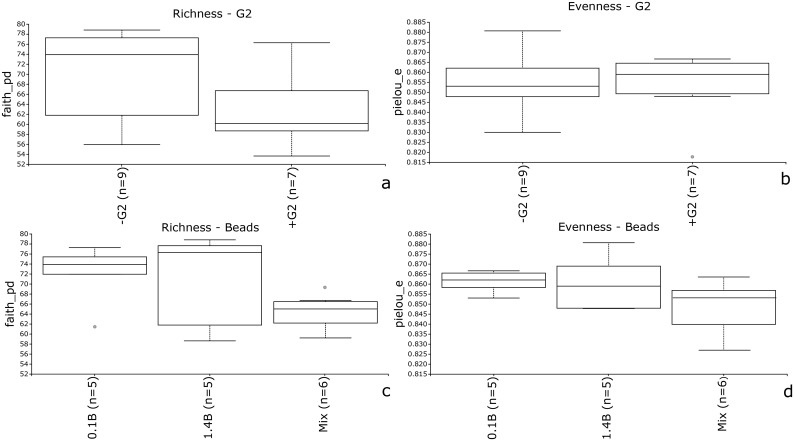
Fig 4. Alpha diversity analyses on G2 and bead impact on richness and evenness.
(a) Boxplot based on the Faith-pd index and comparing all the samples with G2 (n = 7) and without G2 (n = 9). (b) Boxplot based on the Pielou-S score for evenness between samples obtained with G2 (n = 7) and without G2 (n = 9). (c) Boxplot based on the Faith-pd index and comparing all the samples with 0.1 mm (n = 5), 1.4 mm (n = 5) and mixed (n = 6) beads. (d) Boxplot based on the Pielou-S score for evenness on samples obtained with 0.1 mm (n = 5), 1.4 mm (n = 5) and mixed (n = 6) beads.
The presence of G2 did not statistically significant affect the richness (p = 0.08) or evenness (p = 0.874) of the microbial community, as shown in Fig 4A and 4B respectively. The same tests were applied to the results of the bead and tube comparison. While the use of two different plastic tubes did not show any statistically significant effect in terms of richness (p = 0.874) or evenness (p = 0.322), this was not the case for the beads. In particular, the use of mixed bead sizes introduced a significant negative difference in richness when compared to 0.1 mm beads (p = 0.03), and in evenness when performing the same pairwise comparison with 0.1 mm beads (p = 0.01). This change in the microbial community resulted in a smaller amount of sequence variants and evenness when mixed beads were used.
A further analysis was performed of the differences between the samples’ beta diversity using unweighted Unifrac and Bray-Curtis dissimilarity as metrics. These results were visualised using principal coordinates analysis (PCoA) plots and statistically evaluated in PERMANOVA. Fig 5 presents the PCoA plots showing the effects of the use of G2 (Fig 5A) and different beads (Fig 5B). These plots were obtained with the unweighted Unifrac distance matrix. The Bray-Curtis PCoA plot was consistent with this representation (not shown). PERMANOVA tests were performed with 999 permutations on beads, tubes and the G2 effect and the results are summarised in Table 2. These results showed that only mixed beads had a statistically relevant effect on beta diversity in the present study’s samples (p-value = 0.005 and 0.002). A PCoA plot of the tube type used did not allow a clear distribution to be distinguished between the two different tubes (not shown) and this was confirmed by the PERMANOVA analysis (p-value = 0.465) (Table 2).
Fig 5. PCoA plots obtained from the UniFrac distance matrix of the sequenced samples from the different tube preparations.
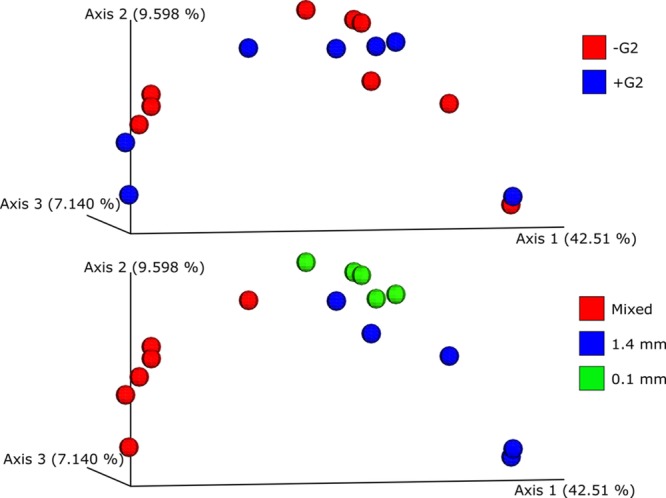
(A) Comparison of data obtained from samples with G2 (blue dots) and without G2 (red dots). (B) Comparison of data obtained from samples using 0.1 mm beads (green dots), 1.4 mm beads (blue dots) and mixed beads (red dots).
Table 2. Results of the PERMANOVA analyses.
| Variable | Groups | Pseudo f- | p-value | q-value |
|---|---|---|---|---|
| G2 | Present /Absent | 0.979 | 0.499 | 0.499 |
| Beads | 0.1 mm / 1.4 mm | 0.907 | 0.787 | 0.787 |
| Beads | 0.1 mm / Mixed | 1.639 | 0.005 | 0.0075* |
| Beads | 1.4 mm / Mixed | 1.715 | 0.002 | 0.006* |
| Tube | Ampliqon/MP-Bio | 0.979 | 0.465 | 0.465 |
The first row refers to the use of G2, the second, third and fourth rows refer to the comparison of different groups of beads, and the fifth row refers to the tube effect.
*Statistically relevant comparisons. Pseudo f- refers to the ratio between cluster variance and within cluster variance; p-value is considered statistically relevant below 0.05; q-value is an adjusted p-value for false discovery rate (FDR) when multiple comparisons are performed.
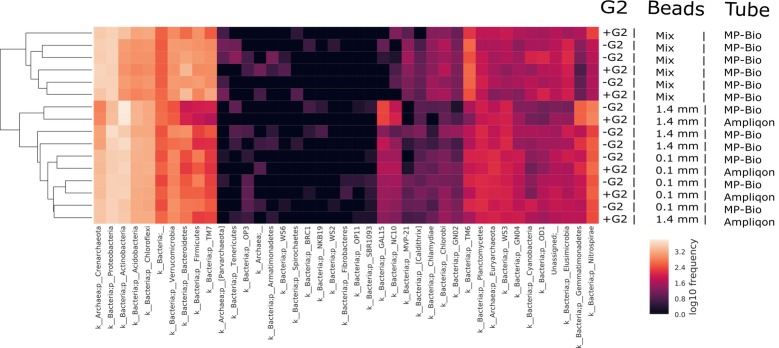
The compositional bar chart made on phylum resolution (S1 Fig) shows that the bacterial communities of the DNA extracts with and without G2 were not visually distinguishable. By contrast, when looking at the profiles using different types of beads, these differences were more evident, especially between the mixed beads compared with 1.4 mm or 0.1 mm beads. All the data reported so far were obtained by excluding the negative control from the alpha and beta diversity analyses in order to maximise the effect of the different variables reducing any false negative results. This could be done after checking the composition of the negative control through a taxa-bar plot. The dominant taxa in the negative control belonged to the genus Ralstonia, already reported and known to be common contaminants of kits and PCR reagents [47]. This taxon was filtered out of the other sample since it was present in low amounts (~0.1% across the different series). Furthermore, the other taxa that appeared in the negative controls were checked and since they were dominants in all the samples and represented only a small fraction of the negative control, it was decided not to remove them. The taxa composition (S1 Fig) of the microbial community in the soil horizon between 1.00 and 2.20 m was shown to be mainly composed of members of the phyla of Proteobacteria, Actinobacteria, Acidobacteria and Chloroflexi. Archaea were also present and the dominant phylum was Crenarcheota, representing 10% of the total relative abundance. For a better visualisation of the differences in terms of phyla attributable to the different variables involved, Fig 6 shows a heatmap of the microbial community composition of the dataset. Looking at the dendrogram on the left of Fig 6, two main groups can be distinguished according to the beads in the second heatmap. The use of mixed beads in particular altered the community profile, whereas none of the other variables produced this effect.
Fig 6. Heatmap at phylum level of the sequencing dataset.
The logarithmic scale in which colour intensity determines the abundance of the taxa can be seen in the bottom right-hand corner. The top heatmap represents the samples grouped by presence/absence of G2, the middle one refers to the different bead sizes used, while the bottom one is related to the two different tube suppliers. The names of the samples are given on the right, while the names of the phylum are stated below. On the left the dendrogram of similarity between all the samples is presented.
Finally, an ANCOM test was run in order to identify differentially abundant microbes in the different groups of samples and determine which variables mostly affected the result.
Specifically, two different group comparisons were performed: one based on the presence/absence of G2 when mixed beads were used, and the other based on the difference between the use of mixed beads compared to 0.1 mm beads when G2 was used. These two tests confirmed that G2 did not statistically alter any taxa among the samples, as reported in S7 Table. However, there were four statistically relevant differences between the use of mixed and 0.1 mm beads as reported in S8 Table. None of these four taxa belonged to the dominant fraction of the microbial community, but they represented around 1% of the microbial community. Three of them were barely represented in the samples when 0.1 mm beads were used, while they were not present at all when mixed beads were used. They belong to the phyla of Acidobacteria and Gemmatimonadetes. Only one of these microbes was represented, at up to 1.36%, when mixed beads were used compared to the 0.1 mm beads and it belongs to the order of Legionellales.
Discussion
The use of G2 allowed the recovery of more DNA in all settings and samples, making it the largest impact variable compared to the other variables of interest (tubes and beads). The increased DNA yield found in the presence of G2 was in accordance with Bælum et al. [48] and Jacobsen et al. [49]. The recovered DNA in the first case [48] was several orders of magnitude higher, probably related to a higher clay content and/or the clay mineral type increasing the retention capacity of the matrix on the released DNA. A 7.5-fold average increase in DNA yield was obtained in the second study, which was an inter-laboratory test [49].
To resolve whether the amount of DNA detected was derived from the microorganisms dwelling in the soil and not to any residual G2, the impact on 16S rRNA gene copy numbers was measured using qPCR (Fig 2). The output confirmed the same trend observed for the Qubit results, meaning a significantly higher recovery of bacterial genes in all tested cases. The combined use of qPCR and Qubit guarantees the best qualitative and quantitative analyses of DNA [48, 50]. The differences between the measured DNA amount and the gene copy number can be explained by the qPCR only being applied to the bacterial population, leading to an underestimation of the total number of cells in the samples. This could partially be compensated for by the copy number variations of the 16S rRNA gene in different bacterial populations possibly leading to an overestimation of the recovered amount of cells within the present samples.
G2 allowed the recovery of the largest amount of DNA when combined with mixed beads, but a relevant increase could also be observed by using just 1.4 mm beads, regardless of the plastic tube used. The number of cells could be estimated from the 16S rRNA gene numbers using the same methodology as Vishnivetskaya et al. [24], in which it was assumed that the average 16S copy number for the microbial community was 3.6, based on the observation of Klappenbach et al. [51]. This led to the calculation of a maximum of 3.7*105 cells g-1 of soil using G2 and 2*105 without G2. This value was lower than other reported cell counts, such as 2.2*108 using the same kit, but in topsoil [24], a difference that can be attributed to a deeper sampled layer. Comparing the present results with a direct DNA extraction from a deep soil layer, such as the one performed by Taylor et al. [52], a more similar value is obtained of around 1.2*106. Differences here could be related to the different soil type and the different kit used for the DNA extraction.
The combination of the two statistical tests, ANOVA and Scheffe, allowed multiple comparison analyses on a single dataset, as reported by Mermillod-Blondin et al. [53] and Brown [54]. In addition to what is reported in S5 Table and visualised in Fig 3, it was interesting to note that although there was not a statistically relevant difference between the use of 1.4 mm or 0.1 mm beads, the results changed when G2 was added and showed a statistical significance (category d in S5 Table). This conversion might be due to the fact that 1.4 mm beads allowed improved lysis of the cells in the sample, probably because of a better dissolution of the soil particles, compared with 0.1 mm beads. However, if G2 was not added, most of the extracted DNA was suddenly adsorbed to the clay particles of the matrix, preventing its recovery.
To verify whether the use of G2 influenced the microbial composition, a subset of the DNA extracted from the soil samples was sequenced for the 16S rRNA gene. An evaluation of alpha diversity showed that evenness and richness did not change when comparing samples obtained with and without G2 or when using different tubes. Non-residual contamination of G2 has also been confirmed by Jacobsen at al. [49], where in-depth sequencing did not show traces of the DNA originating from G2 in the final DNA extract [49]. In contrast, bead size type had a statistically relevant effect on these two parameters. The lower richness and evenness when mixed beads were used could be due to the fact that most of the total DNA extracted came from the dominant fraction of soil bacteria. Since these dominant bacteria were not selectively lysed by the 1.4 and 4.0 mm beads alone, but were by the 0.1 mm beads, this ratio between dominant and rare taxa would be maintained with every bead preparation, although the absolute number of lysed cells would be different. However, the library size from each sample was not proportional to the starting amount of DNA, leading to an uneven representation of rare taxa in the sample with a higher starting amount of DNA. Since the reads belonging to the dominant bacteria were sequenced multiple times for each sample, the richness when mixed beads were used was slightly lower. This was a common issue in all the PCR-based surveys and has also been confirmed by Gonzalez et al. [55]. The lower evenness was also expected. In this case, samples from mixed bead tubes had a lower number of rare taxa, leading to a greater imbalance between dominant and rare taxa and thus to less evenness. With a larger amount of reads per sample, the effect on richness and evenness could probably be cancelled out or reverted due to the sequencing depth effect [56]. Some of the differences caused by uneven sequencing coverage could be reduced by performing a rarefaction on the sample, as was done in this study, but cannot be avoided completely [57].
In terms of beta diversity, only the use of mixed beads had an effect on the different samples. The PCoA distributions presented in Fig 5A show a clear cluster belonging to extracted mixed bead samples. The three bead sizes (0.1 mm, 1.4 mm and 4.0 mm) could act together to provide a better dissolution of the sample and recover more DNA, but if sequencing is not deep enough it could lead to the detection of a reduced number of taxa, as was the case in this study. Furthermore, since commercial products were used here, it is worth noting that the amount of beads differed between the MP-Biochemical tubes and the Ampliqon tubes. In particular MP-Bio tubes have a higher volume of beads, and this could explain some of the differences detected in terms of the amount of DNA and the quality of the taxa. The synergistic bead effect, both positive and negative, was not evaluated in this study. Furthermore, the PCoA plots presented in Fig 5, and then confirmed by the PERMANOVA analyses (Table 2), showed that there was no statistical difference in microbial composition between the samples with or without the use of G2 (Fig 5A) and also irrespective of the plastic tubes used (not shown). Finally, in terms of taxonomic composition of the microbial community (S1 Fig), these results are consistent with Janssen [58] and He [59] with regard to the dominant phyla of Proteobacteria, Actinobacteria and Acidobacteria in soil at different horizon levels.
Conclusions
Based on DNA yield quantification and gene copy-number detection after qPCR, the current study demonstrated that the use of the commercial product G2 DNA/RNA Enhancer (Ampliqon A/S, Odense, Denmark) increased the amount of DNA recovered from composite and homogenised silty clay soil samples. Furthermore, the use of G2 did not introduce any significant differences in the richness or evenness of the bacterial community obtained after amplicon library sequencing when compared with those samples sequenced without the addition of G2. The two plastic lysing tubes tested had no effect on either the yield or the composition of the microbiota.
In contrast, the use of different bead sizes had a significant effect. A higher DNA yield was obtained with the simultaneous presence of differently sized beads. Moreover, the use of mixed beads in this case led to a slightly lower richness and evenness in the taxa distribution, an effect that could be explained by the sequencing depth. In terms of future perspectives coming out of this study, it would be worth applying G2 to other kinds of samples in which DNA recovery could be affected by proteins or other compounds biasing downstream application.
These tests may provide useful information for the improvement of existing commercial products in DNA/RNA extraction kits, raising awareness about the optimal choice of additives.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We acknowledge Jesper S. Pedersen (Ampliqon A/S) for preparing and providing laboratorial material and Patricia Benitez for fieldwork support.
Data Availability
The authors confirm that all data underlying the findings are fully available within the paper and its Supporting Information files. The sequencing data have been deposited in the European Nucleotide Archive (ENA) (http://www.ebi.ac.uk/ena) and are accessible through the study accession number PRJEB24676.
Funding Statement
This study was funded by the Horizon 2020 Programme of the European Commission within the Marie Skłodowska-Curie Innovative Training Network “MicroWine” (grant number 643063) to LHH (https://ec.europa.eu/programmes/horizon2020/en/h2020-section/marie-sklodowska-curie-actions). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ladd JN, Foster RC, Nannipieri P, Oades J. Soil structure and biological activity In: S G, B JM, editors. Soil biochemistry. 9. New York: Marcel Dekker; 1996. p. 23–78. [Google Scholar]
- 2.Nielsen KM, Calamai L, Pietramellara G. Stabilization of extracellular DNA and proteins by transient binding to various soil components In: Nannipieri P, Smalla K, editors. Nucleic acids and proteins in soil. Soil biology: Springer; 2006. p. 141–57. [Google Scholar]
- 3.Greaves M, Wilson M. The adsorption of nucleic acids by montmorillonite. Soil Biology and Biochemistry. 1969;1(4):317–23. [Google Scholar]
- 4.Paget E, Simonet P. On the track of natural transformation in soil. FEMS Microbiology Ecology. 1994;15(1–2):109–17. [Google Scholar]
- 5.Khanna M, Stotzky G. Transformation of Bacillus subtilis by DNA bound on montmorillonite and effect of DNase on the transforming ability of bound DNA. Applied and Environmental Microbiology. 1992;58(6):1930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crecchio C, Stotzky G. Binding of DNA on humic acids: effect on transformation of Bacillus subtilis and resistance to DNase. Soil Biology and Biochemistry. 1998;30(8):1061–7. [Google Scholar]
- 7.Ogram A, Sayler GS, Gustin D, Lewis RJ. DNA adsorption to soils and sediments. Environmental science & technology. 1988;22(8):982–4. [DOI] [PubMed] [Google Scholar]
- 8.Agnelli A, Ascher J, Corti G, Ceccherini MT, Nannipieri P, Pietramellara G. Distribution of microbial communities in a forest soil profile investigated by microbial biomass, soil respiration and DGGE of total and extracellular DNA. Soil Biology and Biochemistry. 2004;36(5):859–68. [Google Scholar]
- 9.Fritze H, Pietikäinen J, Pennanen T. Distribution of microbial biomass and phospholipid fatty acids in Podzol profiles under coniferous forest. European Journal of Soil Science. 2000;51(4):565–73. [Google Scholar]
- 10.Fierer N, Chadwick OA, Trumbore SE. Production of CO 2 in soil profiles of a California annual grassland. Ecosystems. 2005;8(4):412–29. [Google Scholar]
- 11.Madsen EL. Impacts of agricultural practices on subsurface microbial ecology. Advances in agronomy (USA). 1995. [Google Scholar]
- 12.Richter DD, Markewitz D. How deep is soil? BioScience. 1995;45(9):600–9. [Google Scholar]
- 13.Konopka A, Turco R. Biodegradation of organic compounds in vadose zone and aquifer sediments. Applied and Environmental Microbiology. 1991;57(8):2260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiebert FK, Bennett PC. Microbial control of silicate weathering in organic-rich ground water. Science-New York then Washington-. 1992:278-. [DOI] [PubMed] [Google Scholar]
- 15.Holmsgaard PN, Norman A, Hede SC, Poulsen PH, Al-Soud WA, Hansen LH, et al. Bias in bacterial diversity as a result of Nycodenz extraction from bulk soil. Soil Biology and Biochemistry. 2011;43(10):2152–9. [Google Scholar]
- 16.Lombard N, Prestat E, van Elsas JD, Simonet P. Soil-specific limitations for access and analysis of soil microbial communities by metagenomics. FEMS microbiology ecology. 2011;78(1):31–49. 10.1111/j.1574-6941.2011.01140.x [DOI] [PubMed] [Google Scholar]
- 17.Delmont TO, Robe P, Clark I, Simonet P, Vogel TM. Metagenomic comparison of direct and indirect soil DNA extraction approaches. Journal of microbiological methods. 2011;86(3):397–400. 10.1016/j.mimet.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 18.Bürgmann H, Pesaro M, Widmer F, Zeyer J. A strategy for optimizing quality and quantity of DNA extracted from soil. Journal of microbiological methods. 2001;45(1):7–20. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy K, Hall MW, Lynch MD, Moreno-Hagelsieb G, Neufeld JD. Evaluating bias of Illumina-based bacterial 16S rRNA gene profiles. Applied and environmental microbiology. 2014;80(18):5717–22. 10.1128/AEM.01451-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Cheng C, He L, Huang Z, Sheng X. Characterization of depth-related changes in bacterial communities involved in mineral weathering along a mineral-rich soil profile. Geomicrobiology Journal. 2014;31(5):431–44. [Google Scholar]
- 21.Duarte GF, Rosado AS, Seldin L, Keijzer-Wolters AC, van Elsas JD. Extraction of ribosomal RNA and genomic DNA from soil for studying the diversity of the indigenous bacterial community. Journal of Microbiological Methods. 1998;32(1):21–9. [Google Scholar]
- 22.Crecchio C, Gelsomino A, Ambrosoli R, Minati JL, Ruggiero P. Functional and molecular responses of soil microbial communities under differing soil management practices. Soil Biology and Biochemistry. 2004;36(11):1873–83. [Google Scholar]
- 23.Williamson KE, Kan J, Polson SW, Williamson SJ. Optimizing the indirect extraction of prokaryotic DNA from soils. Soil Biology and Biochemistry. 2011;43(4):736–48. [Google Scholar]
- 24.Vishnivetskaya TA, Layton AC, Lau MC, Chauhan A, Cheng KR, Meyers AJ, et al. Commercial DNA extraction kits impact observed microbial community composition in permafrost samples. FEMS microbiology ecology. 2014;87(1):217–30. 10.1111/1574-6941.12219 [DOI] [PubMed] [Google Scholar]
- 25.Bælum J, Jacobsen CS, inventorsImprovement of low-biomass soil DNA/RNA extraction yield and quality. Denmark patent EP 2 443 251 B1. 2012 25.04.2012.
- 26.Ampliqon. G2 DNA/RNA Enhancer 2017 [cited 2017 July 3]. http://ampliqon.com/en/products/pcr-enzymes/dnarna-extraction/g2-dnarna-enhancer/.
- 27.Hansen CHF, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sørensen SJ, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55(8):2285–94. 10.1007/s00125-012-2564-7 [DOI] [PubMed] [Google Scholar]
- 28.Blattner FR, Plunkett G, Bloch CA, Perna NT, Burland V, Riley M, et al. The complete genome sequence of Escherichia coli K-12. science. 1997;277(5331):1453–62. [DOI] [PubMed] [Google Scholar]
- 29.Vestergård M, Bang-Andreasen T, Buss SM, Cruz-Paredes C, Bentzon-Tilia S, Ekelund F, et al. The relative importance of the bacterial pathway and soil inorganic nitrogen increase across an extreme wood-ash application gradient. GCB Bioenergy. 2018;10(5):320–34. [Google Scholar]
- 30.Feld L, Nielsen TK, Hansen LH, Aamand J, Albers CN. Establishment of Bacterial Herbicide Degraders in a Rapid Sand Filter for Bioremediation of Phenoxypropionate-Polluted Groundwater. Applied and environmental microbiology. 2016;82(3):878–87. 10.1128/AEM.02600-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albers CN, Ellegaard-Jensen L, Hansen LH, Sørensen SR. Bioaugmentation of rapid sand filters by microbiome priming with a nitrifying consortium will optimize production of drinking water from groundwater. Water research. 2018;129:1–10. 10.1016/j.watres.2017.11.009 [DOI] [PubMed] [Google Scholar]
- 32.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7(5):335–6. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bokulich NA, Subramanian S, Faith JJ, Gevers D, Gordon JI, Knight R, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nature methods. 2013;10(1):57–9. 10.1038/nmeth.2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nature methods. 2016;13(7):581–3. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald D, Clemente JC, Kuczynski J, Rideout JR, Stombaugh J, Wendel D, et al. The Biological Observation Matrix (BIOM) format or: how I learned to stop worrying and love the ome-ome. GigaScience. 2012;1(1):7 10.1186/2047-217X-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular biology and evolution. 2013;30(4):772–80. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PloS one. 2010;5(3):e9490 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Applied and environmental microbiology. 2005;71(12):8228–35. 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faith DP. Conservation evaluation and phylogenetic diversity. Biological conservation. 1992;61(1):1–10. [Google Scholar]
- 40.Mulder C, Bazeley-White E, Dimitrakopoulos P, Hector A, Scherer-Lorenzen M, Schmid B. Species evenness and productivity in experimental plant communities. Oikos. 2004;107(1):50–63. [Google Scholar]
- 41.Pielou EC. The measurement of diversity in different types of biological collections. Journal of theoretical biology. 1966;13:131–44. [Google Scholar]
- 42.Vázquez-Baeza Y, Pirrung M, Gonzalez A, Knight R. EMPeror: a tool for visualizing high-throughput microbial community data. Gigascience. 2013;2(1):16 10.1186/2047-217X-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.N/A. QIIME 2 plugin supporting taxonomic classification 2017 [cited 2017 02 Dec 2017]. https://github.com/qiime2/q2-feature-classifier.
- 44.Petrinovich LF, Hardyck CD. Error rates for multiple comparison methods: Some evidence concerning the frequency of erroneous conclusions. Psychological Bulletin. 1969;71(1):43. [Google Scholar]
- 45.Aitchison J. The statistical analysis of compositional data. Journal of the Royal Statistical Society Series B (Methodological). 1982:139–77. [Google Scholar]
- 46.Mandal S, Van Treuren W, White RA, Eggesbø M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microbial ecology in health and disease. 2015;26(1):27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salter SJ, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC biology. 2014;12(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bælum J, Scheutz C, Chambon JC, Jensen CM, Brochmann RP, Dennis P, et al. The impact of bioaugmentation on dechlorination kinetics and on microbial dechlorinating communities in subsurface clay till. Environmental pollution. 2014;186:149–57. 10.1016/j.envpol.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 49.Jacobsen CS, Nielsen TK, Vester JK, Stougaard P, Nielsen JL, Voriskova J, et al. Inter-laboratory testing of the effect of DNA blocking reagent G2 on DNA extraction from low-biomass clay samples. Scientific Reports. 2018;8(1):5711 10.1038/s41598-018-24082-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simbolo M, Gottardi M, Corbo V, Fassan M, Mafficini A, Malpeli G, et al. DNA qualification workflow for next generation sequencing of histopathological samples. PLoS One. 2013;8(6):e62692 10.1371/journal.pone.0062692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klappenbach JA, Saxman PR, Cole JR, Schmidt TM. rrndb: the ribosomal RNA operon copy number database. Nucleic acids research. 2001;29(1):181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor J, Wilson B, Mills MS, Burns RG. Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biology and Biochemistry. 2002;34(3):387–401. [Google Scholar]
- 53.Mermillod-Blondin F, Rosenberg R, François-Carcaillet F, Norling K, Mauclaire L. Influence of bioturbation by three benthic infaunal species on microbial communities and biogeochemical processes in marine sediment. Aquatic Microbial Ecology. 2004;36(3):271–84. [Google Scholar]
- 54.Brown AM. A new software for carrying out one-way ANOVA post hoc tests. Computer methods and programs in biomedicine. 2005;79(1):89–95. 10.1016/j.cmpb.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez JM, Portillo MC, Belda-Ferre P, Mira A. Amplification by PCR artificially reduces the proportion of the rare biosphere in microbial communities. PLoS One. 2012;7(1):e29973 10.1371/journal.pone.0029973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith DP, Peay KG. Sequence depth, not PCR replication, improves ecological inference from next generation DNA sequencing. PLoS One. 2014;9(2):e90234 10.1371/journal.pone.0090234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss S, Xu ZZ, Peddada S, Amir A, Bittinger K, Gonzalez A, et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome. 2017;5(1):27 10.1186/s40168-017-0237-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Janssen PH. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Applied and environmental microbiology. 2006;72(3):1719–28. 10.1128/AEM.72.3.1719-1728.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He S, Guo L, Niu M, Miao F, Jiao S, Hu T, et al. Ecological diversity and co-occurrence patterns of bacterial community through soil profile in response to long-term switchgrass cultivation. Scientific Reports. 2017;7(1):3608 10.1038/s41598-017-03778-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available within the paper and its Supporting Information files. The sequencing data have been deposited in the European Nucleotide Archive (ENA) (http://www.ebi.ac.uk/ena) and are accessible through the study accession number PRJEB24676.