Dear Sirs,
High thrombin induced clot strength in whole blood measured by thrombelas tography (TEG) has been associated with increased risk for recurrent thrombotic events after percutaneous coronary intervention (PCI) (1, 2). Whole blood TEG incorporates both the component of platelet aggregation by thrombin, as well as contribution of fibrin to tensile force of whole blood thrombus. We have recently explored the use of plasma fibrin TEG measured in platelet-poor plasma to examine the component of clot strength attributable to fibrin alone in absence of platelets (3–5). We hypothesized that high mechanical strength of cross linked fibrin clot is associated with increased risk of recurrent myocardial infarction (MI) and cardiovascular death (CVD).
We enrolled subjects before or in follow up to a cardiac catheterisation. All subjects had angiographically established coronary artery disease and had undergone percutaneous coronary intervention. Mean time of follow-up was 2.9 years. Kaolin-activated TEG was performed according to the manufacturer’s instructions using platelet-poor citrate plasma (Haemonetics, Brain-tree, MA, USA) as previously described (3, 5, 6). The primary combined clinical endpoint was defined as first occurrence CVD or MI (7). Secondary endpoints included CVD, MI, stent thrombosis, and severe or life-threatening bleeding according to GUSTO criteria (8, 9). Clinical endpoints were evaluated by review of electronic medical records, with last known clinical follow-up as last censored time event. Coronary angiograms were reviewed to ascertain stent thrombosis events, available. The study protocol was approved by the Indiana University institutional review board and written informed consent was obtained from all subjects.
A total of 270 subjects were included the analysis. The mean age of patients was 57.1 ± 9.8 years. Details of clinical variables are available in the Suppl. Material (available online at www.thrombosis-online.com).
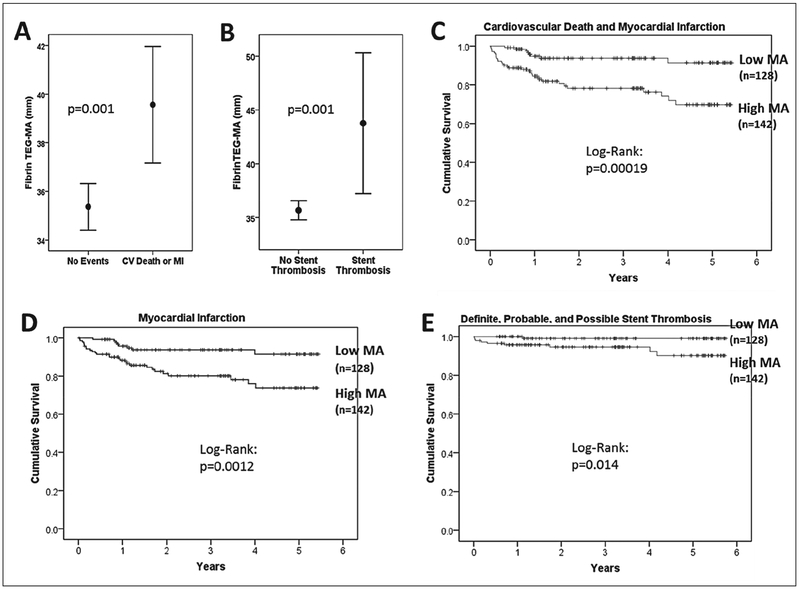
The primary endpoint of CVD and MI occurred in 14.4 % of subjects. Definite stent thrombosis occurred in 3 % and possible, probable, or definite stent thrombosis in 3.7 % of subjects. Subjects with occurrence of the primary endpoint during follow up (CVD or MI) had significantly higher maximal fibrin clot strength (TEGMA: 39.6 ± 7.4 vs 35.4 ± 7.4 mm; p=0.001) and shorter clot formation time (TEG-K: 1.14 ± 0.5 vs 1.49 ± 1.3 min; p=0.002) than patients without events during follow up, while time to fibrin formation was similar (TEG-R: 6.7 ± 2.9 vs 7.0 ± 2.9 min; p=0.55) (▶Figure 1). Patients with possible, probable, or definite stent thrombosis during follow up demonstrated significantly higher fibrin MA than subjects without stent thrombosis (TEG-MA: 43.8 ± 9.1 vs 35.7±7.3 mm; p=0.001), but no significant difference in TEG-K (1.1 ± 0.6 vs 1.45 ± 1.2 min; p=0.1) or TEG-R (6.6 ± 3 vs 7 ± 3 min; p=0.67) (▶Figure 1). Patients with definite stent thrombosis during follow up also demonstrated significantly higher fibrin MA than subjects without stent thrombosis (TEG-MA: 41.5 ± 8.6 vs 35.8 ± 7.6 mm; p=0.037). Fibrinogen levels were not significantly different between subjects with CVD or MI versus those without events (Median [interquartile range]: 3.6 [2.1–5.1] vs 3.6 [2–5.2] mg/ml; p=0.95). Fibrinogen was modestly correlated with plasma fibrin TEG-MA (r=0.2; p=0.028).
Figure 1:
Thrombelastography (TEG) maximal fibrin clot strength (MA) in A) subjects without events vs subjects with cardiovascular death or MI and B) subjects without stent thrombosis vs subjects with possible, probable, or definite stent thrombosis during follow-up. C-E) Kaplan-Meier survival analysis according to high MA (≥35.35 mm) vs low MA (<35.35 mm) for primary endpoint of CVD and MI (C), MI (D), and definite, probable, or possible stent thrombosis (E). Values are mean ± 95 % confidence interval.
Optimal cut-off for high fibrin clot strength (MA≥35.35 mm) was determined by receiver-operating curve (ROC) analysis for the primary endpoint (area under curve (AUC)=0.652, p=0.002). The sensitivity for high MA (≥35.35 mm) was 80 % (CVD and MI) and 90 % (definite, probable, possible stent thrombosis), and the specificity was 52 % (CVD and MI) and 46 % (stent thrombosis). Subjects with high MA (≥35.35 mm) exhibited higher body mass index, and higher prevalence of diabetes mellitus, while there was no significant difference in other baseline variables (see Suppl. Material, available online at www.thrombosis-online.com).
Kaplan-Meier survival analysis and Cox regression analysis was performed with forward conditional adjustment for baseline clinical variables. Subjects with high MA demonstrated significantly increased risk for the primary combined endpoint of CVD and MI (adjusted hazard ratio [HR] (95 % confidence interval [CI]): 3.8 (1.7–8.3);p=0.001), as well as MI (adjusted HR (95 % CI): 3.0 (1.3–6.9); p=0.008) (▶Figure 1). In contrast, bleeding was not significantly different between subjects with high or low MA (adjusted HR (95 % CI): 0.21 (0.04–1.2); p=0.082); however, bleeding events tended to occur more often in subjects with low MA. Four patients with low MA had intracranial bleeding events versus one patient with high MA who was being treated with warfarin at the time. Definite stent thrombosis (adjusted HR (95 % CI): 5.1 (0.7–47); p=0.145; log-rank p=0.041), as well as definite, probable, or possible stent thrombosis (adjusted HR (95 % CI): 6.3 (0.8–51); p=0.08; log-rank p=0.014) occurred more often in subjects with high MA (▶Figure 1).
The results of our study indicate that subjects who experience recurrent ischemic cardiovascular events after PCI show increased maximal fibrin clot strength at baseline as compared to subjects without adverse ischaemic events. The importance of the fibrin component in the prothrombotic environment of atherosclerotic cardiovascular disease is illustrated by the findings of Ang et al., who demonstrated an increased risk of MI after PCI in a cohort of subjects with increased fibrinogen, but not increased platelet reactivity during treatment with clopidogrel (10). Fibrinogen concentration was not significantly different between subjects with and without recurrent ischaemic events in our study; however, fibrinogen was correlated with TEG-MA. Differences in fibrin stabilisation and cross-linking could potentially account for higher event rates in individuals with high plasma clot strength in our study.
The risk of stent thrombosis is significantly reduced with use of more potent P2Y12 platelet inhibitors, such as prasugrel and ticagrelor; however, stent thrombosis continues to occur in a significant number of subjects (11, 12). In addition, increased bleeding risk can be observed in a minority of patients, thus substantiating the need for better characterisation of subjects at risk with current standard medical therapy used in secondary prevention after PCI (11, 13). A validated ex vivo measure of intrinsic coagulation that is strongly associated with clinical risk of thrombosis and/or bleeding may be critical in further developing personalised antithrombotic treatment after PCI. TEG may be able provide such an integrated assessment of thrombotic risk. The functional fibrinogen assay in whole-blood TEG is similar to the measurement of TEG in platelet-poor plasma; however, there are some differences. In particular, in the functional fibrinogen assay, red blood cells are still present, but platelets are inhibited by addition of glycoprotein (GP)IIb/IIIa inhibitor. The functional fibrinogen assay thus measures the strength of clot that includes incorporated red cells, which has been shown to be dependent of factor XIIIa activity and fibrin α-chain crosslinking (14). In contrast, plasma TEG measures cross-linked fibrin strength in absence of red cells and without GPIIb/IIIa antagonist, which could influence the final MA measurement. Functional fibrinogen TEG assays based on GPIIb/IIIa inhibition have also been shown to overestimate fibrin strength in patients with high platelet counts, possibly due to incomplete platelet inhibition (15).Limitations of our study that diminish the strength of our conclusions include the study size, the imbalance of diabetics between subjects with and without high clot strength, and relatively low predictive value of the cut-off for MA.
In conclusion, the results of our analysis suggest that measurement of high plasma fibrin clot strength by TEG may identify subjects at increased risk of recurrent CVD, MI, and stent thrombosis treated with standard dual antiplatelet therapy after PCI. Further studies are needed to examine the utility of plasma TEG in tailored antithrombotic treatment strategies after PCI.
Supplementary Material
Financial support:
This study was supported in part, by the Indiana Clinical and Translational Sciences Institute funded, in part by Grant Number (U54-RR025761. Anantha Shekhar, PI) from the National Institutes of Health, National Center for Research Resources, Clinical and Translational Sciences Award, as well as the Indiana University Health Values Grant, the Indiana University Health – Indiana University School of Medicine Strategic Research Initiative, and internal funding from the Department of Medicine, Indiana University School of Medicine, India napolis. Sample processing and storage was performed in part by the Specimen Storage Facility (SSF) of the Indiana Clinical and Translational Sciences Institute (CTSI) which is supported, in part, by a Clinical and Translational Sciences Award (Grant # UL1TR001108. Anantha Shekhar, PI) and CTSI SSF facility construction was funded in part by grant CO6– RR020128–01. (R. S. Fife, PI, K. Cornetta, Co-I).
Footnotes
Conflicts of interest
RK has served as consultant for Coramed Technologies and Haemonetics Corporation and has received research funding from Haemonetics Corporation.
Supplementary Material to this article is available online at www.thrombosis-online.com.
References
- 1.Gurbel PA, Bliden KP, Guyer K, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol 2005; 46: 1820–1826. [DOI] [PubMed] [Google Scholar]
- 2.Bitar A, Kreutz RP. Role of Thrombelastography (TEG) in Risk Assessment and Guidance of Anti-thrombotic Therapy in Patients with Coronary Artery Disease. Drug Dev Res 2013; 74: 533–540. [Google Scholar]
- 3.Kreutz RP, Owens J, Lu D, et al. Platelet factor XIIIa release during platelet aggregation and plasma clot strength measured by thrombelastography in patients with coronary artery disease treated with clopidogrel. Platelets 2015; 26: 358–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreutz RP, Bitar A, Owens J, et al. Factor XIII Val34Leu polymorphism and recurrent myocardial infarction in patients with coronary artery disease. J Thromb Thrombolysis 2014; 38: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu D, Owens J, Kreutz RP. Plasma and whole blood clot strength measured by thrombelastography in patients treated with clopidogrel during acute coronary syndromes. Thromb Res 2013; 132: e94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreutz RP, Owens J, Breall JA, et al. C-reactive protein and fibrin clot strength measured by thrombelastography after coronary stenting. Blood Coagul Fibrinolysis 2013; 24: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012; 126: 2020–2035. [DOI] [PubMed] [Google Scholar]
- 8.Cutlip DE, Windecker S, Mehran R, et al. ; Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007; 115: 2344–2351. [DOI] [PubMed] [Google Scholar]
- 9.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123: 2736–2747. [DOI] [PubMed] [Google Scholar]
- 10.Ang L, Thani KB, Ilapakurti M, et al. Elevated plasma fibrinogen rather than residual platelet reactivity after clopidogrel pre-treatment is associated with an increased ischemic risk during elective percutaneous coronary intervention. J Am Coll Cardiol 2013; 61: 23–34. [DOI] [PubMed] [Google Scholar]
- 11.Wiviott SD, Braunwald E, McCabe CH, et al. ; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357: 2001–2015. [DOI] [PubMed] [Google Scholar]
- 12.Wallentin L, Becker RC, Budaj A, et al. ; PLATO Investigators, Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045–1057. [DOI] [PubMed] [Google Scholar]
- 13.Bonaca MP, Bhatt DL, Cohen M, et al. ; PEGASUS-TIMI 54 Steering Committee and Investigators. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–1800. [DOI] [PubMed] [Google Scholar]
- 14.Byrnes JR, Duval C, Wang Y, et al. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin -chain crosslinking. Blood 2015; 126: 1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlimp CJ, Solomon C, Ranucci M, et al. The effectiveness of different functional fibrinogen polymerization assays in eliminating platelet contribution to clot strength in thromboelastometry. Anesth Analg 2014; 118: 269–276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.