Abstract
Purpose:
To evaluate cancer incidence in the Agricultural Health Study (AHS), a cohort of private pesticide applicators, their spouses, and commercial applicators, based on 12,420 cancers, adding 5,989 cancers and nine years of follow-up since last evaluation.
Methods:
We calculated age, year, sex, and race-adjusted standardized incidence ratios (SIR) and 95% confidence intervals (CI) for cancer sites in the AHS relative to the general population.
Results:
Overall AHS cancer incidence was lower than the general population (SIRprivate=0.91, CI:0.89–0.93; SIRspouse=0.89, CI:0.86–0.92; SIRcommercial=0.83, CI:0.76–0.92), with notable deficits across applicators and spouses for oral cavity, pancreas, and lung cancers. Cancer excesses included prostate cancer, lip cancer, certain B-cell lymphomas (e.g multiple myeloma), acute myeloid leukemia (AML), thyroid cancer, testicular cancer, and peritoneal cancer. The lung cancer deficit was strongest among applicators reporting potential exposure to endotoxin at study enrollment (tasks such as raising animals and handling stored grain).
Conclusions:
Although an overall deficit in cancer was observed, there were notable exceptions, including newly-observed excesses for AML, thyroid, testicular, and peritoneal cancers. Furthermore, endotoxin exposure may, in part, account for observed lung cancer incidence deficits. Cancer incidence patterns in the AHS suggest farm exposures’ relevance to cancer etiology.
Keywords: Farming, Cancer, Incidence, Pesticides, Endotoxin
BACKGROUND
Agricultural populations in Western countries have lower overall risks of cancer than the general population (1, 2), likely due to lifestyle factors such as lower rates of smoking and a higher level of occupational physical activity. In addition, the healthy worker effect is also thought to, in part, explain observed cancer and mortality deficits in occupational cohorts including agricultural populations (3). However, excesses of certain types of cancer have been reported among agricultural workers and more broadly in farming populations, including prostate, brain, and lip cancer, lymphoma, multiple myeloma, leukemia, and melanoma (1, 4–12). Several farming-related exposures may contribute to these divergent patterns, such as pesticides, diesel engine exhaust, ultraviolet radiation, biologically active dusts, and viral and bacterial exposures via farm animals (13–16).
The Agricultural Health Study (AHS) cohort is a large prospective study of pesticide applicators (mostly male), including private pesticide applicators from Iowa (IA) and North Carolina (NC), USA (mostly farmers), their spouses (mostly women), and commercial pesticide applicators from IA. Cancer incidence in the cohort compared with the general population has been evaluated twice for cancers diagnosed from enrollment through 2002 and through 2006, (4, 7). In these evaluations, overall cancer risk was consistently lower than that in the general population, particularly for smoking-related cancers including esophagus, pancreas, lung, bladder, and kidney. Excesses have also been observed, including cancers of the prostate, ovaries (among female applicators only), and lip, multiple myeloma, and marginal zone lymphoma (MZL) (4, 7). Agricultural exposures and cancer risk have been evaluated extensively within the AHS. For example, certain pesticide exposures have been associated with elevated cancer risk, including prostate cancer (17) and multiple myeloma (18). Additionally, exposure to animals, (e.g. number of livestock) thought to be a proxy for endotoxin exposure, has been inversely associated with lung cancer risk in the AHS (19). Endotoxin, a component of Gram-negative bacteria cell walls, is often present in high concentrations in biologically-active dusts found in grain elevators and animal containment facilities (20).
We have updated the evaluation of cancer incidence in the AHS cohort compared to the general population, extending follow-up nine years and adding 5,989 cancer cases for a total of 12,420.
METHODS
The AHS cohort has been described in detail (21). Briefly, from 1993–1997 52,394 private pesticide applicators (IA and NC) and 4,916 commercial pesticide applicators (IA only) were recruited and completed enrollment questionnaires when they renewed their restricted-use pesticide licenses (82% applicator response rate). A total of 32,346 spouses of private pesticide applicators in IA and NC (an estimated 75% of spouses of married applicators) completed and returned enrollment questionnaires. At enrollment, all study participants provided detailed information about farm and pesticide exposures (e.g. types of crops and animals, self-reported lifetime use of 50 pesticides), demographic information (e.g. age, race), and lifestyle factors (e.g. cigarette smoking, alcohol consumption). AHS study questionnaires are available at https://aghealth.nih.gov/collaboration/questionnaires.html. The study protocol, including implied consent for completion of questionnaires, was approved by all relevant institutional review boards.
AHS Cancer Ascertainment and Classification
We obtained incident cancer cases via linkage with IA and NC state cancer registries. We analyzed malignant first primary cancers diagnosed from enrollment through date of death, movement out of state, or last study follow-up (December 31, 2015 for IA, December 31, 2014 for NC), whichever was earliest. Only 3.4% (n=3,201) of study participants moved out of state; person-time was censored on the date they moved. Cancer site was classified according to the International Classification of Diseases for Oncology, third revision (ICD-O-3) (22). Lymphoid malignancies were classified according to the 2008 Surveillance, Epidemiology, and End Results Lymphoma Subtype Recode (23). Myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPN) diagnosed after 2001, when these cancers became reportable to the US central cancer registries, were classified according to ICD-O-3 (22).
Statistical Methods
We calculated standardized incidence ratios (SIRs) and 95% confidence intervals (CI) to compare the cancer experience in the AHS cohort to the general populations of IA and NC. We first obtained site-specific rates for first primary cancers in IA and NC using the latest releases of the Surveillance and Epidemiology End Results (SEER) and North American Association of Central Cancer Registries (NAACCR) Cancer in North America (CiNA) public use data (24), respectively, by 5-year age and calendar year (1995–2015) categories, sex (male, female), and race (white, all other) strata.
We created an AHS data set with the same stratification variables (age, sex, year, race) and the number of AHS person-years contributed to each stratum. We used the STDRATE command in SAS version 9.4 (SAS Institute, Cary, NC) to calculate age, sex, year, and race-adjusted expected cancer cases for each site. We compared the ratio of observed incident cases within the cohort to the expected number of cases to calculate the SIR. Confidence intervals were calculated using the Breslow and Day method (25).
Previous analyses demonstrated fewer cancers than expected within the cohort (4, 7). To account for this deficit in cancer incidence, we calculated relative SIRs (RSIR) as follows using a modified version of relative SMR methods to account for the comparison of site-specific cancer risks (25–27), where “site” is the specific cancer of interest and “all sites” are all cancers combined:
We calculated SIRs separately for IA and NC for private applicators and spouses; all commercial applicators were from IA (21). For lung cancers, we stratified SIRs by smoking and potential endotoxin exposure at study enrollment to explore the separate contributions of each. Smoking was classified as never (<100 lifetime cigarettes), or ever (100+ lifetime cigarettes). Potential exposure to endotoxin was classified using self-reported farm tasks known to be associated with elevated levels of endotoxin (20). For applicators, this included raising livestock or exposure to grain dust, and for spouses, having direct contact with animals in the past year or occupational grain dust exposure in an off-farm job (16). No information was available for spouses regarding grain dust exposure on the farm. Tests for heterogeneity were calculated using the Breslow and Day method (25). We additionally compared selected characteristics from the AHS, such as current tobacco use, alcohol consumption, and body size, to data from a representative sample of IA and NC adults (ages 18+) in the 1995 Behavioral Risk Factor Surveillance System Survey (BRFSS) (28). All tests were two-sided with α=0.05.
RESULTS
Table 1 describes selected characteristics of the cohort. In general, NC applicators and spouses were older than their IA counterparts, including commercial applicators. Commercial applicators were younger than private applicators and spouses. Applicators were predominantly male, and spouses were predominantly female. NC study participants were more racially diverse and reported fewer years of education. Commercial applicators were most likely to report current smoking and drinking at least once per month at enrollment. IA applicators (private and commercial) were more likely to report alcohol consumption compared to NC applicators. Usual adult body mass index (BMI) for AHS participants ranged from 26 for spouses to 27.6 for applicators. Self-reported lifetime pesticide use (i.e. herbicides, insecticides, fungicides, fumigants) varied by state and applicator type; patterns observed by state were similar for applicators and spouses, though spouses reported less pesticide use. IA private applicators were more likely to be exposed to endotoxin (94.6%) compared to NC private applicators (54.5%) and commercial applicators (49.4%). Similarly, IA spouses were more likely to be exposed to endotoxin (60.4%) compared to NC spouses (31.1%).
Table 1.
Descriptive characteristics of the Agricultural Health Study cohort at enrollment (unless otherwise specified)
| Applicators | Spouses | ||||
|---|---|---|---|---|---|
| Private | Commercial | ||||
| Iowa | North Carolina | Iowa | Iowa | North Carolina | |
| N=31033 | N=20132 | N=4708 | N=20977 | N=10344 | |
| Total person-years contributed | 564,494 | 335,966 | 86,729 | 379,526 | 176,948 |
| N (%) | N (%) | N (%) | N (%) | N (%) | |
| Attained Age | |||||
| <55 | 5933 (19.1) | 3846 (19.1) | 1901 (40.4) | 4349 (20.7) | 1838 (17.8) |
| 55–64 | 10698 (34.5) | 5373 (26.7) | 1654 (35.1) | 7049 (33.6) | 2758 (26.7) |
| 65–74 | 8053 (25.9) | 5500 (27.3) | 801 (17.0) | 5279 (25.2) | 2833 (27.4) |
| 75+ | 6349 (20.5) | 5413 (26.9) | 352 (7.5) | 4300 (20.5) | 2915 (28.2) |
| Gender | |||||
| Male | 30602 (98.6) | 19227 (95.5) | 4513 (95.9) | 65 (0.3) | 147 (1.4) |
| Female | 431 (1.4) | 905 (4.5) | 195 (4.1) | 20912 (99.7) | 10197 (98.6) |
| Race | |||||
| White | 30692 (98.9) | 17949 (89.2) | 4656 (98.9) | 20611 (98.3) | 9351 (90.4) |
| Black/Other | 49 (0.2) | 1439 (7.1) | 22 (0.5) | 50 (0.2) | 489 (4.7) |
| Missing | 292 (0.9) | 744 (3.7) | 30 (0.6) | 316 (1.5) | 504 (4.9) |
| Education | |||||
| < High School | 1619 (5.2) | 3408 (16.9) | 142 (3.0) | 525 (2.5) | 998 (9.6) |
| High school or equivalent | 15304 (49.3) | 8190 (40.7) | 1962 (41.7) | 7250 (34.6) | 3679 (35.6) |
| Vocational/some college | 8180 (26.4) | 3701 (18.4) | 1358 (28.8) | 5976 (28.5) | 2305 (22.3) |
| College graduate | 4441 (14.3) | 2529 (12.6) | 943 (20) | 3394 (16.2) | 1416 (13.7) |
| Graduate school | 698 (2.2) | 758 (3.8) | 155 (3.3) | 1113 (5.3) | 574 (5.5) |
| Something else | 55 (0.2) | 59 (0.3) | 11 (0.2) | 2153 (10.3) | 625 (6.0) |
| Missing | 736 (2.4) | 1487 (7.4) | 137 (2.9) | 566 (2.7) | 747 (7.2) |
| Smoking status | |||||
| Never | 18492 (59.6) | 7885 (39.2) | 2216 (47.1) | 15637 (74.5) | 6604 (63.8) |
| Former | 8590 (27.7) | 6857 (34.1) | 1192 (25.3) | 3413 (16.3) | 1876 (18.1) |
| Current | 3619 (11.7) | 4474 (22.2) | 1228 (26.1) | 1678 (8.0) | 1505 (14.5) |
| Missing | 332 (1.1) | 916 (4.5) | 72 (1.5) | 249 (1.2) | 359 (3.5) |
| Alcohol use (last year) | |||||
| Less than once/month | 11609 (37.4) | 12070 (60.0) | 1205 (25.6) | 13197 (62.9) | 8431 (81.5) |
| At least once/month | 18475 (59.5) | 5508 (27.4) | 3416 (72.6) | 7216 (34.4) | 1170 (11.3) |
| Missing | 949 (3.1) | 2554 (12.7) | 87 (1.8) | 564 (2.7) | 743 (7.2) |
| Farm Exposures | |||||
| Herbicides1 | 30069 (96.9) | 17945 (89.1) | 3960 (84.1) | 8384 (40.0) | 2827 (27.3) |
| Insecticides1 | 28549 (92.0) | 16598 (82.4) | 3543 (75.3) | 8370 (39.9) | 3479 (33.6) |
| Fungicides1 | 5658 (18.2) | 12005 (59.6) | 1014 (21.5) | 643 (3.1) | 846 (8.2) |
| Fumigants1 | 2851 (9.2) | 8430 (41.9) | 808 (17.2) | 197 (0.9) | 381 (3.7) |
| Endotoxin2 | 29346 (94.6) | 10981 (54.5) | 2325 (49.4) | 12674 (60.4) | 3216 (31.1) |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Person years follow-up3 | 18.2 (4.92) | 16.7 (5.47) | 18.4 (5.65) | 18.1 (4.36) | 17.1 (4.41) |
| Body mass index | 27.6 (3.90) | 27.5 (4.24) | 27.4 (4.44) | 26 (4.87) | 26 (4.97) |
| Pack-years (among former smokers) | 12.8 (17.41) | 19.8 (23.63) | 14 (17.28) | 7.9 (12.11) | 10.2 (14.24) |
| Pack-years (among current smokers) | 20.7 (18.55) | 26.6 (23.16) | 19.6 (17.04) | 16.1 (15.38) | 18.6 (15.79) |
Report lifetime ever use of at least one of the 50 pesticides (18 herbicides, 22 insecticides, 6 fungicides, 4 fumigants) queried at enrollment
Applicators: raise farm animals or exposed to grain dust; Spouses: direct contact with farm animals at least once/year or occupationally exposed to grain dust
Iowa participants have one additional year of cancer and mortality follow-up (through 2015) compared to North Carolina participants (through 2014)
We compared lifestyle characteristics of the AHS participants with a representative sample of IA and NC adults aged 18+ (Supplemental Table 1) (28). For context, the BRFSS sample is similar in age for IA private applicators and spouses, younger than NC participants, and older than commercial applicators. AHS study participants were less likely to be current smokers, more were never smokers, and a similar proportion were former smokers compared with the general population. Current smokers in the AHS reported similar numbers of cigarettes/day compared with the general population. Private applicators and spouses were slightly less likely to consume alcoholic beverages compared with the general population, while commercial applicators were more likely. Mean BMI was comparable in AHS and the general population.
Cancer incidence in the cohort was lower than in the general population (SIRprivate=0.91, CI:0.89–0.93, SIRcommercial=0.83, CI:0.76–0.92, SIRspouse=0.89, CI:0.86–0.92), adjusting for age, year, race, and sex (Table 2). This was largely driven by lower rates of respiratory cancers including all lung subtypes and larynx cancer, other smoking-related cancers such as bladder, and digestive system cancers including esophagus, colon, rectum, liver, and pancreas. Among private applicators, the SIRs for lip cancer (SIR=2.22, CI:1.71–2.84), prostate cancer (SIR=1.15, CI:1.11–1.19), B-cell lymphomas overall (SIR=1.12, CI:1.03–1.21), chronic lymphocytic leukemia (CLL; SIR=1.17, CI:1.00–1.36) and acute myeloid leukemia (AML; SIR=1.29, CI:1.03–1.59) were significantly elevated. Among spouses of private applicators, SIRs for peritoneal cancer (SIR=1.80, CI:1.11–2.75), melanoma (SIR=1.21, CI:1.04–1.40), uterine cancer (SIR=1.13, CI:1.01–1.27), papillary thyroid cancer (SIR=1.30, CI:1.07–1.57), and follicular lymphoma (FL; SIR=1.33, CI:1.00–1.74) were significantly elevated. No SIRs were significantly elevated for commercial applicators.
Table 2.
Standardized Incidence Ratios (SIRs) and 95% confidence intervals (CI) adjusted for age, year, race, and sex
| Private Applicators | Commercial Applicators | Spouses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | SIR | CI | N | SIR | CI | N | SIR | CI | |
| All Sites | 8256 | 0.91 | (0.89, 0.93) | 444 | 0.83 | (0.76, 0.92) | 3720 | 0.89 | (0.86, 0.92) |
| Oral Cavity and Pharynx | 198 | 0.69 | (0.60, 0.80) | 10 | 0.50 | (0.24, 0.92) | 44 | 0.72 | (0.52, 0.96) |
| Lip | 63 | 2.22 | (1.71, 2.84) | 4 | – | – | 7 | 1.76 | (0.71, 3.63) |
| Tongue | 35 | 0.46 | (0.32, 0.64) | 2 | – | – | 15 | 0.89 | (0.50, 1.47) |
| Tonsil | 27 | 0.47 | (0.31, 0.68) | 1 | – | – | 3 | – | – |
| Digestive System | 1407 | 0.87 | (0.82, 0.91) | 84 | 0.83 | (0.66, 1.03) | 540 | 0.81 | (0.74, 0.88) |
| Esophagus | 102 | 0.71 | (0.58, 0.86) | 13 | 1.29 | (0.69, 2.21) | 9 | 0.51 | (0.23, 0.97) |
| Stomach | 114 | 0.91 | (0.75, 1.10) | 2 | – | – | 28 | 0.78 | (0.52, 1.12) |
| Small Intestine | 44 | 1.13 | (0.82, 1.52) | 4 | – | – | 11 | 0.61 | (0.30, 1.09) |
| Colon and Rectum | 842 | 0.95 | (0.89, 1.02) | 49 | 0.89 | (0.66, 1.18) | 346 | 0.87 | (0.78, 0.96) |
| Proximal Colon | 310 | 0.94 | (0.84, 1.05) | 17 | 0.89 | (0.52, 1.42) | 169 | 0.93 | (0.80, 1.09) |
| Distal Colon | 226 | 0.99 | (0.87, 1.13) | 13 | 0.93 | (0.49, 1.58) | 84 | 0.91 | (0.73, 1.13) |
| Rectum and Rectosigmoid Junction | 260 | 0.95 | (0.84, 1.08) | 17 | 0.89 | (0.52, 1.42) | 74 | 0.75 | (0.59, 0.94) |
| Anus, Anal Canal and Anorectum | 8 | 0.44 | (0.19, 0.87) | 1 | – | – | 6 | 0.30 | (0.11, 0.66) |
| Liver and Intrahepatic Bile Duct | 78 | 0.56 | (0.45, 0.70) | 4 | – | – | 21 | 0.71 | (0.44, 1.08) |
| Pancreas | 183 | 0.83 | (0.72, 0.96) | 10 | 0.76 | (0.37, 1.41) | 71 | 0.69 | (0.54, 0.87) |
| Peritoneum, Omentum and Mesentery | 1 | -- | – | 0 | – | – | 21 | 1.80 | (1.11, 2.75) |
| Respiratory System | 881 | 0.51 | (0.47, 0.54) | 56 | 0.65 | (0.49, 0.85) | 259 | 0.40 | (0.36, 0.46) |
| Larynx | 66 | 0.48 | (0.37, 0.62) | 4 | – | – | 5 | 0.26 | (0.09, 0.62) |
| Lung and Bronchus | 807 | 0.51 | (0.48, 0.55) | 51 | 0.67 | (0.50, 0.88) | 252 | 0.41 | (0.36, 0.46) |
| Small-Cell Carcinoma | 138 | 0.58 | (0.48, 0.68) | 10 | 0.81 | (0.39, 1.49) | 39 | 0.34 | (0.24, 0.46) |
| Non-Small Cell Carcinoma | 633 | 0.51 | (0.47, 0.55) | 40 | 0.67 | (0.48, 0.91) | 199 | 0.44 | (0.38, 0.50) |
| Squamous Cell Carcinoma | 209 | 0.51 | (0.45, 0.59) | 15 | 0.81 | (0.45, 1.34) | 42 | 0.39 | (0.28, 0.53) |
| Adenocarcinoma | 238 | 0.54 | (0.47, 0.61) | 16 | 0.62 | (0.35, 1.01) | 106 | 0.51 | (0.42, 0.61) |
| Large-Cell Carcinoma | 68 | 0.39 | (0.30, 0.50) | 3 | – | – | 9 | 0.17 | (0.08, 0.31) |
| Melanoma of the Skin | 393 | 1.01 | (0.91, 1.12) | 26 | 1.03 | (0.67, 1.51) | 177 | 1.21 | (1.04, 1.40) |
| Breast | 63 | 0.86 | (0.66, 1.10) | 2 | – | – | 1389 | 1.05 | (0.99, 1.11) |
| Female Genital System | 21 | 0.95 | (0.59, 1.45) | 2 | – | – | 498 | 0.97 | (0.88, 1.05) |
| Cervix Uteri | 3 | -- | – | 0 | – | – | 29 | 0.50 | (0.34, 0.72) |
| Corpus and Uterus | 6 | 0.49 | (0.18, 1.08) | 1 | – | – | 323 | 1.13 | (1.01, 1.27) |
| Ovary and Fallopian Tube | 11 | 1.80 | (0.90, 3.22) | 0 | – | – | 122 | 0.87 | (0.72, 1.04) |
| Male Genital System | 3228 | 1.15 | (1.11, 1.19) | 157 | 1.03 | (0.87, 1.20) | 11 | 0.89 | (0.44, 1.59) |
| Prostate | 3169 | 1.15 | (1.11, 1.19) | 149 | 1.02 | (0.86, 1.19) | 11 | 0.90 | (0.45, 1.61) |
| Testis | 45 | 1.31 | (0.96, 1.75) | 7 | 1.28 | (0.51, 2.65) | 0 | – | – |
| Urinary System | 740 | 0.80 | (0.74, 0.85) | 39 | 0.72 | (0.51, 0.98) | 154 | 0.76 | (0.64, 0.88) |
| Urinary Bladder | 411 | 0.70 | (0.63, 0.77) | 26 | 0.83 | (0.54, 1.22) | 60 | 0.64 | (0.49, 0.82) |
| Kidney and Renal Pelvis | 314 | 0.96 | (0.86, 1.07) | 12 | 0.53 | (0.28, 0.93) | 92 | 0.88 | (0.71, 1.07) |
| Brain | 87 | 0.79 | (0.63, 0.97) | 6 | 0.72 | (0.26, 1.57) | 49 | 1.00 | (0.74, 1.32) |
| Endocrine System | 92 | 1.17 | (0.95, 1.44) | 12 | 1.58 | (0.82, 2.76) | 123 | 1.20 | (1.00, 1.44) |
| Thyroid | 82 | 1.15 | (0.92, 1.43) | 11 | 1.55 | (0.77, 2.77) | 118 | 1.20 | (0.99, 1.44) |
| Papillary Carcinoma | 66 | 1.19 | (0.92, 1.52) | 8 | 1.37 | (0.59, 2.70) | 110 | 1.30 | (1.07, 1.57) |
| All Lymphohematopoietic | 830 | 1.07 | (0.99, 1.14) | 40 | 0.76 | (0.54, 1.04) | 330 | 1.00 | (0.89, 1.11) |
| Lymphoid Malignancies | 700 | 1.08 | (1.00, 1.16) | 36 | 0.82 | (0.57, 1.13) | 290 | 1.04 | (0.92, 1.16) |
| Hodgkin Lymphoma | 27 | 0.99 | (0.65, 1.44) | 2 | – | – | 10 | 0.87 | (0.42, 1.60) |
| B-Cell Lymphoma | 624 | 1.12 | (1.03, 1.21) | 32 | 0.85 | (0.58, 1.20) | 265 | 1.09 | (0.96, 1.23) |
| Chronic Lymphocytic Leukemia | 166 | 1.17 | (1.00, 1.36) | 9 | 0.93 | (0.42, 1.76) | 43 | 0.88 | (0.63, 1.18) |
| Diffuse Large B-Cell Lymphoma | 145 | 1.16 | (0.98, 1.37) | 5 | 0.56 | (0.18, 1.32) | 70 | 1.23 | (0.96, 1.55) |
| Marginal Zone Lymphoma | 16 | 0.61 | (0.35, 0.99) | 2 | – | – | 25 | 1.46 | (0.95, 2.16) |
| Follicular Lymphoma | 81 | 1.14 | (0.91, 1.42) | 7 | 1.25 | (0.50, 2.58) | 54 | 1.33 | (1.00, 1.74) |
| Multiple Myeloma | 146 | 1.18 | (0.99, 1.38) | 5 | 0.71 | (0.23, 1.67) | 57 | 1.04 | (0.79, 1.35) |
| T-Cell Lymphoma | 27 | 0.89 | (0.58, 1.29) | 1 | – | – | 8 | 0.69 | (0.30, 1.36) |
| Leukemia | 145 | 1.10 | (0.93, 1.30) | 7 | 0.82 | (0.33, 1.7) | 45 | 0.88 | (0.65, 1.18) |
| Lymphocytic Leukemia | 14 | 0.77 | (0.42, 1.30) | 2 | – | – | 5 | 0.81 | (0.26, 1.88) |
| Myeloid and Monocytic Leukemia | 115 | 1.12 | (0.92, 1.34) | 4 | – | – | 38 | 0.94 | (0.66, 1.29) |
| Acute Myeloid/Monocytic Leukemia | 86 | 1.29 | (1.03, 1.59) | 3 | – | – | 33 | 1.21 | (0.83, 1.69) |
| Chronic Myeloid Leukemia | 25 | 0.76 | (0.49, 1.13) | 1 | – | – | 5 | 0.41 | (0.13, 0.95) |
| Mesothelioma | 21 | 0.85 | (0.53, 1.30) | 0 | – | – | 4 | – | – |
| Myelodysplastic/Myeloproliferati ve Neoplasm1 | 87 | 0.81 | (0.65, 1.00) | 0 | – | – | 38 | 0.78 | (0.55, 1.07) |
| Myelodysplastic Syndrome | 48 | 0.84 | (0.62, 1.12) | 0 | – | – | 19 | 0.95 | (0.57, 1.48) |
| Myeloproliferative Neoplasm | 36 | 0.75 | (0.53, 1.04) | 0 | – | – | 16 | 0.59 | (0.34, 0.95) |
Analyses restricted to 2001-on when these tumors became reportable to central cancer registries
In addition to the elevated SIRs previously reported among private applicators, RSIRs, the site-specific SIR relative to all other cancer sites, were elevated for several sites (Table 3). These included melanoma (RSIR=1.12, CI:1.01–1.24), serous ovarian cancer (RSIR=2.53, CI:1.20–5.30, n=7, not shown), testicular cancer (RSIR=1.45, CI:1.08–1.94), papillary thyroid cancer (RSIR=1.32, CI:1.03–1.68), CLL (RSIR=1.30, CI:1.11–1.51), diffuse large B-cell lymphoma (DLBCL; RSIR=1.29, CI:1.10–1.52), FL (RSIR=1.27, CI:1.02–1.58), and multiple myeloma (RSIR=1.30, CI:1.11–1.54). Among commercial applicators, RSIRs were significantly elevated for prostate (RSIR=1.33, CI:1.09–1.62) and thyroid cancer (RSIR=1.88, CI:1.03–3.42). In addition to the elevated SIRs previously reported among spouses, RSIRs were elevated for breast cancer (RSIR=1.28, CI:1.20–1.37), DLBCL (RSIR=1.39, CI:1.09–1.76), MZL (RSIR=1.64, CI:1.11–2.44), and FL (RSIR=1.50, CI:1.15–1.96).
Table 3.
Relative standardized Incidence Ratios (RSIRs)1 and 95% confidence intervals (CI) adjusted for age, year, race, and sex
| Private Applicators | Commercial Applicators | Spouses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | RSIR | CI | N | RSIR | CI | N | RSIR | CI | |
| Oral Cavity and Pharynx | 198 | 0.76 | (0.66, 0.88) | 10 | 0.59 | (0.31, 1.10) | 44 | 0.80 | (0.59, 1.08) |
| Lip | 63 | 2.46 | (1.92, 3.16) | 4 | – | – | 7 | 1.98 | (0.94, 4.16) |
| Tongue | 35 | 0.50 | (0.36, 0.70) | 2 | – | – | 15 | 1.00 | (0.60, 1.66) |
| Tonsil | 27 | 0.52 | (0.35, 0.75) | 1 | – | – | 3 | – | – |
| Digestive System | 140 7 | 0.95 | (0.90, 1.01) | 84 | 1.00 | (0.79, 1.27) | 540 | 0.90 | (0.82, 0.98) |
| Esophagus | 102 | 0.78 | (0.64, 0.95) | 13 | 1.57 | (0.90, 2.73) | 9 | 0.57 | (0.30, 1.10) |
| Stomach | 114 | 1.01 | (0.84, 1.21) | 2 | – | – | 28 | 0.87 | (0.60, 1.26) |
| Small Intestine | 44 | 1.25 | (0.93, 1.69) | 4 | – | – | 11 | 0.68 | (0.38, 1.23) |
| Colon and Rectum | 842 | 1.06 | (0.98, 1.13) | 49 | 1.08 | (0.80, 1.45) | 346 | 0.97 | (0.87, 1.08) |
| Proximal Colon | 310 | 1.04 | (0.93, 1.17) | 17 | 1.07 | (0.66, 1.73) | 169 | 1.05 | (0.90, 1.22) |
| Distal Colon | 226 | 1.10 | (0.96, 1.25) | 13 | 1.11 | (0.64, 1.93) | 84 | 1.02 | (0.82, 1.27) |
| Rectum and Rectosigmoid Junction | 260 | 1.05 | (0.93, 1.19) | 17 | 1.07 | (0.66, 1.73) | 74 | 0.84 | (0.67, 1.06) |
| Anus, Anal Canal and Anorectum | 8 | 0.49 | (0.24, 0.97) | 1 | – | – | 6 | 0.34 | (0.15, 0.76) |
| Liver and Intrahepatic Bile Duct | 78 | 0.62 | (0.50, 0.77) | 4 | – | – | 21 | 0.79 | (0.52, 1.22) |
| Pancreas | 183 | 0.92 | (0.79, 1.06) | 10 | 0.92 | (0.49, 1.71) | 71 | 0.77 | (0.61, 0.97) |
| Peritoneum, Omentum and Mesentery | 1 | – | – | 0 | – | – | 21 | 2.02 | (1.32, 3.10) |
| Respiratory System | 881 | 0.51 | (0.47, 0.54) | 56 | 0.75 | (0.57, 0.99) | 259 | 0.41 | (0.36, 0.47) |
| Larynx | 66 | 0.53 | (0.42, 0.68) | 4 | – | – | 5 | 0.30 | (0.12, 0.71) |
| Lung and Bronchus | 807 | 0.52 | (0.48, 0.55) | 51 | 0.78 | (0.58, 1.04) | 252 | 0.42 | (0.37, 0.48) |
| Small-Cell Carcinoma | 138 | 0.63 | (0.53, 0.75) | 10 | 0.97 | (0.52, 1.82) | 39 | 0.37 | (0.27, 0.51) |
| Non-Small Cell Carcinoma | 633 | 0.53 | (0.49, 0.58) | 40 | 0.78 | (0.56, 1.08) | 199 | 0.46 | (0.40, 0.53) |
| Squamous Cell Carcinoma | 209 | 0.55 | (0.48, 0.64) | 15 | 0.97 | (0.58, 1.62) | 42 | 0.44 | (0.32, 0.59) |
| Adenocarcinoma | 238 | 0.58 | (0.51, 0.66) | 16 | 0.74 | (0.45, 1.21) | 106 | 0.56 | (0.46, 0.67) |
| Large-Cell Carcinoma | 68 | 0.43 | (0.34, 0.54) | 3 | – | – | 9 | 0.18 | (0.10, 0.35) |
| Melanoma of the Skin | 393 | 1.12 | (1.01, 1.24) | 26 | 1.25 | (0.84, 1.86) | 177 | 1.37 | (1.18, 1.60) |
| Breast | 63 | 0.95 | (0.74, 1.22) | 2 | – | – | 138 9 | 1.28 | (1.20, 1.37) |
| Female Genital System | 21 | 1.05 | (0.68, 1.61) | 2 | – | – | 498 | 1.10 | (1.00, 1.21) |
| Cervix Uteri | 3 | – | – | 0 | – | – | 29 | 0.56 | (0.39, 0.81) |
| Corpus and Uterus | 6 | 0.54 | (0.24, 1.21) | 1 | – | – | 323 | 1.30 | (1.16, 1.46) |
| Ovary and Fallopian Tube | 11 | 1.99 | (1.10, 3.59) | 0 | – | – | 122 | 0.97 | (0.81, 1.16) |
| Male Genital System | 322 8 | 1.44 | (1.38, 1.51) | 157 | 1.36 | (1.12, 1.65) | 11 | 1.00 | (0.55, 1.80) |
| Prostate | 316 9 | 1.44 | (1.38, 1.50) | 149 | 1.33 | (1.09, 1.62) | 11 | 1.01 | (0.56, 1.83) |
| Testis | 45 | 1.45 | (1.08, 1.94) | 7 | 1.55 | (0.73, 3.27) | 0 | – | – |
| Urinary System | 740 | 0.87 | (0.80, 0.93) | 39 | 0.84 | (0.61, 1.17) | 154 | 0.84 | (0.72, 0.99) |
| Urinary Bladder | 411 | 0.76 | (0.69, 0.84) | 26 | 1.00 | (0.67, 1.49) | 60 | 0.71 | (0.55, 0.92) |
| Kidney and Renal Pelvis | 314 | 1.06 | (0.95, 1.19) | 12 | 0.63 | (0.36, 1.12) | 92 | 0.98 | (0.80, 1.21) |
| Brain | 87 | 0.87 | (0.70, 1.07) | 6 | 0.86 | (0.39, 1.93) | 49 | 1.12 | (0.85, 1.49) |
| Endocrine System | 92 | 1.30 | (1.06, 1.60) | 12 | 1.92 | (1.08, 3.41) | 123 | 1.36 | (1.14, 1.63) |
| Thyroid | 82 | 1.28 | (1.03, 1.59) | 11 | 1.88 | (1.03, 3.42) | 118 | 1.36 | (1.13, 1.63) |
| Papillary Carcinoma | 66 | 1.32 | (1.03, 1.68) | 8 | 1.65 | (0.82, 3.33) | 110 | 1.48 | (1.22, 1.79) |
| All Lymphohematopoietic | 830 | 1.20 | (1.11, 1.29) | 40 | 0.90 | (0.65, 1.25) | 330 | 1.13 | (1.01, 1.27) |
| Lymphoid Malignancies | 700 | 1.21 | (1.12, 1.31) | 36 | 0.98 | (0.70, 1.38) | 290 | 1.18 | (1.04, 1.33) |
| Hodgkin Lymphoma | 27 | 1.09 | (0.75, 1.60) | 2 | – | – | 10 | 0.98 | (0.53, 1.82) |
| B-Cell Lymphoma | 624 | 1.26 | (1.16, 1.36) | 32 | 1.02 | (0.71, 1.46) | 265 | 1.24 | (1.09, 1.41) |
| Chronic Lymphocytic Leukemia | 166 | 1.30 | (1.11, 1.51) | 9 | 1.11 | (0.58, 2.16) | 43 | 0.98 | (0.73, 1.33) |
| Diffuse Large B-Cell Lymphoma | 145 | 1.29 | (1.10, 1.52) | 5 | 0.67 | (0.28, 1.63) | 70 | 1.39 | (1.09, 1.76) |
| Marginal Zone Lymphoma | 16 | 0.67 | (0.41, 1.10) | 2 | – | – | 25 | 1.64 | (1.11, 2.44) |
| Follicular Lymphoma | 81 | 1.27 | (1.02, 1.58) | 7 | 1.51 | (0.72, 3.18) | 54 | 1.50 | (1.15, 1.96) |
| Multiple Myeloma | 146 | 1.30 | (1.11, 1.54) | 5 | 0.86 | (0.35, 2.06) | 57 | 1.17 | (0.90, 1.52) |
| T-Cell Lymphoma | 27 | 0.98 | (0.67, 1.43) | 1 | – | – | 8 | 0.77 | (0.39, 1.55) |
| Leukemia | 145 | 1.22 | (1.04, 1.44) | 7 | 0.99 | (0.47, 2.08) | 45 | 0.99 | (0.74, 1.33) |
| Lymphocytic Leukemia | 14 | 0.85 | (0.50, 1.44) | 2 | – | – | 5 | 0.90 | (0.38, 2.17) |
| Myeloid and Monocytic Leukemia | 115 | 1.24 | (1.03, 1.49) | 4 | – | – | 38 | 1.05 | (0.76, 1.45) |
| Acute Myeloid/Monocytic Leukemia | 86 | 1.42 | (1.15, 1.76) | 3 | – | – | 33 | 1.36 | (0.96, 1.91) |
| Chronic Myeloid Leukemia | 25 | 0.84 | (0.57, 1.25) | 1 | – | – | 5 | 0.46 | (0.19, 1.10) |
| Mesothelioma | 21 | 0.94 | (0.61, 1.44) | 0 | – | – | 4 | – | – |
| Myelodysplastic/Myeloproliferativ e Neoplasm2 | 87 | 0.89 | (0.72, 1.10) | 0 | – | – | 38 | 0.87 | (0.63, 1.20) |
| Myelodysplastic Syndrome | 48 | 0.93 | (0.70, 1.24) | 0 | – | – | 19 | 1.06 | (0.68, 1.67) |
| Myeloproliferative Neoplasm | 36 | 0.83 | (0.60, 1.15) | 0 | – | – | 16 | 0.66 | (0.40, 1.07) |
Ratio of the SIR for the cancer site of interest to the SIR for all cancers except the cause of interest
Analyses restricted to 2001-on when these tumors became reportable to central cancer registries
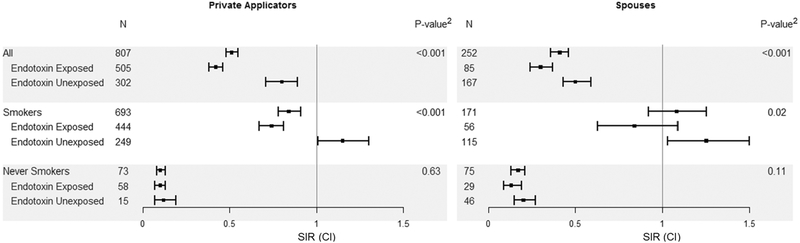
We noted an overall deficit of lung cancers in the cohort (Table 2) for private applicators (SIR=0.51, CI:0.48–0.55), commercial applicators (SIR=0.67, CI:0.50–0.88), and spouses (SIR=0.41, CI:0.36–0.46). Historically, lower incidence of lung cancer in agricultural populations has been attributed to lower rates of smoking, although the literature has also shown that endotoxin exposure is inversely associated with lung cancer risk (29). To evaluate this, we stratified by smoking and computed lung cancer SIRs for private applicators and spouses compared to the general population, which includes smokers and never smokers (Figure 1). For private applicators, SIRs were lower than expected among both ever smokers (SIR=0.84, CI:0.78–0.91) and never smokers (SIR=0.10, CI:0.08–0.13). We further stratified by endotoxin exposure. Among smokers, SIRs were elevated for smokers who did not report endotoxin-related tasks (SIR=1.15, CI:1.01–1.30), while they were lower than expected for smokers reporting endotoxin-related activity (SIR=0.74, CI:0.67–0.81). Similar patterns for endotoxin were observed for spouses, though we did not observe a significant deficit of lung cancers among smokers overall. We also evaluated SIRs stratified by endotoxin separately for former and current smokers. Similarly, SIRs for potentially endotoxin-exposed current and former smokers were significantly lower compared to those who did not report endotoxin-related tasks (p<0.001, not shown).
Figure 1.
Lung cancer standardized incidence ratios (SIRs) and 95% confidence intervals (CI) adjusted for age, year, race, and sex among current and former smoking private applicators and spouses in the AHS cohort, stratified by endotoxin exposure activities at study enrollment1
1Private Applicators: raise farm animals or exposed to grain dust; Spouses: direct contact with farm animals at least once/year or occupationally exposed to grain dust 2Wald test comparing endotoxin exposed and unexposed within categories of smoking
SIRs for private applicators and spouses stratified by state of residence are reported in Supplemental Table 2. There were few sites where the SIRs differed substantially by state. Pancreatic cancer incidence was lower than expected in IA for applicators (SIR=0.72, CI:0.58–0.88) and spouses (SIR=0.61, CI:0.44–0.82). Lung cancer SIRs were lower for IA (SIRprivate=0.34, CI:0.30–0.38) compared to NC (SIRprivate=0.78, CI:0.71–0.85). Among applicators, SIRs for colorectal cancer were lower than expected in IA (SIR=0.83, CI:0.76–0.91) but not NC (SIR=1.07, CI:0.96–1.18), while DLBCL was elevated in NC (SIR=1.38, CI:1.06–1.77) but not IA (SIR=0.96, 96% CI:0.76–1.19). Melanoma, thyroid cancer, and MZL SIRs were elevated in IA spouses only.
DISCUSSION
The lower rate of cancer overall in the AHS cohort is consistent with previous reports (4, 7). Despite this deficit, we found some elevations, including elevated SIRs for prostate and lip cancers, multiple myeloma, DLBCL, and CLL among AHS private applicators, and melanoma, breast cancer, uterine cancer, DLBCL, and MZL among spouses of pesticide applicators that are consistent with previous reports from this cohort. Some elevated SIRs that were previously only observed in RSIR analyses are now apparent in SIR analyses (e.g. uterine cancer among spouses). The previously reported significant excess of ovarian cancer among pesticide applicators was no longer statistically significant, with ten years of additional follow-up and only one additional case (7). We did observe a significant RSIR specifically for the serous subtype. Several sites demonstrated excesses (lip, melanoma, thyroid, FL, AML) or deficits (oral cavity, pancreas, larynx, lung, bladder, kidney) across AHS private applicators, commercial applicators, and spouses.
Contributing to the overall deficit were many cancers associated with known preventable or modifiable risk factors including smoking (e.g. lung, larynx, bladder), HPV (e.g. cervix, oral cavity, anus), obesity (e.g. colorectal [spouses], esophagus, pancreas), and alcohol (e.g. liver, esophagus). Koutros et al. discussed in detail the potential farm-related exposures that may be influencing these findings including pesticides, viral and bacterial exposures related to livestock and poultry, growing up on a farm, sun exposure, and endotoxin (7). Compared to the general population (28), AHS private applicators and spouses report less current smoking (though similar number of cigarettes/day among current smokers), lower alcohol consumption (frequency, number of drinks), and slightly higher BMI, possibly due to greater muscle mass resulting from farming activity. Commercial applicators were similar to the general population in terms of tobacco and alcohol consumption.
Cancer Excesses
This updated analysis also provided the opportunity to evaluate additional cancers with insufficient numbers in previous analyses. We observed elevated SIRs for thyroid cancer, testicular cancer, peritoneal cancer, and AML. Many pesticides have been shown to have thyroid hormone disrupting properties in vivo and/or in vitro (30), suggesting that pesticide exposure may be associated with risk of thyroid cancer. Smoking has been consistently inversely associated with thyroid cancer risk, particularly the most common papillary subtype (31). When results for papillary thyroid cancer in our analysis were stratified by smoking (and compared to the general population including smokers and non-smokers), we noted an elevated SIR among never smoking private applicators (SIR=1.48, 95%CI:1.09–1.99) and no association among current or former smokers (SIR=0.85, 95%CI:0.52–1.30). Thus, it is possible that the elevated SIRs for thyroid cancer in the cohort are largely due to the lower rates of smoking in the AHS compared to the general population, though other occupational risk factors, such as certain pesticides, cannot be completely ruled out. Within the AHS cohort, endocrine disrupting pesticides atrazine (herbicide) and malathion (insecticide) have been associated with thyroid cancer risk in applicators and spouses, respectively (32, 33). A number of pesticide ingredients have also been linked to incident self-reported thyroid disease in AHS applicators (34) and spouses (35, 36). Furthermore, aldrin (insecticide) and pendimethalin (herbicide) have been associated with thyroid hormone dysfunction in AHS male pesticide applicators (37).
Increased incidence of testicular cancer has been suggested in some pesticide-exposed occupational cohorts (38, 39). However, studies examining the effect of specific pesticide exposures on testicular cancer etiology have produced inconsistent findings (40). The evidence is strongest for certain organochlorine insecticides, particularly DDT and its major metabolite, p,p′-DDE, which are thought to have endocrine-disrupting properties (40). In vitro, p,p′-DDE has been shown to completely inhibit binding of [3H]5α-dihydrotestosterone to the androgen receptor (41). The AHS cohort is not an ideal setting to study testicular cancer; the average age of applicators at study enrollment was 45, while the peak incidence occurs at 25–29 for the nonseminomatous germ cell tumors subtype and 35–39 for the seminomatous subtype (40). Despite this, with the most recent update we do observe a statistically significantly elevated RSIR among private applicators with mean age at diagnosis in the AHS of 45 (median=43) for seminomas (n=33) and 42 (median=40) for nonseminomas (n=12).
Peritoneal tumors are rare and often diagnosed at late stage due to a lack of clinical symptoms (42). Among spouses of pesticide applicators, we observed an excess of peritoneal cancers, with 21 incident cases, the majority (n=18) of which were serous peritoneal carcinomas. Recent evidence suggests that most extrauterine high-grade serous carcinomas may be etiologically similar in that they originate from the fimbriated end of fallopian tubes (43). When we evaluated high- and low-grade serous cancers of the ovary, fallopian tube, and peritoneum combined we did not observe significantly elevated incidence (RSIRhigh-grade=1.19, CI:0.89–1.58, RSIRlow-grade=1.17, CI:0.68–2.02). Nearly all peritoneal tumors in AHS spouses were diagnosed in IA (n=18), while incidence for ovarian and fallopian tube tumors was similar for IA and NC, perhaps indicating true regional differences in disease risk or regional variation in pathology practices. While we observe an excess of peritoneal cancers in our analysis, evidence for an etiologic relationship between farm exposures and serous extrauterine tumors based on our findings is relatively weak and requires further examination.
We observed a significantly elevated SIR and RSIR for AML among private applicators, and a non-significantly elevated SIR among spouses. In the previous analysis of cancer incidence in the cohort, Koutros et al. reported elevated AML in RSIR analyses for spouses and applicators, though these elevations did not reach statistical significance (7). A number of occupational risk factors have been associated with AML, such as benzene (44), ionizing radiation (45), and formaldehyde (46, 47), which are unlikely to explain the excess in this cohort. A meta-analysis found that workers exposed to pesticides were at elevated risk of AML, summarizing data from five cohort studies (48). In recent AHS analyses, two herbicides, glyphosate and alachlor, were associated with non-significantly increased risk of AML and myeloid leukemia, respectively (49, 50).
Koutros et al. previously reported an elevated RSIR among private applicators for lip cancer, along with an overall decreased SIR/RSIR for cancers of the oral cavity and pharynx (7). This increase was attributed to potential risk factors such as tobacco use (cigarettes as well as smokeless tobacco) and UV exposure. In our update we calculated SIRs for several specific types of oral cavity cancer, including tongue, tonsil, gum, and pharynx, which all had SIRs less than one, and salivary gland for which we saw no association. We continued to see strong evidence for an excess of lip cancer among private applicators, as well as non-significantly elevated RSIRs for spouses (RSIR=1.98) and commercial applicators (RSIR=1.83, not shown). Compared with the general population, AHS participants were less likely to be ever smokers, but more likely to have used smokeless tobacco products including chewing tobacco, snus, and snuff (51). However, because tobacco is a risk factor for all these cancers, it seems unlikely that differences in tobacco use are alone in explaining the lip cancer excess. Cancers of the external lip (ICD-O-3: C00.0-C00.2) appear to be driving the excess of lip cancers among private applicators (SIR=2.36, CI:1.77–3.08, n=53, not shown), lending support to the hypothesis that UV exposure may, in part, explain the elevated SIR for lip cancer. Additionally, HPV is a known or suspected risk factor for many of the cancers where we see a deficit, including tonsil and base of the tongue, but not for lip (52). We do not have information on HPV status of participants in the cohort, but we suspect HPV prevalence is low based on known risk factors for HPV infection and sociodemographic characteristics of the AHS (53, 54).
Lung Cancer Deficit
The deficit of lung cancer is striking, with SIRs for private applicators ranging from 0.39 for large cell lung cancer to 0.58 for small cell lung cancer. The magnitude of the deficit of lung cancer seemed lower than could be explained solely by differences in smoking. This deficit is seen consistently in farming populations (29), and is similar to that recently reported in a French agricultural cohort (12). We evaluated incident lung cancer in the cohort among smokers (current and former) compared to the general population, hypothesizing that AHS smokers would have an excess of lung cancers compared to the general population comprised of both smokers and non-smokers. Yet even among pesticide applicators who were former or current smokers, there remained significantly lower lung cancer incidence compared to the general population. Therefore, we further explored whether endotoxin exposure may explain this deficit.
Endotoxin has been inversely associated with lung cancer among various occupationally-exposed groups, such as agricultural and textile workers (29). Certain farm activities confer greater probability of endotoxin exposure, for example handling stored grain or working in proximity to farm animals (20). Prior studies in the AHS reported that increasing number of livestock was associated with decreased risk of lung cancer (19), and that endotoxin exposure activities modify the association between diesel exhaust and lung cancer risk (16). Similarly, results from a French agricultural cohort demonstrated that increasing years of farm animal exposure was associated with decreased lung cancer risk in smokers and non-smokers (55). In our analysis, we used information collected at baseline regarding handling of stored grain and proximity to farm animals to assign probable exposure to higher levels of endotoxin (16). Among smokers, those who did not report these high-probability endotoxin exposure activities had elevated lung cancer incidence compared to the general population, which is in line with expectation when comparing a group of smokers to a population which combines smokers and non-smokers. This SIR was small, perhaps reflecting fewer years of smoking or greater time since quitting (among former smokers) compared to the general population. Smoking is a well-established risk factor for lung cancer, with smokers having more than 15 times greater risk of lung cancer diagnosis compared to non-smokers (56); yet, smokers with endotoxin exposure had lower lung cancer risk than expected, even compared to a population comprised of smokers and non-smokers. We did not observe a reduction in lung cancer associated with potential endotoxin exposure among non-smokers. This may be due to very low lung cancer risk in non-smokers (57), or reflect an interaction between endotoxin and tobacco, as has been observed with endotoxin and diesel exhaust in the AHS (16). While these results seem to support the hypothesis that the AHS lung cancer deficit may be tied to both lower rates of smoking and potential endotoxin exposure, some caution is warranted in interpreting these findings. SIRs do not directly evaluate the relationship between endotoxin, smoking, and lung cancer. Due to a limited sample size we did not evaluate SIRS more finely stratified by smoking intensity or duration. Endotoxin-exposed smokers report fewer pack-years compared to unexposed; however, restricting our study population to highly-exposed (>30 pack-year) smokers we still observed significantly lower SIRs among endotoxin exposed farmers compared to unexposed (SIR 1.77 vs. 2.22, respectively, results not shown).
Strengths and Limitations
This updated assessment of cancer incidence in the AHS provided us with the unique ability to evaluate the impact of farm lifestyle and exposures on cancer risk. Since the last cancer incidence evaluation, we have nearly doubled the number of cancer cases in the cohort, allowing for examination of more cancer sites (e.g. testis and thyroid) and finer subtypes of previously evaluated cancer sites (e.g. lung and NHL). The larger number of cases also allowed us to evaluate additional hypotheses, such as whether endotoxin, along with lower rates of smoking, may contribute to the low lung cancer incidence in this agricultural population. However, there are some important, well-known, limitations to the SIR approach. The general population of IA and NC may not represent the cancer experience of AHS participants had they not been living and working on farms. Furthermore, while we were able to standardize the study population according to some important characteristics (i.e., age, sex, race, time period), we were not able to control for other relevant factors such as tobacco use and BMI. We did compare these characteristics in the AHS to data from NHANES to try to understand important demographic and behavioral differences. The differences in age, lifestyle and agricultural exposures among private applicators, spouses, and commercial applicators provides opportunities for hypothesis development for targeted etiologic studies in the future. An important limitation for our lung cancer analysis is classification of smoking status at study enrollment. Prevalence of current smoking in the cohort decreased over time among those who completed enrollment questionnaire and subsequent follow-up interviews, which has implications for lung cancer risk. However, smoking prevalence in the general population also decreased by a similar magnitude during this time (58), and therefore we assume the effect on the SIRs is small. Our evaluation of endotoxin used a classification developed for a prior analysis in the cohort and is based on self-reported activities known to be associated with high levels of endotoxin exposure at study enrollment (16); this self-reported metric is relatively crude and may result in misclassification of endotoxin exposure.
Conclusions
As previously observed, overall cancer incidence in the AHS remains lower than expected compared to the general population. We confirmed excesses of cancers observed in prior analyses, notably prostate cancer, lip cancer, breast cancer, uterine cancer, melanoma, and certain B-cell lymphomas. New in this analysis, we observed elevated SIRs for thyroid cancer, testicular cancer, peritoneal cancer, and AML, as well as differences in lung cancer incidence among smokers that may be due to endotoxin exposure. The observed SIRs are likely due to a range of exposures from behavioral and lifestyle factors to unique occupational and environmental exposures that arise from living and/or working on a farm. These findings will inform future etiologic analyses and focus efforts to better understand the relationship between agricultural exposures and cancer risk.
Supplementary Material
Acknowledgements
This work was supported by the intramural research program of the National Institutes of Health, the National Cancer Institute at the National Institutes of Health (Z01-CP010119), and the National Institute of Environmental Health Sciences at the National Institutes of Health (Z01-ES049030). Data in this analysis are based on Agricultural Health Study releases P1REL201701 and AHSREL201706.
REFERENCES
- 1.Blair A, Zahm SH, Pearce NE, Heineman EF, Fraumeni JF Jr. (1992) Clues to cancer etiology from studies of farmers. Scand J Work Environ Health. 18: 209–15. [DOI] [PubMed] [Google Scholar]
- 2.Acquavella J, Olsen G, Cole P, et al. (1998) Cancer among farmers: a meta-analysis. Ann Epidemiol. 8: 64–74. [DOI] [PubMed] [Google Scholar]
- 3.Kirkeleit J, Riise T, Bjorge T, Christiani DC. (2013) The healthy worker effect in cancer incidence studies. Am J Epidemiol. 177: 1218–24. [DOI] [PubMed] [Google Scholar]
- 4.Alavanja MC, Sandler DP, Lynch CF, et al. (2005) Cancer incidence in the agricultural health study. Scand J Work Environ Health. 31 Suppl 1: 39–45; discussion 5–7. [PubMed] [Google Scholar]
- 5.Eriksson M, Hardell L, Carlberg M, Akerman M. (2008) Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int J Cancer. 123: 1657–63. [DOI] [PubMed] [Google Scholar]
- 6.Fortes C, Mastroeni S, Melchi F, et al. (2007) The association between residential pesticide use and cutaneous melanoma. Eur J Cancer. 43: 1066–75. [DOI] [PubMed] [Google Scholar]
- 7.Koutros S, Alavanja MC, Lubin JH, et al. (2010) An update of cancer incidence in the Agricultural Health Study. J Occup Environ Med. 52: 1098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merhi M, Raynal H, Cahuzac E, Vinson F, Cravedi JP, Gamet-Payrastre L. (2007) Occupational exposure to pesticides and risk of hematopoietic cancers: meta-analysis of case-control studies. Cancer Causes Control. 18: 1209–26. [DOI] [PubMed] [Google Scholar]
- 9.Samanic CM, De Roos AJ, Stewart PA, Rajaraman P, Waters MA, Inskip PD. (2008) Occupational exposure to pesticides and risk of adult brain tumors. Am J Epidemiol. 167: 976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Maele-Fabry G, Libotte V, Willems J, Lison D. (2006) Review and meta-analysis of risk estimates for prostate cancer in pesticide manufacturing workers. Cancer Causes Control. 17: 353–73. [DOI] [PubMed] [Google Scholar]
- 11.Nordby KC, Andersen A, Kristensen P. (2004) Incidence of lip cancer in the male Norwegian agricultural population. Cancer Causes Control. 15: 619–26. [DOI] [PubMed] [Google Scholar]
- 12.Lemarchand C, Tual S, Leveque-Morlais N, et al. (2017) Cancer incidence in the AGRICAN cohort study (2005–2011). Cancer epidemiology. 49: 175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair A, Zahm SH. (1995) Agricultural exposures and cancer. Environ Health Perspect. 103 Suppl 8: 205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramirez CC, Federman DG, Kirsner RS. (2005) Skin cancer as an occupational disease: the effect of ultraviolet and other forms of radiation. International journal of dermatology. 44: 95–100. [DOI] [PubMed] [Google Scholar]
- 15.Merchant J (1987) Agricultural exposures to organic dusts. Occupational medicine (Philadelphia, Pa.). 2: 409–25. [PubMed] [Google Scholar]
- 16.Tual S, Silverman DT, Koutros S, et al. (2016) Use of Dieselized Farm Equipment and Incident Lung Cancer: Findings from the Agricultural Health Study Cohort. Environ Health Perspect. 124: 611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koutros S, Beane Freeman LE, Lubin JH, et al. (2013) Risk of total and aggressive prostate cancer and pesticide use in the Agricultural Health Study. Am J Epidemiol. 177: 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alavanja MC, Hofmann JN, Lynch CF, et al. (2014) Non-hodgkin lymphoma risk and insecticide, fungicide and fumigant use in the agricultural health study. PloS one. 9: e109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman LEB, DeRoos AJ, Koutros S, et al. (2012) Poultry and livestock exposure and cancer risk among farmers in the agricultural health study. Cancer Cause Control. 23: 663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basinas I, Sigsgaard T, Kromhout H, Heederik D, Wouters IM, Schlunssen V. (2015) A comprehensive review of levels and determinants of personal exposure to dust and endotoxin in livestock farming. Journal of exposure science & environmental epidemiology. 25: 123–37. [DOI] [PubMed] [Google Scholar]
- 21.Alavanja MC, Sandler DP, McMaster SB, et al. (1996) The Agricultural Health Study. Environ Health Perspect. 104: 362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz AG. (2000) International Classification of Diseases for Oncology: ICD-O: World Health Organization. [Google Scholar]
- 23.Morton LM, Turner JJ, Cerhan JR, et al. (2007) Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood. 110: 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.NAACCR. (2016) SEER*Stat Database: NAACCR Incidence Data - CiNA Analytic File, 1995–2014, Public Use (which includes data from CDC’s National Program of Cancer Registries (NPCR), CCCR’s Provincial and Territorial Registries, and the NCI’s Surveillance, Epidemiology and End Results (SEER) Registries), certified by the North American Association of Central Cancer Registries (NAACCR) as meeting high-quality incidence data standards for the specified time periods, submitted December 2016.: NAACCR. [Google Scholar]
- 25.Breslow NE, Lubin JH, Marek P, Langholz B. (1983) Multiplicative Models and Cohort Analysis. J Am Stat Assoc. 78: 1–12. [Google Scholar]
- 26.Breslow NE, Day N. (1987) Statistical methods in cancer research Vol II-The design and analysis of cohort studies. [PubMed]
- 27.Pierce DA, Preston DL. (1993) Joint Analysis of Site-Specific Cancer Risks for the Atomic-Bomb Survivors. Radiat Res. 134: 134–42. [PubMed] [Google Scholar]
- 28.CDC. (1995) Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia, USA: Centers for Disease Control and Prevention (CDC). [Google Scholar]
- 29.Lenters V, Basinas I, Beane-Freeman L, et al. (2010) Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Causes Control. 21: 523–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brucker-Davis F (1998) Effects of environmental synthetic chemicals on thyroid function. Thyroid. 8: 827–56. [DOI] [PubMed] [Google Scholar]
- 31.Kitahara CM, Linet MS, Beane Freeman LE, et al. (2012) Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes Control. 23: 1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman LEB, Rusiecki JA, Hoppin JA, et al. (2011) Atrazine and Cancer Incidence Among Pesticide Applicators in the Agricultural Health Study (1994–2007). Environ. Health Perspect. 119: 1253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerro CC, Koutros S, Andreotti G, et al. (2015) Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occupational and environmental medicine. 72: 736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldner WS, Sandler DP, Yu F, et al. (2013) Hypothyroidism and pesticide use among male private pesticide applicators in the agricultural health study. J Occup Environ Med. 55: 1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldner WS, Sandler DP, Yu F, Hoppin JA, Kamel F, Levan TD. (2010) Pesticide use and thyroid disease among women in the Agricultural Health Study. Am J Epidemiol. 171: 455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrestha S, Parks CG, Goldner WS, et al. (2018) Incident thyroid disease in female spouses of private pesticide applicators. Environment international. 118: 282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lerro CC, Beane Freeman LE, Della Valle CT, et al. (2018) Occupational pesticide exposure and subclinical hypothyroidism among male pesticide applicators Occupational and environmental medicine. 75: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleming LE, Bean JA, Rudolph M, Hamilton K. (1999) Cancer incidence in a cohort of licensed pesticide applicators in Florida. J Occup Environ Med. 41: 279–88. [DOI] [PubMed] [Google Scholar]
- 39.Frost G, Brown T, Harding AH. (2011) Mortality and cancer incidence among British agricultural pesticide users. Occupational medicine (Oxford, England). 61: 303–10. [DOI] [PubMed] [Google Scholar]
- 40.McGlynn KA, Trabert B. (2012) Adolescent and adult risk factors for testicular cancer. Nature reviews. Urology. 9: 339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danzo BJ. (1997) Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environ Health Perspect. 105: 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Q, Lin JX, Shi QL, Wu B, Ma HH, Sun GQ. (2011) Primary peritoneal serous papillary carcinoma: a clinical and pathological study. Pathol Oncol Res. 17: 713–9. [DOI] [PubMed] [Google Scholar]
- 43.Liao CI, Chow S, Chen LM, Kapp DS, Mann A, Chan JK. (2018) Trends in the incidence of serous fallopian tube, ovarian, and peritoneal cancer in the US. Gynecol Oncol. 149: 318–23. [DOI] [PubMed] [Google Scholar]
- 44.Loomis D, Guyton KZ, Grosse Y, et al. (2017) Carcinogenicity of benzene. Lancet Oncol. 18: 1574–5. [DOI] [PubMed] [Google Scholar]
- 45.Greaves MF. (1997) Aetiology of acute leukaemia. Lancet. 349: 344–9. [DOI] [PubMed] [Google Scholar]
- 46.Beane Freeman LE, Blair A, Lubin JH, et al. (2009) Mortality from lymphohematopoietic malignancies among workers in formaldehyde industries: the National Cancer Institute Cohort. J Natl Cancer Inst. 101: 751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hauptmann M, Stewart PA, Lubin JH, et al. (2009) Mortality from lymphohematopoietic malignancies and brain cancer among embalmers exposed to formaldehyde. J Natl Cancer Inst. 101: 1696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Maele-Fabry G, Duhayon S, Lison D. (2007) A systematic review of myeloid leukemias and occupational pesticide exposure. Cancer Cause Control. 18: 457–78. [DOI] [PubMed] [Google Scholar]
- 49.Andreotti G, Koutros S, Hofmann JN, et al. (2017) Glyphosate Use and Cancer Incidence in the Agricultural Health Study. J Natl Cancer Inst. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lerro CC, Andreotti G, Koutros S, et al. (2018) Alachlor Use and Cancer Incidence in the Agricultural Health Study: An Updated Analysis. J Natl Cancer Inst. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreotti G, Freedman ND, Silverman DT, et al. (2017) Tobacco Use and Cancer Risk in the Agricultural Health Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 26: 769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blomberg M, Nielsen A, Munk C, Kjaer SK. (2011) Trends in head and neck cancer incidence in Denmark, 1978–2007: focus on human papillomavirus associated sites. Int J Cancer. 129: 733–41. [DOI] [PubMed] [Google Scholar]
- 53.Dunne EF, Unger ER, Sternberg M, et al. (2007) Prevalence of HPV infection among females in the United States. Jama-J Am Med Assoc. 297: 813–9. [DOI] [PubMed] [Google Scholar]
- 54.Han JJ, Beltran TH, Song JW, Klaric J, Choi YS. (2017) Prevalence of Genital Human Papillomavirus Infection and Human Papillomavirus Vaccination Rates Among US Adult Men: National Health and Nutrition Examination Survey (NHANES) 2013–2014. JAMA Oncol. 3: 810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tual S, Lemarchand C, Boulanger M, et al. (2017) Exposure to Farm Animals and Risk of Lung Cancer in the AGRICAN Cohort. Am J Epidemiol. 186: 463–72. [DOI] [PubMed] [Google Scholar]
- 56.Baron JA, Rohan TE. (1996) Tobacco In: Schottenfeld D, Fraumeni JF Jr, eds. Cancer epidemiology and prevention. 2nd ed New York: Oxford University Press; pp. 269–89. [Google Scholar]
- 57.Wakelee HA, Chang ET, Gomez SL, et al. (2007) Lung cancer incidence in never smokers. J Clin Oncol. 25: 472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.CDC. (2009) Cigarette smoking among adults and trends in smoking cessation - United States, 2008 MMWR. Morbidity and mortality weekly report. 2009/November/17 Centers for Disease Control and Prevention (CDC) pp. 1227–32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.