Abstract
Evidence suggests that racial disparities in the HIV care continuum persist in older age groups, particularly among African Americans. The objective of this systematic review was to identify factors that facilitate or hinder older African Americans’ engagement in the HIV care continuum. For studies published between 2003 and 2018, we: (1) searched databases using keywords, (2) excluded non-peer-reviewed studies, (3) limited findings to older African Americans and the HIV care continuum, and (4) retrieved and summarized data focused on barriers and facilitators of the HIV care continuum. Among the 1023 studies extracted, 13 were included: diagnosis/testing (n=1), engagement in care (n=7), and antiretroviral adherence (n=5). Barriers included lack of HIV risk awareness, routine testing, and healthcare access, stigma, and multimorbidities. Social support, health/medication literacy, and increased self-efficacy facilitated engagement. A targeted focus on older African Americans is needed to achieve national goals of improving HIV care and treatment outcomes.
Keywords: Aging, African Americans, health disparities, HIV care continuum, treatment
RESUMEN
La evidencia sugiere que disparidades raciales en el cuidado continuo del VIH persisten en los grupos de mayor edad, particularmente entre los afroamericanos. El objetivo de esta revision sistematica fue identificar los factores que facilitan u obstaculizan la participacion de afroamericanos mayores en el cuidado continuo del VIH. Con respecto a los estudios publicados entre el 2003 y el 2018, nosotros: (1) revisamos bases de datos usando palabras claves, (2) excluimos estudios no revisados por pares, (3) limitamos los resultados a afroamericanos mayores y el cuidado continuo del VIH, y (4) recuperamos y resumimos datos concentrados en las barreras y facilitadores al cuidado continuo del VIH. De los 1023 estudios extraidos, se incluyeron 13: diagnostico / prueba (n = 1), participacion en el cuidado (n = 7) y adhesion antirretroviral (n = 5). Algunas de las barreras incluidas fueron la falta de conciencia de riesgo del VIH, las pruebas rutinarias, y el acceso al cuidado medico, el estigma y las multi-morbosidades. El apoyo social, el conocimiento sobre la salud/medicamentos y una mayor auto-eficacia facilitaron la participacion. Para lograr los objetivos nacionales de mejorar el cuidado del VIH y los resultados de tratamiento es necesario enfocarse en afroamericanos mayores.
INTRODUCTION
Older African Americans in the United States face disproportionate risk of acquiring HIV and suffer from higher rates of HIV morbidity and mortality than older adults of other racial and ethnic groups [1–3]. In 2016, African Americans accounted for 42% of new HIV diagnoses among older adults and constituted the largest proportion (39%) of older people living with HIV (PLWH) [1]. Despite compelling studies indicating that engagement in the HIV care continuum is essential for PLWH to live healthier, longer lives and reduce onward transmission [4–8], older African American PLWH are less likely to engage in the HIV care continuum compared with older populations of other races and ethnicities [9]. The HIV care continuum, also referred to as the HIV treatment cascade, is a model that outlines sequential steps necessary to optimally prevent and treat HIV, from getting tested for HIV and initial diagnosis to achieving viral suppression [10]. Older African American PLWH have higher odds of virologic failure and a mortality risk that is nine times that of older white PLWH [1, 11–12]. Evidence demonstrates that disparities in the engagement in the HIV care continuum have persisted in the antiretroviral treatment (ART) era for African Americans due to multiple social and structural vulnerabilities [13–17]. African Americans suffer disproportionately from poverty, with the lack of economic opportunities and unequal insurance coverage manifesting in delayed entry to HIV care and poorer ART adherence [18–19]. Stigma and discrimination also negatively impact retention in care and viral suppression among African American PLWH [20–21].
The context of aging with HIV is of critical importance since approximately 47% of all PLWH are 50 years of age and older, a growing proportion of whom are African Americans [22]. Although many HIV risk factors remain the same regardless of age, older adults, even if they are sexually active, may not be as aware of their HIV risk compared to younger adults [23]. Additionally, providers are more likely to perceive older adults as at low risk for HIV and as a result, less likely to recommend HIV screening and testing [24–25]. Providers may also mistake signs of HIV infection for common symptoms of aging or age-related conditions [25]. Further, the management of HIV infection is highly complex in the context of multiple treatment effects, multimorbidities, and aging for older adults [26]. Issues related to the loss of social support, HIV stigma, and ageism may pose additional barriers to older PLWH’s engagement in the HIV care continuum [27–31].
Despite older African Americans’ disproportionate risk of HIV infection and lower rates of engagement in the HIV care continuum compared with older adults of other racial and ethnic groups, no literature review exists on factors that facilitate or limit access to HIV treatment and care among this population. Such a review could identify literature available on engagement in the HIV care continuum, inform interventions and services to improve treatment and adherence outcomes, and contribute to the national goal of reducing HIV-related health disparities. The purpose of this review is to identify empirical research that elucidates possible barriers and facilitators related to engagement in the HIV care continuum among older African Americans.
METHODS
The systematic review was conducted utilizing the standard protocol for Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [32]. We used Pubmed/Medline, Academic Search Premier (EBSCO), and Proquest to search for studies published between January 2003 and April 2018. Search terms were: HIV/AIDS, HIV+, African American, Black, older adults, old age, aged, over 50, aging, testing, diagnosis, stigma, retention, adherence, treatment, engagement, and care satisfaction. The second author independently employed these search criteria. The first and third authors independently screened the title and abstract of each article for relevancy. Inclusion was based on agreement between the two reviewers. Discrepancies were discussed between all three authors and then reconciled by the second author. We limited results to those that identified or examined at least one of the five elements of the HIV care continuum, including HIV testing, HIV diagnosis, engagement in care, antiretroviral adherence, and viral suppression. We focused on studies that had a majority representation of older African American PLWH by requiring that the study population was >50% African American/Black and >50% over age 50, with all adults over age 40. We excluded studies if sample demographics were not clearly presented. We also excluded studies that focused on issues such as social support, mental health, substance abuse, and primary HIV prevention without direct linkages to the HIV treatment cascade and care continuum. We excluded editorials, letters, commentaries, literature reviews, and studies conducted outside of the United States. Published peer-reviewed qualitative and quantitative studies in English that met the above inclusion criteria were included in the full-text review.
RESULTS
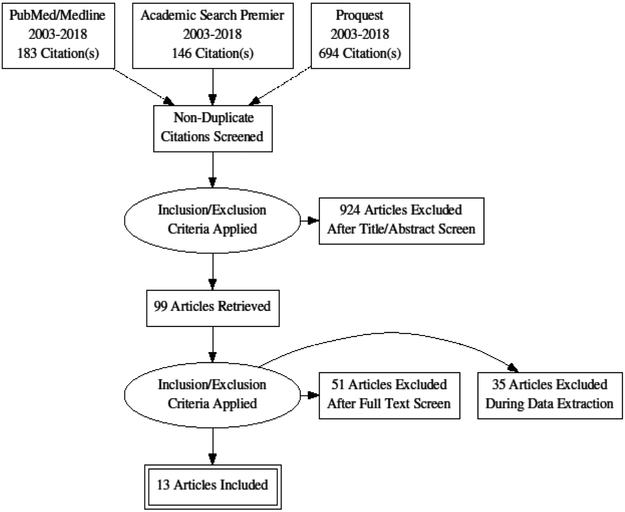
We screened 1,023 relevant titles and abstracts across the three search platforms (Figure 1). 924 studies were excluded as either duplicates or based on screening titles and abstracts. The full text of the remaining 99 papers were reviewed. 86 were excluded. Of these, 51 did not directly relate to the HIV care continuum. 35 were non-empirical studies or did not have a sample that met our inclusion criteria. The remaining 13 papers were reviewed for inclusion in the study: one on testing/diagnosis [33], seven on engagement in care [34–40], and five on ART prescription and adherence [41–45]. Table I outlines the manuscripts included in the review, and identifies and details the sample population, methodology, and key findings of each study.
Figure 1.
PRISMA search strategy
Table I.
Summary of empirical articles on older African Americans and the HIV care continuum in the United States, 2003-2018.
| First Author, Title (Year) | Source of data, location, year(s) | Study Population | N, % AA/Blac k, % male, age, Mean years (SD) | Methodology | HIV Continuum of Care Outcome of Interest | Major Findings |
|---|---|---|---|---|---|---|
| Testing/Diagnosis (1) | ||||||
| Akers et al. (2007) | Primary data collection, Atlanta GA, 2001-2002 | Older women (50 and older) seeking medical care | n =514, 72.6% AA/Black, 0% male, age 50-95, mean age 62.0 (SD 8.1). | Recruitment from existing study, cross-sectional study design, combined qualitative interview and survey. | *Interest in HIV testing *Reason for wanting HIV test |
*Only 22% of older women interested in HIV testing *<25% of women recall ever receiving a provider recommendation *Lack of interest associated with older age, AA race, low HIV knowledge, and low perceived risk of HIV. *Low interest among older, AA women who had moderate-to-high risk of HIV |
| Engagement/Retention in HIV Care (7) | ||||||
| Sangaramoorthy et al. (2017) | Primary data collection, Prince George’s County, MD 2014-2015 | Older Black/AA Women living with HIV | n= 35, 100% AA/Black, 0% male, ages 40-71, mean age 52.3. | Recruitment from local HIV clinics, part of larger mixed methods study, qualitative research design, semi-structured interviews. | *Impact of stigma on engagement in HIV care | *Older, HIV+, African American women experienced intersecting sources of stigma, which negatively impact ability to care for HIV and other comorbid conditions. |
| Warren-Jeanpiere et al. (2017) | Primary data collection, Washington DC, 2011 | Older African American women living with HIV | n= 23, 100% AA/Black, 0% male, ages 53-65, mean age 57. | Recruitment from existing cohort study (Women’s Interagency HIV Study), qualitative study design, focus groups. | *HIV self-management | *Older women struggled to put their own needs in front of others’ needs, especially when prioritizing HIV care. *HIV-stigma described as a barrier that needs to be overcome for women to engage in care. |
| Blake et al. (2017) | Primary data collection, rural Georgia. | Older (50 or older) HIV positive Black men living in rural Georgia, living with HIV for at least 5 years. | n= 35, 100% AA/Black, 100% male, mean age 55.6. | Recruitment from local public health agencies. Qualitative study design, focus groups and semi-structured interviews. | *Coping with Age-related disease/HIV *Access to healthcare resources |
*Aging with comorbid conditions complicated HIV care. *HIV diagnoses commonly viewed as a “wake-up call” to take control of health. *Gaps in services in rural communities can made it difficult to stay engaged in care-particularly related to lack of transportation. *Resources tended to be age and gender specific and may not apply to older men with HIV. |
| DeGrezia & Scrandis (2015) | Primary data collection, unknown location - only “urban”, no dates for data collection. | Older adults living with HIV | n= 40, 88% AA/Black, 42.5% male, age 50-69, mean age 56. | Recruitment from urban HIV support groups. Qualitative study design, focus groups and semi-structured interviews. | * “Stressors” related to HIV management. | *Healthcare related costs, particularly co-pays, made it difficult to manage HIV care and care for multiple comorbid conditions. |
| McDoom et al. (2015) | Primary data collection, Metropolitan Area Boston, 2011 | Older Black/AA Women living with HIV | n= 20, 100% AA/Black, 0% male, age, age 50-63, mean age 56.6. | Recruitment from an HIV clinic in a large hospital, qualitative study design, semi-structured interviews. | * Barriers to staying in HIV care *Facilitators to staying in HIV care |
* Stigma was a barrier to staying in care. *Social support facilitated ability to stay in care. *Providers who expressed understanding of changes associated with aging were found to facilitate greater engagement. |
| Warren-Jeanpiere et al. (2014) | Primary data collection, Washington DC, 2011 | HIV positive African American women over age 50 | n= 23, 100% AA/Black, 0% male, ages 52-65, mean age 57. | Recruitment through existing cohort study (Women’s Interagency HIV Study), qualitative study design, focus groups. | * Barriers and facilitators to managing HIV and co-morbid conditions | *Financial constraints made it difficult to juggle multiple health conditions. *Medication fatigue was a critical concern since so many women were taking multiple medicines for many years. *Newer drugs have made HIV adherence easier. |
| Sheth et al. (2006) | Primary data collection, Johns Hopkins, University Clinic, 1999-2002 | HIV positive patients over age 40 | n=222, 82% AA/Black, 44% male, age, mean age 50.9. | Retrospective cohort design. Random selection of eligible patients from clinic database. Participant information extracted from case files. | * HIV-specific preventative behaviors: vaccination, RPR testing, pap smears (women only) *General health preventive behaviors: mammograms, colon cancer screenings, diabetes related screenings |
* HIV patients were more likely to engage in HIV-specific preventive behaviors (mostly vaccinations) than general screenings. |
| Adherence to ART (5) | ||||||
| Abara et al. 2016 | Secondary data analysis, Medicaid claims from 29 states, 2008-2009 | Older adults (50-64 years) receiving a new HIV or AIDS diagnosis in study period. | n=5,177, 57.6% AA/Black, 62% Male, Age 50-64 (majority under 55). | Retrospective cohort study design, data extracted from Medicare claims records. | * Adherence to ART over 11-month period | *Low levels of optimal adherence (32%) *Males were more adherent than females (APR=1.11,CI 1.02-1.21) *Individuals with co-morbidities were less adherent than those without any co-morbidities. *Rural/small metro areas residents less adherent than those in large metropolitan areas. *No racial differences in optimal adherence. |
| Abara et al. (2014) | Secondary data analysis, Medicaid claims from 14 states (AL, AR, FL, GA, KY, LA, MD, MS, MO, NC, SC, TN, TX, VA), 2005-2007 | Medicaid enrollees age 50-64 diagnosed with HIV or AIDS, 365 days “ART naivete” (no prior use of ART) | n= 801, 75% AA/Black, 55% male, age 50-64, Mean age 54.5 (SD=3.7). | Retrospective cohort design, data extracted from Medicare claims records. | * Time to initiation of ART, measured in days above or below 90. | * About half of sample did not commence ART within 90 days. * Older PLWH with comorbidities were 40% as likely to commence ART promptly compared to older PLWH without comorbidities. *No racial differences in timely initiation of ART were observed. |
| McCoy et al. (2016) | Primary data collection, 9 states (AZ, CA, IL, MA, MI, PA, TX, WA, WI), 2007-2012 | Older adults (50 or older) living with HIV, prescribed ART, history of nonadherence | n= 426, 60% AA/Black, 73% male, age 50-75, mean age 54. | Recruited as part of larger PRIME study, secondary data analysis, cross-sectional study design, telephone-based survey. | *Adherence assessed using self-report missed doses 30 days. *ART Adherence estimated % adherence past 30 days. |
*Mean ART adherence rates averaged between 84% and 92%. *No racial or gender differences in ART adherence rates. *Higher income and increased self-efficacy were associated with being >95% ART adherent. |
| Monroe et al. (2013) | Primary data collection, Maryland, 2011 | Older adults living with HIV and co-morbid diabetes mellitus or hypertension | n = 35, 94% AA/Black, 54% male, age 43-63, mean age 50.8 (SD 5.1). | Recruited from local HIV clinic, cross-sectional mixed-methods study design, focus groups and brief questionnaire. | * Duration of relationship with HIV care provider *Factors affecting ART adherence |
* Comorbid conditions were barriers to adherence. *Simpler regimens and fewer side effects facilitated adherence. *Linking the effects of missed doses to the appearance of symptoms helped promote adherence. *Untreated substance abuse and mental health issues were barriers to adherence. |
| Sangaramoorthy et al. (2017) | Primary data collection, Prince George’s County, MD 2014-2015 | Older Black/AA Women living with HIV | n =35, 100% AA/Black, 0% male, ages 40-71, mean age 52.3. | Recruitment from local HIV clinics, cross-sectional, mixed-methods study design, semi-structured interviews and survey. | *ART Adherence - self-report number of missed doses *Retention in HIV Care - self-report number of missed appointments |
*High-levels of self-reported ART adherence (69% fully adherent). *A majority of sample (86%) reported very high engagement in care, stating that they never missed a HIV-related appointment in the past year. *Experiences of stigma negatively impact both ART adherence and engagement in care. |
Description of Studies:
Among the 13 papers (Table I), there were six qualitative studies (46%) [34–38, 40]. Four studies (31%) were cross-sectional [33, 43–45], three of which used a mixed methods [33, 44–45] research design and one which used a telephone-based survey [43]. The remaining three studies (23%) were retrospective cohort studies [39, 41–42]. Six studies (46%) were conducted with samples that consisted of women only [33–35, 38, 44–45]; one study (8%) was conducted with a sample of only men [36]; and the remaining six studies (46%) were conducted with mixed gender samples [37, 39, 41–44]. Five studies (38%) were conducted with samples consisting of 100% African American PLWH [34–36, 40, 45] and the remaining seven studies (54%) were conducted with mixed race samples that were majority African American [33, 37, 39, 41–44]. The median number of subjects enrolled in each study was 35 with a range of 20–5,177.
Six studies used qualitative interviews and focus groups to understand barriers and facilitators to engagement in the HIV care continuum [34–38, 40]. Three studies used qualitative interviews and validated questionnaires to assess factors that facilitated and impeded engagement in the HIV care continuum [33, 44–45]. Abara et al. used claims for symptomatic HIV disease or AIDS to measure ART initiation [41] and the proportion of prescribed days covered by Medicaid to measure ART adherence [42]. Two studies used self-reports to assess ART adherence [43, 45].
HIV Testing and Diagnosis:
One individual study examined HIV testing and diagnosis among a sample of older women in Atlanta, 73% of whom were African American [33]. Although the study sample had higher HIV testing rates (33%) than older women in national samples (21%), their rates were still low given that more than half of the sample (55%) reported moderate to high-risk factors for lifetime exposure to HIV. Additionally, most women in the study (71%) expressed little to no interest in testing. Factors associated with a lack of interest in testing included low HIV knowledge and low HIV risk perception. Of the women who reported being interested in testing, 39% cited being curious about their HIV status, 23% mentioned concerns related to safety, and 10% indicated reservations about partner’s behaviors as common reasons for expressing interest in testing. Further, less than 25% of the sample reported ever having a provider recommend testing despite national guidelines recommending that providers offer routine testing in areas of high HIV prevalence such as Atlanta [46–47].
Receipt and Retention in Care:
Seven studies explored barriers and facilitators related to receipt and retention in care [34–40]. Only one study measured rates of retention in care among older African American female PLWH, and found that the majority of the sample (86%) reported attending all scheduled HIV-related appointments in the past 12 months, with an average of 4 annual appointments (SD=2)[45]. Participants reported that HIV stigma was a significant barrier to engaging and being retained in care. Older African American PLWH experienced multiple and intersecting stigmas--including those related to HIV, race, gender, sexuality, drug and alcohol use, and incarceration--at intrapersonal, interpersonal, community, and institutional levels which hindered their ability to engage and be retained in care. Additional obstacles to engagement and retention in care included struggling with multimorbidities, lack of money for co-payments, inadequate insurance coverage, being too busy, inflexible work schedules, difficulty accessing transportation, and lack of personalized care (e.g., male-specific education and treatment). Supportive social connections (e.g., family, friends, health care providers, employers, HIV support group members) and HIV clinic-based care (e.g., delivery of disease-specific education and HIV-related health maintenance) facilitated engagement and retention in care among older African American PLWH. Further, social obligations (e.g., caring for family members) and significant life events (e.g., hospitalization, death of a loved one, birth of a grandchild) positively impacted participants’ ability to self-manage HIV. Such responsibilities and incidents prompted an increase in participants’ commitment to self-care in order to stay healthy for themselves and their family members.
Prescription of and Adherence to Antiretroviral Treatment (ART):
Five studies investigated ART initiation and adherence, which varied for samples across the studies [41–45]. One individual study, by Abara et al.[41], evaluated the relationship between race, comorbidity, and timely ART initiation (≤90 days post-HIV/AIDS diagnosis) among a sample of predominantly (75%) older African American PLWH. The authors found that although 48% of older African American ART-eligible participants initiated ART promptly, the median duration to ART initiation was 114 days, the longest of all racial groups [41]. However, they found no racial differences in the timely initiation of ART among the sample; older PLWH who reported a comorbidity were 40% (95% confidence interval = 0.26, 0.61) as likely to initiate ART promptly. In another study, Abara et al. [42] found that among a sample of recently-diagnosed older PLWH between 50 and 64 years of age enrolled in Medicaid, a majority of whom were African American (57%), approximately 32% demonstrated optimal adherence (>95%), with men more likely than women to be optimally adherent to ART.
Two studies found older African American PLWH to be highly ART adherent [43, 45]. McCoy et al. [43] report that in a sample consisting of a majority of older African American PLWH (60%) drawn from an ART adherence intervention trial, 85% of older African American men and 89% older African American women were >95% ART adherent. Race and gender were not associated with ART adherence but higher annual income and increased self-efficacy were associated with being 95% ART adherent. A study among a sample of older African American women found that 33 of the 35 women were actively taking prescribed medication, with 69% reporting that they were fully adherent in the previous week, 26% reporting missing a dose in the previous week, and no one reporting missing more than a single dose in that time [45]. Monroe et al. [44] utilized a qualitative research design based on focus groups and did not directly measure adherence among a sample that consisted of primarily older African American PLWH (94%). Factors that promoted ART adherence included health and medication literacy, simpler ART regimens with fewer side effects, increased self-efficacy as a result of living and aging with HIV, and high annual income. Psychosocial and structural challenges such as untreated substance abuse and mental health issues, medication fatigue, concerns over long-term health effects of ART, stigma, routine financial hardship, the management of co-morbidities, and lack of prescription drug coverage and health care access hindered ART adherence.
None of the included studies specifically measured viral suppression, although one study did report on clinical status of the study sample [39]. Among a sample of largely older African American female PLWH in Baltimore, 80% were on ART and 50% had an HIV-1 RNA less than 400 copies per milliliter suggesting that they were receiving immunologic benefit from ART. In addition, Blank and colleagues [48] reported on a national sample of minority women living with HIV (mean age of 41.9, no breakdown of age range presented). They examined viral suppression among African American/Black (n=226, 63.3%), Hispanic/Latina (n=105, 29.4%), and other (n=26, 7.3%) women living with HIV. Factors associated with increased likelihood of being virally suppressed were reporting good adherence, being Hispanic/Latina, not seeking care, and the impact HIV could have on their families. Reductions in the likelihood of being virally suppressed increased with age, current substance abuse, self-reports of poor health, and reporting 14 or more days of limited activity.
DISCUSSION
We conducted a systematic review of peer-reviewed literature to identify studies that focused on factors that facilitate or hinder older African Americans’ engagement in the HIV care continuum. The studies included highlight several major barriers and facilitators at each step of the HIV care continuum that should be considered in implementing tailored policies and culturally relevant programs that seek to improve engagement in the HIV care continuum for an aging population of African Americans who continue to be disproportionately impacted. These include a targeted focus on older adults within HIV education and prevention programs [33]; providing social, financial, and medical support to those who suffer from multimorbidities [49]; and expanding HIV-clinic based care to improve treatment and adherence outcomes among older African Americans [39].
In their study on HIV testing, Akers et al. underscore the influence of HIV knowledge, risk perception, and risk status on older African American women’s motivation for obtaining HIV testing [33]. Like others, they also emphasize the importance of routine HIV testing for older adults [23, 50]. HIV education and prevention programs and campaigns can play an important role in increasing HIV risk awareness among older adults by shedding the stereotype that older adults are at low risk for HIV [51–54]. For instance, promising educational models such as group education programs delivered by allied health professionals, peer education models, and directly delivered early intervention models (e.g., Senior HIV Intervention Project) could be culturally tailored for older African American PLWH to increase their testing rates and HIV risk awareness [55]. Provider-based interventions also have the potential to increase testing among older African Americans including those that educate providers about up-to-date clinical recommendations for HIV screening and testing, and promote sexual risk assessments, risk-reduction counseling, and HIV testing [56].
The studies that focused on engagement in care and ART adherence described several barriers and facilitators. Social and structural barriers (e.g., intersectional stigma, poverty, lack of access to transportation and healthcare, and low health literacy) as well as multimorbidities were significant barriers. Skill-building and psychotherapeutic-based interventions (e.g., role playing, behavior modeling, social support, and peer-to-peer contact) have been successfully used to reduce HIV stigma and increase self-efficacy among African American PLWH [57–58]. Further work is needed to understand if these existing HIV stigma reduction interventions also reduce perceived barriers to engagement in the HIV care continuum for older adults. It would be useful to also assess whether HIV stigma reduction interventions can be expanded to address multiple dimensions and levels of stigma (e.g., enacted stigma, community-level stigma) or the social norms related to prejudice and discrimination (e.g., race and gender discrimination) that seem to hinder older African Americans’ engagement in the HIV care continuum [59]. Additionally, structural interventions that address poverty, healthcare access, health and medication literacy, and transportation availability should be evaluated and implemented specifically for older African Americans [60–62]. Finally, patient-centered approaches that provide comprehensive HIV care and routinely incorporate chronic management strategies can strengthen efforts to link and retain older PLWH in care and promote adherence to medication [63].
This review has limitations. First, eight of thirteen (62%) studies included small sample sizes (n<50 persons); future studies will need to include larger samples of older African American PLWH to provide more robust analyses and to be more generalizable. Second, social desirability and personal bias may have played a role in participant responses, especially when the study was interviewer-administered rather than computer-assisted. Third, the included studies varied considerably in terms of study design, sample size, and population demographics (e.g., gender, age range, geographic region, etc.). This makes it very difficult to compare the results of these studies with one another and draw definitive conclusions. Fourth, our search was limited to three databases using a discrete list of search terms. Using different terms and searching additional databases might have identified additional studies which could have been included in this review.
CONCLUSION
Clinical and treatment advances have improved the health of PLWH, and as a result, many are living longer. Yet, older African Americans continue to suffer disproportionately from HIV infection, morbidity, and mortality. These disparities persist despite decades of individual-level interventions and an acknowledgement that HIV racial disparities result from structural and social drivers. Designing and implementing tailored policies and culturally relevant interventions that improve treatment and adherence outcomes among older African Americans is essential. There is also a critical need for future research to examine the challenges of managing HIV, as well as conditions traditionally associated with aging, specifically among older African Americans in order to address the complex health and psychosocial issues in this growing but vulnerable population.
Acknowledgments
Funding: The work was supported by a seed grant from the University of Maryland ADVANCE Program [PI: Sangaramoorthy and Dyer] and the US National Institutes of Health’s National Institute of Drug Abuse [grant number R03 DA03713101; PI: Dyer]. The findings and conclusions of this manuscript do not necessarily represent the official views or policies of the University of Maryland or the US National Institutes of Health.
Footnotes
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, Supplemental Report: Diagnoses of HIV Infections Among Adults Aged 50 Years and Older in the United States and Dependent Areas 2011-2016. HIV Surveillance Supplemental Report, 2018;23(5). Available at: http://www.cdc.gov/hiv/library/reports/surveillance/ Accessed November 26, 2018. [Google Scholar]
- 2.Linley L, Prejean J, An Q, Chen M, Hall HI. Racial/ethnic disparities in HIV diagnoses among persons aged 50 years and older in 37 US States, 2005–2008. Am J Public Health. 2012; 102(8): 1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks JT, Buchacz K, Gebo KA, Mermin J. HIV Infection and Older Americans: The Public Health Perspective. Am J Public Health. 2012; 102(8): 1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Vital Signs: HIV Prevention Through Care and Treatment- United States: MMWR, 2011:1618–23. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6047a4.htm. Accesssed November 26, 2018.22129997 [Google Scholar]
- 6.Doshi RK, Milberg J, Isenberg D, et al. High rates of retention and viral suppression in the US HIV safety net system: HIV care continuum in the Ryan White HIV/AIDS Program, 2011. Clin Infect Dis. 2014;60(1): 117–25. [DOI] [PubMed] [Google Scholar]
- 7.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57(8): 1164–71. [DOI] [PubMed] [Google Scholar]
- 8.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588–96. [DOI] [PubMed] [Google Scholar]
- 9.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med. 2013; 173(14): 1337–44. [DOI] [PubMed] [Google Scholar]
- 10.HIV.gov. What is the HIV Care Continuum? Secondary What is the HIV Care Continuum? 2016. Available at:https://www.hiv.gov/federal-response/policies-issues/hiv-aids-care-continuum. Accessed November 26, 2018
- 11.Whiteside OY, Cohen SM, Bradley H, Skarbinski J, Hall I, Lansky A. Progress Along the Continuum of HIV Care Among Blacks with Diagnosed HIV- United States, 2010. MMWR 2014;63(5):85–89. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6305a2.htm. Accessed November 26, 2018. [PMC free article] [PubMed] [Google Scholar]
- 12.Ribaudo HJ, Smith KY, Robbins GK, et al. Racial Differences in Response to Antiretroviral Therapy for HIV Infection: An AIDS Clinical Trials Group (ACTG) Study Analysis. Clin Infect Dis. 2013;57(11): 1607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earnshaw VA, Bogart LM, Dovidio JF, Williams DR. Stigma and racial/ethnic HIV disparities: Moving toward resilience. Am Psychol. 2013;68(4):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brawner BM. A multilevel understanding of HIV/AIDS disease burden among African American women. J Obst Gynecol Neonatal Nurs. 2014;43(5):633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buot M-LG, Docena JP, Ratemo BK, et al. Beyond race and place: distal sociological determinants of HIV disparities. PloS one. 2014;9(4):e91711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis SK, Tucker-Brown A. The effects of social determinants on Black women’s HIV risk: HIV is bigger than biology. J Black Stud. 2013:0021934713481805. [Google Scholar]
- 17.Sharpe TT, Voûte C, Rose MA, Cleveland J, Dean HD, Fenton K. Social determinants of HIV/AIDS and sexually transmitted diseases among black women: implications for health equity. J Womens Health. 2012;21(3):249–54 [DOI] [PubMed] [Google Scholar]
- 18.Nwangwu-Ike N, Frazier EL, Crepaz N, Tie Y, Sutton MY. Racial and Ethnic Differences in Viral Suppression Among HIV-Positive Women in Care. JAIDS. 2018;79(2) [DOI] [PubMed] [Google Scholar]
- 19.Geter A, Sutton MY, Hubbard McCree D. Social and structural determinants of HIV treatment and care among black women living with HIV infection: a systematic review: 2005–2016. AIDS Care. 2018;30(4):409–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turan B, Hatcher AM, Weiser SD, Johnson MO, Rice WS, Turan JM. Framing Mechanisms Linking HIV-Related Stigma, Adherence to Treatment, and Health Outcomes. Am J Public Health. 2017;107(6):863–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brewer RA, Chrestman S, Mukherjee S, et al. Exploring the Correlates of Linkage to HIV Medical Care Among Persons Living with HIV Infection (PLWH) in the Deep South: Results and Lessons Learned from the Louisiana Positive Charge Initiative. AIDS Behav. 2018;22(8):2615–26 [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas, 2016. HIV Surveillance Report. 2017;28 Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-22-2.pdf. Accessed November 26, 2018. [Google Scholar]
- 23.Guo Y, Sims OT. Assessment of recent HIV testing among older adults in the United States. Soc Work Health Care. 2017;56(9):855–64. [DOI] [PubMed] [Google Scholar]
- 24.Davis T, Teaster PB, Watkins JF, Thornton AC, Alexander L, Zanjani F. A Qualitative Approach to Increasing HIV Prevention in Primary Care Settings for Older Adults: Perspectives From Primary Care Providers. J Appl Gerontol. 2016;37(7):840–55. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. HIV Among People Aged 50 and Older, 2018. Available at: https://www.cdc.gov/hiv/group/age/olderamericans/index.html. Accessed November 26, 2018. [Google Scholar]
- 26.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. JAIDS. (1999) 2012;60(Suppl 1):S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emlet CA. “You’re awfully old to have this disease”: Experiences of stigma and ageism in adults 50 years and older living with HIV/AIDS. Gerontologist 2006;46(6):781–90. [DOI] [PubMed] [Google Scholar]
- 28.Emlet CA. An examination of the social networks and social isolation in older and younger adults living with HIV/AIDS. Health & Social Work. 2006;31(4):299–308. [DOI] [PubMed] [Google Scholar]
- 29.Emlet CA. Experiences of stigma in older adults living with HIV/AIDS: A mixed-methods analysis. AIDS Patient Care STDS. 2007;21(10):740–52. [DOI] [PubMed] [Google Scholar]
- 30.Shippy RA, Karpiak SE. The aging HIV/AIDS population: Fragile social networks. Aging Ment Health. 2005;9(3):246–54. [DOI] [PubMed] [Google Scholar]
- 31.Foster PP, Gaskins SW. Older African Americans’ management ofHIV/AFDS stigma. AIDS Care. 2009;21(10): 1306–12. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–69. [DOI] [PubMed] [Google Scholar]
- 33.Akers A, Bernstein L, Henderson S, Doyle J, Corbie-Smith G. Factors associated with lack of interest in HIV testing in older at-risk women. J Womens Health. 2007;16(6):842–58. [DOI] [PubMed] [Google Scholar]
- 34.Sangaramoorthy T, Jamison A, Dyer T. Intersectional stigma among midlife and older Black women living with HIV. Cult Health Sex. 2017;19(12):1329–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warren-Jeanpiere L, Dillaway H, Hamilton P, Young M, Goparaju L. Life begins at 60: Identifying the social support needs of African American women aging with HIV. J Health Care Poor Underserved. 2017;28(1):389–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blake BJ, Taylor GAJ, Sowell RL. Exploring Experiences and Perceptions of Older African American Males Aging With HIV in the Rural Southern United States. Am J Mens Health. 2017;11(2):221–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeGrezia MG, Scrandis D. Successful coping in urban, community-dwelling older adults with HIV. J Assoc Nurses AIDS Care. 2015;26(2):151–63 [DOI] [PubMed] [Google Scholar]
- 38.McDoom MM, Bokhour B, Sullivan M, Drainoni M-L. How older black women perceive the effects of stigma and social support on engagement in HIV care. AIDS Patient Care STDS. 2015;29(2):95–101 [DOI] [PubMed] [Google Scholar]
- 39.Sheth AN, Moore RD, Gebo KA. Provision of general and HIV-specific health maintenance in middle aged and older patients in an urban HIV clinic. AIDS Patient Care STDS. 2006;20(5):318–25 [DOI] [PubMed] [Google Scholar]
- 40.Warren-Jeanpiere L, Dillaway H, Hamilton P, Young M, Goparaju L. Taking It One Day at a Time: African American Women Aging with HIV and Co-Morbidities. AIDS Patient Care STDS. 2014;28(7):372–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abara WE, Smith L, Zhang S, Fairchild AJ, Heiman HJ, Rust G. The influence of race and comorbidity on the timely initiation of antiretroviral therapy among older persons living with HIV/AIDS. Am J Public Health. 2014;104(11):e135–e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abara WE, Adekeye OA, Xu J, Heiman HJ, Rust G. Correlates of combination antiretroviral adherence among recently diagnosed older HIV-infected adults between 50 and 64 years. AIDS Behav. 2016;20(11):2674–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCoy K, Waldrop-Valverde D, Balderson BH, Mahoney C, Catz S. Correlates of antiretroviral therapy adherence among HIV-infected older adults. J Int Assoc Provid AIDS Care. 2016;15(3):248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monroe AK, Rowe TL, Moore RD, Chander G. Medication adherence in HIV-positive patients with diabetes or hypertension: a focus group study. BMC Health Serv Res. 2013;13(1):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sangaramoorthy T, Jamison AM, Dyer TV. HIV stigma, retention in care, and adherence Among older black women living With HIV. J Assoc Nurses AIDS Care 2017;28(4):518–31 [DOI] [PubMed] [Google Scholar]
- 46.United States Preventive Task Force. Human Immunodeficiency Virus (HIV) Infection: Screening, 2013. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/human-immunodeficiency-virus-hiv-infection-screening. Accessed November 26, 2018. [Google Scholar]
- 47.Branson BM, Handsfield HH, Lampe MA, et al. Revised Recommendations for HIV Testing of Adults, Adolescents, and Pregnant Women in Health-Care Settings. MMWR, 2006;55(RR14):1–17. Availabe at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm. November 26, 2018. [PubMed] [Google Scholar]
- 48.Blank AE, Fletcher J, Verdecias N, Garcia I, Blackstock O, Cunningham C. Factors Associated with Retention and Viral Suppression Among a Cohort of HIV+ Women of Color. AIDS Patient Care STDs. 2015;29(Suppl 1):S27–S35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn K, Sanders C, Petroll AE. “HIV is not going to kill me, old age is!”: The intersection of aging and HIV for older HIV-infected adults in rural communities. AIDS Educ Prev. 2017;29(l):62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adekeye OA, Heiman HJ, Onyeabor OS, Hyacinth HI. The New Invincibles: HIV Screening among Older Adults in the U.S. PLoS one. 2012;7(8):e43618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ford CL, Lee S-J, Wallace SP, Nakazono T, Newman PA, Cunningham WE. HIV testing among clients in high HIV prevalence venues: Disparities between older and younger adults. AIDS Care. 2015;27(2): 189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beaulaurier R, Fortuna K, Lind D, Emlet CA. Attitudes and stereotypes regarding older women and HIV risk. J Women Aging. 2014;26(4):351–68. [DOI] [PubMed] [Google Scholar]
- 53.Durvasula R HIV/AIDS in Older Women: Unique Challenges, Unmet Needs. Behav Med. 2014;40(3):85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savasta AM. HIV: associated transmission risks in older adults-an integrative review of the literature. J Assoc Nurses AIDS Care. 2004;15(l):50–59. [DOI] [PubMed] [Google Scholar]
- 55.Milaszewski D, Greto E, Klochkov T, Fuller-Thomson E. A Systematic Review of Education for the Prevention of HIV/AIDS among Older Adults. J Evid Based Soc Work. 2012;9(3):213–30. [DOI] [PubMed] [Google Scholar]
- 56.Bokhour BG, Saifu H, Goetz MB, et al. The role of evidence and context for implementing a multimodal intervention to increase HIV testing. Implement Sci. 2015;10(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosek SG, Lemos D, Harper GW, Telander K. Evaulating the acceptability and feasibility of project ACCEPT: an intervention for youth newly diagnosed with HIV. AIDS Educ Prev. 2011;23(2): 128–44 doi: 10.1521/aeap.2011.23.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rao D, Desmond M, Andrasik M, et al. Feasibility, acceptability, and preliminary efficacy of the unity workshop: an internalized stigma reduction intervention for African American women living with HIV. AIDS Patient Care STDs. 2012;26(10):614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stangl AL, Lloyd JK, Brady LM, Holland CE, Baral S. A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come? J Int AIDS Soc. 2013; 16(3S2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta GR, Parkhurst JO, Ogden JA, Aggleton P, Mahal A. Structural approaches to HIV prevention. Lancet. 2008;372(9640):764–75. [DOI] [PubMed] [Google Scholar]
- 61.Waldrop-Valverde D, Osborn CY, Rodriguez A, Rothman RL, Kumar M, Jones DL. Numeracy Skills Explain Racial Differences in HIV Medication Management. AIDS Behav. 2010;14(4):799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gakumo CA, Enah CC, Vance DE, Sahinoglu E, Raper JL. “Keep it simple”: older African Americans’ preferences for a health literacy intervention in HIV management. Patient Prefer Adherence. 2015;9:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swendeman D, Ingram BL, Rotheram-Borus MJ. Common elements in self-management of HIV and other chronic illnesses: an integrative framework. AIDS Care. 2009;21(10): 1321–34. [DOI] [PMC free article] [PubMed] [Google Scholar]