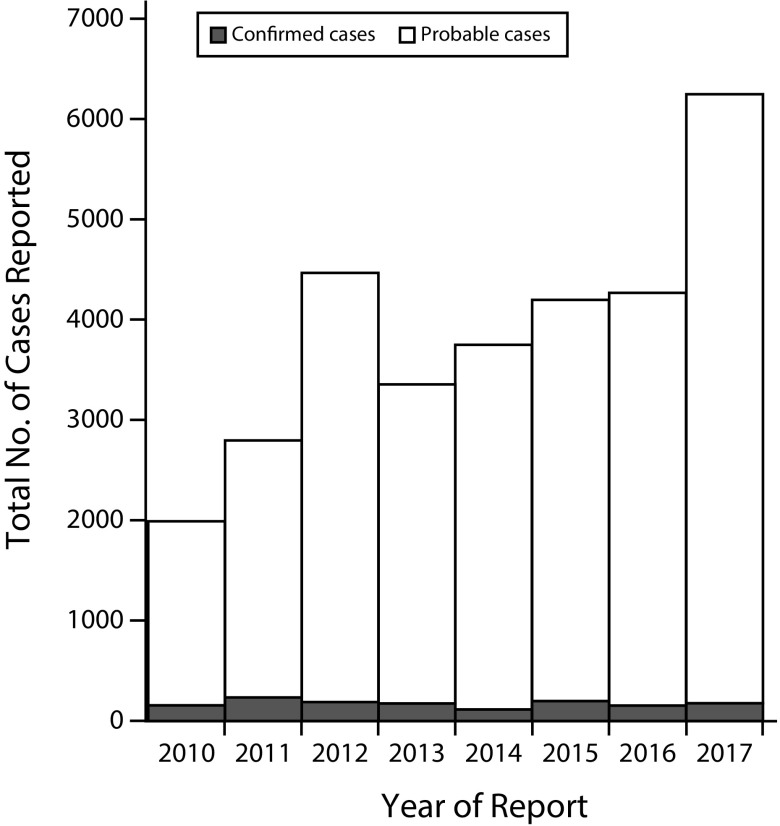
From 2016 through 2017, unprecedented increases in all nationally notifiable tickborne diseases were reported to the Centers for Disease Control and Prevention (CDC). The largest percentage increase was seen in reported cases of spotted fever rickettsiosis (SFR): a 46% increase from 4269 cases to a record 6248.1 Increases were reported in both new areas and in known endemic states. Although the New England, East North Central, and Middle Atlantic regions typically report only a handful of cases each year, in 2017, these areas experienced, a 215%, 78%, and 65% increase in reported cases, respectively. Among tickborne diseases, only anaplasmosis, with a 39% increase during this time, approached the rise seen with SFR. Although this increase raises concerns for elevated disease risk, additional factors are likely contributing to the high number of reports.
REPORTING
Confirmed and probable cases of SFR are reported to the CDC through the National Notifiable Diseases Surveillance System by state and local health departments. In 2017, 176 (3%) cases were reported as confirmed and 6072 (97%) as probable. Although the annual incidence of SFR in the United States increased from 6.4 to 19.2 cases per million persons from 2010 to 2017, there was a corresponding decrease in the proportion of confirmed cases (Figure 1).1,2 Case classification relies on clinical and laboratory data, as defined in the Council of State and Territorial Epidemiologists SFR case definition.3 Confirmed cases are classified using more specific laboratory diagnostics, including polymerase chain reaction assays or demonstration of seroconversion. Classification of probable cases uses less stringent laboratory evidence, including single elevated immunoglobulin G (IgG) antibody titers by indirect immunofluorescence antibody assay or other less specific diagnostic tests. Although the Council of State and Territorial Epidemiologists case definitions are for surveillance purposes only and are not intended for clinical use, surveillance data provide useful information about risk and disease trends.
FIGURE 1—
Probable and Confirmed Cases of Spotted Fever Rickettsiosis Reported to the Centers for Disease Control and Prevention: National Notifiable Diseases Surveillance System, United States, 2010–2017
SFR are caused by infection with spotted fever group Rickettsia (SFGR) species through the bite of an infected tick. Rocky Mountain spotted fever, which is caused by Rickettsia rickettsii, is the most severe and fatal of the SFR, with a case fatality rate of 5% to 10% in the United States.4 Disease caused by other SFGR, including Rickettsia parkeri and Rickettsia species 364D, also contribute to SFR case counts. Infections with these species typically cause less severe illness than does Rocky Mountain spotted fever but may still include fever, rash, and malaise as well as eschars, or areas of necrosis, at the site of the tick bite. Additional SFGR (e.g., Rickettsia amblyommatis, Rickettsia montanensis) are present in human-biting ticks in the United States and are suspected of causing disease, but definitive data are not currently available. Presumptive treatment with doxycycline is the recommended treatment of all rickettsial diseases.
RISING EXPOSURE
Recent studies have suggested that human exposure to less pathogenic SFGR are on the rise.5 Infection with both pathogenic and nonpathogenic SFGR likely induces an antibody response, even without associated symptoms. Although serologic tests for SFR diagnosis use R. rickettsii antigens to detect SFGR antibodies, these tests are nonspecific for Rocky Mountain spotted fever. Because most SFR cases are diagnosed by serology testing, it is unknown what proportion of reported SFR cases are caused by which SFGR.
The rise in reports of SFR cases is multifactorial, and it is clear that more exposures to SFGR are being detected. One possible explanation for this is the expanding geographic distribution of ticks that transmit SFGR. Tick activity can be affected by environmental factors, including temperature, rainfall, and humidity. Ticks can survive a wide range of environments, but milder conditions can extend the length of time ticks engage in host-seeking activities.6 In the United States, reports of expanding range and geographic distribution are documented for the following tick vectors known to transmit SFGR: American dog tick (Dermacentor variabilis) in the Central and Eastern United States, Rocky Mountain wood tick (Dermacentor andersoni) in the Northwest, lone star tick (Amblyomma americanum) in the East and Midwest, and Gulf Coast tick (Amblyomma maculatum) in the Gulf Coast, Midwestern, and South Atlantic states.6 Changing dynamics of tick distributions and environmental suitability can increase tick–human interaction and exposure to ticks and the diseases they carry.
TESTING
Passive surveillance may also be influenced by increased awareness and testing among health care providers. During 2016 through 2017, jurisdictions reported increased educational efforts, including training for providers on clinical diagnosis of rickettsial disease (CDC personal communication, October 2018). In addition to state-level efforts, “Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis—United States” was published in 2016 and provides national guidelines on rickettsial disease diagnosis and reporting practices.4 Activities such as these likely led to increased provider awareness of rickettsial diseases, resulting in increased testing and reporting.
Although it is clear that the surveillance data show increased detection of exposures to SFGR, it is difficult to determine whether the increase in probable cases represents greater numbers of incident SFR. The high percentage of cases reported as probable highlights the limitations of current diagnostic testing practices. The vast majority of reported cases are diagnosed using serologic methods, primarily by immunofluorescence antibody assay. Serologic testing by immunofluorescence antibody assay can provide confirmation of SFR when used correctly; confirmation requires IgG seroconversion (fourfold change) between paired serum samples with one taken in the first week of illness (acute) and a second taken two to four weeks later (convalescent).
A case meets the probable SFR laboratory criteria with a single positive serology result, and most probable cases are supported by report of a single anti-Rickettsia IgG positive result as the only laboratory evidence.2 Because IgG antibodies typically require at least seven days to develop, titers present in the acute phase of illness likely reflect previous exposure rather than incident illness. Studies have shown that background seroprevalence to SFGR may be greater than previously thought, and studies have demonstrated SFGR seropositivity in asymptomatic individuals.7 Thus, nonspecific clinical criteria along with positive serology on acute samples suggests that many cases of probable SFR may be other infections in patients who have previous exposure to SFGR.
Molecular methods, such as polymerase chain reaction, provide species-level specificity and do not require paired specimens for confirmatory diagnosis; however, they are seldom used. Diagnostics that can accurately identify species in the acute phase of illness are most useful in determining true disease burden. The CDC recently received US Food and Drug Administration clearance for the first diagnostic assay for the detection of rickettsial DNA when patients are still experiencing symptoms. These real-time polymerase chain reaction assays are available to certain state and public health laboratories through the Laboratory Response Network.
MOVING FORWARD
With increasing patient and provider awareness and an expanding population of ticks, the burden on local and state public health departments has increased. Additional efforts need to be made to determine whether this increase in reported SFR cases represents an increased risk to the public or if it is an artifact of high seroprevalence in the population and increased testing and reporting practices. Expanding the ability to identify true incident cases of SFR will require additional capacity at state and local levels for tracking, diagnosing, and reporting cases. Without improvements in testing practices, including the use of polymerase chain reaction assays or appropriate serologic testing, the true risk of SFR will remain unknown.
ACKNOWLEDGMENTS
Contributors to this editorial included participating health care providers, laboratorians, and public health partners, including state health departments and public health laboratories.
The authors acknowledge Christopher Paddock for his subject matter expertise in rickettsial disease epidemiology and diagnostics.
CONFLICTS OF INTEREST
The authors of this editorial declare no conflicts of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System: notifiable infectious diseases and conditions data tables. 2018. Available at: https://www.cdc.gov/nndss/infectious-tables.html. Accessed October 3, 2018.
- 2.Drexler NA, Dahlgren FS, Nichols Heitman KN, Massung RF, Paddock CD, Barton Behravesh CB. National surveillance of spotted fever group rickettsioses in the United States, 2008–2012. Am J Trop Med Hyg. 2016;94(1):26–34. doi: 10.4269/ajtmh.15-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Council of State and Territorial Epidemiologists. Position statement 09-ID-16: public health reporting and national notification for spotted fever rickettsiosis (including Rocky Mountain spotted fever). 2009. Available at: http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/09-ID-16.pdf. Accessed May 8, 2018.
- 4.Biggs HM, Behravesh CB, Bradley KK et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain Spotted Fever and other spotted fever group rickettsioses, ehrlichiosis, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65(2):1–44. doi: 10.15585/mmwr.rr6502a1. [DOI] [PubMed] [Google Scholar]
- 5.Dahlgren FS, Paddock CD, Springer YP, Eisen RJ, Behravesh CB. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am J Trop Med Hyg. 2016;94(1):35–42. doi: 10.4269/ajtmh.15-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonenshine DE. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int J Environ Res Public Health. 2018;15(3):E478. doi: 10.3390/ijerph15030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graf PC, Chretien JP, Ung L, Gaydos JC, Richards AL. Prevalence of seropositivity to spotted fever group rickettsiae and Anaplasma phagocytophilum in a large, demographically diverse US sample. Clin Infect Dis. 2008;46(1):70–77. doi: 10.1086/524018. [DOI] [PubMed] [Google Scholar]