Abstract
Background:
We examined the preliminary effectiveness of a computerized counseling session plus post-incarceration text messaging intervention (CARE+ Corrections) to support ART adherence and linkage/engagement in community care among recently incarcerated HIV-infected persons in Washington, DC.
Methods:
Recently incarcerated HIV-infected persons ≥18 years old were recruited from the DC jail or community outreach and randomized to CARE+ Corrections or control arm. Participants completed assessments at baseline, three-months and six-months. Multivariable random effects modeling identified predictors of suppressed viral load (≤200 copies/mL) and engagement in HIV care at six months.
Results:
Participants (N=110) were aged 42 (IQR: 30-49); 58% male, 24% female, 18% transgender, 85% Black, and incarcerated for a median of 7 years (IQR: 2-15). More controls had a regular healthcare provider at baseline. Although not statistically significant, intervention participants had increased odds of viral suppression versus controls at six months (AOR:2.04; 95%CI: 0.62,6.70). Those reporting high ART adherence at baseline had higher odds of viral suppression at follow-up (AOR:10.77; 95%CI: 1.83,63.31). HIV care engagement was similar between the two groups, although both groups reported increased engagement at six months versus baseline.
Conclusion:
We observed a positive but non-significant association of viral suppression in the CARE+ Corrections group, and care engagement increased in both groups after six months. Further attention to increasing viral suppression among CJ-involved HIV-infected persons upon community reentry is warranted.
Keywords: HIV, engagement in care, incarcerated persons, mHealth
Introduction
Criminal justice (CJ)-involved persons bear a disproportionate burden of HIV infection in the United States (U.S.), with the prevalence of HIV in jails and prisons three to five times higher than the general population (1, 2). The majority of these persons have access to HIV care including antiretroviral therapy (ART) during incarceration, and rates of engagement in care and viral suppression during incarceration exceed national averages (3). However, the benefits of HIV treatment during incarceration may be lost after release to the community (3-6). Social and structural challenges such as unstable housing, unemployment, and substance use can disrupt long-term HIV care engagement or re-engagement during community re-entry (1, 3). Release from a correctional facility has been associated with ART interruption (4), failure to maintain adherence to ART (1, 4, 5), and loss of viral control (6). CJ-involved persons are at increased risk of transmitting HIV to sexual and drug-using partners (4, 7). An estimated one in seven of all persons living with HIV in the U.S. cycles through the CJ system every year (8), thus interventions are urgently needed to achieve and maintain viral suppression and engagement in community HIV care in this population after release from incarceration.
Technology-based interventions have been developed for both HIV treatment engagement and prevention. These tools, including information and communication technology (ICT) and text messaging, or “mHealth,” can be implemented at lower cost and with greater fidelity than human-delivered interventions (9, 10). Computer-delivered motivational counseling interventions have been shown to improve ART adherence and reduce viral load among HIV-infected youth and men who have sex with men (11, 12). Similar findings were reported in a meta-analysis of nine mHealth interventions (13), although others found no difference in outcomes (14-16). Most interventions have focused specifically on medication adherence rather than linkage and retention in care (9). Few studies have assessed technology-based interventions in CJ populations. A recent systematic literature review of interventions in correctional settings identified only four linkage to community treatment programs, none of which were technology-based (2). One study found that persons under community supervision who received a computerized motivational intervention had higher rates of HIV testing and were more likely to consider behavior change to reduce the risk of transmission (17). A recent study focused on addiction treatment, with HIV treatment also considered, evaluated the effect of a computerized motivational intervention on treatment initiation, engagement, and retention among individuals on probation (18). This study found that those in the motiviational computer intervention group versus those experiencing standard probation intake were more likely to initiate substance use treatment after two months (19).
In 2010, the National Institutes of Health funded a research initiative to examine the application of the Seek, Test, Treat, and Retain (STTR) strategy to address the HIV epidemic among CJ-involved persons (20). This strategy involves identifying HIV-infected persons through expanded testing, increasing linkage to HIV providers, improving access and adherence to ART, and maintaining long-term retention in care. To address the Treat and Retain components of the STTR strategy, we launched the CARE+ Corrections Study in Washington, D.C. The prevalence of HIV is 1.9% among all Washington, D.C. residents (21) and was estimated to be 5-6% in the DC Department of Corrections (DOC) facilities at the time of this study (22). Formative work we conducted in Washington DC and Rhode Island among HIV-infected persons with a history of criminal justice involvement indicated acceptability of computer-based counseling and text messaging interventions (23). These tools have the potential to be scalable among correctional facilities and community partners who provide supportive services to reentrants. In an effort to increase engagement in care and viral suppression, the CARE+ Corrections study was conducted to evaluate the potential efficacy of a combined ICT and mHealth intervention designed to improve linkage to community HIV providers and ART adherence among CJ-involved HIV-positive persons following release from a correctional facility. Herein, we report the results of the study examining the preliminary effectiveness of this intervention on HIV viral suppression and engagement in care measured at six months.
Methods
Study Design
The CARE+ Corrections Study (ClinicalTrials.gov: NCT01721226) was originally designed as a randomized, controlled trial to evaluate the efficacy of the CARE+ Corrections intervention and has been described elsewhere (24). In the original design, HIV-infected inmates housed in the District of Columbia Department of Corrections (DC DOC) who were scheduled to be released were to be recruited for the CARE+ Corrections study. Due to administrative and municipal delays, study implementation and participant enrollment within DC DOC facilities were delayed. We were therefore unable to enroll the planned number of participants from the DC DOC directly and were underpowered to fully evaluate the efficacy of the intervention. The study objective was modified to focus on an evaluation of feasibility and preliminary efficacy. Subsequently, eligibility was broadened to include persons in the community who were recently released from a correctional facility as well as persons recruited inside the correctional facilities, thus resulting in a convenience sample of HIV-infected persons with recent involvement in the criminal justice system. To ensure recruitment of females within the DOC, recruitment within correctional facilities was stratified to enroll at least 25% females; recruitment in the community was not stratified based upon gender. Enrollment was stratified by gender among those recruited within correctional facilities because men and women were housed separately in two different buildings; therefore, it was necessary to conduct stratified randomization. However, in the community, men and women were recruited by convenience sample from the community and therefore were not subject to differential or stratified enrollment.
Study Population and Eligibility
As described above, the revised target population included HIV-infected individuals currently incarcerated or recently incarcerated. Study eligibility included a) being at least 18 years old; b) HIV-infected by self-report; c) either 1) being incarcerated in the DC DOC with an anticipated release date within six weeks or 2) residing in the community but having been released from any jail, prison or halfway house within the previous six months (confirmed by study staff using public records); d) plans to remain in the geographic area through the end of the study period; and e) able to provide informed consent for study participation.
Recruitment
Recruitment occurred in both DC DOC facilities and in the community, as described elsewhere (24). Briefly, between June 2014 and April 2015, participants were recruited in either the Central Detention Facility (CDF), which houses men and male-to-female transgender persons, or the Correctional Treatment Facility (CTF), which houses men and women and includes a 100-bed treatment facility for persons with substance use disorders. The screening and recruitment occurred in the medical units of each facility where HIV care was provided by a contracted medical provider. In these locations, study staff provided information about the study to potentially interested participants, conducted eligibility assessments, and obtained written informed consent and enrolled eligible participants. HIV-infected persons incarcerated within the DC DOC received comprehensive HIV care including access to ART during their stay, and discharge planning at the time of release that included the provision of a 30-day supply of HIV medications and a referral to community HIV providers.
Individuals from the community were recruited between August 2013 and April 2015 through a combination of street-based recruitment, advertisement with and referrals from local community-based organizations that provide services to returning citizens (such as the Mayor’s Office of Returning Citizens Affairs, shelters, transitional housing programs, female and transgender-friendly agencies, and drug treatment programs), and referrals from other study participants. Persons interested in the study contacted study staff using a toll-free number and were scheduled for eligibility screening, consent, and completion of the baseline study visit in a private office at a study research clinic in the community.
All enrolled participants were assessed at three months and at six months after the completion of the baseline study visit, continuing through the end of December 2015. Comprehensive retention efforts were used among all study participants and included utilizing phone, email, social media, family contacts and field outreach.
Randomization and the CARE+ Corrections Intervention
Study participants were randomized to the CARE+ Corrections Study intervention or a control arm. The CARE+ Corrections intervention consisted of two components. The first was a single computerized counseling session (“CARE+ Corrections”). This was an adapted version of the CARE+ counseling tool, an interactive, computerized motivational interview that assessed a person’s risk and HIV care behaviors (including HIV care utilization, ART adherence, and retention in HIV care) and provided an individualized risk reduction plan for ART adherence or linkage to care (22, 25, 26). The CARE+ intervention consisted of audio-narrated text with the image of an “avatar” who was selected by the participant to be the counselor for the interactive session. The image of the counselor appeared on each screen of the intervention to walk the participant through the CARE+ session. CARE+ Corrections was customized to specifically provide counseling to HIV-infected persons being released from correctional facilities. Counseling content focused on linkage to community HIV care, ART adherence, and reducing risk behaviors (22, 23). Participants received a printout at the end of the session that summarized goals and provided relevant HIV and social service referrals. The session lasted approximately one hour. The second component of the CARE+ Corrections intervention was a text messaging intervention (CARE+ SMS) following release from a correctional facility. The text message library was developed through extensive prior formative research (23) and included supportive behavioral messaging (e.g., “One day at a time. Just for today, don't use.”), barriers to care following release from a correctional facility (e.g., “Holla at your case manager, they’re here to help”) as well as medication and appointment reminder messages. Participants were able to select from nine pre-scripted messages or create their own messages and select frequency and timing of messaging (e.g., daily, weekly, morning, evening). The messages in the texting component were not directly connected to the content from the CARE+ Corrections counseling session risk reduction plan; however, the participant was able to customize the selection of text messages received based on the content of the risk reduction plan. All participants in the intervention group were offered a cell phone by the study in order to ensure the receipt of study-based text messages. Participants had the option to use their own phone to receive the text message plan and to receive a monthly reimbursement to cover CARE-related messaging costs. Of the 57 randomized to the CARE+ Corrections intervention, 50 (88%) opted to receive the study cell phone.
Participants randomized to the control group received an attention control condition by viewing an educational video on opioid overdose prevention (https://www.youtube.com/watch?time_continue=2&v=_QwgxWO4q38) and received a printout of local HIV providers and resources. The length of the video was approximately equal to the time spent by theintervention group participants completing the CARE+ Corrections counseling session. Participants in the control group did not receive a cell phone or the text messaging intervention but received a list of community referrals that included housing, food, and substance treatment resources.
Data Collection
The baseline visit included an interviewer-administered structured assessment covering domains related to demographic characteristics, criminal justice history, HIV care engagement, medication adherence, sexual and substance use behaviors, and mental health and other co-morbid conditions. Participants provided a whole blood specimen obtained by venipuncture to measure the baseline HIV plasma viral load (PVL) and CD4 cell count and completed an in-depth locator form for study retention purposes. Participants enrolled within DC DOC facilities completed the baseline appointment over two visits so that only non-sensitive behavioral data (e.g., demographics, health care utilization and medication adherence prior to current incarceration) were collected inside of the facility. Following release, participants were scheduled within one week of release to complete the baseline visit (including assessment of sexual and substance use behaviors and venipuncture for a blood specimen to conduct the HIV PVL and CD4 count). Specimens were sent to The Miriam Hospital in Providence, Rhode Island for HIV PVL testing conducted by the Roche Cobas AmpliPrep/ Cobas Taqman HIV-1 Test, Version 2.0. CD4 cell count testing was completed by a commercial lab (LabCorp) via the Becton Dickinson Canto II flow cytometer. When study staff were unable to obtain blood from venipuncture, DOC or community medical records from the six-month period prior to the baseline assessment were reviewed to collect HIV PVL and CD4 count data to establish baseline values. Informed consent for medical records abstraction was obtained at the time of study enrollment before study randomization. Randomization into the two study arms was at a 1:1 ratio in blocks of four, stratified by gender.
Variable Definitions
Main outcomes:
The two primary outcomes of the study were HIV viral suppression (defined as a viral load <200 copies/ml) and self-reported engagement in HIV care six months after completion of the baseline assessment. Congruent with the study follow-up period, we defined engagement in care as “having seen an HIV care provider in the community at least once in the past 24 weeks (6 months)”.
Other covariates:
Gender was defined based on a series of questions about gender identity and sex assignment at birth. For the purposes of this analysis, a participant was categorized as transgender if they reported a “transgender” gender identity or if their reported gender differed from their reported sex assignment at birth. Criminal justice experience included the length of the most recent incarceration and the number of times ever been in jail, prison, or juvenile detention. Sexual risk behaviors in the three months prior to their most recent incarceration were assessed, including condomless sex, exchange sex, number of sexual partners, and male to male sex. Substance use was assessed for alcohol and both injection and non-injection drugs during the three months prior to incarceration, as well as lifetime use of injection drugs. Drug dependence, categorized as “not drug dependent” and “drug dependent”, was assessed using the TCU Drug Dependence scale and hazardous alcohol use was determined using the WHO-AUDIT with participants categorized as low, medium or high hazardous harmful alcohol use, both of which have been previously validated (27, 28). Symptoms related to post-traumatic stress disorder (PTSD) and depression were assessed using the Primary Care PTSD Screen (29) and the CES-D instruments (30), respectively. In addition, participants were asked if they had ever been told by a health care provider that they had a mental health disorder diagnosis (schizophrenia, depression, bipolar disorder, personality disorder). Other HIV care indicators including having an HIV medical provider in the community (i.e., linkage to community care) and self-reported adherence to ART 30 days prior to incarceration and during incarceration were assessed. Adherence was measured by a visual analog scale (VAS) where participants estimated the proportion of prescribed ART doses (0-100%) taken during the previous 30 days (31). Optimal self-reported ART adherence was defined as ≥90%.
Statistical Analyses
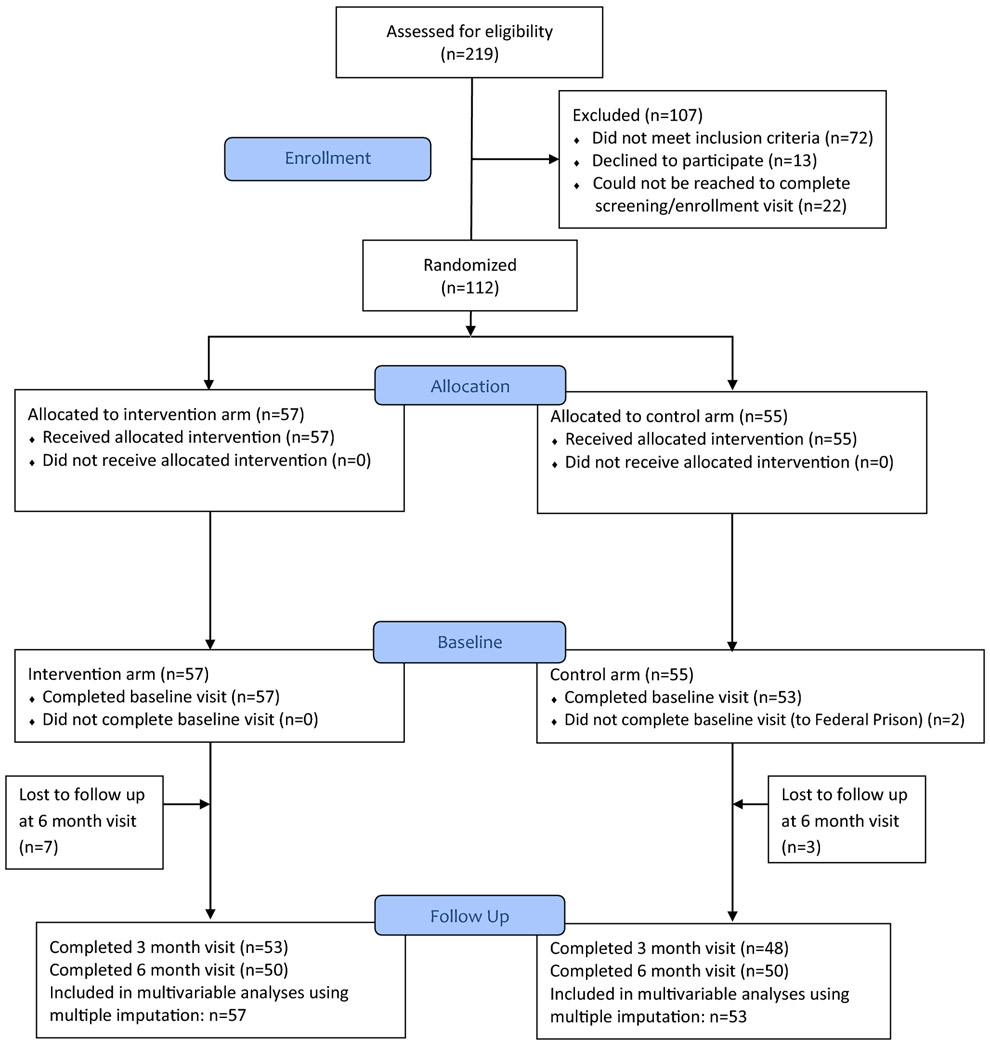
Study enrollment and follow-up were summarized using the recommended Consolidated Standards of Reporting Trials (CONSORT) (32)—see Figure I. Study participants’ demographic characteristics, HIV-related clinical data, and self-reported risk factors at the baseline were summarized by study arms, and further cross-tabulated by study arm and enrollment site. Participants’ baseline characteristics were compared between the two study arms to examine possible chance imbalances. Imbalances were adjusted for in the regression analyses. The primary outcomes (viral suppression and self-reported engagement in care) were summarized by study arm and study visits, including a summary of missing data. The baseline characteristics between study participants having one or more missing values in the primary outcomes and those with complete data were compared.
Figure I:
CONSORT Diagram for CARE+ Corrections Study
To maximize the use of all available follow-up data, we performed multiple imputations using conditional models (33). The conditional models used data from prior follow-up points to impute missing values for subsequent follow-up points, a sequential imputation strategy that mirrors the longitudinal, prospective nature of the study. The sequence of conditional models implicitly led to a fully conditional specification (FCS) model (also known as “chained equations model”) that allowed for imputing intermittent missing outcomes (34). In our multiple imputations, we assumed data were missing at random (MAR) (33), conditional on the study arm, baseline covariates (age, current health status, depression status, unstable housing, VAS, HIV treatment, WHO AUDIT score, and TCU drug dependent score), and the outcomes at the prior visits. The imputations were followed by imputation diagnostics that involved comparing the means, standard deviations and histograms of the imputed data to the observed data, and then by monitoring the Monte Carlo Markov Chains (MCMC) chains while the imputations were processing (35), Through these aforementioned processes, we created K=10 imputed datasets that were used for the subsequent analyses.
Using the imputed datasets, we conducted “univariable” regressions and multivariable regression analyses to compare the two study arms while adjusting for various baseline variables. In the “univariable” regression models, the intervention was “fixed” as a main effect and included one of the remaining baseline covariates each time as a model predictor. From the univariable regression models, the baseline covariates whose effects had a p-value of <0.10 were further included in the multivariable regression model. Imbalanced variables at the baseline also were included in the multivariable model. We used random effects logistic models to compare the viral suppression status at three and six months follow-up between the two study arms, where a random intercept was included to account for the repeated measures from the two follow-up visits. Because engagement in care was defined as having at least one HIV provider visit in the past six months, we fitted logistic models to compare this outcome at the six month visit between the two study arms. The model parameter estimation and standard errors were calculated using the standard formulae for analyses based on multiply imputed datasets (33).
All database management was performed using SAS (version 9.4, Cary, North Carolina) and statistical analyses using STATA (version 14.1, College Station, Texas).
Human Subjects Research
This study was reviewed and approved by the Institutional Review Boards at George Washington University and The Miriam Hospital. Approval from the U.S. Office on Human Research Protections was obtained. All participants provided written, informed consent prior to enrolling in the study. A U.S. Certificate of Confidentiality was also obtained to protect the confidentiality of participant responses.
Results
Of 219 persons assessed for eligibility, 112 (51%) were enrolled and randomized (Figure 1). Among the 107 who did not participate, 72 did not meet eligibility criteria, 13 declined to participate, and 22 were not able to be reached to complete screening and/or enrollment. Of those enrolled, 57 (51%) were randomized to the CARE+ Corrections intervention group and 55 (49%) to the control group; two control participants did not complete the baseline visit, and were therefore dropped from the study, leaving a total of 110 participants who contributed to the analyses. Study retention was 92% at the 3 month visit, 91% at the 6 month visit and similar across both study arms. Although 41 of 110 (37.3%) had experienced reincarceration during the six month follow-up period, 96 of 110 (87.2%) completed all three study visits.
Table I displays descriptive characteristics of the study population, stratified by arm. Overall, 30% of the study population was recruited from the DC DOC, while 70% was recruited through community outreach, and 58% were male, 24% female, and 18% male-to-female transgender (TG). At enrollment, median age was 42 (IQR: 30-49), and nearly two-thirds (63%) of the sample had been in jail/prison five or more times for a median duration of 7 years (IQR: 2-15 years) in their lifetime (data not shown), and one-third (35%) having spent more than 10 years in jail and/or prison. There were no significant differences between participants randomized to the intervention or control groups by location of enrollment, demographic characteristics, and other health care characteristics, except participants in the control arm were significantly more likely to report having a regular community health care provider at study enrollment (88.7% vs. 71.9%, respectively, p=0.03) compared to participants in the intervention arm. The proportion of participants reporting any mental health diagnoses (schizophrenia, personality disorder, depression, or bi-polar) was higher in the control arm than the intervention arm (90.6% vs. 80.7%, p=0.14), although these differences were not statistically significant. “Having a regular health care provider at enrollment” and “any mental health diagnoses” were later adjusted for in our regression analyses.
Table I:
Demographic characteristics and potential confounding variables for the CARE+ Corrections Study, by study arm (N=110)
| Study Arm | Total (N=110) | p- valuea |
|||||
|---|---|---|---|---|---|---|---|
| Control (n=53) |
Intervention (n=57) |
||||||
| n | % | n | % | n | % | ||
| Enrollment location | |||||||
| Community | 40 | 75.47 | 37 | 64.91 | 77 | 70 | 0.23 |
| DOC | 13 | 24.53 | 20 | 35.09 | 33 | 30 | |
| Age, median (IQR), years | 39 | (30-47) | 42 | (30-50) | 41.5 | (30-49) | 0.22 |
| Gender | |||||||
| Male | 31 | 58.49 | 33 | 57.89 | 64 | 58.18 | 0.97 |
| Female | 12 | 22.64 | 14 | 24.56 | 26 | 23.64 | |
| Transgender (MTF) | 10 | 18.87 | 10 | 17.54 | 20 | 18.18 | |
| Race/ethnicity | 2 | 3.77 | 2 | 3.51 | 4 | 3.64 | |
| Non-Hispanic White | 1.00b | ||||||
| Non-Hispanic Black | 45 | 84.91 | 49 | 85.96 | 94 | 85.45 | |
| Hispanic and other | 6 | 11.32 | 6 | 10.53 | 12 | 10.91 | |
| Sexual orientation | |||||||
| Heterosexual or straight | 39 | 73.58 | 45 | 78.95 | 84 | 76.36 | 0.51 |
| Homosexual, gay, lesbian, or Bisexual | 14 | 26.42 | 12 | 21.05 | 26 | 23.64 | |
| Number of times in jail/prison | |||||||
| <=5 | 16 | 30.19 | 23 | 40.35 | 39 | 35.45 | 0.51 |
| 5-10 | 16 | 30.19 | 16 | 28.07 | 32 | 29.09 | |
| >10 | 20 | 37.74 | 17 | 29.82 | 37 | 33.64 | |
| Missing | 1 | 1.89 | 1 | 1.75 | 2 | 1.82 | |
| # of yrs in jail/prison lifetime | |||||||
| <=2 | 15 | 28.3 | 18 | 31.58 | 33 | 30 | 0.32 |
| 2-10 | 21 | 39.62 | 16 | 28.07 | 37 | 33.64 | |
| >10 | 15 | 28.3 | 23 | 40.35 | 38 | 34.55 | |
| Missing | 2 | 3.77 | 0 | 0 | 2 | 1.82 | |
| Current health status (RAND36) | |||||||
| Good-Excellent | 39 | 73.58 | 37 | 64.91 | 76 | 69.09 | 0.33 |
| Poor-fair | 14 | 26.42 | 20 | 35.09 | 34 | 30.91 | |
| Regular community healthcare provider pre-incarceration | |||||||
| No | 6 | 11.32 | 13 | 22.81 | 19 | 17.27 | 0.11 |
| Yes | 47 | 88.68 | 44 | 77.19 | 91 | 82.73 | |
| Regular community healthcare provider at study baseline | |||||||
| No | 6 | 11.32 | 16 | 28.07 | 19 | 17.27 | 0.03 |
| Yes | 47 | 88.68 | 21 | 71.93 | 91 | 82.73 | |
| Any mental health diagnosis* | |||||||
| No | 5 | 9.43 | 11 | 19.3 | 16 | 14.55 | 0.14 |
| Yes | 48 | 90.57 | 46 | 80.7 | 94 | 85.45 | |
| PTSD screener score | |||||||
| Not positive (<3) | 33 | 62.26 | 29 | 50.88 | 62 | 56.36 | 0.23 |
| Positive (3-4) | 20 | 37.74 | 28 | 49.12 | 48 | 43.64 | |
| Unstable housing, past 3 mo** | |||||||
| No | 44 | 83.02 | 45 | 78.95 | 89 | 80.91 | 0.59 |
| Yes | 9 | 16.98 | 12 | 21.05 | 21 | 19.09 | |
| Currently taking HIV treatment(baseline) | |||||||
| No | 7 | 13.21 | 8 | 14.04 | 15 | 13.64 | 0.90 |
| Yes | 46 | 86.79 | 49 | 85.96 | 95 | 86.36 | |
| Ever taken HIV treatment | |||||||
| No | 4 | 7.55 | 6 | 10.53 | 10 | 9.09 | 0.74b |
| Yes | 49 | 92.45 | 51 | 89.47 | 100 | 90.91 | |
| Received ARV prescription before baseline incarceration | |||||||
| N/A (never taken HIV tx) | 4 | 7.55 | 6 | 10.53 | 10 | 9.09 | 0.23 |
| No | 11 | 20.75 | 17 | 29.82 | 28 | 25.45 | |
| Yes | 38 | 71.7 | 34 | 59.65 | 72 | 65.45 | |
| ARV adherence (visual analgog scale) - pre-incarceration | |||||||
| N/A (not currently on HIV tx) | 15 | 28.3 | 23 | 40.35 | 38 | 34.55 | 0.49 |
| 0%-80% | 17 | 32.08 | 18 | 31.58 | 35 | 31.82 | |
| 90%-100% | 21 | 39.62 | 16 | 28.07 | 37 | 33.64 | |
| Hazardous or harmful alcohol use (WHO AUDIT score) | |||||||
| Low (0-7) | 31 | 58.49 | 30 | 52.63 | 61 | 55.45 | 0.30 |
| Medium (8-15) | 10 | 18.87 | 7 | 12.28 | 17 | 15.45 | |
| High (16-40) | 12 | 22.64 | 20 | 35.09 | 32 | 29.09 | |
| TCU± Drug Dependence score | 0.80 | ||||||
| Not drug dependent (0-2) | 22 | 41.51 | 25 | 43.86 | 47 | 42.73 | |
| Drug dependent (3-9) | 31 | 58.49 | 32 | 56.14 | 63 | 57.27 | |
| Ever injected drugs | 0.36 | ||||||
| no | 47 | 88.68 | 47 | 82.46 | 94 | 85.45 | |
| Yes | 6 | 11.32 | 10 | 17.54 | 16 | 14.55 | |
| Recent non-injection drug use (3 months pre-incarceration) | 0.89 | ||||||
| No | 23 | 43.4 | 24 | 42.11 | 47 | 42.73 | |
| Yes | 30 | 56.6 | 33 | 57.89 | 63 | 57.27 | |
Schizophrenia, personality disorder, depression, bi-polar.
Living on the streets or staying in a single room occupancy hotel or shelter.
TCU = Texas Christian University
Based on Pearson’s Chi-square test or Student’s t-test unless otherwise specified
Fisher’s exact test.
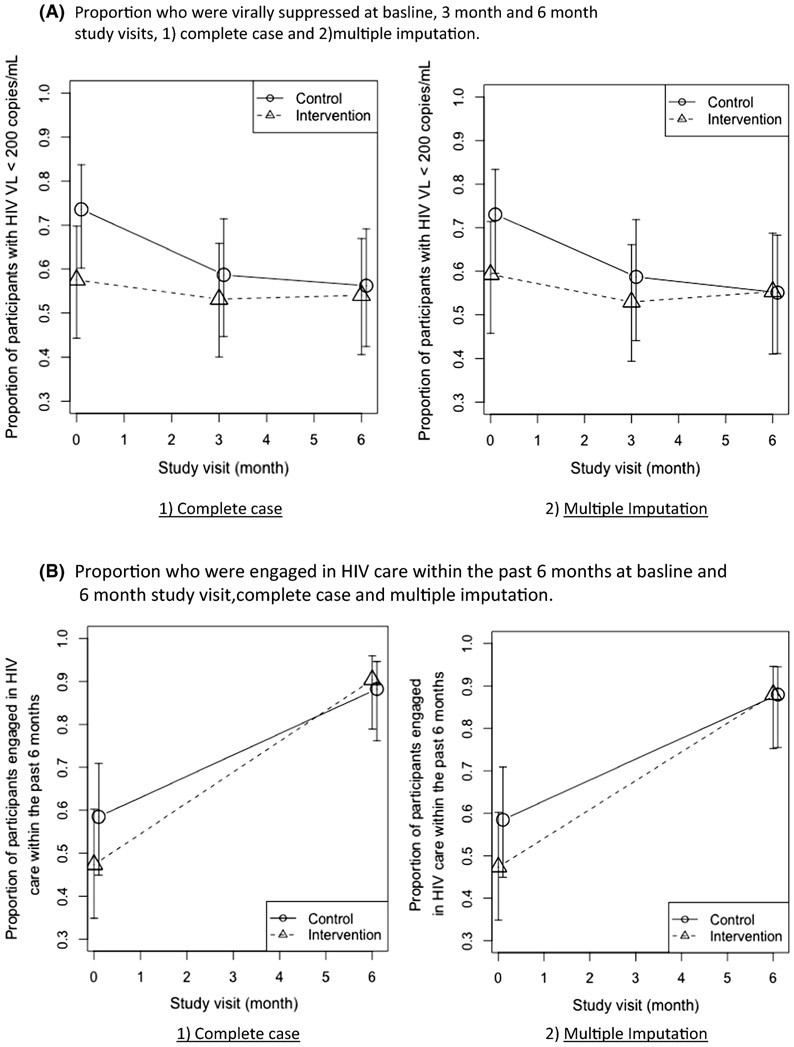
Figure IIA displays the proportions of intervention and control participants who had viral suppression at each study visit. There were no differences in the proportion of persons with viral suppression in the CARE+ Corrections versus the control group at the study enrollment (59% versus 73%, p=0.13, complete case). Similarly, there were no statistically significant differences in viral suppression in either group at the three-month and six month visits (p=0.57 and p=0.98 respectively, complete case). Figure IIB displays the proportions of persons who were engaged in HIV care within the past six months as reported at baseline and at the six month follow up visit. The proportion of persons reporting an HIV-related health care visit in the previous six months increased substantially from the baseline assessment to the six month study visit (47% among the intervention group and 59% among the control group to 90% and 88%, complete case, respectively, both p values < 0.01), but this increase did not differ across the CARE+ Corrections intervention and control groups (p=0.52). These estimates and p-values changed little when the analyses were repeated using imputed data (Table II).
Figure II:
Proportion of participants who were A) virally suppressed (<200 copies/mL) and B) engaged in HIV care at enrollment and across study visits, CARE+ Corrections Study.
Table II:
Proportions of viral suppression and engagement in care in treatment and control groups; 1) complete case and 2) multiple imputation
| Complete Case | Multiple Imputation | ||||
|---|---|---|---|---|---|
| Control | CARE | Control | CARE | ||
| Viral suppression | month 0 | 0.73 (0.59, 0.83); NA=1 |
0.59 (0.46, 0.71); NA=3 |
0.74 (0.602, 0.837) | 0.58 (0.44, 0.70) |
| month 3 | 0.59 (0.44, 0.72); NA=7 |
0.53 (0.39, 0.66); NA=6 |
0.59(0.45, 0.71) | 0.53 (0.40, 0.66) | |
| month 6 | 0.55 (0.41, 0.68); NA=4 |
0.55 (0.41, 0.69); NA=10 |
0.56(0.42, 0.69) | 0.54 (0.41, 0.67) | |
| Engagement in care | month 0 | 0.59 (0.45, 0.71) | 0.47 (0.35, 0.60) | 0.59(0.45, 0.71) | 0.47 (0.35, 0.60) |
| month 6 | 0.88 (0.76, 0.95); NA=2 |
0.90 (0.79, 0.96); NA=5 |
0.88(0.76, 0.95) | 0.88 (0.75, 0.95) | |
NA: # of missing values
Viral suppression at six months
Univariable and multivariable analyses for viral suppression using a random effects logistic regression modeling are displayed in Table III. Gender, race, current health status, having a regular health care provider at baseline, having ever engaged in HIV treatment, and reporting higher ART adherence was associated with viral suppression at six months (p<=0.10) and were therefore adjusted for in the multivariable model. In the multivariable model, those receiving the CARE+ Corrections intervention were twice as likely to have viral suppression at the six month visit compared to those in the control group, but this association was not statistically significant (AOR=2.04; 95% CI: 0.62, 6.70). When combining both study arms, the odds of achieving viral suppression was lower at study follow up visits compared with the baseline visit (AOR=0.29; 95% CI: 0.03, 0.10 at three months; and AOR=0.27; 95% CI: 0.03, 0.9 at six months). Self-reported ART adherence at ≥ 90% on the VAS at the baseline visit compared to not being on ART was also independently associated with viral suppression at six months (AOR=27.8; 95% CI: 1.83, 63.31).
Table III:
Univariable and multivariable random effects logistic model for viral suppression (<200 copies/mL) at the study follow up visits in the CARE+ Corrections Study, N=110
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | p- | 95% CI | A | p- | 95% CI | ||||
| Study arm | Control | ||||||||
| Intervention | 1.5 | 0.47 | 0. | 5.1 | 2.0 | 0.24 | 0. | 6.7 | |
| Visit | Baseline | ||||||||
| 12 wks | 0.3 | 0.06 | 0. | 1.0 | 0.2 | 0.03 | 0. | 0.8 | |
| 24 wks | 0.3 | 0.05 | 0. | 0.9 | 0.2 | 0.03 | 0. | 0.8 | |
| Enrollment location | Community | ||||||||
| DOC | 0.6 | 0.49 | 0. | 2.5 | |||||
| Age | Per 10 years | 1.5 | 0.14 | 0. | 2.9 | ||||
| Gender | Male | ||||||||
| Female | 0.2 | 0.12 | 0. | 1.3 | 0.2 | 0.09 | 0. | 1.2 | |
| Transgender (MTF) | 4.6 | 0.10 | 0. | 29. | 2.2 | 0.39 | 0. | 13. | |
| Race/ethnicity | Non-Hispanic | ||||||||
| Non-Hispanic | 5.4 | 0.08 | 0. | 35. | 5.5 | 0.05 | 0. | 31. | |
| Sexual Orientation | Heterosexual or Homosexual/gay/l | 3.2 | 0.16 | 0. | 15. | ||||
| # of times in jail/prison | <=5 | ||||||||
| 6-10 | 3.0 | 0.19 | 0. | 15. | |||||
| >10 | 1.3 | 0.71 | 0. | 6.6 | |||||
| # of yrs in jail/prison (lifetime) | <=2 | ||||||||
| 2-10 | 0.7 | 0.69 | 0. | 3.7 | |||||
| >10 | 0.6 | 0.57 | 0. | 3.1 | |||||
| Current health status (RAND36) | Good-excellent | ||||||||
| Fair-poor | 0.3 | 0.10 | 0. | 1.2 | 0.7 | 0.65 | 0. | 2.8 | |
| Regular healthcare provider pre-incarceration | No | ||||||||
| Yes | 1.1 | 0.90 | 0. | 6.4 | |||||
| Regular healthcare provider at Baselineb | No | ||||||||
| Yes | 8.1 | 0.02 | 1. | 45. | 1.3 | 0.77 | 0. | 7.6 | |
| Depressive symptoms | No | ||||||||
| Yes | 0.9 | 0.97 | 0. | 3.6 | |||||
| Any MH diagnosis*, b | No | ||||||||
| Yes | 1.1 | 0.90 | 0. | 7.2 | 2.3 | 0.35 | 0. | 13. | |
| PTSD | No | ||||||||
| Yes | 1.4 | 0.59 | 0. | 5.5 | |||||
| Unstable Housing** | No | ||||||||
| Yes | 0.3 | 0.25 | 0. | 2.0 | |||||
| Hazardous/harmful alcohol use | Low to none | ||||||||
| Medium | 0.4 | 0.43 | 0. | 3.0 | |||||
| High | 1.2 | 0.76 | 0. | 5.8 | |||||
| TCU± Drug dependence score | Not drug dependent (0-2) | ||||||||
| Drug dependent | 0.6 | 0.57 | 0. | 2.5 | |||||
| Ever injected drugs | No | ||||||||
| Yes | 1.2 | 0.83 | 0. | 8.1 | |||||
| Recent non-injection drug use (3mos pre-incarceration) | No | ||||||||
| Yes | 0.5 | 0.39 | 0. | 2.1 | |||||
| Currently taking HIV treatment (baseline) | No | ||||||||
| Yes | 27. | 0.00 | 3. | 241 | 6.5 | 0.08 | 0. | 54. | |
| Ever taken HIV treatment | No | ||||||||
| Yes | 31. | 0.01 | 2. | 380 | |||||
| Had ARV prescription pre-incarceration | No | ||||||||
| Yes | 7.9 | 0.01 | 1. | 39. | |||||
| ARV adherence (visual analogscale)—pre-incarceration | Not currently taking ARVs | ||||||||
| 0-80% | 4.1 | 0.07 | 0. | 19. | 1.5 | 0.63 | 0. | 8.0 | |
| 90-100% | 27. | 0.00 | 5. | 153 | 10. | 0.01 | 1. | 63. | |
Independent variables having a p-value <0.10 were included in the multivariable regression model;
“Regular healthcare provider” and “Any MD diagnosis” exhibited some imbalance at baseline (p<0.15; see Table I) and were included in the multivariable regression model.
Schizophrenia, personality disorder, depression, bi-polar.
Living on the streets or staying in a single room occupancy hotel or shelter.
TCU = Texas Christian University
Engagement in HIV care at six months
In univariate analyses, the location of study enrollment, gender, sexual orientation, depressive symptomatology, and positive PTSD score were associated with being engaged in HIV care in the past six months at the final study visit (Table IV). Adjusting for these characteristics, the odds of being engaged in HIV care at the final study visit was not significantly different between those in the CARE+ Corrections arm versus the control arm (AOR=1.18; 95% CI: 0.25, 5.53). Adjusting for study arm, persons recruited from within the DC DOC were significantly less likely to be engaged in HIV care at six months (AOR=0.07; 95% CI: 0.02, 0.29) versus those recruited from within the community. In addition, screening positive for PTSD was independently associated with being engaged in HIV care at six months compared to those who screened negative for PTSD (AOR=3.37; 95% CI: 1.14, 9.97).
Table IV:
Univariable and multivariable random effects logistic model for engagement in HIV care within the past 6 months at the 6 month visit in the CARE+ Corrections Study, N=110.
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | p- | 95% CI | A | p- | 95% CI | ||||
| Study arm | Control | ||||||||
| Intervention | 1.2 | 0.76 | 0. | 6.5 | 1.1 | 0.83 | 0. | 5.5 | |
| Visit | Baseline | ||||||||
| 6 months | 14. | 0.00 | 2. | 75. | 15. | 0.00 | 3. | 81. | |
| Enrollment location | Community | ||||||||
| DOC | 0.0 | 0.00 | 0. | 0.2 | 0.0 | 0.00 | 0. | 0.2 | |
| Age | Per 10 years | 0.8 | 0.56 | 0. | 1.4 | ||||
| Gender | Male | ||||||||
| Female | 1.2 | 0.77 | 0. | 4.0 | 0.6 | 0.43 | 0. | 1.8 | |
| Transgender (MTF) | 3.8 | 0.08 | 0. | 17. | 1.9 | 0.34 | 0. | 7.1 | |
| Race/ethnicity | Non-Hispanic | ||||||||
| Non-Hispanic Black | 1.9 | 0.36 | 0. | 8.6 | |||||
| Sexual Orientation | Heterosexual or | ||||||||
| Homosexual/gay/l | 3.1 | 0.09 | 0. | 11. | 1.4 | 0.47 | 0. | 4.4 | |
| # of times in jail/prison | <=5 | ||||||||
| 6-10 | 1.0 | 0.94 | 0. | 3.8 | |||||
| >10 | 0.7 | 0.58 | 0. | 2.4 | |||||
| # of yrs in jail/prison (lifetime) | <=2 | ||||||||
| 2-10 | 2.2 | 0.22 | 0. | 8.2 | |||||
| >10 | 1.3 | 0.67 | 0. | 4.6 | |||||
| Current health status (RAND36) | Good-excellent | ||||||||
| Fair-poor | 1.3 | 0.57 | 0. | 4.2 | |||||
| Regular healthcare provider pre-incarceration | No | ||||||||
| Yes | 2.0 | 0.29 | 0. | 8.0 | |||||
| Regular healthcare provider at baselineb | No | ||||||||
| Yes | 2.1 | 0.25 | 0. | 7.8 | 1.1 | 0.81 | 0. | 3.1 | |
| Depressive symptoms | 0-9 | ||||||||
| 10-30 | 2.4 | 0.10 | 0. | 7.2 | 1.0 | 0.92 | 0. | 2.6 | |
| Any mental health diagnosis*, b | No | ||||||||
| Yes | 2.4 | 0.23 | 0. | 10. | 0.8 | 0.80 | 0. | 2.9 | |
| PTSD | No | ||||||||
| Yes | 4.6 | 0.01 | 1. | 15. | 3.3 | 0.03 | 1. | 9.9 | |
| Unstable Housing** | No | ||||||||
| Yes | 1.0 | 0.98 | 0. | 3.7 | |||||
| Hazardous/harmful alcohol use (WHO AUDIT) | Low to none | ||||||||
| Medium | 0.9 | 0.92 | 0. | 4.0 | |||||
| High | 0.4 | 0.18 | 0. | 1.4 | |||||
| TCU± Drug Dependence Score | Not drug dependent (0-2) | ||||||||
| Drug dependent | 2.1 | 0.14 | 0. | 6.2 | |||||
| Ever injected drugs | No | ||||||||
| Yes | 1.5 | 0.57 | 0. | 7.0 | |||||
| Recent non-injection drug use | No | ||||||||
| Yes | 0.4 | 0.20 | 0. | 1.4 | |||||
| Currently taking HIV treatment (baseline) | No | ||||||||
| Yes | 1.5 | 0.56 | 0. | 6.9 | |||||
| Ever taken HIV treatment | No | ||||||||
| Yes | 1.8 | 0.51 | 0. | 10. | |||||
| Had ARV prescription pre-incarceration | No | ||||||||
| Yes | 0.9 | 0.93 | 0. | 3.7 | |||||
| ARV adherence (visual analog scale)—pre-incarceration | No on ARV | ||||||||
| 0-80% | 1.2 | 0.78 | 0. | 4.5 | |||||
| 90-100% | 1.0 | 0.91 | 0. | 3.6 | |||||
Independent variables having a p-value <0.10 were included in the multivariable regression model;
“Regular healthcare provider” and “Any MD diagnosis” exhibited some imbalance at baseline (p<0.15; see Table I) and were included in the multivariable regression model.
Schizophrenia, personality disorder, depression, bi-polar.
Living on the streets or staying in a single room occupancy hotel or shelter.
TCU = Texas Christian University
Discussion
In this pilot study that evaluated the impact of an mHealth intervention on viral suppression among recently incarcerated HIV-positive individuals, the proportion of those experiencing viral suppression at the six months study visit did not differ significantly among those receiving the CARE+ Corrections intervention versus those receiving standard discharge planning. Although the odds of achieving viral suppression was not significantly higher for those randomized to the CARE+ Corrections intervention compared to those in the control group, the sample size for this study was lower than expected due to regulatory and recruitment barriers, and thus, the lack of statistical significance may be a result of being underpowered to detect the difference between the two groups.
Our null findings are consistent with recent reports from another studies that tested mHealth interventions among recently incarcerated HIV-positive individuals and vulnerable populations (36, 37), in which no statistically significant differences were demonstrated between the intervention and standard of care groups over time, although in both studies a signal of positive effect was seen. We did not observe any differences in being engaged in community-based HIV care at the six months study visit across groups.
We observed that the proportions of virally suppressed individuals both in the control and intervention groups that had suppression were relatively high at the baseline assessment (73% and 59%, respectively), yet these proportions declined steadily across study visits in both groups, confirming findings of previous studies among HIV-positive criminal justice populations that community re-entry is associated with loss of viral suppression and failure to engage in community-based HIV care (3, 4, 6, 38-40). Of note, our viral suppression rate at six months was consistent with rates of viral suppression reported in other similar intervention studies in HIV-positive persons released from CJ institutions (36, 41). This observation continues to underscore the critical need for effective interventions to mitigate the loss of viral suppression status as this vulnerable population returns to the community.
Conversely, the proportion of those engaged in community-based HIV care was relatively low for both groups (approximately 50%) at study enrollment, but increased significantly to 88% and 92% across both groups, regardless of exposure to the intervention. Despite the observed increase in engagement in community-based care at the six month study visit, this did not translate to increased or even the same level of viral suppression in this population at the end of the study, a phenomenon which was also observed in a similar study in this population (36). However, we observed that the decrease in viral suppression among the intervention group appeared to be mitigated (i.e, less rapid decline) compared to that of the control group. Engagement in care was measured by self-report, and recent research has suggested that self-reported engagement in care does not always correlate well with medical records and public health surveillance data (42, 43), which may help to explain this discrepancy.
PTSD has been reported to negatively impact medication adherence (44, 45); however, we did not observe any differences by PTSD experience on viral suppression in our sample, despite the finding that those screening positive for PTSD had a significantly higher odds of being engaged in HIV care. In this case, PTSD may act as a marker of being engaged in regular health care, yet engagement in care did not equate to viral suppression in our sample.
There are limitations to our findings. First, the overall study was underpowered due to a smaller sample size than originally planned. Due to unforeseen administrative and regulatory challenges resulting from administrative turnover among the DC Department of Corrections director and mid-level staff, these delays resulted in a lower than planned sample size, and the implementation of a de novo community-based recruitment scheme of recently released incarcerated persons. To address these challenges, we connected and engaged with community-based organizations that served this population. Through these partnerships with non-profit organizations working with criminal justice-involved populations, we observed and learned about the stigma encountered by HIV-positive individuals in the CJ environment and their reluctance to disclose their status even during the re-entry period. In addition, often other subsistence needs, such as food security, housing and employment, take priority over seeking or being re-engaged in HIV care (5, 46-48). These observations underscore the challenges of conducting HIV research among persons involved in the criminal justice system. Second, although we were able to confirm viral load through laboratory testing or medical records, engagement in care was self-reported and may be over-estimated due to social desirable reporting and also due to conflicting interpretations of engagement in care as described above. Third, the sample enrolled in this study may be more likely to have disclosed their status and to prioritize HIV care and may not fully represent the true population of HIV-infected persons leaving CJ institutions. Among those who did choose to enroll in the study, our overall retention rate in the study was high (>90%) , so we do not anticipate much selection bias from losses-to-follow-up. Lastly, at the time the study was conducted, the use of a cell phone for the delivery of an SMS intervention might have been a drawback in terms of limiting the scalability of the intervention. However, given the present day ubiquity of mobile devices in most populations, we do not believe that the lack of an SMS-enabled device would be a barrier to implementation or scale-up.
In conclusion, our mHealth intervention tailored to HIV-infected persons involved in the criminal justice system did not significantly increase or maintain viral suppression after release from a CJ institution. Engagement in care increased after release but did not differ by treatment group. However, given that we observed a positive signal from the intervention in terms of achieving viral suppression in the adjusted model, further research with adequately powered studies is needed to investigate the potential usefulness of mHealth interventions among CJ-involved persons. Future studies should test the individual mHealth components of the CARE+ Corrections intervention (computerized motivational interview versus the SMS intervention) with and without additional intervention components such as in-person counseling or navigation sessions to better understand how each of these components interact with one another to support viral suppression and retention in care. In addition, given that rates of ART use and viral suppression were sub-optimal at study baseline, interventions also need to focus on community-based HIV treatment for this marginalized population. Future research is needed to better understand how to best engage this population in care and to develop more innovative methods of achieving and maintaining viral suppression to improve HIV morbidity and decrease HIV transmission.
Acknowledgements:
We would like to acknowledge funding from the National Institutes of Health, National Institute on Drug Abuse (R01DA030747), National Institute on Allergy and Infectious Diseases and institutional support from the Providence/Boston Center for AIDS Research (P30AI42853) and the District of Columbia Center for AIDS Research (P30AI117970). We would also like to acknowledge former study staff members (Avery Barber, Alice Cates, Halli Olsen, Anthony Rawls, and Hannah Yellin), our District of Columbia Department of Corrections partners (Drs. Beth Mynett and Reena Chakraborty) and community-based partners and advisory board for their support and assistance in conducting this work. Lastly, we would like to give our deepest thanks to the study participants without whom we could not do this work.
Funding: This study was funded by the National Institutes of Health National Institute on Drug Abuse (R01DA030747). The authors also received institutional support from the Providence/Boston Center for AIDS Research (P30AI42853) and the District of Columbia Center for AIDS Research (P30AI117970).
Footnotes
Conflicts of Interest: Irene Kuo, Tao Liu, Rudy Patrick, Claudia Trezza, Lauri Bazerman, Breana Uhrig Castonguay, James Peterson and Ann Kurth have all declared no conflicts of interest. Curt Beckwith has received research support from Gilead Sciences.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Dennis AC, Barrington C, Hino S, Gould M, Wohl D, Golin CE. “You're in a world of chaos”: experiences accessing HIV care and adhering to medications after incarceration. J Assoc Nurses AIDS Care. 2015;26(5):542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elkington KS, Jaiswal J, Spector AY, et al. Can TasP Approaches Be Implemented in Correctional Settings?: A review of HIV testing and linkage to community HIV treatment programs. Journal of health care for the poor and underserved. 2016;27(2a):71–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iroh PA, Mayo H, Nijhawan AE. The HIV Care Cascade Before, During, and After Incarceration: A Systematic Review and Data Synthesis. Am J Public Health. 2015;105(7):e5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baillargeon J, Giordano TP, Rich JD, et al. Accessing antiretroviral therapy following release from prison. Jama. 2009;301(8):848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements-Nolle K, Marx R, Pendo M, Loughran E, Estes M, Katz M. Highly active antiretroviral therapy use and HIV transmission risk behaviors among individuals who are HIV infected and were recently released from jail. Am J Public Health. 2008;98(4):661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson BL, Wohl DA, Golin CE, Tien HC, Stewart P, Kaplan AH. Effect of release from prison and re-incarceration on the viral loads of HIV-infected individuals. Public Health Rep. 2005;120(1):84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westergaard RP, Spaulding AC, Flanigan TP. HIV among persons incarcerated in the USA: a review of evolving concepts in testing, treatment, and linkage to community care. Current opinion in infectious diseases. 2013;26(1):10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS One. 2009;4(11):e7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simoni JM, Kutner BA, Horvath KJ. Opportunities and Challenges of Digital Technology for HIV Treatment and Prevention. Curr HIV/AIDS Rep. 2015;12(4):437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012(3):CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naar-King S, Parsons JT, Johnson AM. Motivational interviewing targeting risk reduction for people with HIV: a systematic review. Curr HIV/AIDS Rep. 2012;9(4):335–43. [DOI] [PubMed] [Google Scholar]
- 12.Kurth AE, Spielberg F, Cleland CM, et al. Computerized counseling reduces HIV-1 viral load and sexual transmission risk: findings from a randomized controlled trial. J Acquir Immune Defic Syndr. 2014;65(5):611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One. 2014;9(2):e88166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalichman SC, Kalichman MO, Cherry C, Eaton LA, Cruess D, Schinazi RF. Randomized Factorial Trial of Phone-Delivered Support Counseling and Daily Text Message Reminders for HIV Treatment Adherence. J Acquir Immune Defic Syndr. 2016;73(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orrell C, Cohen K, Mauff K, Bangsberg DR, Maartens G, Wood R. A Randomized Controlled Trial of Real-Time Electronic Adherence Monitoring With Text Message Dosing Reminders in People Starting First-Line Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2015;70(5):495–502. [DOI] [PubMed] [Google Scholar]
- 16.Shet A, De Costa A, Kumarasamy N, et al. Effect of mobile telephone reminders on treatment outcome in HIV: evidence from a randomised controlled trial in India. Bmj. 2014;349:g5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alemagno SA, Stephens RC, Stephens P, Shaffer-King P, White P. Brief motivational intervention to reduce HIV risk and to increase HIV testing among offenders under community supervision. Journal of correctional health care : the official journal of the National Commission on Correctional Health Care. 2009;15(3):210–21. [DOI] [PubMed] [Google Scholar]
- 18.Taxman FS, Walters ST, Sloas LB, Lerch J, Rodriguez M. Motivational tools to improve probationer treatment outcomes. Contemporary clinical trials. 2015;43:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerch J, Walters ST, Tang L, Taxman FS. Effectiveness of a computerized motivational intervention on treatment initiation and substance use: Results from a randomized trial. J Subst Abuse Treat. 2017;80:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandler RK, Kahana SY, Fletcher B, et al. Data Collection and Harmonization in HIV Research: The Seek, Test, Treat, and Retain Initiative at the National Institute on Drug Abuse. Am J Public Health. 2015;105(12):2416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.District of Columbia Department of Health HIV/AIDS Hepatitis STDs and Tuberculosis Administration. District of Columbia Department of Health HIV/AIDS, Hepatitis, STD and TB Epidemiology Annual Report, 2015. Washington, DC: District of Columbia Department of Health, 2016. [Google Scholar]
- 22.Kurth A, Kuo I, Peterson J, et al. Information and Communication Technology to Link Criminal Justice Reentrants to HIV Care in the Community. AIDS Res Treat. 2013;2013:547381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson J, Cota M, Gray H, et al. Technology use in linking criminal justice reentrants to HIV care in the community: a qualitative formative research study. Journal of health communication. 2015;20(3):245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckwith C, Castonguay BU, Trezza C, et al. Gender Differences in HIV Care among Criminal Justice-Involved Persons: Baseline Data from the CARE+ Corrections Study. PLoS One. 2017;12(1):e0169078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skeels MM, Kurth A, Clausen M, Severynen A, Garcia-Smith H. CARE+ user study: usability and attitudes towards a tablet pc computer counseling tool for HIV+ men and women. AMIA Annual Symposium proceedings AMIA Symposium 2006:729–33. [PMC free article] [PubMed] [Google Scholar]
- 26.Spielberg F, Kurth AE, Severynen A, et al. Computer-facilitated rapid HIV testing in emergency care settings: provider and patient usability and acceptability. AIDS Educ Prev. 2011;23(3):206–21. [DOI] [PubMed] [Google Scholar]
- 27.Daeppen JB, Yersin B, Landry U, Pecoud A, Decrey H. Reliability and validity of the Alcohol Use Disorders Identification Test (AUDIT) imbedded within a general health risk screening questionnaire: results of a survey in 332 primary care patients. Alcohol Clin Exp Res. 2000;24(5):659–65. [PubMed] [Google Scholar]
- 28.Pankow J, Simpson DD, Joe GW, Rowan-Szal GA, Knight K, Meason P. Examining Concurrent Validity and Predictive Utility for the Addiction Severity Index and Texas Christian University (TCU) Short Forms. Journal of offender rehabilitation. 2012;51(1-2):78–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prins A, Ouimette P, Kimerling R, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Primary Care Psychiatry. 2003;9:9–14. [Google Scholar]
- 30.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 31.Buscher A, Hartman C, Kallen MA, Giordano TP. Validity of self-report measures in assessing antiretroviral adherence of newly diagnosed, HAART-naive, HIV patients. HIV clinical trials. 2011;12(5):244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Clinical oral investigations. 2003;7(1):2–7. [DOI] [PubMed] [Google Scholar]
- 33.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Second ed. New York: John Wiley and Sons, Inc; 2002. [Google Scholar]
- 34.van Buuren S Flexible Imputation of Missing Data. First ed. Boca Raton: Chapman and Hall/CRC; 2012. [Google Scholar]
- 35.Gelman A, Van Mechelen I, Verbeke G, Heitjan DF, Meulders M. Multiple imputation for model checking: completed-data plots with missing and latent data. Biometrics. 2005;61(1):74–85 [DOI] [PubMed] [Google Scholar]
- 36.Wohl DA, Golin CE, Knight K, et al. Randomized Controlled Trial of an Intervention to Maintain Suppression of HIV Viremia After Prison Release: The imPACT Trial. J Acquir Immune Defic Syndr. 2017;75(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christopoulos KA, Riley ED, Carrico AW, et al. A Randomized Controlled Trial of a Text Messaging Intervention to Promote Virologic Suppression and Retention in Care in an Urban Safety-Net Human Immunodeficiency Virus Clinic: The Connect4Care Trial. Clin Infect Dis. 2018;67(5):751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer JP, Cepeda J, Springer SA, Wu J, Trestman RL, Altice FL. HIV in people reincarcerated in Connecticut prisons and jails: an observational cohort study. The lancet HIV. 2014;1(2):e77–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wohl DA, Scheyett A, Golin CE, et al. Intensive case management before and after prison release is no more effective than comprehensive pre-release discharge planning in linking HIV-infected prisoners to care: a randomized trial. AIDS Behav. 2011;15(2):356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spaulding AC, Messina LC, Kim BI, et al. Planning for success predicts virus suppressed: results of a non-controlled, observational study of factors associated with viral suppression among HIV-positive persons following jail release. AIDS Behav. 2013;17 Suppl 2:S203–11. [DOI] [PubMed] [Google Scholar]
- 41.Springer SA, Qiu J, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PLoS One. 2012;7(5):e38335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christopoulos KA, Scheer S, Steward WT, et al. Examining clinic-based and public health approaches to ascertainment of HIV care status. J Acquir Immune Defic Syndr. 2015;69 Suppl 1:S56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castel AD, Tang W, Peterson J, et al. Sorting through the lost and found: are patient perceptions of engagement in care consistent with standard continuum of care measures? J Acquir Immune Defic Syndr. 2015;69 Suppl 1:S44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machtinger EL, Wilson TC, Haberer JE, Weiss DS. Psychological trauma and PTSD in HIV-positive women: a meta-analysis. AIDS Behav. 2012;16(8):2091–100. [DOI] [PubMed] [Google Scholar]
- 45.Applebaum AJ, Bedoya CA, Hendriksen ES, Wilkinson JL, Safren SA, O’Cleirigh C. Future directions for interventions targeting PTSD in HIV-infected adults. J Assoc Nurses AIDS Care. 2015;26(2):127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunningham WE, Andersen RM, Katz MH, et al. The impact of competing subsistence needs and barriers on access to medical care for persons with human immunodeficiency virus receiving care in the United States. Med Care. 1999;37(12):1270–81. [DOI] [PubMed] [Google Scholar]
- 47.Milloy MJ, Montaner JS, Wood E. Incarceration of people living with HIV/AIDS: implications for treatment-as-prevention. Curr HIV/AIDS Rep. 2014;11(3):308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Booker CA, Flygare CT, Solomon L, et al. Linkage to HIV care for jail detainees: findings from detention to the first 30 days after release. AIDS Behav. 2013;17 Suppl 2:S128–36. [DOI] [PubMed] [Google Scholar]