Abstract
Objective:
The contribution of depression to mortality in adults with and without HIV is unclear. We hypothesized that depression increases mortality risk and this association is stronger among those with HIV.
Methods:
Veterans Aging Cohort Study (VACS) data were analyzed from the first clinic visit on/after 4/1/2003 (baseline) to 9/30/2015. Depression definitions: 1) major depressive disorder by ICD-9 codes; 2) depressive symptoms by Patient Health Questionnaire (PHQ)-9 scores ≥ 10. Outcome: all-cause mortality. Covariates: demographics, comorbid conditions, health behaviors.
Results:
Among 129,140 eligible participants, 30% had HIV, 16% had major depressive disorder diagnosis, and 24% died over a median follow-up of 11 years. The death rate (95% confidence interval [CI]) was 25.3 [25.0–25.6] deaths per 1000 person-years. Major depressive disorder was associated with mortality: hazard ratio (HR) [95% CI] = 1.04 [1.01, 1.07]. This association was modified by HIV status (interaction p-value=0.02). In HIV-stratified analyses, depression was significantly associated with mortality among HIV uninfected veterans but not among those with HIV. Among those with PHQ-9 data (N=7372), 50% had HIV, 22% had PHQ-9 scores ≥ 10, and 28% died over a median follow-up of 12 years. The death rate was 27.3 (26.1–28.5) per 1000 person-years. Depressive symptoms were associated with mortality (1.16 [1.04, 1.28]). This association was modified by HIV status (interaction p-value=0.05). In HIV-stratified analyses, depressive symptoms were significantly associated with mortality among veterans with HIV but not among those without HIV.
Conclusion:
Depression was associated with all-cause mortality. This association was modified by HIV status and method of depression ascertainment.
Keywords: HIV, depression, mortality
Introduction
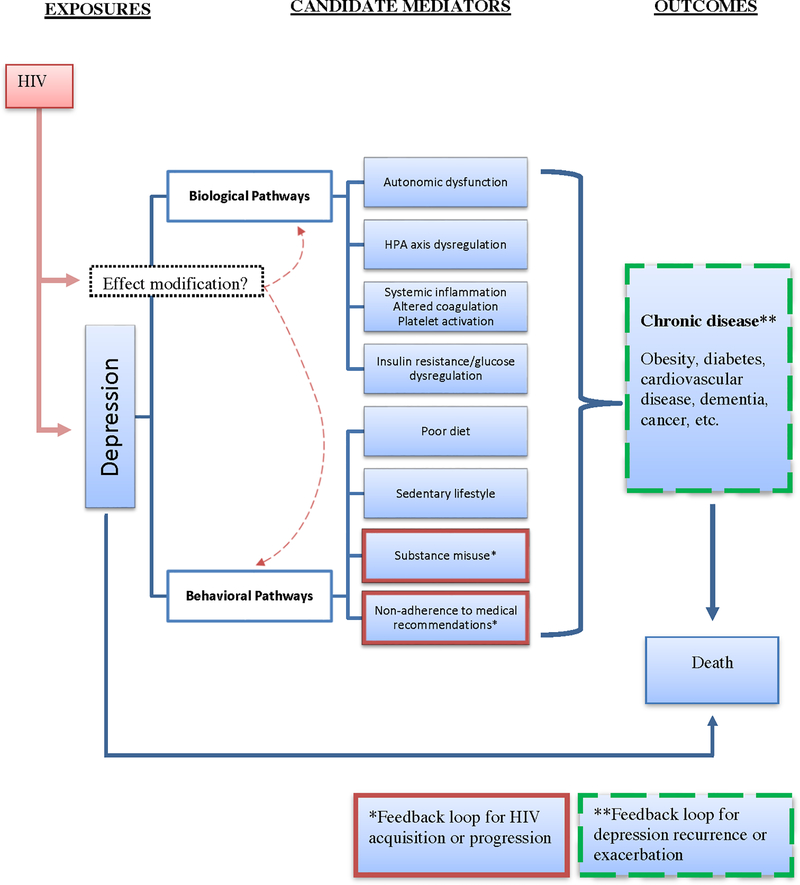
Depression is the most frequently reported mental health condition in people living with HIV in the United States (U.S.), with prevalence ranging from 20–40%.(1–5) Depressive disorders have been associated with increased mortality in persons with chronic diseases, including heart disease, end stage renal disease, and diabetes.(6–8) Several studies have described similar associations in the context of HIV infection, although conflicting data exist. Limitations in prior work include absence of a comparator group without HIV that would inform conclusions about whether HIV infection modifies the association between depression and mortality. If HIV infection strengthens the association between depression and mortality, this would suggest that among people with HIV, greater emphasis on depression screening, better synchronization of HIV and depression treatment, and more research into the mechanisms underlying the interaction of HIV and depression on mortality risk are warranted. Thus, it is important to understand the interplay among HIV infection, depression and all-cause mortality (Figure 1). Our objectives were to assess the independent association of depression and mortality and to determine whether and how HIV modifies this association.
Figure 1.
Conceptual model linking depression and mortality and highlighting a potential role for HIV as an effect modifier.
Methods
Study design and participants
We used the Veterans Aging Cohort Study (VACS) to assess the relationships between depression, HIV infection, and all-cause mortality. The VACS is a prospective, multisite, longitudinal cohort of HIV-infected and age, race/ethnicity, and geographic region matched uninfected adults in the U.S. Department of Veterans Affairs (VA) system.(9) In the VACS each HIV-infected veteran is matched to two uninfected veterans also in clinical care. The baseline date of participation was defined as the date of a participant’s first clinic visit on or after April 1, 2003.
A subset of VACS participants completed comprehensive annual health and behavior surveys. These surveys included the PHQ-9, a validated screening tool for major depressive disorder as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria.(10) For this subset of participants (PHQ-9 subset), an additional baseline was defined as the date on which they completed their baseline survey. This was to enable analyses using two different definitions for depression status i.e. diagnostic codes and formal depression screening.
All participants were followed from their baseline date until date of death, the last follow-up date, or the censoring date of February 11, 2016.
The University of Pittsburgh, Yale University, and West Haven VA Medical Center institutional review boards approved this study.
Outcome variable
The primary outcome variable was all-cause mortality, which was determined from the VA vital status file, the Social Security Administration death master file, the Beneficiary Identification and Records Locator Subsystem, and the Veterans Health Administration Medical Statistical Analysis Systems inpatient data sets.
Exposure variables
The primary exposure variable was depression. We defined depression at baseline as having at least one inpatient or two outpatient International Classification of Diseases, Ninth Revision [ICD-9] codes for major depressive disorder (296.2 or 296.3).(11, 12) Among those with PHQ-9 data available at baseline (PHQ-9 subset), we also defined clinically significant depressive symptoms as a PHQ-9 score ≥ 10.(13) PHQ-9 scores ≥ 10 indicate the presence of depressive symptoms with strong reliability and validity for diagnosing major depressive disorder.(14)
Effect modifier and covariates
HIV status was the primary effect modifier. HIV infection was defined as at least one inpatient or two outpatient ICD-9 codes for HIV in the VA Immunology Case Registry as previously described.(15)
HIV-specific data included in these analyses were CD4 cell count, HIV-1 RNA level, and antiretroviral therapy (ART) regimen obtained as part of clinical care at baseline (defined as 180 days prior to through 7 days after the baseline enrollment date). Baseline CD4 cell counts were categorized as ≥500/mm3, 200–499/mm3, or <200/mm3 and HIV-1 RNA levels as <500 or ≥500 copies/mL. Being on any ART regimen (yes/no) and the use of efavirenz (which was specifically included due to its potential neuropsychiatric effects and higher risk of suicidality(16)), were obtained through pharmacy data.
Covariates were obtained closest to baseline date as previously described.(17, 18) Sociodemographic variables of age, self-reported sex, and self-reported race/ethnicity were determined through administrative data. History of cardiovascular disease, chronic obstructive pulmonary disease, and cancer (either AIDS-defining or non-AIDS-defining) were assessed by ICD-9 codes as previously defined.(19–24) History of an AIDS-defining illness was also defined by ICD-9 codes (Supplementary Table 1). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were defined by averaging the three routine outpatient systolic and diastolic blood pressure measurements performed closest to the baseline date. Hypertension was categorized as no hypertension (blood pressure <140/90mmHg and no antihypertensive medication), controlled hypertension (<140/90 mmHg with antihypertensive medication), or uncontrolled hypertension (≥140/90 mmHg). GFR was estimated using the CKD-EPI equation(25) and was categorized as <60 or ≥60 mL/min/1.73m2. Hemoglobin levels were categorized as either <12g/dL or ≥12g/dL. Diabetes was identified using a previously validated metric that incorporates glucose measurements, antidiabetic agent use, and/or at least one inpatient or two outpatient ICD-9 codes for diabetes.(26) Hepatitis C virus seropositivity was defined as a positive hepatitis C virus antibody test result or at least one inpatient or two outpatient ICD-9 codes for this diagnosis.(27)
Smoking and body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) were assessed using the VA electronic medical record health factor data collected through standardized clinical reminders to VA clinicians.(28) Smoking was categorized as never, current, or past, and BMI was dichotomously categorized (<30 or ≥30 kg/m2). History of alcohol abuse or dependence and history of cocaine use were defined using ICD-9 codes.(29)
For analyses restricted to the PHQ-9 subset, smoking data were from self-report, BMI data were from laboratory results, alcohol use data were from both Alcohol Use Disorders Identification Test (AUDIT-C) and ICD-9 codes (28, 30). For analysis using AUDIT-C data, alcohol use was categorized as not current, not hazardous, hazardous, at-risk or heavy episodic, or alcohol abuse or dependence.
Baseline antidepressant use was assessed using VA pharmacy records documenting a filled prescription for the following selective serotonin reuptake inhibitors: citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline; and tricyclic antidepressants: amitriptyline, amitriptyline/perphenazine, clomipramine, desipramine, doxepin, imipramine, and nortriptyline. ICD-9 codes were used to define schizophrenia, bipolar disorder, and post-traumatic stress disorder (PTSD) (Supplementary Table 1).
Statistical Analysis
Baseline characteristics were stratified by depression status and presented as median (interquartile range) for continuous variables and frequencies with percentages for categorical variables.
Cox proportional hazards models were used to estimate the association of depression status with all-cause mortality. We applied restricted cubic splines on continuous predictors in order to allow a nonlinear relationship between the covariate and outcome. Interactions between depression and HIV status were assessed in each model. The proportional hazards assumption was tested by the Schoenfeld residuals and including the interaction term of the covariates by time as evaluated by the “cox.zph” function in R. We also constructed Kaplan-Meier curves showing survival distributions over follow-up time.
We constructed three Cox models to estimate the association of depressive disorder diagnoses or depression symptoms, defined using either ICD-9 codes or PHQ-9 scores respectively, and mortality. Model 1 was adjusted for HIV status. Model 2 adjusted for demographics (age, sex, race/ethnicity) and HIV status. Model 3 built upon Model 2 and additionally adjusted for co-morbid conditions that could confound the association between depression and mortality including prevalent cardiovascular disease, AIDS-defining illnesses, chronic obstructive pulmonary disease, cancer, diabetes, hypertension, hypercholesterolemia, renal disease, anemia and hepatitis C seropositivity. Model 3 was our primary model. Model 3B included an interaction term for depression and HIV status. Interactions were considered statistically significant at the p<0.10 threshold and HIV-stratified analyses were performed. HIV-stratified analyses were further adjusted for HIV-1 RNA, CD4+ T-cell count and antiretroviral therapy prescription among those with HIV.
We then performed several exploratory analyses to assess the effects of other potential confounding or mediating influences on the relationships between depression and all-cause mortality. First, we added behavioral factors including baseline body mass index (a surrogate of diet and physical activity), smoking status, alcohol use, and cocaine use to Model 3. Then, we adjusted Model 3 for other mental health diagnoses present at baseline, i.e., schizophrenia, bipolar disorder, and post-traumatic stress disorder. Lastly, we examined the role of antidepressant medication use by adjusting Model 3 for use of selective serotonin reuptake inhibitor or tricyclic antidepressant medications prescribed at baseline.
Missing data methods are described in the Online Supplement.
Results
Characteristics of the VACS participants
There were 129,140 VACS participants included in this study with a mean age of 50 years, >90% men, 50% black or African American, and median (IQR) duration of follow-up was 10.7 (6.3–12.7) years (Table 1). Of these participants, 16% had a diagnosis of major depressive disorder at baseline. Among those with major depressive disorder, 32% had HIV compared to an HIV prevalence of 30% among those without major depressive disorder. Those with major depressive disorder had a higher prevalence of cardiovascular disease, chronic obstructive pulmonary disease, hepatitis C, current smoking, alcohol or cocaine abuse/dependence diagnoses, and history of schizophrenia, bipolar disorder, or post-traumatic stress disorder (Table 1). These characteristics were similar in the PHQ-9 subset (median (IQR) follow-up time 12.2 (7.4–13.0) years) with the exception of prevalent cardiovascular disease, which had similar prevalence among those with PHQ-9< 10 compared to those with PHQ-9≥10.
Table 1.
Baseline Characteristics of the Veterans Aging Cohort Study (VACS)
| VACS (n=129140) | PHQ-9 Subset (n=7372) | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-depressed (n=108136) | Depressed (n=21004) | No depressive symptoms (n=5779) | Depressive symptoms (n=1593) | |||||
| HIV-negative (n=75646) | HIV-positive (n=32490) | HIV-negative (n=14280) | HIV-positive (n=6724) | HIV-negative (n=2932) | HIV-positive (n=2847) | HIV-negative (n=765) | HIV-positive (n=828) | |
| Baseline year | ||||||||
| 2003–2006 | 56523 (74.7) | 23456 (72.2) | 11600 (81.2) | 5501 (81.8) | 2530 (86.3) | 2470 (86.8) | 642 (83.9) | 700 (84.5) |
| 2007–2012 | 19123 (25.3) | 9034 (27.8) | 2680 (18.8) | 1223 (18.2) | 402 (13.7) | 377 (13.2) | 123 (16.1) | 128 (15.5) |
| Age, y | 50.6 (44.2–56.9) | 50.0 (43.3–56.4) | 50.22 (44.9–55.2) | 49.1 (43.6–54.6) | 51.1 (44.9–57.0) | 49.8 (44.1–55.4) | 49.9 (44.9–54.4) | 49.0 (44.1–54.2) |
| Male sex | 73805 (97.6) | 31742 (97.7) | 13659 (95.7) | 6419 (95.5) | 2718 (92.7) | 2772 (97.4) | 700 (91.5) | 805 (97.2) |
| Race/ethnicity | ||||||||
| White | 29292 (38.7) | 12409 (38.2) | 5903 (41.3) | 2886 (42.9) | 664 (22.6) | 530 (18.6) | 195 (25.5) | 186 (22.5) |
| Black or African-American | 36051 (47.7) | 15986 (49.2) | 6764 (47.4) | 3157 (47.0) | 1887 (64.4) | 1966 (69.1) | 445 (58.2) | 497 (60.0) |
| Hispanic | 5695 (7.5) | 2185 (6.7) | 1307 (9.2) | 524 (7.8) | 290 (9.9) | 244 (8.6) | 88 (11.5) | 103 (12.4) |
| Other | 4608 (6.1) | 1910 (5.9) | 306 (2.1) | 157 (2.3) | 91 (3.1) | 107 (3.8) | 37 (4.8) | 42 (5.1) |
| History of CVD | 12470 (16.5) | 3923 (12.1) | 2990 (20.9) | 1141 (17.0) | 503 (17.2) | 377 (13.2) | 152 (19.9) | 127 (15.3) |
| History of AIDS | 5976 (18.4) | 1648 (24.5) | 767 (26.9) | 281 (33.9) | ||||
| History of cancer | 1785 (2.4) | 1070 (3.3) | 349 (2.4) | 285 (4.3) | 103 (3.4) | 133 (4.7) | 25 (3.3) | 29 (3.5) |
| History of diabetes | 13510 (17.9) | 3918 (12.1) | 3061 (21.4) | 1013 (15.1) | 632 (21.6) | 435 (15.3) | 190 (24.8) | 144 (17.4) |
| History of COPD | 7867 (10.4) | 3136 (9.7) | 2557 (17.9) | 1215 (18.1) | 292 (10.0) | 294 (10.3) | 102 (13.3) | 137 (16.5) |
| SBP, mm Hg | 132.0 (123.0–142.0) | 128.7 (119.0–138.7) | 131.0 (121.7–141.3) | 128.3 (119.3–138.3) | 131.3 (121.3–141.7) | 128.0 (118.3–138.0) | 130.3 (120.7–140.7) | 127.3 (118.0–136.7) |
| DBP, mm Hg | 79.0 (73.0–85.7) | 77.7 (71.3–84.3) | 79.3 (73.0–85.7) | 78.0 (72.0–84.3) | 78.7 (72.7–85.0) | 77.7 (71.7–84.3) | 78.3 (72.3–84.0) | 77.7 (72.3–84.0) |
| Total cholesterol ≥200 mg/dL | 23383 (38.0) | 8226 (29.7) | 4811 (38.8) | 1801 (29.9) | 915 (33.6) | 770 (28.3) | 233 (33.1) | 181 (23.2) |
| eGFR <60 mL/min/1.73 m2 | 5455 (8.2) | 2736 (9.0) | 853 (6.4) | 459 (7.1) | 213 (7.5) | 213 (7.5) | 42 (5.6) | 47 (5.7) |
| Seropositive for Hepatitis C | 8309 (11.0) | 9243 (28.4) | 3009 (21.1) | 2701 (40.2) | 628 (21.4) | 1236 (43.4) | 263 (34.4) | 435 (52.5) |
| Hemoglobin <12 g/dL | 3043 (4.6) | 4274 (13.9) | 654 (4.9) | 795 (12.1) | 154 (5.5) | 374 (13.2) | 47 (6.3) | 130 (15.8) |
| BMI ≥30 kg/m2 | 28616 (40.6) | 4914 (15.6) | 5769 (41.3) | 1209 (18.2) | 1181 (40.6) | 404 (14.2) | 338 (44.3) | 132 (16.0) |
| Smoking | ||||||||
| Current | 24367 (48.0) | 11839 (54.5) | 6484 (59.4) | 3216 (64.3) | 1242 (43.0) | 1417 (50.0) | 438 (58.5) | 531 (64.3) |
| Former | 9663 (19.0) | 3573 (16.4) | 1807 (16.6) | 730 (14.6) | 821 (28.4) | 723 (25.5) | 151 (20.2) | 147 (17.8) |
| Never | 16731 (33.0) | 6321 (29.1) | 2625 (24.0) | 1059 (21.2) | 828 (28.6) | 693 (24.5) | 160 (21.4) | 148 (17.9) |
| Alcohol | ||||||||
| Not current | N/A | N/A | N/A | N/A | 1053 (36.0) | 1032 (36.4) | 306 (40.1) | 294 (35.6) |
| Not hazardous | N/A | N/A | N/A | N/A | 727 (24.8) | 767 (27.0) | 121 (15.8) | 161 (19.5) |
| Hazardous | N/A | N/A | N/A | N/A | 86 (2.9) | 60 (2.1) | 9 (1.2) | 11 (1.3) |
| At-risk or heavy episodic | N/A | N/A | N/A | N/A | 444 (15.2) | 431 (15.2) | 91 (11.9) | 106 (12.8) |
| Abuse/dependence | 16470 (21.8) | 6958 (21.4) | 7483 (52.4) | 3522 (52.4) | 616 (21.1) | 547 (19.3) | 237 (31.0) | 255 (30.8) |
| Cocaine use | 8691 (11.5) | 5116 (15.7) | 4689 (32.8) | 2731 (40.6) | 733 (25.0) | 761 (26.7) | 315 (41.2) | 330 (39.9) |
| History of schizophrenia | 5170 (6.8) | 1210 (3.7) | 2830 (19.8) | 986 (14.7) | 202 (6.9) | 176 (6.2) | 113 (14.8) | 89 (10.7) |
| History of bipolar disorder | 3121 (4.1) | 1163 (3.6) | 2974 (20.8) | 1182 (17.6) | 154 (5.3) | 150 (5.3) | 116 (15.2) | 106 (12.8) |
| History of post-traumatic stress disorder | 8093 (10.7) | 2058 (6.3) | 6019 (42.1) | 1949 (29.0) | 378 (12.9) | 273 (9.6) | 283 (37.0) | 183 (22.1) |
| Antidepressant medication use | ||||||||
| SSRI use | 15953 (21.1) | 7144 (22.0) | 10912 (76.4) | 5094 (75.8) | 729 (24.9) | 770 (27.0) | 470 (61.4) | 497 (60.0) |
| TCA use | 8877 (11.7) | 4316 (13.3) | 3852 (27.0) | 1952 (29.0) | 367 (12.5) | 527 (18.5) | 193 (25.2) | 238 (28.7) |
| HIV-1 RNA ≥500 copies/mL | N/A | 14822 (54.8) | N/A | 3239 (57.1) | N/A | 1287 (45.3) | N/A | 449 (54.4) |
| CD4 cell count/mm3 | N/A | N/A | N/A | N/A | ||||
| <200 | N/A | 6473 (24.7) | N/A | 1194 (21.6) | N/A | 596 (21.0) | N/A | 214 (26.0) |
| 211–499 | N/A | 11059 (42.1) | N/A | 2295 (41.6) | N/A | 1327 (46.8) | N/A | 370 (45.0) |
| ≥500 | N/A | 8724 (33.2) | N/A | 2031 (36.8) | N/A | 911 (32.1) | N/A | 238 (29.0) |
| Receiving ART regimen | N/A | 14308 (44.0) | N/A | 3345 (49.7) | N/A | 2304 (80.9) | N/A | 648 (78.3) |
| Receiving efavirenz | N/A | 5871 (18.1) | N/A | 1116 (16.6) | N/A | 699 (27.3) | N/A | 189 (25.1) |
Abbreviations: CVD, cardiovascular disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; COPD, chronic obstructive pulmonary disease; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; N/A, not applicable or not available.
Notes: All data presented as n (%) or median (IQR). In the VACS, the following characteristics include fewer than 129140 patients because of missing baseline data (n, %): BMI (6556, 5.1%), SBP (4698, 3.6%), DBP (4698, 3.6%), total cholesterol (21392, 16.6%), hypertension (4698, 3.6%), smoking status (40725, 31.5%), eGFR (11994, 9.3%), hemoglobin (12542, 9.7%), HIV-1 RNA (6496, 16.6% of HIV-positive), CD4 cell count (7438, 19.0% of HIV-positive), receiving efavirenz (11663, 29.4% of HIV-positive). In the PHQ-9 subset, the following characteristics include fewer than 7372 patients because of missing baseline data (n, %): BMI (36, 0.5%), SBP (12, 0.2%), DBP (12, 0.2%), total cholesterol (443, 6%), hypertension (12, 0.2%), smoking status (73, 1%), eGFR (107, 1.5%), hemoglobin (158, 2.1%), alcohol use (18, 0.2%), HIV-1 RNA (10, 0.3% of HIV-positive), CD4 (19, 0.5% of HIV-positive), receiving efavirenz (366, 9.9% of HIV-positive).
Unadjusted mortality rates
At the end of follow-up, almost 24% of the VACS cohort had died, giving a mortality rate of 25 deaths per 1000 person years. Among HIV uninfected people, mortality rates (95% confidence interval) were 20.2 (19.9–20.5) among those without major depressive disorder and 22.7 (21.0–23.5) among those with major depressive disorder. Among those with HIV, these rates were 36.9 (36.2–37.7) and 39.4 (37.8–41.0), respectively (Table 2). In the PHQ-9 subset, mortality rates were similar among uninfected people with PHQ-9 score <10 compared to their counterparts with PHQ-9 score ≥10. However, mortality rates were higher among those with HIV with PHQ-9≥10 (43.2 [38.6–48.1]) versus PHQ-9<10 (33.7 [31.6–36.0]) (Table 2).
Table 2.
All-cause Mortality Rates of the Veterans Aging Cohort Study (VACS) and PHQ-9 subset
| Characteristic | VACS (major depressive disorder defined by ICD-9 code) | PHQ-9 subset (depressive symptoms defined by PHQ-9) | ||||||
|---|---|---|---|---|---|---|---|---|
| HIV and depression status at baseline | Number at baseline | All-cause mortality over follow-up, n (%) | Total person-years of follow-up | Mortality rate/1000 person-years (95% CI) | Number at baseline | All-cause mortality over follow-up, n (%) | Total person-years of follow-up | Mortality rate/1000 person-years (95% CI) |
| HIV-negative without depression | 75646 | 14617 (19.3) | 723508 | 20.2 (19.9–20.5) | 2932 | 622 (21.2) | 31423 | 19.8 (18.3–21.4) |
| HIV-negative with depression | 14280 | 3211 (22.5) | 141288 | 22.7 (21.0–23.5) | 765 | 159 (20.8) | 8001 | 19.9 (17.0–23.2) |
| HIV-positive without depression | 32490 | 10145 (31.2) | 274695 | 36.9 (36.2–37.7) | 2847 | 932 (32.7) | 27625 | 33.7 (31.6–36.0) |
| HIV-positive with depression | 6724 | 2359 (35.1) | 59907 | 39.4 (37.8–41.0) | 828 | 320 (38.6) | 7412 | 43.2 (38.6–48.1) |
| Total | 129140 | 30332 (23.5) | 1199398 | 25.3 (25.0–25.6) | 7372 | 2033 (27.6) | 74461 | 27.3 (26.1–28.5) |
Associations between depression and mortality
A diagnosis of major depressive disorder was associated with a 9% increased risk of mortality adjusting for HIV status only (Table 3a). This association persisted after further adjustment for demographics and comorbid conditions that could confound the association between major depressive disorder and mortality (Table 3a). The hazard ratio (95% confidence interval) for major depressive disorder in the primary model (Model 3a) was 1.04 (1.01–1.07). We detected a significant interaction between major depressive disorder and HIV status (p=0.02). Thus, we stratified our analyses by HIV status and detected modestly increased mortality risk associated with major depressive disorder that reached statistical significance among HIV uninfected people (1.06 [1.02–1.11]) but not among people with HIV (1.04 [0.99–1.09]; Table 3b). Exploratory analyses adjusting for behavioral factors, mental health diagnoses, and depression treatment completely attenuated the modest associations observed in the primary analyses regardless of HIV status (Table 4a).
Table 3a:
Mortality risk by diagnosis of major depressive disorder (defined by ICD-9 code) in the Veterans Aging Cohort Study (VACS)
| Model 1 | Model 2 | Model 3* | ||||
|---|---|---|---|---|---|---|
| Hazard ratio [95% CI] | p-value | Hazard ratio [95% CI] | p-value | Hazard ratio [95% CI] | p-value | |
| No major depressive disorder | 1 (ref) | <0.01 | 1 (ref) | <0.01 | 1 (ref) | 0.02 |
| Major depressive disorder | 1.09 [1.06, 1.13] | 1.23 [1.20, 1.27] | 1.04 [1.01, 1.07] | |||
Model 3B includes an interaction term for major depressive disorder diagnosis and HIV status. The p-value for this interaction term was 0.02
Model 1 is adjusted for HIV status only. Model 2 additionally adjusted Model 1 for age, sex, and race. Model 3 additionally adjusted Model 2 for baseline cardiovascular disease, chronic obstructive pulmonary disease, cancer, diabetes, systolic blood pressure, diastolic blood pressure, total cholesterol, estimated glomerular filtration rate, hemoglobin, and hepatitis C seropositivity.
Table 3b:
HIV-stratified mortality risk by diagnosis of major depressive disorder (defined by ICD-9 code) in the Veterans Aging Cohort Study (VACS)
| Hazard ratio [95% CI] | |||
|---|---|---|---|
| HIV uninfected | HIV infected | ||
| Model 3 | No major depressive disorder | 1 (ref) | 1 (ref) |
| Major depressive disorder | 1.06 (1.02, 1.11) | 1.04 (0.99, 1.09) | |
| Model 4 | No major depressive disorder | 1 (ref) | 1 (ref) |
| Major depressive disorder | 0.98 (0.94, 1.02) | 0.99 (0.95, 1.04) | |
| Model 5 | No major depressive disorder | 1 (ref) | 1 (ref) |
| Major depressive disorder | 1.02 (0.98, 1.07) | 1.01 (0.96, 1.06) | |
| Model 6 | No major depressive disorder | 1 (ref) | 1 (ref) |
| Major depressive disorder | 1.01 (0.96, 1.05) | 0.97 (0.92, 1.02) | |
Model 3 adjusted age, sex, race, prevalent cardiovascular disease, chronic obstructive pulmonary disease, cancer, diabetes, systolic blood pressure, diastolic blood pressure, total cholesterol, estimated glomerular filtration rate, hemoglobin, and hepatitis C seropositivity. Model 4 adjusted for 3 for body mass index, smoking status, alcohol use, cocaine use. Model 5 adjusted Model 3 for baseline diagnosis of schizophrenia, bipolar disorder, and post-traumatic stress disorder. Model 6 adjusted Model 3 for baseline prescription of selective serotonin reuptake inhibitors and tricyclic antidepressant medications. All Models for the HIV-infected participants were then further adjusted for baseline use of antiretroviral therapy, CD4 cell count, and HIV-1 RNA level
Table 4a:
Mortality risk by depressive symptoms (defined by PHQ-9) in the PHQ-9 subset
| Model 1 | Model 2 | Model 3* | ||||
|---|---|---|---|---|---|---|
| Hazard ratio [95% CI] | p-value | Hazard ratio [95% CI] | p-value | Hazard ratio [95% CI] | p-value | |
| No depressive symptoms (PHQ-9) | 1 (ref) | <0.01 | 1 (ref) | <0.01 | 1 (ref) | <0.01 |
| Depressive symptoms present (PHQ-9) | 1.17 [1.06, 1.30] | 1.34 [1.21, 1.49] | 1.16 [1.04, 1.28] | |||
Model 3B includes an interaction term for depressive symptoms and HIV status. The p-value for this interaction term was 0.05
Model 1 is adjusted for HIV status only. Model 2 additionally adjusted Model 1 for age, sex, and race. Model 3 additionally adjusted Model 2 for baseline cardiovascular disease, chronic obstructive pulmonary disease, cancer, diabetes, systolic blood pressure, diastolic blood pressure, total cholesterol, estimated glomerular filtration rate, hemoglobin, and hepatitis C seropositivity.
In the PHQ-9 subset, compared to those with PHQ-9<10, veterans with PHQ-9≥10 had a 17% increased risk of mortality adjusting for HIV status only (Table 4a). This association persisted in fully adjusted models (Table 4a). The hazard ratio (95% confidence interval) PHQ-9≥10 in the primary model was 1.16 (1.04–1.28). We detected a significant interaction between depressive symptoms and HIV status (p=0.05). Thus, we stratified our analyses by HIV status and detected no increased mortality risk associated with PHQ-9≥10 among HIV uninfected people (1.00 [0.83–1.20]) but significantly increased mortality risk associated with PHQ-9≥10 among people with HIV (1.24 [1.09–1.42]; Table 4b). Exploratory analyses adjusting for behavioral factors, mental health diagnoses, and depression treatment had little impact on the associations observed in the primary analyses (Table 4b).
Table 4b:
HIV-stratified mortality risk by depressive symptoms (defined by PHQ-9) in the PHQ-9 subset
| Hazard ratio [95% CI] | |||
|---|---|---|---|
| HIV uninfected | HIV infected | ||
| Model 3 | No moderate/severe depression by PHQ-9 | 1 (ref) | 1 (ref) |
| Moderate or severe depression by PHQ-9 | 1.00 (0.83, 1.20) | 1.23 (1.08, 1.41) | |
| Model 4 | No moderate/severe depression by PHQ-9 | 1 (ref) | 1 (ref) |
| Moderate or severe depression by PHQ-9 | 0.89 (0.74, 1.07) | 1.17 (1.02, 1.33) | |
| Model 5 | No moderate/severe depression by PHQ-9 | 1 (ref) | 1 (ref) |
| Moderate or severe depression by PHQ-9 | 0.98 (0.81, 1.18) | 1.22 (1.07, 1.39) | |
| Model 6 | No moderate/severe depression by PHQ-9 | 1 (ref) | 1 (ref) |
| Moderate or severe depression by PHQ-9 | 0.96 (0.80, 1.16) | 1.22 (1.07, 1.40) | |
Model 3 adjusted age, sex, race, prevalent cardiovascular disease, chronic obstructive pulmonary disease, cancer, diabetes, systolic blood pressure, diastolic blood pressure, total cholesterol, estimated glomerular filtration rate, hemoglobin, and hepatitis C seropositivity. Model 4 adjusted for 3 for body mass index, smoking status, alcohol use, cocaine use. Model 5 adjusted Model 3 for baseline diagnosis of schizophrenia, bipolar disorder, and post-traumatic stress disorder. Model 6 adjusted Model 3 for baseline prescription of selective serotonin reuptake inhibitors and tricyclic antidepressant medications. All Models for the HIV-infected participants were then further adjusted for baseline use of antiretroviral therapy, CD4 cell count, and HIV-1 RNA level.
Discussion
To our knowledge, this is the largest cohort study to date investigating the relationships among depressive disorders or symptoms, HIV status, and all-cause mortality. We report a moderate association of depressive symptoms and a modest association of depression diagnoses with mortality, which appear to differ by HIV status. Among people with HIV, there was a 24% increased mortality risk associated with elevated depressive symptoms ascertained by PHQ-9 but no significant increase in risk for depressive disorders ascertained by ICD-9 codes. For HIV uninfected people, there was a 6% increased mortlity risk associated with depressive disorders ascertained by ICD-9 codes and a non-significant 4% increased mortality risk for elevated depressive symptoms ascertained by PHQ-9.
Prior studies investigating depression and mortality among people with HIV have reported increased mortality risk among those with depression diagnoses or questionnaire-based depressive symptoms (2, 31–37) with exceptions.(1, 38) Some studies have focused on multiple mental health and/or substance use disorders (39), while others investigated joint effects of depression and antiretroviral therapy adherance on mortality.(40, 41) None of these studies included a group of people without HIV to determine whether HIV status modifies the effect of depression on mortality. The current study therefore extends the existing literature with the finding of an associaiton of depressive symptoms with mortality among people with HIV but not among HIV uninfected people.
The discrepancy in our findings between depression diagnoses (ICD-9) and depressive symptoms (PHQ-9) may have multiple explanations. First, it may reflect a positive effect of depression treatment on mortality risk. If people with a clinical diagnosis are more likely to receive treatment for – and thus improvement in – their depression than people with depressive symptoms without a clinical diagnosis, this would explain why depressive symptoms but not depression diagnoses were associated with mortality among people with HIV. Second, it may reflect lower risk of depression missclassification in the PHQ-9 subset versus the full VACS cohort. This is because the PHQ-9 was administered to everyone in the PHQ-9 subset (i.e., systematic assessment), reducing the likelihood of depression misclassification compared to ICD-9 codes recorded as part of routine clinical care (i.e. non-systematic assessment). Additionally, most of the above-referenced HIV studies used questionnaires to measure depressive symptoms and reported associations of depressive symptoms and mortality consistent with our findings among those with HIV. The two studies investigating major depressive disorder diagnoses (ICD-9 codes) and mortality among people with HIV reported conflicting results. The first (also from the VA) found no association of depressive disorders with mortality (1) while the second (from Kaiser Permanente Northern California) found psychiatric diagnoses (including major depressive disorder) were associated with increased mortality risk.(39) Our results are consistent with the other VA study and difficult to compare to the Kaiser Permanente study, which did not specifically report associations for major depressive disorders alone.
Analyses in most of the prior HIV studies had limited adjustment for potential confounders beyond demographics, HIV, HIV treatment and immune function characteristics. Our primary analysis included demographics, cardiovascular and pulmonary diseases, cancer, diabetes, and hepatitis C. These comorbidities are known to be associated with both depression and mortality,(42–47) and, as such, need to be adjusted for as potential confounders. Although several previous meta-analyses(48–50) in the general population have reported a positive association between depression and mortality, few of the included studies accounted for potential confounders beyond demographics and calendar time. For example, in a 2015 meta-analysis of 203 studies of mortality in mental disorders in the general population, seven adjusted for smoking and two adjusted for diabetes.(50) Thus, our results extend the literature by providing effect sizes that may be more reflective of the independent effect of depression on mortality among HIV uninfected people.
Although our study was not designed to assess the mechanisms by which HIV infection modifies the association between depressive symptoms and mortality, we offer potential explanations for this result. It is possible that AIDS-related mortality drives a substantial proportion of the association between depression and mortality among those with HIV. This idea is supported by prior work supporting a positive associations between depression and AIDS-related mortality and no association between depressive symptoms and mortality among people initiating antiretroviral therapy who were adherent to this therapy.(1, 34, 36, 37, 40, 41) Second, HIV infection is associated with other detrimental behaviors (e.g., substance misuse, polypharmcy leading to reduced treatment adherence, social isolation),(51–53) which could feedback to depression symptom recurrence or exacerbation and amplify the association of depression with mortality (Figure 1). A third potential explanation involves the association of both HIV infection and depression with increased chronic systemic inflammation, (17, 54, 55) an immunologic phenomenon associated with increased mortality risk.(56–58) In this case, we speculate that combined effect of chronic systemic inflammation from both HIV and depression on mortality is greater than would have been expected from their individual effects.(59).
If HIV infection increases the risk of death associated with depressive symptoms, this has important clinical and research implications. As people with HIV are living longer, their risk for diseases of aging (e.g., cancer, cardiovascular disease) increases, and this risk is accentuated (higher risk at all ages) among people with HIV compared to those without HIV infection.(60) These diseases of aging are associated with depression and may, combined with HIV, further increase mortality risk in this population (i.e., three-way interaction between HIV, depression and diseases of aging). Despite clinical guidelines recommending routine screening for depressive symptoms, there is varying success in implementation and there is underdiagnosis of depression in HIV.(61–63) This needs to be improved; better understanding of barriers and facilitators to effective depression screening and integrating depression treatment into HIV primary care is needed. Even with effective screening, depression treatment is challenging,(64) particularly in the context of HIV infection. Many people with HIV in the combination antiretroviral therapy era are on at least five medications.(65) Adding depression therapy to this background increases risk of harm from polypharmacy including drug drug interactions and decreased treatment adherence. To reduce mortality risk associated with depression among people with HIV, future studies compare watchful waiting, pharmacotherapy, psychotherapy and their appropriate combination paying close attention to the adverse effects of polypharmacy.
There were limitations to this analysis. First, we did not perform time-updated analyses. This may have led to an underestimation of effects depression on mortality because new onset depression was not captured. Second, these results may not be generalizable to women. Third, we cannot exclude the possibility that even with our extensive adjustments, unmeasured confounding due to other variables differing between the HIV and depressed subgroups led to discordant results.
In conclusion, we found that depressive symptoms were moderately associated with all-cause mortality among U.S. veterans with HIV but not among their counterparts without HIV infection. Depression diagnoses were modestly associated with mortality among those without HIV but not among those with HIV infection. To reduce mortality risk, these findings reinforce the need to assess and treat depressive symptoms and major depressive disorder in patients with and without HIV infection; a strategy supported by a recently published trial showing depression treatment is associated with long-term cardiac outcomes (including mortality) among people with recent acute coronary syndrome.(66) Particularly among people with HIV, there is a need to compare the effectiveness of different depression treatment modalities (e.g., watchful waiting, pharmacotherpy, psychotherapy) accounting for health consequences of polypharmacy.
Supplementary Material
Key points:
Depressive symptoms were associated with all-cause mortality among those with HIV but not among their counterparts without HIV infection. Depression diagnoses were modestly associated with mortality among those without HIV but not among those with HIV infection.
Research in context.
Evidence before this study:
Depressive disorders have been associated with increased mortality in persons with chronic diseases, including heart disease, end stage renal disease, and diabetes. Several studies have described similar associations in the context of HIV infection, although conflicting data exist. Limitations in prior work include absence of a comparator group without HIV that would inform conclusions about whether HIV infection modifies the association between depression and mortality.
Added value of this study:
This is the largest cohort study to date investigating the relationships among depressive disorders or symptoms, HIV status (including HIV uninfected comparators), and all-cause mortality.
Implications of available evidence:
To reduce mortality risk, our findings reinforce the need to assess and treat depressive symptoms and major depressive disorder in patients with and without HIV infection.
Acknowledgements
Funding/Support
This work was supported by a grant from the National Heart Lung and Blood Institute at the National Institutes of Health (grant numbers R01 HL126557 to S.K.G. and K01 HL134147 to K.S.).
The funder/sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures
Dr. So-Armah reports funding from the National Institues of Health. Dr. Gupta reports funding from the National Institutes of Health, Indiana University, and Gilead Sciences; advisory board fees from Gilead Sciences and GlaxoSmithKline/ViiV; and travel support to present data at scientific conferences from Gilead Sciences and Bristol-Myers Squibb. Dr. Kundu reports funding from the National Institutes of Health. Dr. Stewart reports funding from the National Institutes of Health. Dr. Goulet reports funding from the National Institutes of Health, the Department of Veterans Affairs, and the Department of Defense. Dr. Budoff reports funding from the National Institutes of Health. Dr. Rodriguez-Barradas reports funding from the National Institutes of Health and Kowa Pharmaceuticals. Dr. Marconi reports funding from the National Institues of Health, Centers for Disease Control and Prevention, the Department of Veterans Affairs, GlaxoSmithKline/ViiV, Gilead Sciences, and Bayer. Dr. Freiberg reports funding from the National Institutes of Health. Dr. Crystal reports funding from the Agency for Healthcare Quality and Research, the National Institutes of Health, and the Patient-Centered Outcomes Research Institute. Dr. Gibert reports funding from the National Institutes of Health. Dr. Bedimo reports grant funding from Merck & Co, Bristol-Myers Squibb, Theratechnologies, and Gilead Sciences. Dr. Chang reports funding from the National Institutes of Health. Dr. Sico has no disclosures to report.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs.
This work was previously presented as a poster at IDWeek 2016, New Orleans, LA, USA.
Contributor Information
DR Kaku So-Armah, Boston University School of Medicine, Boston, MA, USA; kaku@bu.edu.
Samir K Gupta, Indiana University School of Medicine, Indianapolis (IUPUI), IN, USA; sgupta1@iu.edu.
Suman Kundu, Vanderbilt University School of Medicine, Nashville, TN, USA; suman.kundu@vanderbilt.edu.
Jesse C Stewart, Indiana University-Purdue University Indianapolis, Indianapolis, IN, USA; jstew@iupui.edu.
Joseph L Goulet, VA Connecticut Healthcare System, West Haven, CT, Yale School of Medicine, New Haven, CT, USA; joseph.goulet@va.gov.
Adeel A Butt, VA Pittsburgh Healthcare System, Pittsburgh, PA; Weill Cornell Medical College, NY, USA and Doha, Qatar; Hamad Medical Corporation, Doha, Qatar; aab2005@qatar-med.cornell.edu.
Jason J Sico, VA Connecticut Healthcare System, West Haven, CT, Yale School of Medicine, New Haven, CT, USA; jason.sico@yale.edu.
Vincent C Marconi, Emory University School of Medicine and Rollins School of Public Health, Atlanta VA Medical Center, Atlanta, GA, USA; vcmarco@emory.edu.
Stephen Crystal, Center for Health Services Research, Institute for Health, Rutgers University, New Brunswick, NJ, USA; scrystal@rutgers.edu.
Maria C Rodriguez-Barradas, Infectious Diseases Section, Michael E. DeBakey VAMC, and Department of Medicine, Baylor College of Medicine, Houston, TX; Maria.Rodriguez-Barradas2@va.gov.
Matthew Budoff, Los Angeles Biomedical Research Institute at Harbor-UCLA, Torrance, CA, USA; mbudoff@labiomed.org.
Cynthia L. Gibert, Washington DC Veterans Affairs Medical Center and George Washington University School of Medicine and Health Sciences, Washington, DC, USA; cynthia.gibert@va.gov.
Chung-Chou H Chang, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; changjh@upmc.edu.
Roger Bedimo, VA North Texas Health Care System, Dallas, TX, USA; roger.bedimo@va.gov.
Matthew S Freiberg, Vanderbilt University School of Medicine, VA Tennessee Valley Healthcare System, Nashville, TN, USA; matthew.s.freiberg@vanderbilt.edu.
References
- 1.Nurutdinova D, Chrusciel T, Zeringue A, Scherrer JF, Al-Aly Z, McDonald JR, et al. Mental health disorders and the risk of AIDS-defining illness and death in HIV-infected veterans. AIDS. 2012;26(2):229–34. [DOI] [PubMed] [Google Scholar]
- 2.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–74. [DOI] [PubMed] [Google Scholar]
- 3.Williams P, Narciso L, Browne G, Roberts J, Weir R, Gafni A. The prevalence, correlates, and costs of depression in people living with HIV/AIDS in Ontario: implications for service directions. AIDS Education & Prevention. 2005;17(2):119–30. [DOI] [PubMed] [Google Scholar]
- 4.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–8. [DOI] [PubMed] [Google Scholar]
- 5.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. The American journal of psychiatry. 2001;158(5):725–30. [DOI] [PubMed] [Google Scholar]
- 6.Fan H, Yu W, Zhang Q, Cao H, Li J, Wang J, et al. Depression after heart failure and risk of cardiovascular and all-cause mortality: a meta-analysis. Preventive Medicine. 2014;63:36–42. [DOI] [PubMed] [Google Scholar]
- 7.Farrokhi F, Abedi N, Beyene J, Kurdyak P, Jassal SV. Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. American Journal of Kidney Diseases. 2014;63(4):623–35. [DOI] [PubMed] [Google Scholar]
- 8.van Dooren FE, Nefs G, Schram MT, Verhey FR, Denollet J, Pouwer F. Depression and risk of mortality in people with diabetes mellitus: a systematic review and meta-analysis. PLoS ONE [Electronic Resource]. 2013;8(3):e57058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 Suppl 2):S25–30. [DOI] [PubMed] [Google Scholar]
- 10.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–44. [DOI] [PubMed] [Google Scholar]
- 11.White JR, Chang CC, So-Armah KA, Stewart JC, Gupta SK, Butt AA, et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: Veterans Aging Cohort Study. Circulation. 2015;132(17):1630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khambaty T, Stewart JC, Gupta SK, Chang CH, Bedimo RJ, Budoff MJ, et al. Association Between Depressive Disorders and Incident Acute Myocardial Infarction in Human Immunodeficiency Virus-Infected Adults: Veterans Aging Cohort Study. JAMA Cardiol. 2016;1(8):929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 Suppl 2):S25–30. [DOI] [PubMed] [Google Scholar]
- 16.Mollan KR, Smurzynski M, Eron JJ, et al. Association between efavirenz as initial therapy for hiv-1 infection and increased risk for suicidal ideation or attempted or completed suicide: An analysis of trial data. Annals of Internal Medicine. 2014;161(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55(1):126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173(8):614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freiberg MS, Chang CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA internal medicine. 2013;173(8):614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–85. [DOI] [PubMed] [Google Scholar]
- 21.Petersen LA, Wright S, Normand SL, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med. 1999;14(9):555–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sico JJ, Chang CC, So-Armah K, Justice AC, Hylek E, Skanderson M, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84(19):1933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183(3):388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park LS, Tate JP, Rodriguez-Barradas MC, Rimland D, Goetz MB, Gibert C, et al. Cancer Incidence in HIV-Infected Versus Uninfected Veterans: Comparison of Cancer Registry and ICD-9 Code Diagnoses. Journal of AIDS & clinical research. 2014;5(7):1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49(2):225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, et al. Validating Smoking Data From the Veteran’s Affairs Health Factors Dataset, an Electronic Data Source. Nicotine Tob Res. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraemer KL, McGinnis KA, Skanderson M, Cook R, Gordon A, Conigliaro J, et al. Alcohol problems and health care services use in human immunodeficiency virus (HIV)-infected and HIV-uninfected veterans. Medical Care. 2006;44(8 Suppl 2):S44–51. [DOI] [PubMed] [Google Scholar]
- 30.Freiberg MS, McGinnis KA, Kraemer K, Samet JH, Conigliaro J, Curtis Ellison R, et al. The association between alcohol consumption and prevalent cardiovascular diseases among HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2010;53(2):247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills JC, Pence BW, Todd JV, Bengtson AM, Breger TL, Edmonds A, et al. Cumulative Burden of Depression and All-Cause Mortality in Women Living with HIV. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pence BW, Mills JC, Bengtson AM, Gaynes BN, Breger TL, Cook RL, et al. Association of Increased Chronicity of Depression With HIV Appointment Attendance, Treatment Failure, and Mortality Among HIV-Infected Adults in the United States. JAMA Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Todd JV, Cole SR, Pence BW, Lesko CR, Bacchetti P, Cohen MH, et al. Effects of Antiretroviral Therapy and Depressive Symptoms on All-Cause Mortality Among HIV-Infected Women. Am J Epidemiol. 2017;185(10):869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leserman J, Pence BW, Whetten K, Mugavero MJ, Thielman NM, Swartz MS, et al. Relation of lifetime trauma and depressive symptoms to mortality in HIV. The American journal of psychiatry. 2007;164(11):1707–13. [DOI] [PubMed] [Google Scholar]
- 35.Antelman G, Kaaya S, Wei R, Mbwambo J, Msamanga GI, Fawzi WW, et al. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J Acquir Immune Defic Syndr. 2007;44(4):470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94(7):1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farinpour R, Miller EN, Satz P, Selnes OA, Cohen BA, Becker JT, et al. Psychosocial risk factors of HIV morbidity and mortality: findings from the Multicenter AIDS Cohort Study (MACS). J Clin Exp Neuropsychol. 2003;25(5):654–70. [DOI] [PubMed] [Google Scholar]
- 38.Bengtson AM, Pence BW, Moore R, Mimiaga MJ, Mathews WC, Heine A, et al. Relationship between ever reporting depressive symptoms and all-cause mortality in a cohort of HIV-infected adults in routine care. AIDS. 2017;31(7):1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeLorenze GN, Satre DD, Quesenberry CP, Tsai AL, Weisner CM. Mortality after diagnosis of psychiatric disorders and co-occurring substance use disorders among HIV-infected patients. AIDS Patient Care STDS. 2010;24(11):705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villes V, Spire B, Lewden C, Perronne C, Besnier JM, Garre M, et al. The effect of depressive symptoms at ART initiation on HIV clinical progression and mortality: implications in clinical practice. Antivir Ther. 2007;12(7):1067–74. [PubMed] [Google Scholar]
- 41.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21(9):1175–83. [DOI] [PubMed] [Google Scholar]
- 42.Khambaty T, Stewart JC, Gupta SK, et al. Association between depressive disorders and incident acute myocardial infarction in human immunodeficiency virus–infected adults: Veterans aging cohort study. JAMA Cardiology. 2016;1(8):929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang C-Q, Dong B-R, Lu Z-C, Yue J-R, Liu Q-X. Chronic diseases and risk for depression in old age: A meta-analysis of published literature. Ageing Research Reviews. 2010;9(2):131–41. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, Johansen C, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. The Lancet Oncology. 2011;12(2):160–74. [DOI] [PubMed] [Google Scholar]
- 45.Hofmann M, Kohler B, Leichsenring F, Kruse J. Depression as a risk factor for mortality in individuals with diabetes: a meta-analysis of prospective studies. PLoS One. 2013;8(11):e79809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer SC, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, et al. Association between depression and death in people with CKD: a meta-analysis of cohort studies. Am J Kidney Dis. 2013;62(3):493–505. [DOI] [PubMed] [Google Scholar]
- 47.Butt AA, Evans R, Skanderson M, Shakil AO. Comorbid medical and psychiatric conditions and substance abuse in HCV infected persons on dialysis. J Hepatol. 2006;44(5):864–8. [DOI] [PubMed] [Google Scholar]
- 48.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. The British journal of psychiatry : the journal of mental science. 2013;202(1):22–7. [DOI] [PubMed] [Google Scholar]
- 49.Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. The American journal of psychiatry. 2014;171(4):453–62. [DOI] [PubMed] [Google Scholar]
- 50.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. Journal of studies on alcohol. 2002;63(2):179–86. [DOI] [PubMed] [Google Scholar]
- 52.Nahvi S, Cooperman NA. Review: the need for smoking cessation among HIV-positive smokers. AIDS Educ Prev. 2009;21(3 Suppl):14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green TC, Kershaw T, Lin H, Heimer R, Goulet JL, Kraemer KL, et al. Patterns of drug use and abuse among aging adults with and without HIV: a latent class analysis of a US Veteran cohort. Drug Alcohol Depend. 2010;110(3):208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, et al. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89(4):419–24. [DOI] [PubMed] [Google Scholar]
- 55.Vogelzangs N, Duivis HE, Beekman AT, Kluft C, Neuteboom J, Hoogendijk W, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;2:e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.So-Armah KA, Tate JP, Chang C-CH, Butt AA, Gerschenson M, Gibert CL, et al. Do Biomarkers Of Inflammation, Monocyte Activation And Altered Coagulation Explain Excess Mortality Between HIV Infected and Uninfected People? JAIDS Journal of Acquired Immune Deficiency Syndromes. 9000;Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh-Manoux A, Shipley MJ, Bell JA, Canonico M, Elbaz A, Kivimaki M. Association between inflammatory biomarkers and all-cause, cardiovascular and cancer-related mortality. CMAJ. 2017;189(10):E384–E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polanka BM, Berntson J, Vrany EA, Stewart JC. Are Cardiovascular Risk Factors Stronger Predictors of Incident Cardiovascular Disease in U.S. Adults With Versus Without a History of Clinical Depression? Annals of Behavioral Medicine. 2018:kay007-kay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis. 2015;60(4):627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asch SM, Kilbourne AM, Gifford AL, Burnam MA, Turner B, Shapiro MF, et al. Underdiagnosis of depression in HIV: who are we missing? J Gen Intern Med. 2003;18(6):450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodkjaer L, Laursen T, Balle N, Sodemann M. Depression in patients with HIV is under-diagnosed: a cross-sectional study in Denmark. HIV Med. 2010;11(1):46–53. [DOI] [PubMed] [Google Scholar]
- 63.Parry S, Zetler S, Kentridge A, Petrak J, Barber T. Simple screening for neurocognitive impairment in routine HIV outpatient care: is it deliverable? AIDS Care. 2017;29(10):1275–9. [DOI] [PubMed] [Google Scholar]
- 64.Cuijpers P The Challenges of Improving Treatments for Depression. JAMA. 2018. [DOI] [PubMed] [Google Scholar]
- 65.Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs & aging. 2013;30(8):613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J, Stewart R, Lee Y, et al. Effect of escitalopram vs placebo treatment for depression on long-term cardiac outcomes in patients with acute coronary syndrome: A randomized clinical trial. JAMA. 2018;320(4):350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barnard J, B. Rubin D. Small-sample degrees of freedom with multiple imputation1999. 948 p.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.