Abstract
Although the impacts of climate change on biodiversity are increasing worldwide, few studies have attempted to forecast these impacts on Amazon Tropical Forest. In this study, we estimated the impact of climate change on Amazonian avian assemblages considering range shifts, species loss, vulnerability of ecosystem functioning, future effectiveness of current protected areas and potential climatically stable areas for conservation actions. Species distribution modelling based on two algorithms and three different scenarios of climate change was used to forecast 501 avian species, organized on main ecosystem functions (frugivores, insectivores and nectarivores) for years 2050 and 2070. Considering the entire study area, we estimated that between 4 and 19% of the species will find no suitable habitat. Inside the currently established protected areas, species loss could be over 70%. Our results suggest that frugivores are the most sensitive guild, which could bring consequences on seed dispersal functions and on natural regeneration. Moreover, we identified the western and northern parts of the study area as climatically stable. Climate change will potentially affect avian assemblages in southeastern Amazonia with detrimental consequences to their ecosystem functions. Information provided here is essential to conservation practitioners and decision makers to help on planning their actions.
Introduction
The average global surface temperature has increased by approximately 0.8°C during the last century and is expected to continue increasing [1]. Species have always reacted to climatic changes throughout their evolutionary history [2–4]; however, currently, the main concern is the unprecedented rapidity of the observed changes [1]. Although, until now, habitat loss and fragmentation have represented the highest threat to biodiversity [5,6], some studies have suggested that climate change is likely to outweigh habitat loss as a global threat in the coming decades [7]. In fact, even though climate change constitutes its own set of risks, it may interact with habitat loss and increase shifts in species distributions, extinctions, and hence compositional changes in communities [8]. This potential distribution reshuffling of biodiversity may affect the structure, dynamics and functioning of ecosystems and the contributions they provide [9].
Nature’s contributions to people (NCP) is the concept of “the benefits that people derive from nature [to provide] a good quality of life” proposed by the Intergovernmental Platform on Biodiversity and Ecosystem Services [10,11]. Similar to the more popular ecosystem services concept [5], nature components (biodiversity) interact in complex processes that control flows of energy, nutrients and organic matter (ecosystem functioning) that, in turn, produce environmental goods and services (e.g., clean air, fresh water, climate stabilization) that contribute to health and human well-being.
Birds are good biological indicators of climate change impacts on NCP, since they occupy all terrestrial habitats, consume virtually all type of resources [12] and therefore provide key ecosystem functions and services such as pollination, seed and nutrient dispersion, predation, and scavenging [13]. Besides, they are one of the most well-known and studied groups, with a huge amount of data available. Nonetheless, even for birds, there is a deficiency of data in terms of describing and identifying the biodiversity of the Amazon rainforest and providing high-quality species distribution data [14,15]. For example, according to the last available survey based simply on georeferenced occurrence maps [16], this biome harbours 1778 resident bird species. However, this number continues to rise as new species are discovered [17], and to date, large areas have not been inventoried in Amazonia [14].
Amazonia is a complex biome, and, just in floristic terms, it consists of areas covered either by forest (FT; terra-firme), periodically inundated forested environments (várzea, igapó), as well as open-area vegetation patches (OAV; cerrado, campinas/campinaranas, cangas). This environmental heterogeneity is one of the leading factors responsible for its high species richness [18,19]. Moreover, despite being recognized as providing key NCP [20], Brazilian Amazonia has already lost approximately 19.6% of its forest [21,22]. Land-use change caused by agricultural expansion, logging, mining and energy production are responsible for more than 90% of Brazil’s total greenhouse gas emissions [23]. Therefore, the rapidity of environmental changes has increased the urgency of collecting, organizing and analysing biodiversity data to guide decision-making in conservation. Moreover, the NCPs are essential to human activities, and a better understanding of the range of ecosystem responses to climate change will help to improve the determination and the implementation of effective ecosystems management in a manner that promotes resilience [24]. Although several studies have found a strong negative impact of forest fragmentation on the biodiversity in Amazonia [6,8,25], very few studies have evaluated how species will be affected by climate change in the near future [26,27] and fewer studies have examined the consequences of climate change on functional diversity [26].
In this study, we gathered extensive species occurrence data representative of southeastern (SE) Amazonia to assess the potential climate change impact on avian assemblages. Our work encompasses 501 species (representing more than 50% of the known avian diversity of the focal area [17] and 199,250 occurrence records (S1 Table). Using Species Distribution Modeling (SDM), we analysed how different scenarios of climate change could affect the pattern of species distributions and assemblage compositions. By grouping species based on their main diet (frugivores, insectivores, nectarivores and others [28]) as a proxy to NCPs (seed dispersion, pest control and pollination), we were able to indicate the most susceptible functional group to climate change. In addition, we evaluated the future effectiveness of current Protected Areas (PAs; Conservation Units [CUs] and Indigenous Lands [ILs]) and presented focal areas for different conservation planning considering the dynamics of climate scenarios.
Material and methods
Study system
The species list was derived from those recorded in Carajás, currently one of the biologically best known areas in the SE Amazonia due to over three decades of periodic inventories and sporadic observations [29,30]. It was updated from Vale’s internal database (bdbio), internet repositories (e.g., http://wikiaves.com.br and http://xeno-canto.org) and Museu Paraense Emilio Goeldi ornithological collection, achieving 620 species. The projections and analysis focuses mainly on SE Amazonia, an area between the right margin of Tapajós and Juruena Rivers (59°W) and the Amazonia east limit (44°W), and from Marajó island (0°) to the Amazonia south limit (14°S). The south and east limits of this area correspond to the Amazonia-Cerrado transition zone, encompassing FT and OAV environments, and it is naturally close to the climatic limit of tropical forests [31,32]. In fact, several climate model simulations confirm that SE Amazonia is the most sensitive region to global climate change [33,34] and it has the most deforested area and the highest deforestation rates considering all Amazonia [22]. It is important to mention that our set of species corresponds to ca. 63% of the total known avian taxa of the focal area, and, although some avifauna turnover may occur between the interfluves of major rivers, most species have wide distributions and less than 1% represent taxa exclusively endemic to the respective interfluves. In addition, the list includes FT and OAV species, which allow testing if and how these groups will potentially respond differently to climate changes. Moreover, to assess changes in ecosystem functioning, we also assign each species to one of three trophic guilds based on the main diet of the species [28]: frugivores (FR [seed dispersion]), insectivores (IN [pest control]) and nectarivores (NE [pollination]). Species with other diets (e.g., omnivores, granivores, carnivores, and scavengers) were assigned as "others" (OT).
Data collection
As above mentioned almost all species analysed present wide distributions, thus the total known distribution area of each species in the Neotropics was used and then later projected to the focal area. Our occurrence data were compiled from the open access platform Global Biodiversity Information Facility (available from http://gbif.org; accessed 19 July 2017), using the R package rgbif [35]. We applied a quality control to minimize sample bias by using only georeferenced data, ensuring a minimum distance of 10 km between consecutive presence records (lower spatial aggregation). We also excluded dubious/disparate records, i.e., those that were clearly outside the expected bird distribution, grounding our decision in the Handbook of the Birds of the World and BirdLife International [17]). Our database includes 228,298 nonduplicate presence records (ranging from 9-1297/taxa) for 547 species (introduced, migratory and aquatic species were excluded). Of these species, 23 are Brazilian endemic, 49 are facing some degree of threat [36–38] and only four species have a limited availability of occurrence data (<15 records; all endemic and/or rare). Species taxonomy follows the latest updated checklist of the Brazilian Ornithological Records Committee [39].
Current climate data and projections for 2050 and 2070 were obtained from the WorldClim database (available at: http://worldclim.org; accessed 24 November 2016) at a resolution of 5 arcmin (~10km at the equator) for the Neotropical region (spatial limits of 110° to 30°W; 25°N to 60°S). This resolution was chosen due to the high computational power demanded for modelling such a high number of species, their broad distributional area and high number of scenarios. After performing a pairwise Pearson correlation test for all 20 variables (19 bioclimatic and altitude) to remove those that were highly correlated (r>0.85), we selected 11 as our predictors: annual mean temperature, mean diurnal range, temperature seasonality, temperature annual range, mean temperature of warmest quarter, annual precipitation, precipitation of driest month, precipitation seasonality, precipitation of warmest quarter, precipitation of coldest quarter and altitude. Future climatic projections were derived from general circulation model (GCM) simulations from the Community Climate System Model (CCSM4), a well-known and frequently used GCM (e.g., [40,41]). In addition, among the various GCMs, the Amazonia rainfall and its seasonality have highly variable bias (which account to the uncertainty in forecasting future atmospheric CO2 concentration and climate change), yet the CCSM4 is one of the models that best represent the main climatic drivers on the region [42,43]. Three representative concentration pathway scenarios (RCPs 2.6, 6.0 and 8.5) were projected, representing a broad range of climate outcomes from the most to the least optimistic scenario.
Species distribution modelling
The SDMs were estimated by running two different algorithms within the biomod2 package on R [44]: generalised linear models (GLM [45]) and maximum entropy (Maxent [46]). We generated three sets of pseudo-absence data containing ten times the number of presence data points randomly distributed for each species [47]. The data sets were then partitioned so that 80% were used to calibrate the models, while the remaining 20% were used for the evaluation. Model performance was assessed with the True Skill Statistics (TSS; threshold-dependent [48]) and the receiver operating characteristic curve analysis (ROC; threshold-independent [46]), this procedure was repeated ten times for cross-validation, and the models with poor performance (TSS<0.5) were eliminated from the ensemble process. We also report sensitivity (percentage of true positives), specificity (percentage of true negatives) and assessed the predictor variable importance using the tables of permutation importance.
Finally, we summarized the ensemble of predicted species distributions (two algorithms x three pseudo-absences x models with TSS>0.5) using the committee average method, where probabilities from different models were first transformed into binary according to a threshold (here, the TSS cut-off threshold) and then averaged [44]. In the end, seven consensual maps were generated per species (one for each scenario: current, and RCPs 2.6, 6.0, 8.5 for 2050 and 2070).
Analyses
First, our continuous probability consensual maps were converted into a binary classification by selecting threshold values that maximized the TSS (cutoff; S1 Table). The projected occurrence area was calculated by multiplying the cell area (10x10 = 100km2) with the cell count. Species with projected current occurrence areas <100 grid cells were excluded from the analyses, to avoid overestimating the predicted effects within the focal area. Therefore, the final dataset included 501 species and 199,250 records (S1 Table). Our data include species that occur in both FT and OAV environments, and we treat them separately. The total species richness (SR) was computed by stacking the maps of all individual species for each scenario (and for each algorithm to estimate uncertainties, see next). Uncertainties were calculated as the contribution of different sources (here, the algorithms and future scenarios) to variation around the consensus maps, through the proportional sum of squares of each cell in relation to the total sum of squares using the SR maps as response [49]. We estimated the species range change dynamics (in terms of area gain/loss) based on the differences between the projected area for each future climate scenario and current. To highlight the impact on ecosystem functioning, these results were grouped by the trophic guild (see above) to reflect the most affected function in the study area. We also assessed the future effectiveness of current PAs by accounting for the differences in the estimated SR in each PA over the different climate scenarios. Although there are 57 PAs (37 CUs and 20 ILs) in the focal area, we grouped them in 13, since they form continuous blocks of protected area and were considered together. Thus, for this analysis we kept only blocks with an area greater than 1,000 km2 (S2 Table; data for Brazilian PAs available from Instituto Chico Mendes de Conservação da Biodiversidade: http://icmbio.gov.br). Finally, for the FT species only, we calculated the size of climatically stable areas considering grid cells containing at least 20% of each trophic guild and with an increment of +10% (until 80%) in each scenario. We first stacked each series of percentage/guild on one binary map, and stacked this map with each scenario to show the suitable habitats across scenarios. Areas were considered climatically stable if the guilds were predicted to be present in six to seven out the seven scenarios. Also, a deforestation layer (data for Amazon for the year 2015 available from Instituto Nacional de Pesquisas Espaciais [22]: http://www.obt.inpe.br) was downscaled from the original resolution (60 m) to the topoclimatic variables resolution (10 km) and then, overlaid to create a final decision-making oriented map. The climatically stable areas, outside PAs, that potentially will maximize suitable habitats for biodiversity and having natural vegetation were considered important to conservation, and the same areas with no natural vegetation, important for restoration programs. All analyses were performed using R [50] and QGIS [51].
Results
The quality of the models, according to TSS (0.68 ± 0.11) and ROC (0.90 ± 0.05; all values above 0.80), had a high levels of accuracy (S1 Table), indicating good model fit. Temperature Seasonality (BIO4, for 80% of the species), Annual Mean Temperature (BIO1, for 67%) and Mean Temperature of Warmest Quarter (BIO10, for 46%) were important drivers (mean importance > 30%) for many species distributions; and all species (except for Myiozetetes cayanensis) have at least one predictor variable with importance greater than 30% (S1 Table). The sum of squares obtained to each cell showed distinct contributions to variation around the SR maps (S1 Fig). Algorithms are responsible for the greatest proportion of variation (FT median [min-max] = 79% [5–100%]; and OAV median [min-max] = 87%, [1–100%]), widely spread in the study area. The proportion of variation attributable to future scenarios was lower than the former (FT median [min-max] = 62% [1–100%]; and OAV median [min-max] = 41%, [1–100%]).
Species richness and range change dynamics
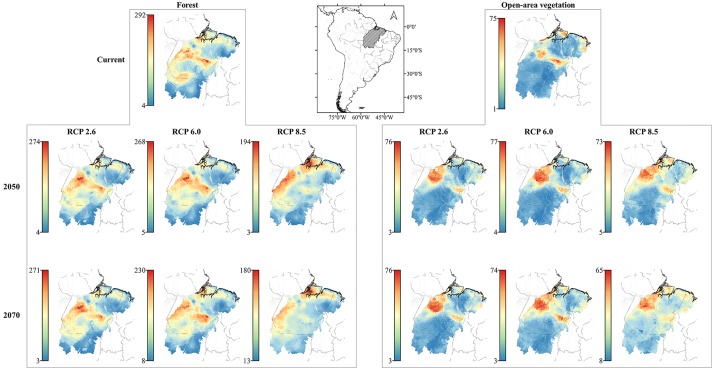
For FT species (N = 382), our current projections indicated high SR in the centre and western portion of the focal area (≥50%, reaching 76% by cell), and low SR in the south eastern portion (<30%). Under the future scenarios, climate change may result in significant loss in SR (reaching 47% at its maximum in the worst case scenario) besides a distributional shift from west-central richest area to north towards the Marajó island (Fig 1). The current projected areas with high SR for OAV species (N = 119) were the central portion of focal area (reaching 59%), and east of Marajó island (46%). Our future scenarios forecasted a northward shift from the core occurrence region (not shown; see the Discussion) resulting in increased SR (Fig 1).
Fig 1. Maps of projected species richness.
Avian assemblage for current and future (2050 and 2070) climate change scenarios (RCP 2.6, 6.0 and 8.5). Hotter colours represent higher numbers of species with forecasted suitable habitats for each scenario.
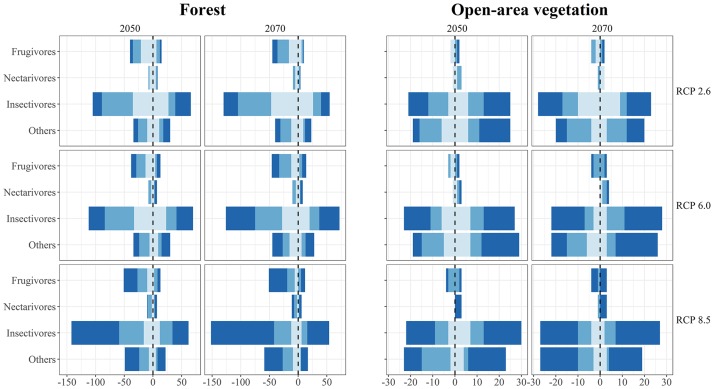
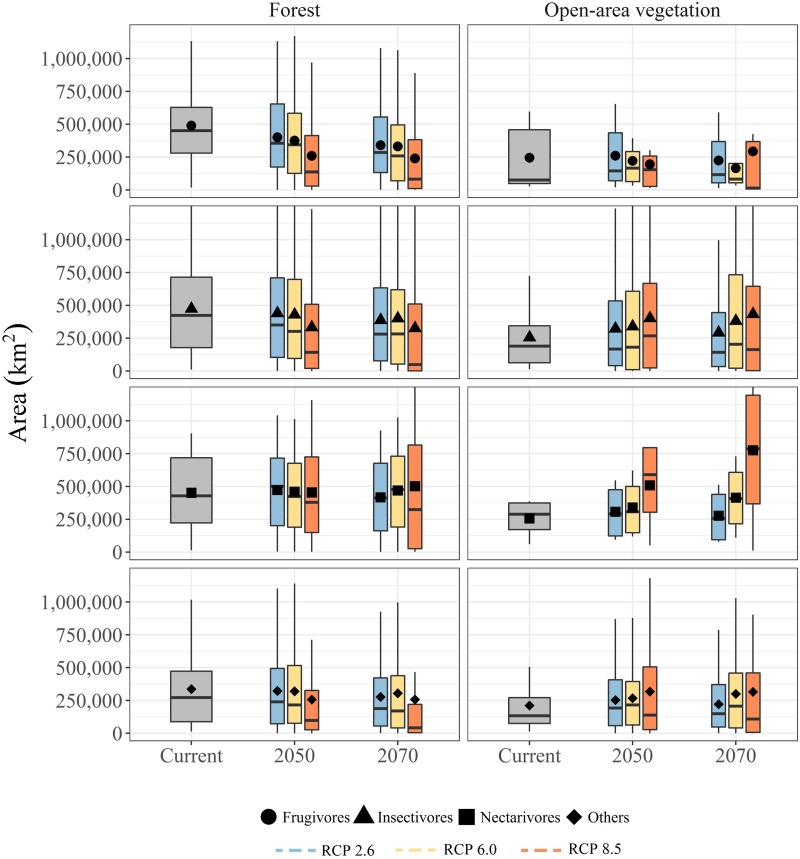
Our results indicate that a greater number of species were projected to show a range loss rather than a range gain (Fig 2). Independent of trophic guild, approximately 187 (49% for FT) and 43 (36% for OAV) species will potentially lose ≥20% of their current suitable area in the next 20 years in the most optimistic scenario (2050; RCP 2.6), and between 72 (19% for FT) and 13 (11% for OAV) species will no longer find suitable habitat in the focal area in the worst case scenario (RCP 8.5; 2070; Fig 2, S3 Table). In absolute numbers, FTIN accounts for most of the projected loss of geographic range size (out of the 219 species between 105 [48%] and 152 [69%] are expected to lose at least 20% of current projected suitable habitats depending on the scenario), but proportionally the FTFR species will potentially be more negatively affected (out of the 66 species between 38 [58%] and 51 [77%] are expected to lose at least 20% of current projected suitable habitats depending on the scenario). Most of the species projected to gain geographic range sizes are from OAV group with an average increase of 234% (SD 467%; Fig 3, S3 Table). All guilds, for FT species, will potentially experience a gradual decline on mean range size (Fig 3), except for FTNE from which four species presented an increase of range size far greater than 100% pulling up the mean (S3 Table). Our models also projected that OAVFR species would experience a decline on mean range size (from near 245,000 km2 in current projections on average, to less than 195,000 km2 considering the RCP 8.5 for 2050; Fig 3, S3 Table), while for the remaining groups, an increase (Fig 3, S3 Table).
Fig 2. Potential climate change effects on forecasted occurrence area per species.
Negative values represent the number of species predicted to lose suitable habitats under different scenarios at each guild class. Positive values represent the number of species predicted to gain suitable habitat. Light blue: ≥20%, blue: ≥ 50%, and dark blue: ≥ 90%.
Fig 3. Potential change in mean area per trophic guild.
Mean area for frugivores (circles), insectivores (triangles), nectarivores (squares), and other guilds (diamonds). Centre lines show the medians; box limits the 25%-75% percentiles in current (gray) and different future (2050 and 2070) climate change scenarios—RCP 2.6 (light blue), RCP 6.0 (orange), and RCP 8.5 (red); and whiskers 1.5x interquartile range of the data.
Effectiveness of current protected areas and priority areas
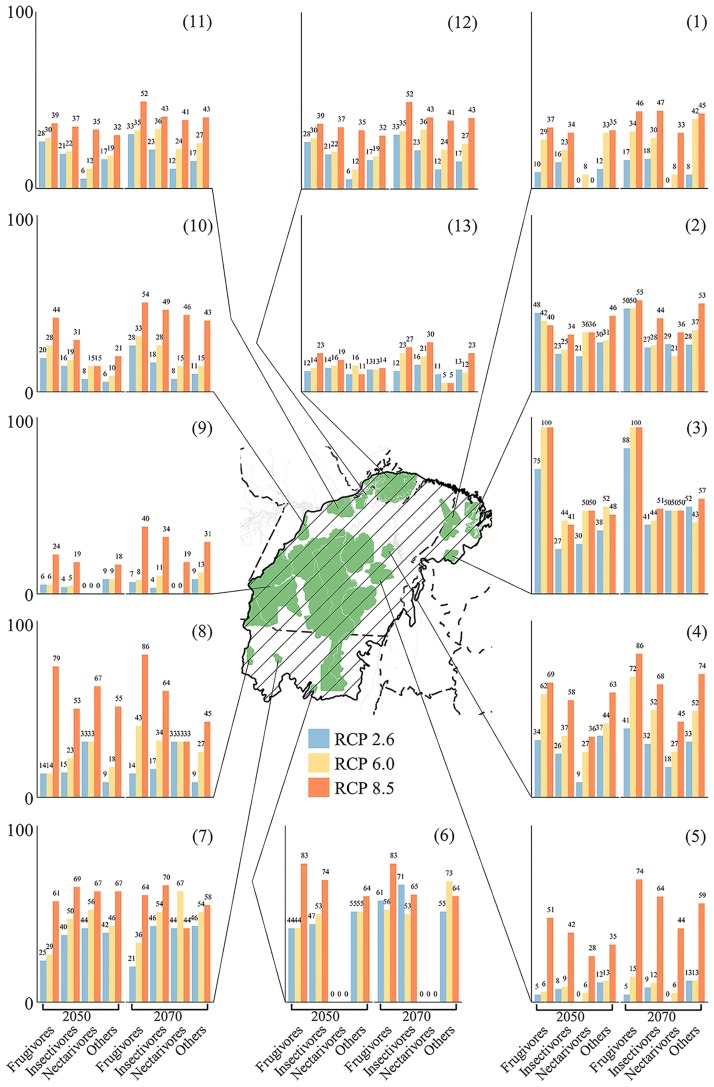
The 13 mosaics of PAs in the focal area potentially protect on average 53% (SD 29%) of the species, according to current projections (S4 Table; the lowest values occurred at the most southern PAs [ESEC Rio Ronuro, 13% and IL Rikbaktsá, 15%]; and the highest values occurred at the centre [Carajás Mosaic, 82%] and western [Terra do Meio Mosaic, 98%] PAs). Under future scenarios, approximately 34% (SD 14%) of the current forecasted species in each PA will potentially not find suitable habitats (disregarding species turnover), and the projected species loss displayed distinct patterns depending on the trophic guild, scenario, and PA position and size (Fig 4, S4 Table).
Fig 4. Percentage of species predicted to disappear in each protected area (PA).
Each bar plot represents the percentage of species loss in relation to current projected species richness and assuming no species turnover. The central map and numbers therein indicate the PA names: (1) Gurupi Mosaic; (2) APA Baixada Maranhense; (3) IL Guajajara; (4) IL Parakana; (5) Carajás Mosaic; (6) ESEC Rio Ronuro; (7) IL Kayabi; (8) IL Erikbakts; (9) Terra do Meio Mosaic; (10) FLONA Tapajós; (11) RESEX Renascer and Verde para sempre; (12) RESEX Gurupá-Melgaço and FLONA Caxiuanã; (13) Marajó Mosaic. For more details see S2 and S4 Tables.
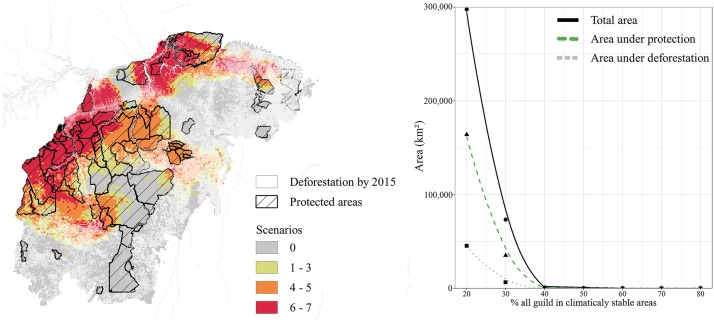
Our analyses suggest that the majority of the northern and western parts of the focal area are climatically stable suitable habitats for FT species, indicating that suitable habitats coincided with six to seven of the seven scenarios (Fig 5). Climatically stable grid cells which were considered suitable habitats for at least 20% of each trophic guild correspond to 297,500 km2. Nearly half of this area is within PAs (164,000 km2 [55%]), yet another significant part has native vegetation but unprotected (88,100 km2 [30%]) or overlaps with degraded areas (45,400 km2 [15%]; Fig 5). Also, there is a drastic reduction in the stable area when considering a higher proportion of each trophic guild per grid cell, for instance, climatically stable cells that were considered suitable habitats for at least 30% of each trophic guild correspond to only 73,400 km2 (a decrease of about 75%) and there is no single cell that maintains at least 50% of each guild in most scenarios (Fig 5).
Fig 5. Potential climatically stable areas for conservation actions.
Map indicating climatically stable suitable areas for at least 20% of each trophic guild across seven climatic scenarios. Colour scales represent the overlap of the models. All the priority areas are plotted with currently existing Protected Areas (PAs) and deforestation layers. Graphs represents the amount of climatically stable suitable areas for different percentages of a minimum guild class (solid line); the amount of area overlapped with PAs (dashed green line) and overlapped with deforested area (dotted grey line).
Discussion
Climate change will impact the biodiversity across the globe [7] and it is important to forecast the effects not only on range shifts but also on the functionalities [52]. Through our SDMs we show that avian assemblage from SE Amazonia will be potentially strongly affected by climate change in the near future, even under the most optimistic scenario. As expected, species occupying different habitats will respond differently, but invariably frugivores will be more negatively affected. Considering only FT species, currently, the centre and western portion of SE Amazonia are potentially the richest areas, and future projections suggest a northern shift with an expressive depletion in SR. The OAV species have a disjunct distribution, where the occurrence core is in the “dry diagonal” (out of the scope of this work) and isolated patches of the Amazonia [53]. As our study system includes a transition zone encompassing both environments (FT and OAV), the OAV species occur naturally at low numbers (<30% in almost all areas) but potentially could reach 46% east of Marajó Island and 59% in patches at the central and northwestern portion of the focal area based on our current projections. Under the future scenarios, potential SR was predicted to increase due to a northward shift (from the core region, not shown) that becomes uniform along all of the focal area, maintaining its maximum value (~55%) at the northwestern portion.
According to our results, the majority of species were projected to show a range loss. In addition, in terms of trends in mean area change per trophic guild, the FT species presented a decrease in the mean projected area under all future scenarios, resulting in a progressive range retraction through time. It is worth pointing out the range expansion in some FT species that exceed in more the 100%. This could be due to the fact that SE Amazonia represents the distribution margin of some FT species and may not express its climatic affinities precisely [54]. However, it is also likely that other factors besides climate, such as those related to species ecological characteristics, could play a role in the distribution ranges. Therefore, finer resolution models and/or the inclusion of species-specific environmental factors may be needed to address these issues. Conversely, the OAV species presented an overall trend to increase progressively their distributional range. We hypothesized that FT species would have the greatest impact, and likely, climate change would favour OAV species, based on the expected "savannization" process [32,33,55] (but see [56]). Our models partially corroborate this idea, showing an increase in mean area of OAV species, except for OAVFR species. Based on our models, frugivores seem to be the most sensitive functional group (FTFR mean area loss ranging between 18.2% [2050; RCP 2.6] and 51% [2070; RCP 8.5], and OAVFR ranging between 9.7% [2050; RCP 6.0] and 32.8% [2070; RCP 6.0]) for the study area as a whole.
In terms of current effectiveness of PAs based on SDM, our results are in accordance with those from recently analysed Brazilian protected areas [57,58]. Here, we also provide an evaluation of future effectiveness of the currently established PAs for avian assemblages in SE Amazonia. Our SDMs project a significant loss of species considering future warming climate scenarios in the focal area, and this is even worse considering each PA apart, in which species loss can reach 100% (e.g., Frugivores in IL Guajajara, RCP 6.0 and 8.5). In fact, considering future effectiveness based only on the differentiation between current and future mean temperatures, it was estimated that approximately 19–67% of all current protected areas in Brazil will potentially not have similar temperature regimes in the future [40]. In addition, the frugivores are the trophic guild class that shows the strongest loss (ranging between 5–100% species loss, in Carajás Mosaic, RCP 2.6, 2050, and IL Guajajara, RCP 6.0, 2050, respectively), and other studies have already suggested this guild is more extinction-prone [59]. Frugivores play a key role in providing seed dispersal services [60], mainly in tropical rainforests (70–94% of woody plants [61]). Additionally, studies have demonstrated that species may not vanish from the ecosystem, but large population declines are sufficient to diminish the amount and quality of seed dispersal to such an extent that plant recruitment is no longer feasible, triggering negative feedback impacts on plant populations, community dynamics and hence ecosystem functions [62–64]. Proportionally, our analysis shows that insectivores constitute the second most sensitive guild, despite including more species loss than any other group in absolute terms (between 4–74% species loss, in Terra do Meio Mosaic, RCP 2.6, 2050, and ESEC Rio Ronuro, RCP 8.5, 2050, respectively). Regardless of the low number of studies that have been conducted, the importance of insectivorous birds in reducing plant damage from herbivory has been demonstrated in Neotropical forest canopies [65]. Although less sensitive than other guilds, nectarivores could potentially lose up to 60% of their current projected diversity (e.g., IL Kayabi and Rikbaktsá, RCP 8.5, 2050). A recent estimation found that >90% of angiosperm species in tropical communities are animal pollinated [66] and over 111 avian species pollinate plants in Brazil (effectively or potentially), but their importance in pollination is not well documented [67]. The possible impact of climate change on reducing bird-pollination services is unknown and could include decrease in gene flow among plants, as well in fruit and seed set [68]. Using knowledge of biological interactions coupled with current and expected future climate conditions is a key strategy to improve the efficiency of conservation plans in terms of restoration and strengthening landscape connectivity [69,70]. Notwithstanding, as noted previously, such information need to be more deep- and locally accessed to better evaluate the consequences for other communities of species that interact with avian species. Moreover, the species losses mentioned above did not consider species turnover (i.e., the amount of arriving species), and when it was considered, the SR in each PA did not change substantially. At first glance, this could be considered resilience if it is interpreted as a community response to perturbation, but a large part of this turnover was due to generalists. Several questions remain unanswered about "functional homogenization" in terms of if generalists represent a new degraded state, a transition that will return to the initial equilibrium, or a transition to a novel ecosystem; regardless, generalists invariably affect functioning and productivity, reducing resistance and resilience (see [71] for a review).
Our study identified a large, almost connected, and, in great part, intact block in the focal area as climatically stable for at least 20% of each FT trophic guild. Approximately the same area was forecasted for bats using the same parameters [26]. Our analysis shows that about 55% of our modelled climatically stable area overlap with currently established PAs. Despite of indigenous lands are legally not regarded as conservation units by the Brazilian law, these areas strongly ensure biodiversity protection [72,73]. Notably, the westernmost areas are the best preserved and protected, even under heavy pressure from illegal logging and mining, as well as recent government attempts to reduce protected areas (provisional measures MP 756/2016 and MP 758/2016 [74]) and undermine environmental laws to facilitate the implementation of large infrastructure projects (constitutional amendment–PEC65 [75]). On the other hand, approximately 15% of the area overlaps with degraded areas, and the remaining 30% still have natural vegetation but no protection. Together, these areas could form a large continuous block of suitable habitat for avian species. Several studies have demonstrated that large, connected, and intact ecosystems ensure the representativeness of multiple landscape gradients, ecological effectiveness of species population size, and intraspecific genetic diversity ([76] and references therein). Also, the contribution undertaken by Brazil at the 2015 Paris Conference of the Parties is to restore 120,000 km2 of forests by 2030. Most of this restoration should be in sites potentially suitable for as many species as possible, such as those pointed out by our models.
Several studies have already analysed and described how methodological uncertainty could complicate predicting the impacts of climate change [77–80]. In fact, each stage of the modelling method provides variation that generate uncertainties (e.g., collinearity and variable selection [81]; GCMs [82]; future emission scenarios; algorithms [83]; threshold selection [84], without mentioning the uncertainties from the data which is hard to fully explore). Recent works that have partitioned and measured the contribution of the sources of variation inherent to the models, show that the algorithms are responsible for most of the uncertainty, being greater than the climatic models and the future emission scenarios [49,77,81]. Our results are in line with those showing that our algorithms contributed the most to uncertainty when compared with emission scenarios. Although our conclusions are based upon simulation derived from only one GCM, significant efforts have been made between the IPCC's Assessment Reports to reduce biases in climate models [34] and, as above mentioned, our choice were based in recent analysis showing that CCSM4 best represent the main features over northern South America [42,43]. Here, our main goal is not to compare computational methods but instead to provide decision makers with an array of possible outcomes related to the potential impacts of climate change on avian assemblages, considering our large set of species data, the resolution of multi-algorithm, multi-timescale and multi-emission scenario and computational feasibility. There are two more important points that relate to our analysis. Firstly, the dependence of model accuracy methods on sample/species prevalence, highlighting that without reliable presence—absence data, no metrics yield proper estimates (even for TSS as recently demonstrated) [85–87]. True absences already are very tough to obtain because they demand high sampling effort to ensure their exactitude, which is much harder in the neotropics where species records’ data (true presences) have spatial, temporal and taxonomic biases [14,15]. In addition, expecting for a flawless evaluation metric is unworthy when we can use the available ones in a complementary way. Therefore, we believe that our consensus maps are robust in the projections of possible distribution/composition changes of avian assemblages in the study area. Another important point is the idiosyncratic response of each species to climate change, stressing out that different species may not react in the same way and for this reason, we focus on the general inferred patterns for guild groups. Finally, conservation actions based on our results must assume a flexible strategy (i.e., accept the risks, reassess conditions in front of new evidence, adapt/change strategy) keeping in mind managing in the face of uncertainty [69].
Conclusions
Throughout our habitat suitability models, we show that (1) birds in SE Amazonia will be affected by climate change even under the most optimistic scenario; (2) frugivores are the most sensitive group facing climate change in coming decades which could bring consequences on seed dispersion and plant recruitment; and (3) the current set of protected areas has the potential to protect half of current projected biodiversity, and that 55% of climatically stable areas identified in this study overlapped with PAs. In the context of decision-making, our models are important in suggesting insightful conservation strategies that involve not only improving currently established PAs but also demonstrating which areas to maintain and which to restore to optimize the potential for natural processes, such as dispersal and adaptation.
Supporting information
Uncertainty proportion associated to algorithms and future scenarios, based on the total sum of squares.
(TIF)
Nrec: number of occurrence; Habitat: Forest (FT) and Open-area vegetation (OAV); Guild: Frugivores (FR), Insectivores (IN), Nectarivores (NE), Others (OT); Status: Brazilian Endemic (ED), Near Threathened (NT), Vulnerable (VU), Endangered (EN), Critically Endangered (CR), *IUCN, ‡MMA, †SEMAS-PA; Model performance: ROC, TSS, Sensitivity, Specificity; Cutoff: threshold values maximized TSS for binarization; Variables importance: mean (sd) between runs; bold numbers represent mean values higher than 30% of importance.
(XLSX)
Conservation Units (CU) and Indigenous Lands (IL) and the mosaics of which they are part.
(XLSX)
Projected species occurrence area and differences between scenarios.
(XLSX)
Projected species richness and species turnover in Protected Areas.
(XLSX)
occurrence records acquisition, modeling procedures and analysis.
(R)
Acknowledgments
We wish to thank Letícia Guimarães and Alexandre Castilho for data access; André Luis Acosta, Wilian França Costa, Marcelo Awade for their assistance with scripting in R; Rafael Melo de Brito for help with QGIS; and to Julianna Fernandes for help with figures.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by Conselho Nacional de Desenvolvimento Científico eTecnológico (CNPq) post-doctoral fellowship to LSM [301215/2016-2; 300712/2017-0]; and research grant to TCG [446167/2015-0]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.IPCC. Summary for policymakers Climate Change 2013: The Physical Science Basis. United Kingdom and New York, NY, USA,: Cambridge University Press; 2013 [Google Scholar]
- 2.Dynesius M, Jansson R. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc Natl Acad Sci. 2000;97: 9115–9120. 10.1073/pnas.97.16.9115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansson R. Global patterns in endemism explained by past climatic change. Proc R Soc B Biol Sci. 2003;270: 583–590. 10.1098/rspb.2002.2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandel B, Arge L, Dalsgaard B, Davies RG, Gaston KJ, Sutherland WJ, et al. The influence of late quaternary climate-change velocity on species endemism. Science. 2011;334: 660–664. 10.1126/science.1210173 [DOI] [PubMed] [Google Scholar]
- 5.MA (Millenium Ecosystem Assessment). Ecosystems and Human Well-being Synthesis. Island Press; 2005. [Google Scholar]
- 6.Barlow J, Lennox GD, Ferreira J, Berenguer E, Lees AC, Nally R Mac, et al. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature. 2016;535: 144–147. 10.1038/nature18326 [DOI] [PubMed] [Google Scholar]
- 7.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. Impacts of climate change on the future of biodiversity. Ecol Lett. 2012;15: 365–377. 10.1111/j.1461-0248.2011.01736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurance WF, Useche DC. Environmental synergisms and extinctions of tropical species. Conserv Biol. 2009;23: 1427–1437. 10.1111/j.1523-1739.2009.01336.x [DOI] [PubMed] [Google Scholar]
- 9.Gallagher R V., Hughes L, Leishman MR. Species loss and gain in communities under future climate change: Consequences for functional diversity. Ecography (Cop). 2013;36: 531–540. 10.1111/j.1600-0587.2012.07514.x [DOI] [Google Scholar]
- 10.Díaz S, Demissew S, Carabias J, Joly C, Lonsdale M, Ash N, et al. The IPBES Conceptual Framework—connecting nature and people. Curr Opin Environ Sustain. 2015;14: 1–16. 10.1016/j.cosust.2014.11.002 [DOI] [Google Scholar]
- 11.Díaz S, Pascual U, Stenseke M, Martín-López B, Watson RT, Molnár Z, et al. Assessing nature’s contributions to people. Science. 2018;359: 270–272. 10.1126/science.aap8826 [DOI] [PubMed] [Google Scholar]
- 12.Birdlife International. State of the world’s birds: indicators for our changing world. Cambridge: BirdLife International; 2013 [Google Scholar]
- 13.Whelan CJ, Wenny DG, Marquis RJ. Ecosystem services provided by birds. Ann N Y Acad Sci. 2008;1134: 25–60. 10.1196/annals.1439.003 [DOI] [PubMed] [Google Scholar]
- 14.Aleixo A. Knowledge gaps, research priorities, and future perspectives on bird conservation in the Brazilian Amazon In: Luca AC, Develey PF, Bencke GA, Goerck JM, editors. Áreas importantes para a Conservação das Aves no Brasil Parte II-Amazônia, Cerrado e Pantanal. SAVE Brasil; 2009. pp. 55–69 [Google Scholar]
- 15.Oliveira U, Paglia AP, Brescovit AD, de Carvalho CJB, Silva DP, Rezende DT, et al. The strong influence of collection bias on biodiversity knowledge shortfalls of Brazilian terrestrial biodiversity. Divers Distrib. 2016;22: 1232–1244. 10.1111/ddi.12489 [DOI] [Google Scholar]
- 16.Vale MM, Cohn-Haft M, Bergen S, Pimm SL. Effects of future infrastructure development on threat status and occurrence of Amazonian birds. Conserv Biol. 2008;22: 1006–1015. 10.1111/j.1523-1739.2008.00939.x [DOI] [PubMed] [Google Scholar]
- 17.Birdlife International and Handbook of the Birds of the World. Bird species distribution maps of the world. Version 9.0 [Internet]. 2017. http://www.birdlife.org/datazone/info/taxonomy
- 18.Pomara LY, Ruokolainen K, Tuomisto H, Young KR. Avian Composition Co-varies with Floristic Composition and Soil Nutrient Concentration in Amazonian Upland Forests. Biotropica. 2012;44: 545–553. 10.1111/j.1744-7429.2011.00851.x [DOI] [Google Scholar]
- 19.Stein A, Gerstner K, Kreft H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett. 2014;17: 866–880. 10.1111/ele.12277 [DOI] [PubMed] [Google Scholar]
- 20.Betts RA, Malhi Y, Roberts JT. The future of the Amazon: new perspectives from climate, ecosystem and social sciences. Philos Trans R Soc B Biol Sci. 2008;363: 1729–1735. 10.1098/rstb.2008.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fearnside PM. Deforestation in Brazilian Amazonia: History, rates, and consequences. Conserv Biol. 2005;19: 680–688. 10.1111/j.1523-1739.2005.00697.x [DOI] [Google Scholar]
- 22.INPE. PRODES: Assessment of Deforestation in Brazilian Amazonia [Internet]. 2018. http://www.obt.inpe.br/prodes/index.php
- 23.Observatório do Clima. Sistema de Estimativas de Emissões e Remoções de Gases de Efeito Estufa—SEEG [Internet]. 2018. http://seeg.eco.br/
- 24.Lo V. Synthesis report on experiences with ecosystem- based approaches to climate change adaptation and disaster risk reduction Convention on Biological Diversity. Montreal: Secretariat of the Convention on Biological Diversity; 2016 [Google Scholar]
- 25.Gardner TA, Ferreira J, Barlow J, Lees AC, Parry L, Vieira ICG. et al. A social and ecological assessment of tropical land uses at multiple scales: the Sustainable Amazon Network. Philos Trans R Soc Lond B Biol Sci. 2013;368: 20120166 10.1098/rstb.2012.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa WF, Ribeiro M, Saraiva AM, Imperatriz-Fonseca VL, Giannini TC. Bat diversity in Carajás National Forest (Eastern Amazon) and potential impacts on ecosystem services under climate change. Biol Conserv. 2018;218: 200–210. 10.1016/j.biocon.2017.12.034 [DOI] [Google Scholar]
- 27.Ribeiro BR, Sales LP, Loyola R. Strategies for mammal conservation under climate change in the Amazon. Biodivers Conserv. 2018. 10.1007/s10531-018-1518-x [DOI] [Google Scholar]
- 28.Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology. 2014;95: 2027–2027. 10.1890/13-1917.1 [DOI] [Google Scholar]
- 29.Pacheco JF, Kirwan GM, Aleixo A, Whitney BM, Whittaker A, Whittaker A, et al. An avifaunal inventory of the CVRD Serra dos Carajás project, Pará, Brazil. Cotinga. 2007;27: 15–30 [Google Scholar]
- 30.Martins FD, Castilho AF, Campos J, Hatano FM, Rolim SG. Fauna da Floresta Nacional de Carajás Nitro Editorial, São Paulo: 2012 [Google Scholar]
- 31.Malhi Y, Wright J. Spatial patterns and recent trends in the climate of tropical rainforest regions. Philos Trans R Soc B Biol Sci. 2004;359: 311–329. 10.1098/rstb.2003.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malhi Y, Aragao LEOC, Galbraith D, Huntingford C, Fisher R, Zelazowski P, et al. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc Natl Acad Sci. 2009;106: 20610–20615. 10.1073/pnas.0804619106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salazar LF, Nobre CA, Oyama MD. Climate change consequences on the biome distribution in tropical South America. Geophys Res Lett. 2007;34 10.1029/2007GL029695 [DOI] [Google Scholar]
- 34.IPCC. Climate Change 2013: The Physical Science Basis Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. United Kingdom and New York, NY, USA,: Cambridge University Press; 2013 [Google Scholar]
- 35.Chamberlain S, Ram K, Barve V, Mcglinn D, Oldoni D, Geffert L, Ram K. Package ‘rgbif’: Interface to the Global ‘Biodiversity’ Information Facility ‘API’ [Internet]. 2015. http://cran.r-project.org/package=rgbif
- 36.SEMAS (Secretaria de Estado de Meio Ambiente e Sustentabilidade do Pará). Lista de Espécies da Flora e da Fauna Ameaçadas no Estado do Pará [Internet]. 2007. http://semas.pa.gov.br/2009/03/27/9439/
- 37.MMA (Ministério do Meio Ambiente). Lista Nacional das Espécies da Fauna Brasileira Ameaçada de Extinção [Internet]. 2014. http://mma.gov.br/biodiversidade/especies-ameacadas-de-extincao/fauna-ameacada
- 38.IUCN (International Union for Conservation of Nature). The IUCN Red List of Threatened Species [Internet]. 2017. http://www.iucn.org/ [Google Scholar]
- 39.Piacentini V de Q, Aleixo A, Agne CEQ, Maurício GN, Pacheco JF, Bravo GA, et al. Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee / Lista comentada das aves do Brasil pelo Comitê Brasileiro de Registros Ornitológicos. Rev Bras Ornitol. 2015;23: 91–298 [Google Scholar]
- 40.Feeley KJ, Silman MR. Disappearing climates will limit the efficacy of Amazonian protected areas. Divers Distrib. 2016;22: 1081–1084. 10.1111/ddi.12475 [DOI] [Google Scholar]
- 41.Coxen CL, Frey JK, Carleton SA, Collins DP. Species distribution models for a migratory bird based on citizen science and satellite tracking data. Glob Ecol Conserv. 2017;11: 298–311. 10.1016/j.gecco.2017.08.001 [DOI] [Google Scholar]
- 42.Yin L, Fu R, Shevliakova E, Dickinson RE. How well can CMIP5 simulate precipitation and its controlling processes over tropical South America? Clim Dyn. 2013;41: 3127–3143. 10.1007/s00382-012-1582-y [DOI] [Google Scholar]
- 43.Sierra JP, Arias PA, Vieira SC. Precipitation over Northern South America and its seasonal variability as simulated by the CMIP5 models. Adv Meteorol. 2015;2015: 634720 10.1155/2015/634720 [DOI] [Google Scholar]
- 44.Thuiller W, Lafourcade B, Engler R, Araújo MB. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography. 2009;32: 369–373. 10.1111/j.1600-0587.2008.05742.x [DOI] [Google Scholar]
- 45.McCullagh P, Nelder JA. Generalized Linear Models, Second Edition. 2nd ed Chapman and Hall; 1989 [Google Scholar]
- 46.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006;190: 231–259. 10.1016/j.ecolmodel.2005.03.026 [DOI] [Google Scholar]
- 47.Chefaoui RM, Lobo JM. Assessing the effects of pseudo-absences on predictive distribution model performance. Ecol Modell. 2008;210: 478–486. 10.1016/j.ecolmodel.2007.08.010 [DOI] [Google Scholar]
- 48.Allouche O, Tsoar A, Kadmon R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J Appl Ecol. 2006;43: 1223–1232. 10.1111/j.1365-2664.2006.01214.x [DOI] [Google Scholar]
- 49.Diniz-Filho JAF, Bini LM, Rangel TF, Loyola RD, Hof C, Nogués-Bravo D, et al. Partitioning and mapping uncertainties in ensembles of forecasts of species turnover under climate change. Ecography. 2009;32: 897–906. 10.1111/j.1600-0587.2009.06196.x [DOI] [Google Scholar]
- 50.R Development Core Team. R: A Language and Environment for Statistical Computing Viena: R Foundation for Statistical Computing; 2016 [Google Scholar]
- 51.QGIS Development Team. QGIS Geographic Information System Open Source Geospatial Foundation Project; 2018 [Google Scholar]
- 52.Thuiller W. Patterns and uncertainties of species’ range shifts under climate change Glob Chang Biol. 2004;10: 2020–2027. 10.1111/j.1365-2486.2004.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Werneck FP. The diversification of eastern South American open vegetation biomes: Historical biogeography and perspectives. Quat Sci Rev. 2011;30: 1630–1648. 10.1016/j.quascirev.2011.03.009 [DOI] [Google Scholar]
- 54.Vale CG, Tarroso P, Brito JC. Predicting species distribution at range margins: Testing the effects of study area extent, resolution and threshold selection in the Sahara-Sahel transition zone. Divers Distrib. 2014;20: 20–33. 10.1111/ddi.12115 [DOI] [Google Scholar]
- 55.Nobre CA, Borma LDS. “Tipping points” for the Amazon forest. Curr Opin Environ Sustain. 2009;1: 28–36. 10.1016/j.cosust.2009.07.003 [DOI] [Google Scholar]
- 56.Marini MÂ, Barbet-Massin M, Lopes LE, Jiguet F. Predicted climate-driven bird distribution changes and forecasted conservation conflicts in a neotropical savanna. Conserv Biol. 2009;23: 1558–1567. 10.1111/j.1523-1739.2009.01258.x [DOI] [PubMed] [Google Scholar]
- 57.Carvalho DL, Sousa-neves T, Cerqueira PV, Gonsioroski G, Silva SM, Silva DP, et al. Delimiting priority areas for the conservation of endemic and threatened Neotropical birds using a niche-based gap analysis. PLOS ONE. 2017;12: e0171838 10.1371/journal.pone.0171838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliveira U, Soares-Filho BS, Paglia AP, Brescovit AD, De Carvalho CJB, Silva DP, et al. Biodiversity conservation gaps in the Brazilian protected areas. Sci Rep. 2017;7: 9141 10.1038/s41598-017-08707-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Şekercioğlu ÇH, Daily GC, Ehrlich PR, Daily GC, Ehrlich PR. Ecosystem Consequences of Bird Declines. Proc Natl Acad Sci. 2004;101: 18042–18047 10.1073/pnas.0408049101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang BC, Smith TB. Closing the seed dispersal loop. Trends Ecol Evol. 2002;17: 379–385. 10.1016/S0169-5347(02)02541-7 [DOI] [Google Scholar]
- 61.Jordano P. Fruits and frugivory In: Gallagherl RS, editor. Seeds: The Ecology of Regeneration in Plant Communities. Wallingford: CABI; 2013. pp. 18–61 [Google Scholar]
- 62.Galetti M, Guevara R, Côrtes MC, Fadini R, Matter S Von, Leite AB, et al. Functional Extinction of Birds Drives Rapid Evolutionary Changes in Seed Size. Science. 2013;340: 1086–1090 10.1126/science.1233774 [DOI] [PubMed] [Google Scholar]
- 63.McConkey KR, O’Farrill G. Loss of seed dispersal before the loss of seed dispersers. Biol Conserv. 2016;201: 38–49. 10.1016/j.biocon.2016.06.024 [DOI] [Google Scholar]
- 64.Pérez-Méndez N, Jordano P, García C, Valido A. The signatures of Anthropocene defaunation: Cascading effects of the seed dispersal collapse. Sci Rep. 2016;6: 24820 10.1038/srep24820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Bael SA, Brawn JD, Robinson SK. Birds defend trees from herbivores in a Neotropical forest canopy. Proc Natl Acad Sci. 2003;100: 8304–8307. 10.1073/pnas.1431621100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals? Oikos. 2011;120: 321–326. 10.1111/j.1600-0706.2010.18644.x [DOI] [Google Scholar]
- 67.Buzato S, Giannini TC, Machado IC, Sazima M, Sazima I. Polinizadores Vertebrados: uma visão geral para as espécies brasileiras In: Fonseca VLI, Canhos DAL, Alves DA, Saraiva AM, editors. Polinizadores no Brasil Contribuição e perspectivas para a biodiversidade, uso sustentável, conservação e serviços ambientais. São Paulo: Editora da Universidade de São Paulo; 2012. pp. 119–141 [Google Scholar]
- 68.Anderson SH, Kelly D, Ladley JJ, Molloy S, Terry J. Cascading effects of bird functional extinction reduce pollination and plant density. Science. 2011;331: 1068–1071. 10.1126/science.1199092 [DOI] [PubMed] [Google Scholar]
- 69.Millar CI, Stephenson NL, Stephens SL. Climate change and forest of the future: Managing in the face of uncertanity. Ecol Appl. 2007;17: 2145–2151. 10.1890/06-1715.1 [DOI] [PubMed] [Google Scholar]
- 70.Tulloch AIT, Sutcliffe P, Naujokaitis-Lewis I, Tingley R, Brotons L, Ferraz KMPMB, et al. Conservation planners tend to ignore improved accuracy of modelled species distributions to focus on multiple threats and ecological processes. Biol Conserv. 2016;199: 157–171. 10.1016/j.biocon.2016.04.023 [DOI] [Google Scholar]
- 71.Clavel J, Julliard R, Devictor V. Worldwide decline of specialist species: Toward a global functional homogenization? Front Ecol Environ. 2011;9: 222–228. 10.1890/080216 [DOI] [Google Scholar]
- 72.Nepstad D, Schwartzman S, Bamberger B, Santilli M, Ray D, Schlesinger P, et al. Inhibition of Amazon deforestation and fire by parks and indigenous lands. Conserv Biol. 2006;20: 65–73. 10.1111/j.1523-1739.2006.00351.x [DOI] [PubMed] [Google Scholar]
- 73.Soares-Filho B, Moutinho P, Nepstad D, Anderson A, Rodrigues H, Garcia R, et al. Role of Brazilian Amazon protected areas in climate change mitigation. Proc Natl Acad Sci. 2010;107: 10821–10826. 10.1073/pnas.0913048107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brandford S. Brazil moves to cut Amazon conservation units by 1.2 million hectares. In: Mongabay [Internet]. 2017. Available: http://news.mongabay.com/2017/04/brazil-moves-to-cut-amazon-conservation-units-by-1-2-million-hectares/ [Google Scholar]
- 75.Fearnside PM. Brazilian politics threaten environmental policies. Science. 2016;353: 746–748. 10.1126/science.aag0254 [DOI] [PubMed] [Google Scholar]
- 76.Watson JEM, Evans T, Venter O, Williams B, Tulloch A, Stewart C, et al. The exceptional value of intact forest ecosystems. Nat Ecol Evol. 2018; 10.1038/s41559-018-0490-x [DOI] [PubMed] [Google Scholar]
- 77.Thuiller W. Patterns and uncertainties of species’ range shifts under climate change. Glob Chang Biol. 2004;10: 2020–2027. 10.1111/j.1365-2486.2004.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Araújo MB, Whittaker RJ, Ladle RJ, Erhard M. Reducing uncertainty in projections of extinction risk from climate change. Glob Ecol Biogeogr. 2005;14: 529–538. 10.1111/j.1466-822X.2005.00182.x [DOI] [Google Scholar]
- 79.Beaumont LJ, Hughes L, Pitman AJ. Why is the choice of future climate scenarios for species distribution modelling important? Ecol Lett. 2008;11: 1135–1146. 10.1111/j.1461-0248.2008.01231.x [DOI] [PubMed] [Google Scholar]
- 80.Wiens JA, Stralberg D, Jongsomjit D, Howell CA, Snyder MA. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proc Natl Acad Sci. 2009;106: 19729–19736. 10.1073/pnas.0901639106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dormann CF, Purschke O, Márquez JRG, Lautenbach S, Schröder B. Components of uncertainty in species distribution analysis: a case study of the Great grey shrike. Ecology. 2008;89: 3371–3386. 10.1890/07-1772.1 [DOI] [PubMed] [Google Scholar]
- 82.Goberville E, Beaugrand G, Hautekèete NC, Piquot Y, Luczak C. Uncertainties in the projection of species distributions related to general circulation models. Ecol Evol. 2015;5: 1100–1116. 10.1002/ece3.1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heikkinen RK, Luoto M, Araújo MB, Virkkala R, Thuiller W, Sykes MT. Methods and uncertainties in bioclimatic envelope modelling under climate change Risto. Prog Phys Geogr. 2006;30: 751–777. 10.1177/0309133306071957 [DOI] [Google Scholar]
- 84.Liu C, White M, Newell G. Selecting thresholds for the prediction of species occurrence with presence-only data. J Biogeogr. 2013;40: 778–789. 10.1111/jbi.12058 [DOI] [Google Scholar]
- 85.Leroy B, Delsol R, Hugueny B, Meynard CN, Barhoumi C, Barbet-Massin M, et al. Without quality presence-absence data, discrimination metrics such as TSS can be misleading measures of model performance. J Biogeogr. 2018;45: 1994–2002. 10.1111/jbi.13402 [DOI] [Google Scholar]
- 86.Fernandes RF, Scherrer D, Guisan A. Effects of simulated observation errors on the performance of species distribution models. Divers Distrib. 2018; 400–413. 10.1111/ddi.12868 [DOI] [Google Scholar]
- 87.Lobo JM, Jiménez-Valverde A, Hortal J. The uncertain nature of absences and their importance in species distribution modelling. Ecography. 2010;33: 103–114 10.1111/j.1600-0587.2009.06039.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Uncertainty proportion associated to algorithms and future scenarios, based on the total sum of squares.
(TIF)
Nrec: number of occurrence; Habitat: Forest (FT) and Open-area vegetation (OAV); Guild: Frugivores (FR), Insectivores (IN), Nectarivores (NE), Others (OT); Status: Brazilian Endemic (ED), Near Threathened (NT), Vulnerable (VU), Endangered (EN), Critically Endangered (CR), *IUCN, ‡MMA, †SEMAS-PA; Model performance: ROC, TSS, Sensitivity, Specificity; Cutoff: threshold values maximized TSS for binarization; Variables importance: mean (sd) between runs; bold numbers represent mean values higher than 30% of importance.
(XLSX)
Conservation Units (CU) and Indigenous Lands (IL) and the mosaics of which they are part.
(XLSX)
Projected species occurrence area and differences between scenarios.
(XLSX)
Projected species richness and species turnover in Protected Areas.
(XLSX)
occurrence records acquisition, modeling procedures and analysis.
(R)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.