Abstract
Enantiomerically enriched phthalans were synthesized efficiently via an enantioselective copper-catalyzed alkene carboetherification reaction. In this reaction, 2-vinylbenzyl alcohols enantioselectively cyclize then couple with vinylarenes. The utility of the method was demonstrated by the enantioselective synthesis of (R)-fluspidine, a σ1 receptor ligand.
Graphical Abstract

Phthalides and phthalans make up the core of important bioactive compounds of both natural and unnatural origins (Figure 1).1 Their attractive biological profiles have inspired the development of a number of creative approaches toward their de novo synthesis.1a, 2
Figure 1.
Bioactive phthalides and phthalans.
One surprisingly underexplored direct approach to the synthesis of phthalides and phthalans is the intramolecular cyclization of readily available 2-vinyl benzoic acids and alcohols (Scheme 1).4 Along these lines, Kobayashi has reported an iodine-promoted cycloetherification,3 while more recently Waser has reported a palladium-catalyzed carboetherification/cyclization (Scheme 1a) and Zhu has reported a copper-catalyzed carboetherification/cyclization (Scheme 1a).4 The synthesis of enantioenriched phthalides from 2-vinyl-benzoic acids has been achieved by Sharpless asymmetric dihydroxylation and subsequent lactonization.5 More recently, progress has been made toward enantioselective organo-catalytic bromolactonization and fluorolactonization, reported by Yeung6 and Rueping,7 respectively. A catalytic enantioselective cyclization of benzyl alcohols onto pendant ortho-α,β-unsaturated ketones via intramolecular oxa-Michael reactions has also been demonstrated by Ghorai (Scheme 1b).8 Herein we disclose a copper-catalyzed enantioselective alkene carboetherification as an approach to the synthesis of enantioenriched phthalans from unactivated 2-vinyl benzyl alcohols (Scheme 1c). Application of the method to the synthesis of (R)-fluspidine, a σ1 receptor ligand, is also demonstrated.
Scheme 1.
Phthalides and Phthalans from 2-Vinylbenzyl Alcohols and 2-Vinylbenzoic Acids
We have disclosed enantioselective copper-catalyzed alkene carboetherifications for the synthesis of chiral tetrahydrofurans.9a,b Related alkene carboetherification methods have also been disclosed by other groups.9c–f We aimed to extend the scope of this powerful methodology to the synthesis of chiral phthalans and phthalides. Using this approach, requisite substrates are 2-vinylbenzyl alcohols and benzoic acids 1, whose application in metal-catalyzed alkene cyclization/difunctionalization is less common, possibly due to loss of conjugation in the exo-cyclization transition state. The envisioned reaction sequence for phthalan formation, analogous to our prior tetrahydrofuran synthesis method,9a is illustrated in Scheme 2. The benzylalcohol 1 is proposed to coordinate to the copper(II) center followed by intramolecular cis-oxycupration. Homolysis of the resulting alkyl-Cu(II) bond provides a methyl radical that can add to vinyl arenes. Oxidation of the resulting benzylic radical then results in formation of the higher substituted vinyl arene 2.
Scheme 2.
Proposed Copper-Catalyzed Carboetherification Mechanism
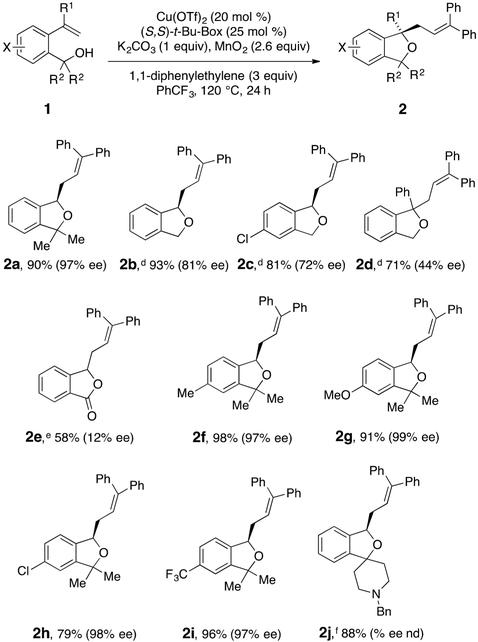
We investigated the optimal conditions for enantioselective carboetherification of 2-vinylbenzyl alcohols 1a and 1b with 1,1-diphenylethylene, as shown in Table 1. Subjecting 1a to Cu(OTf)2 (20 mol %), (S,S)-t-Bu-Box (25 mol %), and 1,1-diphenylethylene (3 equiv) in PhCF3 at 120 °C in the presence of K2CO3 (1 equiv)9 provided phthalan 2a in 90% yield and 97% ee (Table 1, entry 1). An attempt to reduce loading of the Cu(OTf)2 and (S,S)-t-Bu-Box to 15 and 18 mol %, respectively, decreased the isolated yield to 74% (Table 1, entry 2). Reducing the reaction temperature to 100 °C resulted in reduced isolated yield (Table 1, entry 3). The reaction of 1a under optimal conditions (Table 1, entry 1) was performed on 1 mmol scale and resulted in 70% isolated yield of 2a (>99% ee, Table 1, entry 4). 2-Vinylbenzyl alcohol 1b, a primary benzyl alcohol, was next investigated. Under the conditions deemed optimal for 1a (Table 1, entry 1), cycloetherification occurred to provide a 2:2:1 ratio (crude 1H NMR) of phthalan 2b, the benzaldehyde derived from 1b, and benzyl alcohol 1b, respectively (Table 1, entry 5). The competitive oxidation of the primary benzyl alcohol under these conditions, which employ MnO2 as the stoichiometric oxidant, was not surprising. Fortunately, upon changing to a milder oxidant, Ag2CO3 (200 mol %), 71% of phthalan 2b could be obtained in 80% ee (Table 1, entry 6). Reducing the amount of Ag2CO3 to 100 mol % provided 2b in even higher yield (93%, Table 1, entry 7). Reducing the reaction temperature to 100 °C resulted in lower conversion (Table 1, entry 8). It should be noted that no reaction was observed when Cu(OTf)2 was omitted and minimal reaction (ca. 5% conversion) was detected when MnO2 was omitted in the reaction of 1a (not shown). Thus, with the optimal conditions in hand (Table 1, entries 1 and 7), the 2-vinylbenzyl alcohol scope was further explored (Scheme 3).
Table 1.
Reaction Optimization a
 | ||||
|---|---|---|---|---|
| entry | substrate | oxidant (mol %)b | yield (%)c | ee (%)d |
| 1 | 1a | MnO2 (260) | 90 | 97 |
| 2e | 1a | MnO2 (260) | 74 | 98 |
| 3f | 1a | MnO2 (260) | 78 | |
| 4g | 1a | MnO2 (260) | 70 | >99 |
| 5h | 1b | MnO2 (260) | ca. 40 | |
| 6 | 1b | Ag2CO3 (200) | 71 | 80 |
| 7 | 1b | Ag2CO3 (100) | 93 | 81 |
| 8f,h | 1b | Ag2CO3 (100) | ca. 56 | |
Reaction conditions: Cu(OTf)2 (0.037 mmol, 20 mol %) and (S,S)-t-Bu-Box (25 mol %) were complexed in PhCF3 (1 mL) at 60 °C. After 2 h, oxidant, alcohol 1 (0.185 mmol) in PhCF3 (1 mL), K2CO3 (1 equiv), 1,1-diphenylethylene (3 equiv), and 4 Å mol. sieves were added at rt and the mixture was heated and stirred for 24 h at 120 °C in a sealed tube.
MnO2 (85%, <5 μ was used).
Isolated yield unless otherwise noted.
Enantioselectivity determined by chiral HPLC.
Reaction run with 15 mol % Cu(OTf)2 and 18 mol % (S,S)-t-Bu-Box.
Reaction run at 100 °C.
This reaction used substrate 1a at 1 mmol scale.
Yield estimated by crude 1H NMR. Ca. 40% of the benzaldehyde derived from 1b was also formed.
Scheme 3.
Scope with Respect to 2-Vinylbenzyl Alcohol or 2-Vinylbenzoic Acida
a(a) The reaction conditions described in Table 1, entry 1 were used unless otherwise noted. (b) Yield is reported for product isolated by chromatography on silica gel. (c) Enantiomeric excess was determined by chiral HPLC. (d) Ag2CO3 (1 equiv) was used instead of MnO2. (e) 2,6-Di-t-butyl-4-methylpyridine was used instead of K2CO3. (f) The enantiomers were not separable on a number of chiral columns. nd = not determined.
A number of substrate functionalities including Cl, Me, OMe, CF3, and a tertiary amine were tolerated under the reaction conditions (Scheme 3). Tertiary alcohol substrates uniformly provided higher levels of enantioselectivity than primary benzyl alcohol substrates (e.g., compare 2a and 2h to 2b and 2c). A benzoic acid was able to cyclize to give phthalide 2e, albeit with low enantioselectivity. In this case, the soluble base 2,6-di-tert-butyl-4-methylpyridine provided higher conversion than when K2CO3 was used, possibly due to the difference in solubility of the intermediate corresponding benzoate salt. (2-(1-Phenylvinyl)phenyl)methanol provided phthalan 2d, but the enantioselectivity was greatly diminished. The absolute stereochemistry of the products was assigned by conversion of phthalan 2c to its known corresponding primary alcohol (using OsO4 and PhI(OAc)2, then NaBH4) and optical rotation comparison (see Supporting Information).8
A survey of vinylarene coupling partners was also investigated (Scheme 4). While 1,1-disubstituted vinyl arenes are generally the best partners due to their greater ability to react with the presumed carbon radical intermediates, 4-methylstyrene also provided phthalan 2k in 99% yield and 96% ee. Similarly, reaction with 4-methoxystyrene and unsubstituted styrene (5 equiv) provided 65% and 95% yields of adducts 2l and 2m in 99% ee each. The less electron-rich 4-chlorostyrene (5 equiv) underwent the reaction with alcohol 1a in 55% yield and 99% ee, while the 4-cyanostyrene was unreactive under these conditions. Reaction of 1a with 2-methoxystyrene provided 2p in 52% yield and 99% ee, while reaction with 2-bromostyrene provided 2q in 33% yield and 99% ee. In these reactions with styrenes, use of the soluble 2,6-di-tert-butyl-4-methylpyridine instead of K2CO3 significantly reduced styrene polymerization. Reaction of 1a with 1,1-(4-chlorophenyl)ethylene provided phthalan 2r in 90% yield and 93% ee. Vinyl arenes with exocyclic alkenes provided endocyclic alkenes 2s and 2t as major isomers.
Scheme 4.
Scope of the Alkene Coupling Partnera
a(a) The reaction conditions described in Table 1, entry 1 were used unless otherwise noted. (b) Yield is reported for products isolated by chromatography on silica gel. (c) Enantiomeric excesses were determined by chiral HPLC. (d) 2,6-Di-tert-butyl-4-methylpyridine was used instead of K2CO3. (e) Five equiv of styrene was used.
The synthetic utility of this enantioselective phthalan synthesis method is illustrated in the enantioselective synthesis of (R)-fluspidine, a σ1 receptor probe (Scheme 5).1b–e This compound was previously obtained in enantioenriched form via chiral HPLC resolution,1c and more recently by enantioselective synthesis using a catalytic asymmetric reduction as the key step.1e Our synthesis began with commercially available 2-bromostyrene, which was subjected to lithium–halogen exchange followed by treatment with N-benzoyl-piperidine-4-one, providing the corresponding 2-vinylbenzyl alcohol in 78% yield. Enantioselective carboetherification with 1,1-diphenylethylene provided chiral phthalan 2u in 81% yield and 86% ee. Alternatively, reaction with 4-methylstyrene provided adduct 2v in 87% yield and 89% ee. Oxidative cleavage of the alkene followed by reduction of the resulting aldehyde and concomitant reduction of its amide provided the known primary alcohol 4.1c,e Application of 2v instead of 2u in this synthesis is slightly more atom economical (less carbons removed in oxidation step). Direct fluorination of 4 with DAST as previously reported1e provided (R)-fluspidine. The optical rotation of (R)-fluspidine (5) was similar to the reported value (Scheme 5).1c,e Our enantioselective synthesis fluspidine is two steps shorter than the racemic synthesis1c and five steps shorter than the previously reported enantioselective synthesis.1e
Scheme 5.
Enantioselective Synthesis of (R)-Fluspidine
In summary, copper-catalyzed alkene carboetherification is an effective method for the synthesis of enantioenriched phthalans from 2-vinyl benzylalcohols. Further demonstrations of the scope of the copper-catalyzed enantioselective alkene carboetherification are underway and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institutes of Health (GM078383) for financial support of this work.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:10.1021/acs.orglett.8b02766.
Experimental procedures, tabulated characterization data for all new compounds, HPLC traces of chiral products (PDF)
1H and 13C NMR spectra for all new compounds (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1) (a).Karmakar R; Pahari P; Mal D Chem. Rev 2014, 114, 6213–6284. [DOI] [PubMed] [Google Scholar]; (b) Maestrup EG; Wiese C; Schepmann D; Brust P; Wunsch B Bioorg. Med. Chem 2011, 19, 393–405. [DOI] [PubMed] [Google Scholar]; (c) Holl K; Falck E; Kohler J; Schepmann D; Humpf H-U; Brust P; Wunsch B ChemMedChem 2013, 8, 2047–2056. [DOI] [PubMed] [Google Scholar]; (d) Brust P; Deuther-Conrad W; Becker G; Patt M; Donat CK; Stittsworth S; Fischer S; Hiller A; Wenzel B; Dukic-Stefanovic S; Hesse S; Steinbach J; Wunsch B; Lever SZ; Sabri OJ Nucl. Med 2014, 55, 1730–1736. [DOI] [PubMed] [Google Scholar]; (e) Nakane S; Yoshinaka S; Iwase S; Shuto Y; Bunse P; Wunsch B; Tanaka S; Kitamura M Tetrahedron 2018, 74, 5069–5084. [Google Scholar]
- (2) (a).Weldy NM; Schafer AG; Owens CP; Herting CJ; Varela-Alvarez A; Chen S; Niemeyer Z; Musaev DG; Sigman MS; Davies HML; Blakey SB Chem. Sci 2016, 7, 3142–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Phan DHT; Kim B; Dong VM J. Am. Chem. Soc 2009, 131, 15608–15609. [DOI] [PubMed] [Google Scholar]
- (3).Kobayashi K; Shikata K; Fukamachi S; Konishi H Heterocycles 2008, 75, 599–609. [Google Scholar]
- (4) (a).Nicolai S; Erard S; Gonzalez DF; Waser J Org. Lett 2010, 12, 384–387. [DOI] [PubMed] [Google Scholar]; (b) Ha TM; Wang Q; Zhu J Chem. Commun 2016, 52, 11100–11103. [DOI] [PubMed] [Google Scholar]; (c) Xie J; Wang Y-W; Qi L-W; Zhang B Org. Lett 2017, 19, 1148–1151 Reviews of alkene carboetherification by oxymetallation: . [DOI] [PubMed] [Google Scholar]; (d) Wolfe JP Synlett 2008, 2008, 2913–2937. [Google Scholar]; (e) Garlets ZJ; White DR; Wolfe JP Asian J. Org. Chem 2017, 6, 636–653. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) McDonald RI; Liu G; Stahl SS Chem. Rev 2011, 111, 2981–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chemler SR; Karyakarte SD; Khoder ZM J. Org. Chem 2017, 82, 11311–11325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Reddy RS; Kiran INC; Sudalai A Org. Biomol. Chem 2012, 10, 3655–3661. [DOI] [PubMed] [Google Scholar]
- (6).Chen J; Zhou L; Tan CK; Yeung Y-Y J.Org. Chem 2012, 77, 999–1009. [DOI] [PubMed] [Google Scholar]
- (7).Parmar D; Maji MS; Rueping M Chem. - Eur. J 2014, 20, 83–86. [DOI] [PubMed] [Google Scholar]
- (8).Ravindra B; Das BG; Ghorai P Org. Lett 2014, 16, 5580–5583. [DOI] [PubMed] [Google Scholar]
- (9).(a) Bovino MT; Liwosz TW; Kendel NE; Miller Y; Tyminska N; Zurek E; Chemler SR Angew. Chem., Int. Ed 2014, 53, 6383–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Karyakarte SD; Um C; Berhane IA; Chemler SR Angew. Chem., Int. Ed 2018, 57, 12921–12924. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) For related palladium-catalyzed carboetherifcations, see: Hopkins BA; Garlets ZJ; Wolfe JP Angew. Chem., Int. Ed 2015, 54, 13390–13392. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hu NF; Li K; Wang Z; Tang W Angew. Chem., Int. Ed 2016, 55, 5044–5048. [DOI] [PubMed] [Google Scholar]; (e) Zhang G; Cui L; Wang Y; Zhang LJ Am. Chem. Soc. 2010, 132, 1474–1475. [DOI] [PubMed] [Google Scholar]; (f) For an alternative copper-catalyzed carboetherification strategy, see: Cheng Y-F; Dong X-Y; Gu Q-S; Yu ZL; Liu XY Angew. Chem., Int. Ed 2017, 56, 8883–8886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.