Introduction
Blood cultures are essential for the evaluation of sepsis. However, they may sometimes be obtained inappropriately, leading to high false positive rates, largely due to contamination.1 As a quality improvement project, clinician decision support tools for evaluating patients with fever or signs and symptoms of sepsis were implemented in April 2014 in our Pediatric Intensive Care Unit (PICU). This initiative resulted in a 46% decrease in blood culture obtainment2 and has been replicated in other institutions3. It is important to evaluate antibiotic use as a balancing measure since a reduction in blood cultures could lead to an increase in antibiotic treatment days if clinicians continued empiric treatment in scenarios when blood culture results were not available. The objective of this study was to evaluate whether antibiotic use in the PICU changed in association with a reduction in blood culture utilization.
Methods
We conducted a retrospective observational study examining antibiotics administered to children admitted to the PICU at The Johns Hopkins Hospital during the 12 months before and during implementation of a locally developed blood culture clinical practice guideline.2 The antibiotic data reflects medication administered to patients while admitted to the PICU. Broad-spectrum antibiotics (BSA) commonly administered for the empiric treatment of sepsis were evaluated including cefepime, piperacillin-tazobactam, meropenem, imipenem-cilastatin, and vancomycin. The intervention primarily targeted hospital-onset events, for which ceftriaxone or fluoroquinolones were not typically prescribed. Antibiotic use was evaluated as: 1) monthly antibiotic days of therapy (DOT) per 1,000 patient-days (PD),4 and 2) monthly number of antibiotic initiations per 1,000 PD. Initiations were defined as the start of a BSA with at least 48 hours elapsed from the last time the patient received a BSA.
The rate of antibiotic DOT per 1,000 PD and antibiotic initiations per 1,000 PD before and after the intervention were compared using a standard incident rate ratio (IRR) and by an interrupted time-series (ITS) model using log-transformed monthly antibiotic DOT and antibiotic initiations5. A preexisting pre-approval antibiotic stewardship program restricted all the antibiotics evaluated, and there were no notable changes in antibiotic stewardship practices during the study period. This evaluation was acknowledged as part of a quality improvement project by the Johns Hopkins Institutional Review Board.
Results
In the year preceding implementation of the guideline, there were 11,196 PD, 6,255 antibiotic DOT and 701 initiations. The proportion of total antibiotic DOT contributed by each medication were as follows: cefepime 36%, vancomycin 31%, piperacillin-tazobactam 23%, meropenem 10%, and imipenem-cilastatin 0.6% with similar distribution in the pre and post years. Compared to the pre-implementation year, there were no changes in the overall antibiotic DOT per 1,000 PD (559 vs 556; IRR 0.99; 95% CI 0.96–1.03). In the ITS analysis (Figure 1, Panel A), the monthly rate of antibiotic DOT per 1,000 PD during the year preceding (IRR 1.00, 95% CI 0.98–1.02) and the year during implementation (IRR 1.00; 95%CI 0.97–1.02) were similar (p=0.90).
Figure 1.
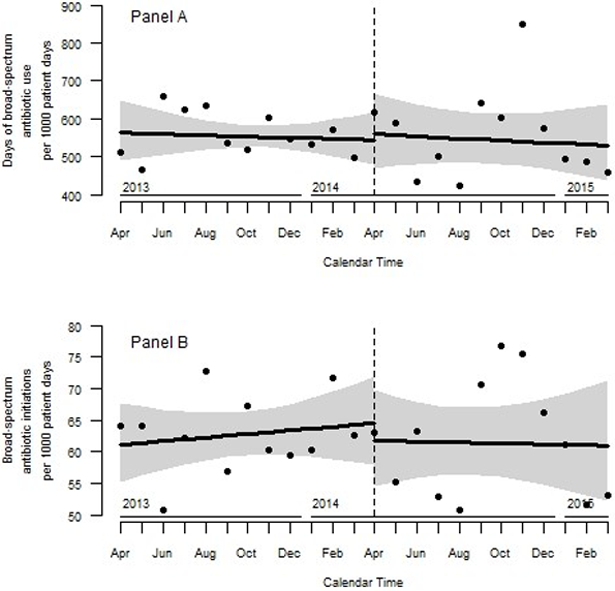
Monthly broad-spectrum antibiotic days of therapy (Panel A) and broad-spectrum antibiotic initiations (Panel B) per 1,000 patient days in the pediatric intensive care unit. Antibiotic use depicted twelve months before and after introduction of a quality improvement initiative in April 2014 to optimize the use of blood cultures in the pediatric intensive care unit.
There were no changes in overall antibiotic initiations per 1,000 PD (63 vs. 62, IRR 0.98; 95% CI 0.89–1.10) in the post-implementation year. Similarly, in the ITS analysis (Figure 1, Panel B), there was no change in the monthly rate of initiations during the year prior to (IRR 1.00; 95% CI 0.99–1.02) and in year during implementation (IRR 1.00; 95%CI 0.98–1.02) (p=0.66).
Discussion
We examined broad-spectrum antibiotic use in the setting of a quality improvement project to optimize blood culture use. Despite a 46% decline in blood cultures following program implementation, there was no change in antibiotic use. A priori, there was concern that some clinicians who complied with the guidelines may have feared “missing” bacteremia and thus increased empiric antibiotic prescribing in scenarios when blood cultures were not obtained. Similarly, there was concern that clinicians would initiate empiric antibiotic therapy and in the absence of blood culture results to follow, would not discontinue therapy after 48–72 hours. Our findings indicate that there was not a significant increase in antibiotic DOT with the reduction in blood culture obtainment.
Prior diagnostic stewardship interventions to improve urine culture testing have demonstrated a reduction in the frequency of urine cultures,6,7 and reduced urine culture utilization was associated with reduced antibiotic use.8,9 In contrast to these findings, we did not observe a decline in antibiotic DOT or initiations associated with a reduction in blood culture utilization. Reasons for this are unclear; however, it is possible the reduction in blood cultures was primarily driven by decreasing the number of cultures obtained from each patient rather than the number of patients from whom blood cultures were obtained. For example, obtaining only a peripheral culture instead of peripheral and central line cultures from the same patient, or obtaining initial blood cultures but not daily follow-up cultures.
A limitation of this study is that we used aggregate antibiotic data. As a result, we were unable to adjudicate indication or appropriateness of antibiotic treatment for individual patients. Perhaps there was a reduction of antibiotic use for the indication of ruling out bacteremia; however, this was coupled with an increase in use of antibiotics for another indication leading to an overall equal rate of use. Alternatively, we may not have had the power to detect a small reduction in antibiotic use in this population given the variability in monthly use. Nevertheless, antibiotic use related to changes in blood culture practice remains an important balancing measure to evaluate. Additional larger, multicenter analyses are needed to better understand the association of improved blood culture use and antibiotic prescribing.
Acknowledgements:
We thank the staff of the Johns Hopkins Children’s Center Pediatric Intensive Care Unit.
Funding: This work was funded in part by National Institutes of Health grant numbers T32-A1052071 (ACS) and K24AI141580 (AM), the Agency for Healthcare Research and Quality grant number R18HS025642 (AMM), and the MITRE Corporation, an independent, not for profit organization that operates federally funded research and development centers (AMM, JCF, SAK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflicts of Interest: AM – Becton, Dickinson and Company - consulting.
References:
- 1.Lamy B, Dargere S, Arendrup MC, Parienti JJ, Tattevin P. How to Optimize the Use of Blood Cultures for the Diagnosis of Bloodstream Infections? A State-of-the Art. Front Microbiol 2016;7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woods-Hill CZ, Fackler J, Nelson McMillan K, et al. Association of a Clinical Practice Guideline With Blood Culture Use in Critically Ill Children. JAMA Pediatr. 2017;171(2):157–164. [DOI] [PubMed] [Google Scholar]
- 3.Woods-Hill CZ, Lee L, Xie A, et al. Dissemination of a Novel Framework to Improve Blood Culture Use in Pediatric Critical Care. Pediatric Quality & Safety. 2018;3(5):e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass G, Willson V, Gottman J. Deisgn and Analysis of Time-Series Experiments. Boulder, Colorado: Associated University Press; 1975. [Google Scholar]
- 6.Epstein L, Edwards JR, Halpin AL, et al. Evaluation of a Novel Intervention to Reduce Unnecessary Urine Cultures in Intensive Care Units at a Tertiary Care Hospital in Maryland, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(5):606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullin KM, Kovacs CS, Fatica C, et al. A Multifaceted Approach to Reduction of Catheter-Associated Urinary Tract Infections in the Intensive Care Unit With an Emphasis on “Stewardship of Culturing”. Infect Control Hosp Epidemiol. 2017;38(2):186–188. [DOI] [PubMed] [Google Scholar]
- 8.Hartley SE, Kuhn L, Valley S, et al. Evaluating a Hospitalist-Based Intervention to Decrease Unnecessary Antimicrobial Use in Patients With Asymptomatic Bacteriuria. Infect Control Hosp Epidemiol. 2016;37(9):1044–1051. [DOI] [PubMed] [Google Scholar]
- 9.Stagg A, Lutz H, Kirpalaney S, et al. Impact of two-step urine culture ordering in the emergency department: a time series analysis. BMJ Qual Saf. 2018;27(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]


