Abstract
Background:
Canagliflozin, a sodium-glucose cotransporter-2 inhibitor (SGLT2i), was associated with an approximately two-fold increase in amputation risk in a recent cardiovascular outcome trial. Observational studies in real-world data have yielded mixed or inconclusive results.
Methods:
We conducted a retrospective cohort study using the MarketScan Commercial Claims and Encounters Database (2013–2015) to compare the incidence of lower-extremity amputation (LEA) between initiators of SGLT2i and initiators of two second-line drugs, dipeptidyl-peptidase 4 inhibitors (DPP4i) and sulfonylureas (SU). We estimated crude incidence rates (IR) and adjusted hazard ratios (aHR), with 95% confidence intervals (CI), before and after propensity score weighting. We additionally conducted sensitivity analyses using a comparator group of all non-metformin, non-SGLT2i glucose-lowering drugs, as previous studies used this approach.
Results:
In a cohort of 328,150 individuals aged 18–64, the IR of LEA ranged from 1.5 to 2.4 per 1,000 person-years. In as-treated analysis, the estimated hazard of LEA was increased among SGLT2i initiators compared to DPP4i initiators (aHR 1.69, 95% CI 1.20–2.38), but not compared to SU initiators (aHR 1.02, 95% CI 0.67–1.55) or non-metformin, non-SGLT2i initiators (aHR 1.02, 95% CI 0.54–1.93). Results were consistent in intention-to-treat analysis and across a number of sensitivity analyses.
Conclusions:
Among commercially-insured patients in the US, results suggest that initiation of SGLT2i may increase the risk of LEA compared to initiation of DPP4i. Contrasting results when comparing SGLT2i initiators to DPP4i and SU initiators highlight the importance of choosing appropriate comparator drugs when addressing comparative effectiveness and safety questions that can inform clinical decision-making.
INTRODUCTION
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) reduce blood glucose in Type 2 diabetes mellitus (T2DM) by inhibiting renal glucose reabsorption in the proximal tubule, increasing urinary glucose excretion.1,2 In addition to glycemic efficacy,1,3 observational and cardiovascular outcome studies demonstrate that SGLT2i have cardio-renal protective effects vs. placebo and other glucose-lowering drugs (GLDs).4–7
Safety concerns, however, were raised by the Canagliflozin Cardiovascular Assessment Study (CANVAS) program, which revealed a significant increase in the risk of overall amputation (hazard ratio (HR) 1.97, 95% confidence interval (CI) 1.41–2.75), primarily of the toe or forefoot, among patients randomized to canagliflozin with median follow-up of 126 weeks.5 This represents an important finding given that T2DM is the leading cause of non-traumatic lower-extremity amputations (LEA).8 In response, the FDA issued a bulletin regarding amputation risk in May 20169 and a drug labeling change in July 2017.10 Recently, several observational studies have sought to corroborate this finding in broader populations, with mixed conclusions.11–16
To address the variation in observed risk of amputation with SGLT2i, we implemented an active comparator, new user (ACNU) study17 to estimate and compare the risk of LEA between patients initiating SGLT2i versus patients initiating two second-line GLDs, dipeptidyl peptidase-4 inhibitors (DPP4i) and sulfonylureas (SU), which are prescribed as alternatives to SGLT2i. Specifically, we explored how selection of different analytic populations and comparators impacts risk estimates of LEA with SGLT2i, and how such choices may have contributed to differences in published findings in this domain.
METHODS:
Data Source
We conducted a cohort study using the Truven Analytics’ IBM® Watson Health MarketScan® Commercial Claims and Encounters (CCAE) Database from 2013 to 2015. MarketScan data contain inpatient, outpatient, and pharmaceutical claims and encounters data linked with demographic and enrollment information from approximately 350 insurance payers in the U.S.18 This database is representative of the U.S. population with employer-based insurance and offers longitudinal follow-up of patients while under the same employer.19 The study was determined to be exempt from full Institutional Review Board review by the University of North Carolina at Chapel Hill. The study protocol was registered with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) on October 23, 2017 (EU PAS Register Number 21368, http://www.encepp.eu/encepp/viewResource.htm?id=25456).
Eligible Population
The base population consisted of all MarketScan beneficiaries, aged 18–64, with at least one prescription dispensing claim for a SGLT2i, DPP4i, or SU between March 1, 2013 and September 30, 2015. To ensure new use of either comparator drug, we required subjects to have ≥12 months of continuous MarketScan enrollment prior to the first eligible prescription dispensing claim, during which no prescription for any of the study drug classes could be observed (washout period).
Exposure Definition
Exposure to a SGLT2i, DPP4i, or SU drug (Appendix Table 1) was defined by at least two same-class drug prescription dispensing claims observed within a pre-defined window. This “prescription window” was calculated as the first prescription’s recorded days’ supply, plus a 30-day grace period (GP). By requiring a second prescription and using that prescription date as the index date, we restricted the analysis to a cohort for whom we were reasonably confident were taking the cohort drug. Patients were excluded if they 1) received a prescription for either study drug prior to index date; 2) switched to the comparator drug between the first and second prescriptions; 3) received both study drugs on index date; or 4) received an empagliflozin-linagliptin combination drug on index date (Appendix FSUPP1).
Outcome Definitions
The primary outcome of LEA was defined using a combination of International Classification of Diseases, Ninth Revision, Clinical Modification and Common Procedural Terminology procedure codes. Patients who experienced LEA between the first and second prescriptions were included in the analysis, with those events captured in the baseline amputation history. Secondary outcomes included other diabetes-related conditions and procedures (tissue and bone debridement, peripheral vascular disease, and diabetic foot ulcer) commonly considered to be direct precursors or alternatives to LEA. Codes for all outcome definitions were informed through prior literature20–30 and clinical guidance (Appendix Table 2). For secondary analyses, we excluded patients with baseline history of any outcome.
Follow-up
The primary analysis was performed in “as-treated” fashion (Appendix Figure 2). Follow-up began at index date (date of second prescription) and ended when an individual experienced either an outcome of interest or censoring event (treatment discontinuation, switch, or augmentation; disenrollment; or September 30, 2015), whichever came first. Secondary outcome analyses additionally censored patients if LEA was observed prior to occurrence of a secondary outcome.
Confounding Control
We used propensity scores to control for measured confounding. We incorporated covariate groupings for patient demographics, diabetes-related comorbidities, general health comorbidities, medication use history (including prior use of other GLDs), and measures of healthcare system use in the propensity score model, measured in the year prior to cohort entry (Table 1). We also applied the adjusted diabetes complication severity index (aDCSI)31,32 as a proxy to control for diabetes disease severity.
Table 1.
Baseline Characteristics of the Eligible Initiators of SGLT2i, Compared to Initiators of DPP4i and SU, Before and After Implementation of Standardized Mortality Ratio (SMR) Weightinga (365-Day Washout Period)b
| SGLT2i vs. DPP4i | SGLT2i vs. SU | |||||
|---|---|---|---|---|---|---|
| Characteristic | SGLT2i initiators (n = 49,324) | DPP4i initiators (n = 116,439) | Weighted DPP4i initiators (n=50,189) | SGLT2i initiators (n = 46,878) | SU initiators (n = 149,623) | Weighted SU initiators (n =48,954) |
| Canagliflozin | 34700 (70.3) | --- | --- | 32452 (69.2) | --- | --- |
| Dapagliflozin | 11041 (22.4) | --- | --- | 10815 (23.1) | --- | --- |
| Empagliflozin | 3583 (7.3) | --- | --- | 3611 (7.7) | --- | --- |
| Age, mean (std. dev.) | 52.5 (8.2) | 52.8 (8.2) | 52.3 (8.2) | 52.3 (8.2) | 51.0 (9.7) | 52.3 (8.1) |
| Male | 26039 (52.8) | 65941 (56.6) | 25,499 (50.8) | 24468 (52.2) | 78710 (52.6) | 25,019 (51.1) |
| Diabetes Comorbiditiesb | ||||||
| Adjusted DCSI, mean (std. dev.) | 0.7 (1.1) | 0.6 (1.1) | 0.7 (1.2) | 0.7 (1.1) | 0.6 (1.1) | 0.7 (1.1) |
| Nephropathy | 2274 (4.6) | 4480 (3.8) | 2,360 (4.7) | 1928 (4.1) | 4714 (3.2) | 2,089 (4.3) |
| Neuropathy | 5644 (11.4) | 7848 (6.7) | 6,006 (12.0) | 4941 (10.5) | 8065 (5.4) | 5,445 (11.1) |
| Retinopathy | 4910 (10.0) | 7593 (6.5) | 4,986 (9.9) | 4351 (9.3) | 7153 (4.8) | 4,816 (9.8) |
| Peripheral vascular disease | 1396 (2.8) | 3254 (2.8) | 1,459 (2.9) | 1320 (2.8) | 3364 (2.2) | 1,401 (2.9) |
| Prior LEA | 76 (0.2) | 204 (0.2) | 82 (0.2) | 60 (0.1) | 234 (0.2) | 59 (0.1) |
| Debridement | 1107 (2.2) | 2190 (1.9) | 1,180 (2.4) | 993 (2.1) | 2393 (1.6) | 1,091 (2.2) |
| Diabetic ulcer | 1031 (2.1) | 2007 (1.7) | 1,105 (2.2) | 950 (2.0) | 2370 (1.6) | 965 (2.0) |
| General Health Comorbiditiesb | ||||||
| Hypertension | 35035 (71.0) | 76079 (65.3) | 35,864 (71.5) | 32882 (70.1) | 90326 (60.4) | 34,576 (70.6) |
| Dyslipidemia | 36678 (74.4) | 76080 (65.3) | 37,260 (74.2) | 34736 (74.1) | 83601 (55.9) | 36,655 (74.9) |
| Ischemic heart disease | 5019 (10.2) | 11658 (10.0) | 5,032 (10.0) | 4606 (9.8) | 13082 (8.7) | 4,884 (10.0) |
| Cerebrovascular disease | 1754 (3.6) | 4494 (3.9) | 1,765 (3.5) | 1645 (3.5) | 5402 (3.6) | 1,739 (3.6) |
| Congestive heart failure | 1075 (2.2) | 3231 (2.8) | 1,094 (2.2) | 940 (2.0) | 3980 (2.7) | 963 (2.0) |
| COPD | 1292 (2.6) | 3405 (2.9) | 1,382 (2.8) | 1186 (2.5) | 4307 (2.9) | 1,281 (2.6) |
| Depression | 4829 (9.8) | 9414 (8.1) | 5,173 (10.3) | 4746 (10.1) | 12097 (8.1) | 5,139 (10.5) |
| History of cancer | 2398 (4.9) | 6054 (5.2) | 2,456 (4.9) | 2315 (4.9) | 7486 (5.0) | 2,392 (4.9) |
| Chronic kidney disease | 4245 (8.6) | 11037 (9.5) | 4,494 (9.0) | 3730 (8.0) | 13049 (8.7) | 3,975 (8.1) |
| Alcohol abuse | 323 (0.7) | 884 (0.8) | 325 (0.6) | 305 (0.7) | 1461 (1.0) | 294 (0.6) |
| Smoking | 2462 (5.0) | 6077 (5.2) | 2,551 (5.1) | 2266 (4.8) | 8625 (5.8) | 2,326 (4.8) |
| Prior Medication Useb | ||||||
| ACEI | 22535 (45.7) | 52698 (45.3) | 22,957 (45.7) | 20266 (43.2) | 62629 (41.9) | 20,984 (42.9) |
| ARB | 13898 (28.2) | 28923 (24.8) | 14,255 (28.4) | 13581 (29.0) | 29055 (19.4) | 14,711 (30.1) |
| Aspirin | 2779 (5.6) | 4594 (3.9) | 3,027 (6.0) | 2655 (5.7) | 5029 (3.4) | 2,883 (5.9) |
| Beta blockers | 13763 (27.9) | 30812 (26.5) | 14,054 (28.0) | 12277 (26.2) | 42967 (28.7) | 12,594 (25.7) |
| Calcium channel blockers | 9680(19.6) | 23313 (20.0) | 9,911 (19.7) | 8872 (18.9) | 28005 (18.7) | 9,260 (18.9) |
| Statins | 31312 (63.5) | 69582 (59.8) | 31,499 (62.8) | 29479 (62.9) | 75044 (50.2) | 30,989 (63.3) |
| Loop diuretics | 3615 (7.3) | 7651 (6.6) | 3,891 (7.8) | 3181 (6.8) | 8910 (6.0) | 3,515 (7.2) |
| Non-loop diuretics | 16887 (34.2) | 37665 (32.3) | 17,493 (34.9) | 15707 (33.5) | 44452 (29.7) | 16,615 (33.9) |
| Digoxin | 277 (0.6) | 888 (0.8) | 261 (0.5) | 267 (0.6) | 1003 (0.7) | 265 (0.5) |
| Cilostazol | 93 (0.2) | 185 (0.2) | 91 (0.2) | 80 (0.2) | 212 (0.1) | 90 (0.2) |
| Gabapentin | 4573 (9.3) | 7965 (6.8) | 4,866 (9.7) | 4137 (8.8) | 9382 (6.3) | 4,509 (9.2) |
| Pregabalin | 1390 (2.8) | 2177 (1.9) | 1,487 (3.0) | 1310 (2.8) | 2153 (1.4) | 1,579 (3.2) |
| Duloxetine | 2117 (4.3) | 3710 (3.2) | 2,357 (4.7) | 2097 (4.5) | 3643 (2.4) | 2,415 (4.9) |
| Metformin | 38231 (77.5) | 98790 (84.8) | 38,552 (76.8) | 36429 (77.7) | 108068 (72.2) | 38,529 (78.7) |
| GLP-1 Receptor Agonist | 13950 (28.3) | 5941 (5.1) | 15,168 (30.2) | 10492 (22.4) | 7372 (4.9) | 12,640 (25.8) |
| DPP4i | --- | --- | --- | 14769 (31.5) | 28227 (18.9) | 17,187 (35.1) |
| SU | 17381 (35.2) | 42590 (36.6) | 17,992 (35.8) | --- | --- | --- |
| Thiazolidinediones | 4787 (9.7) | 14850 (12.8) | 4,903 (9.8) | 4278 (9.1) | 9930 (6.6) | 4,788 (9.8) |
| Insulin | 16162 (32.8) | 14687 (12.6) | 17,150 (34.2) | 14685 (31.3) | 13259 (8.9) | 16,488 (33.7) |
| Measures of Healthcare Utilization in Year Prior to Index Dateb | ||||||
| N HbAlc tests in past year | ||||||
| 0 | 4101 (8.3) | 13706 (11.8) | 3,959 (7.9) | 3925 (8.4) | 31605 (21.1) | 4,174 (8.5) |
| 1 | 9564 (19.4) | 31526 (27.1) | 9,975 (19.9) | 9295 (19.8) | 44163 (29.5) | 9,014 (18.4) |
| 2 | 13699 (27.8) | 33432 (28.7) | 14,085 (28.1) | 13232 (28.2) | 37179 (24.8) | 13,296 (27.2) |
| 3 | 21960 (44.5) | 37775 (32.4) | 22,170 (44.2) | 20426 (43.6) | 36676 (24.5) | 22,469 (45.9) |
| N LDL tests in past year | ||||||
| 0 | 7040 (14.3) | 20606 (17.7) | 7,345 (14.6) | 6670(14.2) | 42324 (28.3) | 7,171 (14.6) |
| 1 | 16478 (33.4) | 42377 (36.4) | 16,522 (32.9) | 15532 (33.1) | 54070 (36.1) | 15,061 (30.8) |
| 2 | 14185 (28.8) | 31861 (27.4) | 14,720 (29.3) | 13438 (28.7) | 33046 (22.1) | 14,191 (29.0) |
| 3 | 11621 (23.6) | 21595 (18.5) | 11,602 (23.1) | 11238 (24.0) | 20183 (13.5) | 12,531 (25.6) |
| Flu shot received in past year | 16077 (32.6) | 34584 (29.7) | 16,374 (32.6) | 15218 (32.5) | 42827 (28.6) | 15,911 (32.5) |
| N hospitalizations | ||||||
| 0 | 45735 (92.7) | 105388 (90.5) | 46,412 (92.5) | 43543 (92.9) | 133177 (89.0) | 45,552 (93.1) |
| 1 | 2976 (6.0) | 8678 (7.5) | 3,122 (6.2) | 2757 (5.9) | 12963 (8.7) | 2,839 (5.8) |
| 2 | 450 (0.9) | 1615 (1.4) | 475 (0.9) | 426 (0.9) | 2392 (1.6) | 399 (0.8) |
| ≥3 | 163 (0.3) | 758 (0.7) | 180 (0.4) | 152 (0.3) | 1091 (0.7) | 163 (0.3) |
| N days in hospital | ||||||
| 0 | 45735 (92.7) | 105388 (90.5) | 46,412 (92.5) | 43543 (92.9) | 133177 (89.0) | 45,552 (93.1) |
| 1–2 | 1471 (3.0) | 3988 (3.4) | 1,542 (3.1) | 1346 (2.9) | 5822 (3.9) | 1,363 (2.8) |
| 3–5 | 1268 (2.6) | 3730 (3.2) | 1,334 (2.7) | 1215 (2.6) | 5657 (3.8) | 1,252 (2.6) |
| 5–10 | 501 (1.0) | 1739 (1.5) | 519 (1.0) | 461 (1.0) | 2641 (1.8) | 478 (1.0) |
| >10 | 349 (0.7) | 1594 (1.4) | 382 (0.8) | 313 (0.7) | 2326 (1.6) | 309 (0.6) |
| N emergency department visits | ||||||
| 0 | 39544 (80.2) | 92730 (79.6) | 39,938 (79.6) | 37775 (80.6) | 115887 (77.5) | 39,563 (80.8) |
| 1 | 6751 (13.7) | 16338 (14.0) | 6,949 (13.8) | 6280 (13.4) | 22788 (15.2) | 6,436 (13.1) |
| ≥2 | 3029 (6.1) | 7371 (6.3) | 3,302 (6.6) | 2823 (6.0) | 10948 (7.3) | 2,955 (6.0) |
| N physician encounters | ||||||
| 0 | 162 (0.3) | 662 (0.6) | 155 (0.3) | 162 (0.3) | 1365 (0.9) | 162 (0.3) |
| 1–3 | 3152 (6.4) | 12227 (10.5) | 3,131 (6.2) | 3019 (6.4) | 20497 (13.7) | 2,952 (6.0) |
| 4–6 | 7665 (15.5) | 22349 (19.2) | 7,695 (15.3) | 7333 (15.6) | 28773 (19.2) | 7,365 (15.0) |
| ≥7 | 38345 (77.7) | 81201 (69.7) | 39,208 (78.1) | 36364 (77.6) | 98988 (66.2) | 38,475 (78.6) |
| N podiatrist visits | ||||||
| 0 | 44392 (90.0) | 107032 (91.9) | 45,105 (89.9) | 42275 (90.2) | 139833 (93.5) | 44,034 (89.9) |
| 1 | 2309 (4.7) | 4547 (3.9) | 2,401 (4.8) | 2144 (4.6) | 4904 (3.3) | 2,306 (4.7) |
| 2 | 1078 (2.2) | 2102 (1.8) | 1,145 (2.3) | 1063 (2.3) | 2210 (1.5) | 1,108 (2.3) |
| ≥3 | 1545 (3.1) | 2758 (2.4) | 1,538 (3.1) | 1396 (3.0) | 2676 (1.8) | 1,506 (3.1) |
Abbreviations: SGLT2i, sodium-glucose cotransporter-2 inhibitors; DPP4i, dipeptidyl peptidase-4 inhibitors; SU, sulfonylureas; DCSI, diabetes complication severity index; LEA, lower-extremity amputation; COPD, chronic obstructive pulmonary disease; ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blockers; GLP, glucagon-like peptide; HbA1c, hemoglobin A1c; LDL, low-density lipoprotein
Weighted by standardizing the comparator drug initiators to the population of SGLT2i initiators, using the propensity score odds (PS/(1-PS)), to estimate the treatment effect in the treated (ATT).
All baseline characteristics measured in the one year (365 days) prior to date of cohort drug initiation
Statistical Analysis
We estimated propensity scores using multivariable logistic regression to model each patient’s predicted probability of receiving SGLT2i, conditional on his or her baseline covariates. We then weighted comparator cohorts by the propensity score odds (PS/(1-PS)) to standardize the distribution of measured covariates to the SGLT2i cohorts, and estimated the average treatment effect in the treated.33 This approach aims to address the question, “would LEA incidence have changed had all SGLT2i initiators instead initiated a comparator drug?” Covariate balance was assessed using the standardized mean difference (SMD).34
For all outcomes, we estimated crude incidence rates (IR) using Poisson regression, and crude and adjusted HR (aHR) and 95% CIs using weighted Cox proportional hazards models. We used weighted Kaplan-Meier methods to estimate cumulative incidence of LEA, as well as risk differences (RD) and 95% CIs in the 1–30, 31–90, 91–180, and 180+ days after drug initiation in each study cohort. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Sensitivity and Subgroup Analyses
We conducted multiple sensitivity analyses. First, we re-analyzed the data using an intention-to-treatment (ITT) follow-up approach for up to one year, where patients were censored only for disenrollment or at the administrative study end date. Second, following previous studies,11–14 we performed an analysis requiring only a single prescription claim to be considered exposed to a study drug. Third, to replicate comparator choices in previous studies, we conducted an analysis using any non-metformin, non-SGLT2i GLD (SU, thiazolidinediones, DPP4i, glucagon-like peptide-1 receptor agonists, and insulins) as the comparator group, excluding all patients with baseline use of these agents. Fourth, because LEA risk was specifically noted in the CANVAS program, we repeated the analysis restricting to canagliflozin users.
To assess the impact of various design specifications, we repeated the analyses using 15-, 60-, and 90-day grace periods; 30-, 60-, 90-, and 180-day induction periods; 30-, 60-, 90-, and 180-day latency periods; and a 6-month washout period. We specified a range of induction and latency periods a priori to determine possible durations of time required for treatment initiation to contribute to LEA occurrence; both periods were extended to 6 months to reflect CANVAS results that reported emerging amputation risk by 6 months of follow-up. We additionally repeated the analysis with 1%, 2.5%, and 5% asymmetric trimming of the propensity score distribution.35
The CANVAS program reported particularly elevated risk of LEA among patients with prior LEA history, which could have important implications for clinical decision-making. To validate this result in our cohort, we estimated LEA risk within subgroups with and without prior amputation in the year prior to cohort entry. Because baseline metformin use predicts diabetes-related cardiovascular endpoints among users of second-line GLDs,36 we estimated and compared stratum-specific HR estimates among patients with and without baseline metformin use in the year prior to cohort entry. Finally, to investigate the impact of other baseline GLD use and to compare results to previous literature, we performed subgroup analyses by baseline SU use, baseline insulin use, baseline cardiovascular disease (CVD) status, and baseline chronic kidney disease (CKD) status.
RESULTS:
Eligible Cohort
We identified a total of 328,150 eligible users of at least one study drug in the MarketScan database between March 1, 2013 and September 30, 2015. Among study patients, we identified a total of 165,763 eligible new users in the SGLT2i (n=49,324) vs. DPP4i (n=116,439) comparison and 196,501 eligible new users for the SGLT2i (n=46,878) vs. SU (n=149,623) comparison. Canagliflozin, dapagliflozin, and empagliflozin accounted for 69–70%, 22–23%, and 7–8% of SGLT2i use, respectively (Table 1). Median (interquartile range (IQR)) follow-up (Table 2) in as-treated analyses was slightly longer among comparator drug initiators, and ranged from 0.42 (0.21–0.76) years among SGLT2i initiators to 0.47 (0.25–0.94) years among DPP4i initiators. Median follow-up was longer in ITT analysis, again among comparator drug initiators, ranging from 0.64 (0.31–1.05) years in SGLT2i initiators to 1.06 (0.46–2.01) years among DPP4i initiators and 1.03 (0.46–1.86) years among SU initiators. Follow-up was capped at one year after index date to minimize this observed difference in person-time available.
Table 2.
Crude and Adjusted Hazard Ratio (HR) Estimates for Lower-Extremity Amputation (LEA) and with Initiation of SGLT2i compared to Initiation of Other Second-Line Glucose Lowering Drugs, 365-Day Washout Period, As-Treated Analysis
| Comparison | Cohort | Number of Patients | Median (IQR) Follow-up Time, Yearsa | Total Person-Years | Number of Eventsb | Crude Incidence Rate, per 1,000 Person-Years | Crude HR (95% CI) | PS-weighted HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| SGLT2i vs. DPP4i | ||||||||
| AT Analysis | SGLT2i | 49324 | 0.42 (0.21–0.76) | 26751 | 63 | 2.4 (1.8–3.0) | 1.54 (1.13–2.10) | 1.69 (1.20–2.38) |
| DPP4i | 116439 | 0.47 (0.25–0.94) | 82947 | 122 | 1.5 (1.2–1.8) | |||
| ITT Analysis | SGLT2i | 49324 | 0.64 (0.31–1.00) | 30362 | 70 | 2.3 (1.8–2.9) | 1.45 (1.08–1.93) | 1.43 (1.01–2.03) |
| DPP4i | 116439 | 1.00 (0.46–1.00) | 86187 | 136 | 1.6 (1.3–1.9) | |||
| SGLT2i vs. SU | ||||||||
| AT Analysis | SGLT2i | 46878 | 0.42 (0.21–0.76) | 25427 | 50 | 2.0 (1.5–2.6) | 1.06 (0.77–1.45) | 1.02 (0.67–1.55) |
| SU | 149623 | 0.43 (0.22–0.91) | 102058 | 184 | 1.8 (1.6–2.1) | |||
| ITT Analysis | SGLT2i | 46878 | 0.64 (0.31–1.00) | 28900 | 56 | 1.9 (1.5–2.5) | 1.01 (0.75–1.36) | 1.02 (0.69–1.50) |
| SU | 149623 | 1.00 (0.46–1.00) | 110352 | 207 | 1.9 (1.6–2.1) | |||
| SGLT2i vs. All Non-Metformin, Non-SGLT2i | ||||||||
| AT Analysis | SGLT2i | 15687 | 0.35 (0.17–0.64) | 7275 | 10 | 1.4 (0.7–2.6) | 0.66 (0.35–1.24) | 1.02 (0.54–1.93) |
| Non-SGLT2i | 224363 | 0.41 (0.20–0.86) | 148171 | 282 | 1.9 (1.7–2.1) | |||
| ITT Analysis | SGLT2i | 15687 | 0.57 (0.28–0.97) | 9152 | 13 | 1.4 (0.8–2.4) | 0.66 (0.38–1.16) | 1.06 (0.60–1.86) |
| Non-SGLT2i | 224363 | 0.99 (0.44–1.00) | 162958 | 340 | 2.1 (1.9–2.3) | |||
Abbreviations: SGLT2i, sodium-glucose cotransporter-2 inhibitors; DPP4i, dipeptidyl peptidase-4 inhibitors; SU, sulfonylureas; IQR, interquartile range; PS, propensity score; AT, as-treated; ITT, intention-to-treat
Follow-up in ITT analyses were capped at 1 year following index date.
We observed 51, 59, and 102 lower-extremity amputation events between the 1st and 2nd prescription fills in the DPP4i, SU, and non-SGLT2i comparisons, respectively. These patients were not excluded from the primary analysis, but were considered as prior amputations for the assessment of subgroup effects for patients with and without prior amputation history. These amputations were re-introduced as analysis endpoints for the sensitivity analysis where we include patients with at least 1 prescription fill.
Age and gender distributions were similar across cohorts (Table 1). Prior to SMR weighting, we observed imbalances in several baseline comorbidities, including diabetic nephropathy, neuropathy, hypertension, dyslipidemia, ischemic heart disease, and baseline use of angiotensin-receptor blockers, statins, and metformin; baseline imbalances were generally more pronounced in the SGLT2i vs. SU comparison. All measured covariates were balanced after SMR weighting (Appendix Figure 4).
Primary and Secondary Outcomes
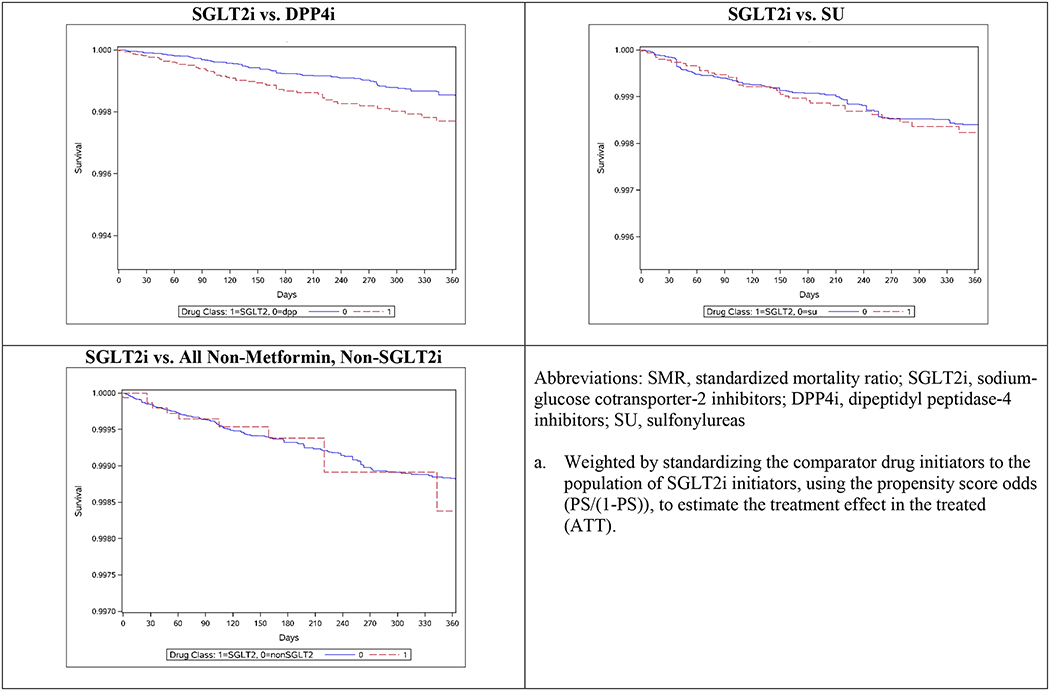
In the as-treated analysis (Table 2), we identified 185 LEA events in the SGLT2i (n=63) vs. DPP4i (n=122) comparison, and 234 LEA events in the SGLT2i (n=50) vs. SU (n=184) comparison. The majority of amputations were for toe and metatarsal (83%), foot and ankle (7%), and lower leg (9%). Absolute LEA risks were low in all cohorts (Figure 1, Appendix Table 3). Crude IRs were higher among SGLT2i initiators (2.4 per 1,000 person-years, 95% CI 1.8–3.0) than among DPP4i initiators (1.5 per 1,000 person-years, 95% CI 1.2–1.8), but were similar between SGLT2i (2.0 per 1,000 person-years, 95% CI 1.5–2.6) and SU (1.8 per 1,000 person-years, 95% CI 1.6–2.1) initiators.
Figure 1.
SMR-Weighteda Kaplan-Meier Curves for Lower-Extremity Amputation following Drug Initiation, 365-Day Washout Period
Hazard ratio estimates were similar between crude and adjusted analyses. After controlling for baseline confounding via SMR weighting, we observed an increased hazard of LEA among SGLT2i initiators vs. DPP4i initiators (aHR 1.69, 95% CI 1.20–2.38). However, no association was observed when comparing SGLT2i to SU initiators (aHR 1.02, 95% CI 0.67–1.55). To further aid clinical decision-making around second-line therapies, we performed a post-hoc analysis comparing DPP4i initiators to SU initiators, and observed a reduced hazard of LEA among DPP4i initiators (aHR 0.67, 95% CI 0.49–0.91), which is consistent with primary analysis findings. Finally, no increased hazard of LEA was observed when comparing initiators of SGLT2i to initiators of all non-metformin, non-SGLT2i GLDs (aHR 1.02, 95% CI 0.54–1.93), although we observed notable differences between crude and adjusted estimates in this comparison. For secondary outcomes, we observed modest to no increases in LEA hazard (Appendix Table 4), with HR estimates ranging from 0.96–1.22 and all 95% CIs containing the null.
Sensitivity and Subgroup Analyses
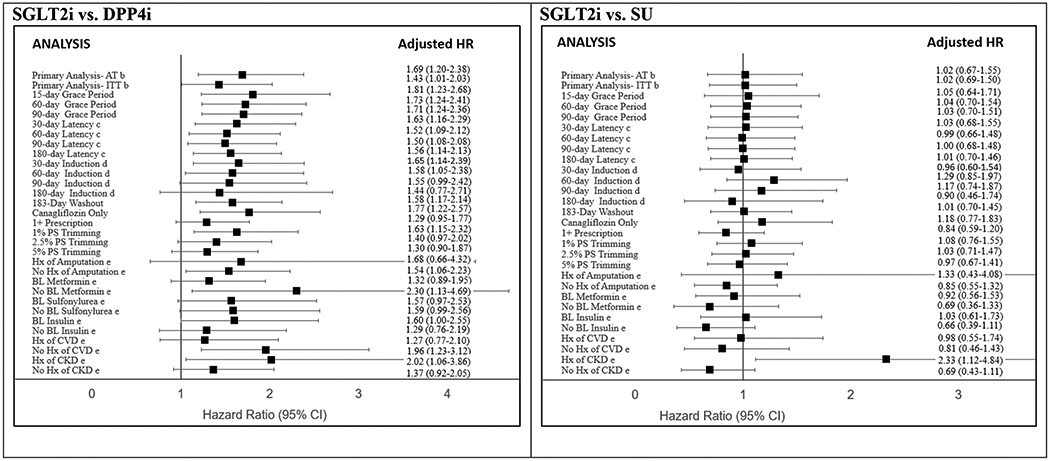
Results were consistent across a number of sensitivity analyses (Figure 2, Appendix Tables 5 and 6). We observed a downward trend in HR estimates for ITT analysis, when requiring only one prescription to define exposure, when using longer GPs, latency, and induction periods, and when increased trimming was performed. In the SU comparison, HR estimates increased when using 60- and 90-day induction periods, although estimates remained non-significant. Requiring a shorter (6-month) washout period did not substantially change HR estimates. Finally, restricting to just canagliflozin initiators yielded higher HR estimates in both the DPP4i and SU comparisons, but lower HR estimates vs. non-metformin, non-SGLT2i initiators (aHR 0.85, 95% CI 0.38–1.91).
Figure 2. Crude and Adjusted Hazard Ratio (HR) Estimates for Lower-Extremity Amputation (LEA) with Initiation of SGLT2i compared to Initiation of Other Second-Line Glucose Lowering Drugs, Sensitivity and Subgroup Analysesb.
Abbreviations: SGLT2i, sodium-glucose cotransporter-2 inhibitors; DPP4i, dipeptidyl peptidase-4 inhibitors; SU, sulfonylureas; IQR, interquartile range; SMR, standardized mortality ratio; HR, hazard ratio; AT, as-treated; ITT, intention-to-treat; Hx, history
a. Weighted by standardizing the comparator drug initiators to the population of SGLT2i initiators, using the propensity score odds (PS/(1-PS)), to estimate the treatment effect in the treated (ATT).
b. Primary analyses performed with both induction and latency periods = 0
c. Holding induction period constant (=0)
d. Holding latency period constant (=0)
e. Subgroup analyses adjusted for age, sex, baseline neuropathy, baseline peripheral vascular disease, baseline congestive heart failure, baseline chronic kidney disease, baseline ischemic heart disease, and baseline metformin use.
Subgroup analysis results suggested possible differences in LEA risk by baseline metformin and insulin use, and prior history of amputation, CVD, and CKD (Figure 2). In both DPP4i and SU comparisons, we observed higher HR estimates among patients with history of amputation, with baseline insulin use, and with history of CKD. Baseline metformin use and history of CVD were associated with higher HR estimates in the SU comparison, but lower HR estimates in the DPP4i comparison. We did not observe evidence of modification by baseline SU use.
DISCUSSION:
In this large, ACNU cohort study among commercially-insured U.S. patients, we observed a statistically significant elevation in LEA risk among SGLT2i initiators vs. DPP4i initiators (aHR 1.69, 95% CI 1.20–2.38), but not vs. SU initiators (aHR 1.02, 95% 0.67–1.55). Primary analysis results remained consistent across a number of sensitivity analyses. We observed modest to no increases in LEA risk for the secondary outcomes of debridement, peripheral vascular disease, and diabetic foot ulcer. Finally, risk appeared elevated in patients with prior history of amputation and CKD, and baseline insulin use, though these estimates were not statistically significant.
Results from this analysis join a growing body of evidence surrounding the risk of LEA associated with use of SGLT2i (Table 3). Recent ITT analyses conducted in the MarketScan CCAE population by Yuan et al (aHR 0.98, 95% CI 0.68–1.41) and Ryan et al (aHR 1.01, 95% CI 0.80–1.28) reported null associations of below-knee amputation risk among new users of canagliflozin vs. new users of all non-SGLT2i GLDs.11,12 Using as-treated analysis, Dawwas et al16 obtained a similar result vs. both DPP4i (aHR 0.88, 95% CI 0.65–1.15) and SU (aHR 0.74, 95% CI 0.57–0.96) users, and Ryan et al12 reported a protective effect of SGLT2i vs. all non-SGLT2i GLDs (aHR 0.56, 95% CI 0.32–0.92).
Table 3.
Study Design Comparison of Published Literature Assessing the Association between SGLT2i Use and Amputation Risk
| Author/Year (Database) |
Exclusions: Prior insulin, prior amputation, baseline CKD/ESRD, baseline CVD |
Mean Age (years) | Index Drug | Comparator Drug | Exposure Definition | Amputation Site (includes above-knee?) | Confounding Control | Analytic Approach | HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
|
Neal 2017 (CANVAS) |
None | 63.3 | CANA | Placebo | --- | Yes | Randomization | ITT | 1.97 (1.41–2.75) |
| None | 63.3 | CANA | Placebo | --- | No | Randomization | ITT | 1.94 (1.31–2.88) | |
| Amputation | 63.3 | CANA | Placebo | --- | Yes | Randomization | ITT | 1.88 (1.27–2.78) | |
| CVD | 63.3 | CANA | Placebo | --- | Yes | Randomization | ITT | 2.34 (1.53–3.58) | |
|
Yuan 2017 (MarketScan CCAE) |
Amputation | 53.3 | SGLT2i | All non-SGLT2i AHAb | 1+ Rx | No | PS matching | ITT | 0.98 (0.68–1.41) |
|
Ryan 2018 (MarketScan CCAE)a |
None | 55 | CANA | All non-SGLT2i AHA | 1+ Rx | No | PS matching | AT | 0.56 (0.32–0.92) |
| None | 55 | CANA | All non-SGLT2i AHA | 1+ Rx | No | PS matching | ITT | 1.01 (0.80–1.28) | |
| Amputation | 55 | CANA | All non-SGLT2i AHA | 1+ Rx | No | PS matching | AT | 0.47 (0.25–0.83) | |
| Amputation | 55 | CANA | All non-SGLT2i AHA | 1+ Rx | No | PS matching | ITT | 0.99 (0.77–1.27) | |
| Insulin | 55 | CANA | All non-SGLT2i AHA | 1+ Rx | No | PS matching | AT | 1.10 (0.38–2.51) | |
| Insulin | 55 | CANA | All non-SGLT2i AHA | 1+ Rx | No | PS matching | ITT | 1.00 (0.59–1.60) | |
| Insulin, Amputation | 55 | CANA | All non-SGLT2i AHA | 1+ Rx | No | PS matching | AT | 0.87 (0.26–2.15) | |
| Insulin, Amputation | 55 | CANA | All non-SGLT2i AHA | 1+ Rx | No | PS matching | ITT | 1.00 (0.58–1.63) | |
| None | 55 | CANA | Select non-SGLT2i AHAc | 1+ Rx | No | PS matching | AT | 1.08 (0.65–1.75) | |
| None | 55 | CANA | Select non-SGLT2i AHA | 1+ Rx | No | PS matching | ITT | 0.96 (0.77–1.21) | |
| Amputation | 55 | CANA | Select non-SGLT2i AHA | 1+ Rx | No | PS matching | AT | 0.85 (0.47–1.46) | |
| Amputation | 55 | CANA | Select non-SGLT2i AHA | 1+ Rx | No | PS matching | ITT | 0.93 (0.72–1.18) | |
| Insulin | 55 | CANA | Select non-SGLT2i AHA | 1+ Rx | No | PS matching | AT | 1.12 (0.57–2.04) | |
| Insulin | 55 | CANA | Select non-SGLT2i AHA | 1+ Rx | No | PS matching | ITT | 1.20 (0.91–1.57) | |
| Insulin, Amputation | 55 | CANA | Select non-SGLT2i AHA | 1+ Rx | No | PS matching | AT | 0.82 (0.36–1.68) | |
| Insulin, Amputation | 55 | CANA | Select non-SGLT2i AHA | 1+ Rx | No | PS matching | ITT | 1.07 (0.79–1.42) | |
|
Chang 2018 (MarketScan CCAE) |
Insulin, Amputation | 54.2 | SGLT2i | DPP4i | 1+ Rx | Yes | SMR weighting | AT | 1.50 (0.85–2.67) |
| Insulin | 54.2 | SGLT2i | DPP4i | 1+ Rx | Yes | SMR weighting | AT | 1.73 (1.01–2.98) | |
| Insulin, Amputation | 52.7 | SGLT2i | GLP-1 receptor agonist | 1+ Rx | Yes | SMR weighting | AT | 1.47 (0.64–3.36) | |
| Insulin | 52.7 | SGLT2i | GLP-1 receptor agonist | 1+ Rx | Yes | SMR weighting | AT | 1.53 (0.70–3.34) | |
| Insulin, Amputation | 51.5 | SGLT2i | SU/metformin/TZD | 1+ Rx | Yes | SMR weighting | AT | 2.12 (1.19–3.77) | |
| Insulin | 51.5 | SGLT2i | SU/metformin/TZD | 1+ Rx | Yes | SMR weighting | AT | 2.19 (1.27–3.77) | |
|
Adimadhyam 2018 (MarketScan CCAE) |
Insulin, CKD | 54.7 | SGLT2i | DPP4i | 1+ Rx | Yes | PS matching | AT | 1.38 (0.83–2.31) |
| Insulin, CKD, Amputation | 54.7 | SGLT2i | DPP4i | 1+ Rx | Yes | PS matching | AT | 1.04 (0.63–1.73) | |
| Insulin, CKD, CVD | 54.7 | SGLT2i | DPP4i | 1+ Rx | Yes | PS matching | AT | 1.50 (0.76–2.97) | |
| Insulin, CKD | 54.7 | CANA | DPP4i | 1+ Rx | Yes | PS matching | AT | 1.15 (0.64–2.07) | |
| Insulin, CKD | 54.7 | SGLT2i | DPP4i | 1+ Rx | No | PS matching | AT | 1.38 (0.79–2.42) | |
|
Dawwas 2018 (MarketScan CCAE) |
Amputation, ESRD | 54.5 | SGLT2i | DPP4i | 1+ Rx | No | PS matching | AT | 0.88 (0.65–1.15) |
| Amputation, ESRD | 54 | SGLT2i | SU | 1+ Rx | No | PS matching | AT | 0.74 (0.57–0.96) | |
|
Our Study (MarketScan CCAE) |
None | 52.5 | SGLT2i | DPP4i | 2+ Rx | No | SMR weighting | AT | 1.69 (1.20–2.38) |
| None | 52.5 | SGLT2i | DPP4i | 2+ Rx | No | SMR weighting | ITT | 1.43 (1.01–2.03) | |
| None | 52.5 | SGLT2i | SU | 2+ Rx | No | SMR weighting | AT | 1.02 (0.67–1.55) | |
| None | 52.5 | SGLT2i | SU | 2+ Rx | No | SMR weighting | ITT | 1.02 (0.69–1.50) | |
| None | 52.5 | SGLT2i | All non-SGLT2i AHA | 2+ Rx | No | SMR weighting | AT | 1.02 (0.54–1.93) | |
| None | 52.5 | SGLT2i | All non-SGLT2i AHA | 2+ Rx | No | SMR weighting | ITT | 1.06 (0.60–1.86) | |
| None | 52.5 | CANA | DPP4i | 2+ Rx | No | SMR weighting | AT | 1.77 (1.22–2.57) | |
| None | 52.5 | CANA | SU | 2+ Rx | No | SMR weighting | AT | 1.18 (0.77–1.83) | |
| None | 52.5 | CANA | All non-SGLT2i AHA | 2+ Rx | No | SMR weighting | AT | 0.85 (0.38–1.91) | |
| None | 52.5 | SGLT2i | DPP4i | 1+ Rx | No | SMR weighting | AT | 1.29 (0.95–1.77) | |
| None | 52.5 | SGLT2i | SU | 1+ Rx | No | SMR weighting | AT | 0.84 (0.59–1.20) | |
| None | 52.5 | SGLT2i | All non-SGLT2i AHA | 1+ Rx | No | SMR weighting | AT | 0.86 (0.49–1.50) | |
| Insulin | 52.5 | SGLT2i | DPP4i | 2+ Rx | No | SMR weighting | AT | 1.54 (1.06–2.23) | |
| Insulin | 52.5 | SGLT2i | SU | 2+ Rx | No | SMR weighting | AT | 0.85 (0.55–1.32) | |
| Amputation | 52.5 | SGLT2i | DPP4i | 2+ Rx | No | SMR weighting | AT | 1.54 (1.06–2.23) | |
| Amputation | 52.5 | SGLT2i | SU | 2+ Rx | No | SMR weighting | AT | 0.84 (0.54–1.31) | |
| CKD | 52.5 | SGLT2i | DPP4i | 2+ Rx | No | SMR weighting | AT | 1.37 (0.92–2.05) | |
| CKD | 52.5 | SGLT2i | SU | 2+ Rx | No | SMR weighting | AT | 0.69 (0.43–1.11) | |
| CVD | 52.5 | SGLT2i | DPP4i | 2+ Rx | No | SMR weighting | AT | 1.96 (1.23–3.12) | |
| CVD | 52.5 | SGLT2i | SU | 2+ Rx | No | SMR weighting | AT | 0.81 (0.46–1.43) | |
|
Udell 2018 (US DoD Health System) |
Amputation | 65.8 | SGLT2i | All non-SGLT2i AHA | 1+ Rx | No | PS matching | AT | 2.01 (0.89–4.53) |
| Amputation | 65.8 | SGLT2i | All non-SGLT2i AHA | 1+ Rx | No | PS matching | ITT | 1.99 (1.12–3.51) | |
|
Ueda 2018 (Swedish/Danish National Register) |
ESRD | 61 | SGLT2i | GLP-1 receptor agonist | 1+ Rx | No | PS matching | AT | 2.32 (1.37–3.91) |
| ESRD | 61 | SGLT2i | GLP-1 receptor agonist | 1+ Rx | No | PS matching | ITT | 1.90 (1.25–2.87) | |
| ESRD, CVD | 61 | SGLT2i | GLP-1 receptor agonist | 1+ Rx | No | PS matching | AT | 2.12 (0.96–4.69) | |
| ESRD, Amputation, | 61 | SGLT2i | GLP-1 receptor agonist | 1+ Rx | No | PS matching | AT | 2.48 (1.14–5.40) |
Abbreviations: CCAE, Commercial Claims and Encounters; DoD, Department of Defense; CKD, chronic kidney disease; ESRD, end-stage renal disease; CVD, cardiovascular disease; SGLT2i, sodium-glucose cotransporter-2 inhibitor; CANA, canagliflozin; DPP4i, dipeptidyl peptidase-4 inhibitor; GLP, glucagon-like peptide; SU, sulfonylurea; TZD, thiazolidinedione; AHA, anti-hyperglycemic agents; Rx, prescription; PS, propensity score; SMR, standardized mortality ratio; ITT, intention-to-treat; AT, as-treated; CI, confidence interval
Only MarketScan CCAE results shown for Ryan et al. Other results, not shown, were reported for MarketScan Medicare Supplement, MarketScan Medicaid Supplement, and Optum, and are made available by authors at http://data.ohdsi.org/AhasHfBkleAmputation/
All non-SGLT2i AHA: DPP4i, GLP-1 RA, TZD, SU, insulin, other AHA (acarbose, bromocriptine, miglitol, nateglinide, repaglinide)
Select non-SGLT2i AHA: DPP4i, GLP-1 RA, other AHA
On the other hand, also using MarketScan CCAE, Chang et al (aHR 1.50, 95% CI 0.85–2.67)13 and Adimadhyam et al (aHR 1.38, 95% CI 0.83–2.31)14 have suggested the possibility of increased LEA risk associated with new use of SGLT2i agents, vs. new use of DPP4i. However, low event counts and more rigid cohort exclusions in both studies contributed to limited precision and CIs that contained the null. Finally, studies by Udell et al15 (aHR 2.01, 95% CI 0.89–4.53) and Ueda37 et al (aHR 2.32, 95% CI 1.37–3.91) obtained similar results as the CANVAS program in different populations, though older mean age of patients in all three studies could be an underlying contributor.
Our present study, which was planned and pre-specified in 2017 prior to publication of other pharmacoepidemiological analyses, observed a similarly elevated risk of LEA among SGLT2i initiators vs. DPP4i comparator, and similarly null result vs. initiators of all non-metformin, non-SGLT2i GLDs (aHR 1.02, 95% CI 0.54–1.93). However, results from the SU and non-SGLT2i comparisons were both substantively different from the results obtained by Chang et al, who compared against a suite of older GLDs including metformin, SU, and thiazolidinediones (aHR 2.12, 95% CI 1.19–3.77).13 We attribute these differences to our simultaneous inclusion of insulin (typically a marker of more severe diabetes and higher amputation risk) and exclusion of metformin (often prescribed to patients with less severe diabetes and lower amputation risk) in the non-SGLT2i comparator group, which mirrors a commonly used comparator in previous studies.11,12,15
Because the potential mechanisms behind SGLT2i and amputation risk remain undetermined – current hypotheses relate to volume depletion and reduced tissue perfusion11 – we assert that heterogeneity of findings results primarily from differences in study design decisions. We demonstrate that inference regarding comparative LEA risk changes meaningfully when different active comparators (DPP4i vs. SU vs. non-metformin, non-SGLT2i GLDs) are employed. We highlight the important differences between patients prescribed these drugs in practice, which is underscored by the crude baseline differences observed between SGLT2i and SU initiators (Table 1), and between SGLT2i and non-metformin, non-SGLT2i initiators (Appendix Table 7). Sulfonylureas, for example, while often categorized as a second-line GLD in current diabetes pharmacotherapy guidelines, may not be an ideal analytic alternative to SGLT2i because they are less costly and may be more frequently prescribed to patients with lower socioeconomic status and health care system access.38–40 Furthermore, often in restrictive, protocol-driven health systems, approaches are adopted that spare the use of more recent diabetes therapies such as SGLT2i until after SU has failed. This has previously been demonstrated in the Medicare population, where SU is often used as a first-line therapy as an alternative to metformin.41,42 Crude imbalances between SGLT2i and non-metformin, non-SGLT2i initiators were even more severe (Appendix Table 7). In particular, baseline CKD imbalances suggest the possibility of channeling by contraindication. Although CKD was balanced after SMR weighting, this crude imbalance suggests that “catch-all” comparator groups that combine different-line diabetes therapies are not recommended for comparative effectiveness and safety studies. Proper selection of appropriate clinical alternatives can enhance the ability of comparative safety studies to inform clinical decision-making around prescribing of similar-line medications to treat T2DM.
Other study design decisions have also contributed to the heterogeneous results of studies conducted in the MarketScan CCAE (Table 3). First, our study, which is the first on this topic to define diabetes drug exposure using at least 2 prescriptions, demonstrates that analyses defining exposure with only one prescription claim tend to yield reduced HR estimates. Second, we demonstrate that canagliflozin-only analyses yielded higher HR estimates vs. DPP4i and SU initiators, but lower HR estimates vs. non-metformin, non-SGLT2i initiators. This last result is more consistent with the canagliflozin-specific HR estimates observed by Yuan et al and Ryan et al. Third, analyses using ITT follow-up typically yielded HR estimates closer to the null than those using as-treated approaches, which may contribute to observed differences between Yuan et al and Ryan et al, vs. Chang et al, Adimadhyam et al, and our study. Fourth, our subgroup analyses demonstrated that excluding patients with prior amputation and prior CKD reduced HR estimates, whereas excluding patients with baseline insulin use increased HR estimates.
Finally, differences in propensity-score methods and confounder sets can impact the extent to which confounding by indication and diabetes severity is controlled. Kaiser et al43 have previously demonstrated that potential for residual confounding can vary substantially depending on the outcome under study. Given that studies typically define a single set of confounders, one implication is that confounding control may be more complete in studies that apply these confounders to assess amputation risk alone,11,13,14 versus those that use the same confounders to assess both beneficial and adverse outcomes5,15,16 or to assess multiple adverse outcomes with different mechanisms.37 In our study, we aimed to minimize residual confounding by including confounders specific to amputation risk, and pre-specified secondary outcomes that we believe share a similar mechanism to amputation risk.
Ultimately, authors should justify study design choices to reflect the specific clinical question(s) under study, and provide transparency when communicating data for clinical and regulatory decision-making. Our study aimed to address the question, “would LEA incidence have changed had all initiators of SGLT2i instead initiated a similar, second-line diabetes drug?” To reflect this aim, we pre-specified our primary analysis to 1) focus on new users; 2) compare to only similar second-line therapies (DPP4i and SU, rather than all non-SGLT2i GLDs); 3) require at least 2 prescription dispensing claims as an indicator of adherence; 4) focus on below-knee amputation for consistency with CANVAS findings; 5) employ as-treated follow-up; and 6) perform confounding control via SMR weighting to estimate the average treatment effect in the treated. We then performed a number of sensitivity and subgroup analyses to test the robustness of our primary analysis to different study design choices and specifications, and to quantify impacts of excluding and stratifying by prior comorbidity and GLD use histories. As analytic approaches continue to evolve around questions of LEA risk with SGLT2i use, our understanding of SGLT2i effectiveness and safety will continue to deepen.
This study had several strengths. First, the ACNU study design helps to address issues related to confounding by indication by comparing patients treated with similar second-line T2DM therapies, and provides additional control for other unmeasured confounders and for various time-related biases.17 Second, propensity-score weighting adequately controlled for remaining measured baseline confounding, as demonstrated in Table 1.We controlled for baseline use of other oral GLDs, including metformin and insulin, which were not controlled for in several previous studies.11,13 Third, by restricting to patients with at least two prescriptions in close proximity, we gain confidence that patients are actually taking the study drugs. The commonly used approach of defining exposure through a single prescription claim alone can include patients who are not actually on therapy, since prescription dispensing claims do not necessarily reflect whether patients actually take the study drugs after filling the prescription. In our analysis, over 30% of patients with one study drug prescription did not fill a second prescription within the allotted prescription window (Appendix Figure 1, Appendix Tables 5 and 6). These patients, who contributed 26% of follow-up time in time-to-event analyses, may represent misclassified “exposed” time. Our results indicate that requiring the second prescription, which has not previously been implemented in similar studies, reduces bias towards the null.44
Finally, we were able to leverage the size of the MarketScan CCAE database to assess subgroup differences by a number of baseline characteristics. Multiple studies have assessed possible differences in amputation risk by CVD status, but no prior observational studies have, to our knowledge, assessed differences in HR by prior amputation history and baseline metformin use. Although differences in subgroup analyses were modest and precision of estimates was limited due to low event counts, these analyses can nevertheless offer important insight to aid physician decision-making when weighing clinical alternatives among patients with specific comorbidity and diabetes medication use histories.
Our study also exhibited limitations characteristic of observational studies using administrative data. First, although the MarketScan CCAE database contains data for patients aged ≥65, data for these patients are inconsistently reported in MarketScan due to the priority of Medicare coverage among such patients. To account for these potentially missing claims data, we limited our analysis to employer-insured patients aged <65, for whom we are confident data are relatively complete. Thus, our findings may not generalize to older or unemployed populations, which may have different amputation risk. Second, although we restricted to patients aged ≥18 as a proxy for patients with T2DM, patients with Type I diabetes mellitus (T1DM) may also be present in the study cohort. However, the treatment patterns assessed in this study are more indicative of T2DM, since oral GLDs are uncommonly prescribed in T1DM.45 Third, the MarketScan CCAE database does not report patient vital status, which precluded modeling of death as a competing risk. However, we did not expect death to be a major competing risk given the short (<1 year) follow-up in our cohort and relatively low mortality expected among the younger, privately-insured population. Fourth, because canagliflozin has dominated SGLT2i use in the US thus far, we did not have sufficient power to study dapagliflozin and empagliflozin individually, and were unable to determine to what extent the class effect observed in this study extends to dapagliflozin and empagliflozin.46 However, use of dapagliflozin and empagliflozin continues to grow, and evidence should emerge in coming years. Finally, we acknowledge the possibility of unmeasured confounding due to the observational nature of this study and the lack of data on race/ethnicity and socioeconomic status. However, we were able to provide partial control through claims-based definitions of dyslipidemia, aDCSI, smoking cessation, and COPD, as proxies for patient body-mass index, diabetes severity and duration, and smoking status, respectively.
CONCLUSIONS:
This cohort study adds to, and contextualizes, existing evidence by demonstrating that initiation of SGLT2i may be associated with an increased LEA risk vs. initiation of DPP4i, but not SU. We provide evidence that risk may be greater in patients with history of LEA, history of CKD, or baseline insulin use. Study design decisions, particularly the choice of active comparator drug, can meaningfully impact HR estimates and downstream conclusions about the comparative safety of SGLT2i and its clinical alternatives. As data continue to accrue around SGLT2i prescribing in real-world patient populations, analytic approaches must continue to evolve to appropriately address questions that deepen our clinical understanding of benefits and risks with SGLT2i use.
Supplementary Material
Acknowledgments
Grant Support:
This research was supported, in part, by grants from the National Institutes of Health R01 AG056479, T32 DK007634, UL1TR002489, and by the University of North Carolina Royster Society of Fellows.
Disclosures:
Yang JY: No conflicts to disclose
Wang T: No conflicts to disclose
Gower EW: No conflicts to disclose
Pate V: VP receives salary support from R01 AG056479 (National Institute on Aging), R01 HL118255 (National Institutes of Health, NIH), and the National Center for Advancing Translational Sciences (NCATS, UL1TR002489), NIH.
Crowley MJ: MJC is supported by a Career Development Award from Veterans Affairs Health Services Research and Development (CDA 13–261) and by the Center of Innovation to Accelerate Discovery and Practice Transformation (CIN 13–410).
Buse JB: JBB’s contracted consulting fees are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Eli Lilly, MannKind, NovaTarg, Novo Nordisk, Senseonics, vTv Therapeutics, and Zafgen; grant support from Novo Nordisk, Sanofi, and vTv Therapeutics. He is a consultant to Neurimmune AG. He holds stock options in Mellitus Health, PhaseBio and Stability Health. He is supported by a grant from the National Institutes of Health (UL1TR002489).
Stürmer T: TS receives investigator-initiated research funding and support as Principal Investigator (R01 AG056479) from the National Institute on Aging (NIA), and as Co-Investigator (R01 CA174453; R01 HL118255, R21-HD080214), National Institutes of Health (NIH). He also receives salary support as Director of Comparative Effectiveness Research (CER), NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR002489), the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck, Shire), and from pharmaceutical companies (Amgen, AstraZeneca, Novo Nordisk) to the Department of Epidemiology, University of North Carolina at Chapel Hill. Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk.
The database infrastructure used for this project was funded by the Department of Epidemiology, UNC Gillings School of Global Public Health; the Cecil G. Sheps Center for Health Services Research, UNC; the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1TR002489); and the UNC School of Medicine.
Footnotes
Source of the Data: IBM® Watson Health;
REFERENCES:
- 1.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8(8):495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 2.Fujita Y, Inagaki N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes : Clinical data and mechanism of action. J Diabetes Investig. 2014;5(3):265–275. doi: 10.1111/jdi.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsenic O Glucose Control by the Kidney: An Emerging Target in Diabetes. Am J Kidney Dis. 2009;53(5):875–883. doi: 10.1053/j.ajkd.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 5.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 6.Kosiborod M, Cavender MA, Fu AZ, et al. Lower Risk of Heart Failure and Death in Patients Initiated on SGLT-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study. Circulation. 2017;136(3):249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2018:NEJMoa1812389. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Diabetes at a Glance 2016. OECD Indic. 2016;(September):1–14. doi: 10.1787/eag-2013-en. [DOI] [Google Scholar]
- 9.Administration UF and D. FDA Drug Safety Communication: Interim clinical trial results find increased risk of leg and foot amputations, mostly affecting the toes, with the diabetes medicine canagliflozin (Invokana, Invokamet); FDA to investigate. https://www.fda.gov/Drugs/DrugSafety/ucm500965.htm. Accessed October 1, 2018.
- 10.FDA. FDA Confirms Increased Risk of Leg and Foot Amputations with the Diabetes Medicine Canagliflozin (Invokana, Invokamet, Invokamet XR). Vol 2; 2017. [Google Scholar]
- 11.Yuan Z, Defalco FJ, Ryan PB, et al. Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium-glucose co-transporter-2 inhibitors in the USA : A retrospective cohort study. Diabetes, Obes Metab. 2018;20(3):582–589. doi: 10.1111/dom.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan PB, Buse JB, Schuemie MJ, et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: A real-world meta-analysis of 4 observational databases (OBSER. Diabetes, Obes Metab. 2018;20(11):2585–2597. doi: 10.1111/dom.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang H-Y, Singh S, Mansour O, Baksh S, Alexander GC. Association Between Sodium-Glucose Cotransporter 2 Inhibitors and Lower Extremity Amputation Among Patients With Type 2 Diabetes. JAMA Intern Med. 2018;178(9):1190–1198. doi: 10.1001/jamainternmed.2018.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adimadhyam S, Lee TA, Calip GS, Marsh DES, Layden BT, Schumock GT. Risk of amputations associated with SGLT2 inhibitors compared to DPP-4 inhibitors: A propensity-matched cohort study. Diabetes, Obes Metab. 2018;20(12):2792–2799. doi: 10.1111/dom.13459. [DOI] [PubMed] [Google Scholar]
- 15.Udell JA, Yuan Z, Rush T, Sicignano NM, Galitz M, Rosenthal N. Cardiovascular Outcomes and Risks After Initiation of a Sodium Glucose Cotransporter 2 Inhibitor Results From the EASEL Population-Based Cohort Study (Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World). Circulation. 2018;137(14):1450–1459. doi: 10.1161/CIRCULATIONAHA.117.031227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawwas GK, Smith SM, Park H. Cardiovascular outcomes of sodium glucose cotransporter-2 inhibitors in patients with type 2 diabetes. Diabetes, Obes Metab. 2018:0–3. doi: 10.1111/dom.13477. [DOI] [PubMed] [Google Scholar]
- 17.Lund JL, Richardson DB, Sturmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–228. doi: 10.1007/s40471-015-0053-5.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quint J Health Research Data for the Real World: The MarketScan Databases Truven Health Analytics, Inc., Ann Arbor, MI. [Google Scholar]
- 19.Hansen L, Chang S. 2011. Health research data for the real world: the MarketScan Databases. Available at: http://truvenhealth.com/portals/0/assets/PH_11238_0612_TEMP_MarketScan_WP_FINAL.pdf.
- 20.Agarwal S, Pitcavage JM, Sud K, Thakkar B. Burden of Readmissions Among Patients With Critical Limb Ischemia. J Am Coll Cardiol. 2017;69(15):1897–1908. doi: 10.1016/j.jacc.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 21.Brennan MB, Allen GO, Ferguson PD, McBride JA, Crnich CJ, Smith MA. The Association Between Geographic Density of Infectious Disease Physicians and Limb Preservation in Patients With Diabetic Foot Ulcers. Open Forum Infect Dis. 2017;4(1):1–8. doi: 10.1093/ofid/ofx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turley RS, Mi X, Qualls LG, et al. The Effect of Clinical Care Location on Clinical Outcomes After Peripheral Vascular Intervention in Medicare Beneficiaries. JACC Cardiovasc Interv. 2017;10(11):1161–1171. doi: 10.1016/j.jcin.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Eggers PW, Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999;56(4):1524–1533. doi: 10.1046/j.1523-1755.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg JB, Goodney PP, Cronenwett JL, Baker F. The effect of risk and race on lower extremity amputations among Medicare diabetic patients. J Vasc Surg. 2012;56(6):1663–1668. doi: 10.1016/j.jvs.2012.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinson M, Martinson N. A comparative analysis of skin substitutes used in the management of diabetic foot ulcers. J Wound Care. 2016;25(Sup10):S8–S17. doi: 10.12968/jowc.2016.25.Sup10.S8. [DOI] [PubMed] [Google Scholar]
- 26.McEwen LN, Ylitalo KR, Munson M, Herman WH, Wrobel JS. Foot Complications and Mortality: Results from Translating Research Into Action for Diabetes (TRIAD). J Am Podiatr Med Assoc. 2016;106(1):7–14. doi: 10.7547/14-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medhekar AN, Mix DS, Aquina CT, et al. Outcomes for critical limb ischemia are driven by lower extremity revascularization volume, not distance to hospital. J Vasc Surg. 2017;66(2):476–487. doi: 10.1016/j.jvs.2017.01.062. [DOI] [PubMed] [Google Scholar]
- 28.Newhall K, Spangler E, Dzebisashvili N, Goodman DC, Goodney P. Amputation rates for patients with diabetes and peripheral arterial disease: the effects of race and region. Ann Vasc Surg. 2016;30:292–298. doi: 10.1007/s00401-015-1526-9.Pathological. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newhall K, Stone D, Svoboda R, Goodney P. Possible consequences of regionally based bundled payments for diabetic amputations for safety net hospitals in Texas. J Vasc Surg. 2016;64(6):1756–1762. doi: 10.1016/j.jvs.2016.06.098. [DOI] [PubMed] [Google Scholar]
- 30.Sargen MR, Hoffstad O, Margolis DJ. Geographic variation in Medicare spending and mortality for diabetic patients with foot ulcers and amputations. J Diabetes Complications. 2013;27(2):128–133. doi: 10.1016/j.jdiacomp.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young BA, Lin E, Von Korff M, et al. Diabetes Complications Severity Index and Risk of Mortality, Hospitalization, and Healthcare Utilization. Am J Manag Care. 2008;14(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- 32.Chang H, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the Adapted Diabetes Complications Severity Index in Claims Data. Am J Manag Care. 2012;18(11):721–726. [PubMed] [Google Scholar]
- 33.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 34.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and monte carlo simulations. Biometrical J. 2009;51(1):171–184. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 35.Stürmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: Dealing with observations in the tails of the propensity score distribution-A simulation study. Am J Epidemiol. 2010;172(7):843–854. doi: 10.1093/aje/kwq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowley MJ, Williams JW Jr, Kosinski AS, D’Alessio DA, Buse JB. Metformin Use May Moderate the Effect of DPP-4 Inhibitors on Cardiovascular Outcomes. Diabetes Care. 2017;40(12):1787–1789. doi: 10.2337/dc17-1528. [DOI] [PubMed] [Google Scholar]
- 37.Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018;363:k4365. doi: 10.1136/bmj.k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chahal H Comparative Review of Oral Hypoglycemic Agents in Adults.
- 39.Zhang Y, Mccoy RG, Mason JE, Smith SA, Shah ND, Denton BT. Second-line Agents for Glycemic Control for Type 2 Diabetes : Are Newer Agents Better ? 2014;(1):1–8. doi: 10.2337/dc13-1901. [DOI] [PubMed] [Google Scholar]
- 40.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National Trends in Treatment of Type 2 Diabetes Mellitus, 1994– 2007. Arch Intern Med. 2008;168(19):2088–2094. doi: 10.1001/archinte.168.19.2088.National. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong J-L, Jonsson Funk M, Buse J, et al. Comparative effect of Initiating Metformin versus Sulfonylureas on Breast Cancer Risk in Older Women. Epidemiology. 2017;28(3):446–454. doi: 10.1097/EDE.0000000000000635.Comparative. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ko EM, Stürmer T, Hong JL, Castillo WC, Bae-Jump V, Funk MJ. Metformin and the risk of endometrial cancer: A population-based cohort study. Gynecol Oncol. 2015;136(2):341–347. doi: 10.1016/j.ygyno.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Kaiser P, Arnold AM, Benkeser D, et al. Comparing methods to address bias in observational data: Statin use and cardiovascular events in a US cohort. Int J Epidemiol. 2018;47(1):246–254. doi: 10.1093/ije/dyx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suissa S, Nystr T, Bodeg J, et al. Lower Risk of Death With SGLT2 Inhibitors in Observational Studies : Real. 2018;41(June):104–105. doi: 10.2337/dci18-0015. [DOI] [PubMed] [Google Scholar]
- 45.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, Maahs DM TW. Type 1 Diabetes Exchange Clinic Network. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 38(6):971–978. doi:doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 46.Khouri C, Cracowski J, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation : Is this a class effect ? Diabetes, Obes Metab. 2018;(January):1531–1534. doi: 10.1111/dom.13255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.