Abstract
Rationale:
Regeneration of denuded or injured endothelium is an important component of vascular injury response. Cell-cell communication between endothelial cells (ECs) and smooth muscle cells (SMCs) plays a critical role not only in vascular homeostasis but also in disease. We have previously demonstrated that protein kinase C-delta (PKCδ) regulates multiple components of vascular injury response including apoptosis of SMCs and production of chemokines, thus is an attractive candidate for a role in SMC-EC communication.
Objective:
To test whether PKCδ-mediated paracrine functions of SMCs influence reendothelialization in rodent models of arterial injury.
Methods and Results:
Femoral artery wire injury was performed in SMC-conditional Prkcd knockout mice, and carotid angioplasty was conducted in rats receiving transient Prkcd knockdown or overexpression. SMC-specific knockout of Prkcd impaired reendothelialization, reflected by a smaller Evans blue-excluding area in the knockout compared to the wildtype controls. A similar impediment to reendothelialization was observed in rats with SMC-specific knockdown of Prkcd. In contrast, SMC-specific gene transfer of Prkcd accelerated reendothelialization. In vitro, medium conditioned by AdPKCδ-infected SMCs increased endothelial wound closure without affecting their proliferation. A PCR-based array analysis identified Cxcl1 and Cxcl7 among others as PKCδ-mediated chemokines produced by SMCs. Mechanistically, we postulated that PKCδ regulates Cxcl7 expression through signal transducer and activator of transcription 3 (STAT3) as knockdown of STAT3 abolished Cxcl7 expression. The role of CXCL7 in SMC-EC communication was demonstrated by blocking CXCL7 or its receptor CXCR2, both significantly inhibited endothelial wound closure. Furthermore, insertion of a Cxcl7 cDNA in the lentiviral vector that carries a Prkcd shRNA overcame the adverse effects of Prkcd knockdown on reendothelialization.
Conclusions:
SMCs promote reendothelialization in a PKCδ-dependent paracrine mechanism, likely through CXCL7-mediated recruitment of ECs from uninjured endothelium.
Keywords: Reendothelialization, restenosis, smooth muscle cells (SMCs), protein kinase C-delta (PKCδ), chemokine (C-X-C Motif) receptor 2 (CXCR2) ligands, signal transducer and activator of transcription 3 (STAT3)
Subject Terms: Endothelium, Peripheral Vascular Disease, Restenosis, Vascular Biology
INTRODUCTION
A healthy endothelium is necessary for vascular homeostasis and blood flow. By the production of bioactive substances, endothelial cells (ECs) regulate vascular tone, proliferative state of the underlying vascular smooth muscle cells (SMCs) and maintain a non-thrombogenic blood-tissue interface that has a limited permeability 1. Damaged or dysfunctional endothelium drives vascular inflammation through expression of adhesion molecules and enhanced permeability to leukocytes 2. In addition, endothelial injury diminishes the production of nitric oxide (NO), a potent inhibitor of SMC proliferation, and induces intimal hyperplasia (IH) 3. Thus, endothelial dysfunction plays a key role in various vascular diseases, such as atherosclerotic cardiovascular disease, the leading cause of death worldwide 4, 5. Angioplasty followed by stenting effectively restores blood flow by compressing atherosclerotic plaques against the artery wall. However, this procedure causes regional damage to endothelium as well as SMCs and triggers the development of restenosis, which hampers the long-term success of vascular interventions 6, 7. Endothelial regeneration or reendothelialization correlates inversely with the growth of intimal lesion 8. Furthermore, improved reendothelialization prevents thrombotic events and IH triggered by vascular interventions 9, 10. The rate of reendothelialization is thus critical in vascular repair.
Reendothelialization after arterial injury involves multiple types of resident vascular cells and circulating cells in a coordinated fashion 11. Denuded endothelium is immediately covered by a layer of platelets and leukocytes 12. In the hours following arterial injury, resident ECs surrounding damaged arterial endothelium rapidly enter the replication cycle to restore endothelial continuity 12. Arrays of arterial injury studies demonstrate that the damaged endothelium is repaired by proliferation and migration of ECs in regions bordering the denuded area 13–16. Experimental manipulations that inhibit endothelial proliferation such as gene deletion of endothelial microRNA-126 impair reendothelialization after denudation 17. Conversely, administration of microRNA-126–5p or deletion of genes that negatively regulate EC proliferation/migration, such as a disintegrin and metalloproteinase with thrombospondin motifs 7 (ADAMTS7), promote endothelial repair and limit the growth of atherosclerotic plaques 17, 18.
As a major component of the arterial wall, SMCs are critical in orchestrating vascular injury response. Vascular injury induces a phenotypic switch of SMCs toward the synthetic phenotype characterized by increased rate of proliferation, migration, and synthesis of extracellular matrix components 6. This phenotypic switch is generally believed to underlie the development of IH 7. Proliferation and migration of synthetic SMCs are further powered by the loss of endothelial inhibition until endothelium is functionally recovered. Numerous studies have shown that SMCs are an important source of cytokines and chemokines in the vessel wall 19–22. Recent data from our laboratory demonstrated that injured medial SMCs signal to adventitial fibroblasts via the Protein kinase C-delta (PKCδ)-mediated release of MCP-1 23. Whether and how injured SMCs may influence endothelial repair remains largely unknown.
PKCδ is a member of the PKC family of serine-threonine kinases. Expressed by all major vascular cell types, PKCδ plays an essential role in the regulation of multiple cellular functions such as apoptosis and chemokine expression 22, 24. We have previously reported that levels of PKCδ are elevated in human restenotic lesions as well as balloon-injured rat carotid arteries 25. Mice deficient in Prkcd develop exacerbated injury- and vein graft-related IH, which is associated with diminished medial SMC apoptosis 25, 26. Conversely, gene transfer of Prkcd to balloon-injured carotid arteries inhibits IH, which is associated with a profound upregulation of apoptotic activity within medial SMCs 25. In cultured SMCs, PKCδ promotes cytokine expression and apoptosis but suppresses proliferation and migration 27, 28. In ECs, PKCδ is necessary for proliferation and migration 29. Bai et al. showed the exaggerated neointimal lesions found in Prkcd gene-deficient mice are in part caused by compromised EC proliferation and migration during the reendothelialization process following arterial injury 29. In this current study, we report that SMC-specific upregulation of PKCδ promotes reendothelialization. Specifically, SMCs facilitate EC recovery in a PKCδ-dependent manner, likely in part involving the release of CXCR2 ligands which promotes EC migration.
METHODS
The authors declare that all supporting data are available within the article and its Online Data Supplement. Detailed Methods are available in the Online Data Supplement.
Animal models.
All animal procedures were performed under protocols approved by the Institute Animal Care and Use Committee at the University of Wisconsin-Madison (#M002285) and conformed to the Guide for the Care and Use of Laboratory Animals. The inducible SMC-specific Prkcd knockout mice were generated by breeding Prkcdfl/fl mice with SMMHC-CreERT2 mice. The congenic Prkcdfl/fl mice on a predominantly C57BL/6 background were generated by Bezy et al. (a kind gift from Dr. C. Ronald Kahn at Harvard Medical School) 30. The SMMHC-CreERT2 mice were obtained from The Jackson Laboratory (Stock No: 019079) in which the expression of Cre-ERT2 recombinase is driven by smooth muscle myosin heavy chain promoter and is activated by tamoxifen (TM). 8–12 weeks male Myh11-Cre/ERT2-Prkcdwt/wt, Myh11-Cre/ERT2-Prkcdfl/fl, and Prkcdfl/fl mice received TM (75 mg/kg/day, i.p.) for five consecutive days. Three weeks after TM injections, mouse femoral artery wire injury was performed blindly as described previously 31. Evans blue staining was performed on 7 days post wire-injury and arteries were harvested and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD). Arterial denudation was performed in male Sprague-Dawley rats (8~12 weeks) obtained from Charles River Laboratories through carotid balloon angioplasty as described before 25, 32. Then, rats were randomly assigned to different groups. Gene transfer to medial SMCs was achieved by intraluminal perfusion with adenoviral vectors or lentiviral vectors within the injured segment after balloon-injury as described previously 25. A sham group underwent surgery without balloon angioplasty/viral infection. Evans blue staining was performed on 14 days or 21 days post wire-injury and arteries were harvested and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD). Evans blue staining was performed on 14 days or 21 days post injury and arteries were harvested and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD). Reendothelialization was determined by the percentage of Evans blue negative area over the total denuded areas.
Cell culture.
Primary SMCs were isolated from rat carotid arteries according to a method described previously 33 and maintained in DMEM supplemented with 10% FBS, and penicillin-streptomycin. Mouse aortic ECs were isolated from C57BL/6J immorto mice as described previously using anti-CD31 conjugated magnetic beads 34. ECs were grown on gelatin-coated dishes in DMEM containing 10% FBS, 2 mM L-glutamine, 2 mM sodium pyruvate, 20 mM HEPES, 1% nonessential amino acids, 100 μg/ml streptomycin, 100 U/ml penicillin, freshly added heparin at 55 U/ml (Sigma, St. Louis, MO), endothelial growth supplement 100 μg/ml (Sigma), and the murine recombinant interferon-γ (R&D, Minneapolis, MN) at 44 U/ml. The endothelial identity was confirmed by FACS analysis for CD31, VE-cadherin, and B4-lectin.
Immunostaining.
Immunostaining was performed on cryosections of 4% PFA fixed arteries with the indicated antibodies. Quantification of immunostaining was performed using ImageJ Software (National Institutes of Health, Bethesda, MD).
Statistical analysis.
Results are presented as mean ± SEM. Data were assessed for normality using the Shapiro-Wilk normality test. Data not exhibiting a normal distribution were log2-transformed and retested for normality. Two-tailed Student’s t test for normally distributed data and Mann–Whitney nonparametric test for skewed data that remained deviate from normality after transformation were used to compare between two conditions. One-way Analysis of Variance (ANOVA) with Tukey post hoc test for normally distributed data and Kruskal–Wallis nonparametric test for skewed data after transformation were used to compare ≥ three means. Statistical analyses were performed with GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA). Experiments were repeated as indicated. Differences with P<0.05 were considered statistically significant.
RESULTS
Inhibition of PKCδ in SMCs impairs endothelial regeneration and increases IH.
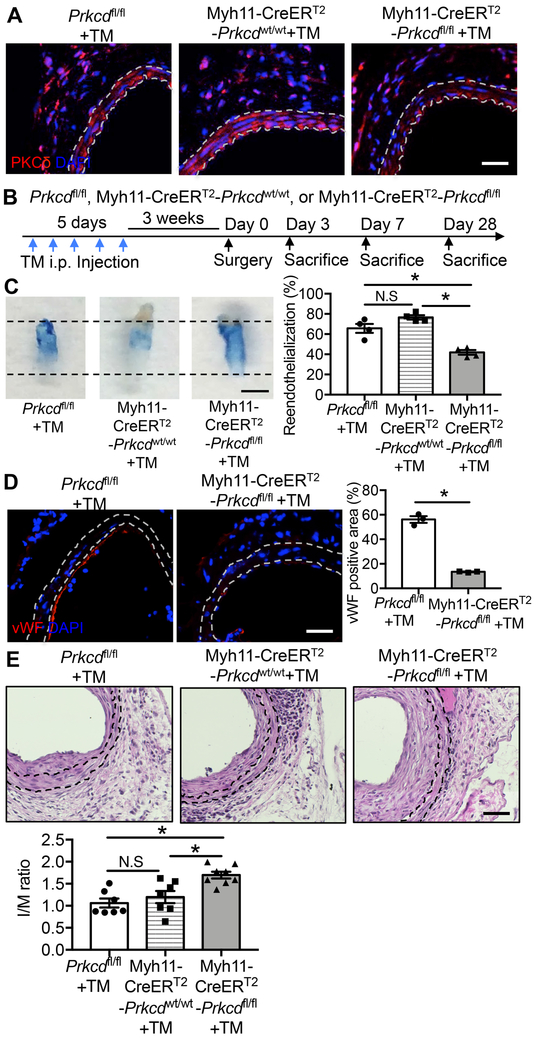
We have previously reported that levels of PKCδ protein are upregulated in the arterial wall following injury 25. To delineate the function of PKCδ specifically in SMCs, we bred Myh11-CreERT2 mice with Prkcdfl/fl mice to generate a tamoxifen-inducible Prkcd gene deficiency in vascular SMCs. Five-day continuous injection of Tamoxifen (TM) (75 mg/kg, i.p.) was administered to all mice, which produced a ~70% reduction of arterial PKCδ levels in Myh11-CreERT2-Prkcdfl/fl mice as compared to Prkcdfl/fl or Myh11-CreERT2-Prkcdwt/wt mice (Figure 1A). Three weeks after TM injection, we performed wire injury in mice of the three genotypes (Figure 1B). The procedure denudes the endothelium and triggers a regeneration process that reaches completion at least 7 days after wire injury 35, 36. We, therefore, euthanized the mice 7 days after injury to evaluate endothelial regeneration using en face staining with Evans blue dye. Because Evans blue is excluded by uninjured or regenerated functional endothelium 37, the dye-free area of denuded arterial segments reflects the degree of endothelial repair. Deletion of PKCδ in SMCs (Myh11-CreERT2-Prkcdfl/fl mice) showed 41.90±2.36% reendothelialization, which was significantly less compared to 65.66±4.46% and 76.56±2.18% in Prkcdfl/fl and Myh11-CreERT2-Prkcdwt/wt mice, respectively (Figure 1C). Consistently, the injured segment of Myh11-CreERT2-Prkcdfl/fl mice showed significantly diminished von Willebrand factor (vWF) positivity compared to Prkcdfl/fl mice (positive area: 13.48±0.29% vs 56.14±2.68%, respectively) (Figure 1D). Together, these data suggest that SMC-specific loss of PKCδ impedes endothelial regeneration. In response to wire injury, mice of all three genotypes developed IH. However, the delayed reendothelialization in Myh11-CreERT2-Prkcdfl/fl mice was associated with significantly larger IH. The intima/media (I/M) ratio measured 28 days after injury was 59.72% and 41.76% higher in Myh11-CreERT2-Prkcdfl/fl mice than Prkcdfl/fl and Myh11-CreERT2-Prkcdwt/wt mice, respectively (Figure 1E).
Figure 1. PKCδ signaling in SMCs regulates re-endothelialization.
(A) Representative images of uninjured femoral arteries harvested from Prkcdfl/fl mice, Myh11-CreERT2-Prkcdwt/wt, and Myh11-CreERT2-Prkcdfl/fl mice 3 weeks after tamoxifen (TM) injection. Sections were immunostained for PKCδ (red). Nuclei were stained with DAPI (blue). The locations of the internal and external elastic lamina defining the boundaries of the media are shown as white dashed lines. Scale bar = 50 μm. (B) Schematic design of wire injury study. (C) Representative Evans blue dye-stained carotid arteries harvested 7 days after wire-injury to Prkcdfl/fl+TM, Myh11-CreERT2-Prkcdwt/wt+TM, and Myh11-CreERT2-Prkcdfl/fl+TM mice. Boundaries of injured areas are indicated by dashed lines. Reendothelialization was determined by the percentage of Evans blue negative area over the total injured area using ImageJ software. Scale bar = 1 mm. Results are expressed as mean±SEM. n=4, *p<0.05, One-way ANOVA. (D) Representative images and quantifications of wire-injured femoral arteries harvested from Prkcdfl/fl mice and Myh11-CreERT2-Prkcdfl/fl mice 3 days post-injury. Sections were immunostained for vWF (red). Nuclei were stained with DAPI (blue). The locations of the internal and external elastic lamina defining boundaries of the media are shown as white dashed lines. Scale bar = 50 μm. Results are expressed as mean±SEM. n=3, *p<0.05, Two-tailed Student’s t-test. (E) Representative images and quantifications of wire-injured femoral arteries harvested from Prkcdfl/fl, Myh11-CreERT2-Prkcdwt/wt, and Myh11-CreERT2-Prkcdfl/fl mice 28 days post-injury. Sections were immunostained for Haemotoxylin and Eosin (H&E). The locations of the internal and external elastic lamina defining boundaries of the media are shown as black dashed lines. Scale bar = 50 μm. The intima area to media area ratio (I/M ratio) was measured as described in the Methods. Results are expressed as mean±SEM. n= 7-8, *p<0.05, One-way ANOVA.
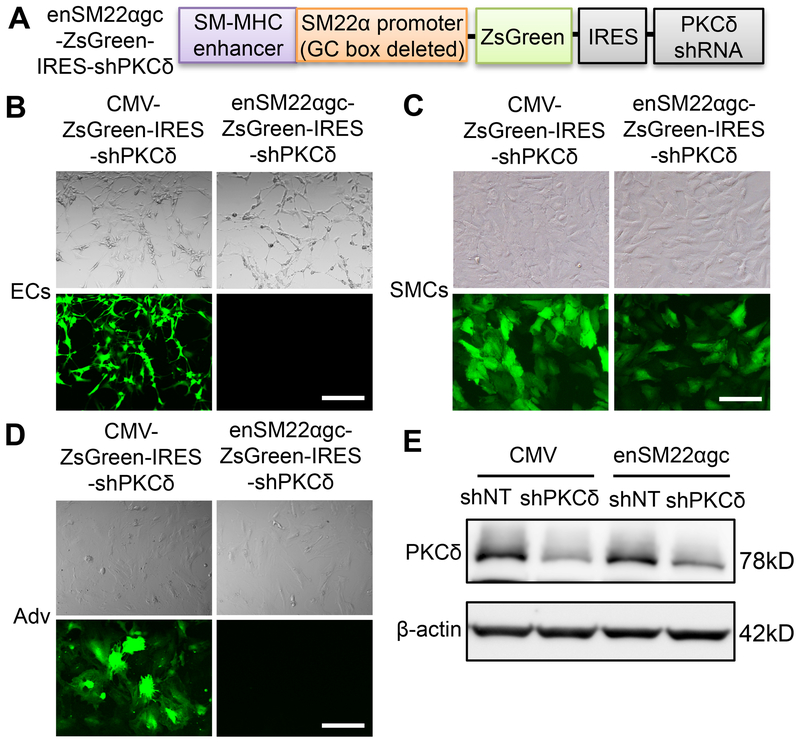
To investigate whether PKCδ plays a similar role in rat endothelial regeneration, we employed the carotid balloon injury model, in which reendothelialization typically requires 28 days to complete 37, 38. We constructed a lentiviral vector to express a Prkcd shRNA driven by a chimeric promoter (enSM22αgc) that contains a smooth muscle-myosin heavy chain (SM-MHC) enhancer and a modified SM22α promoter whose G/C-rich cis-element is deleted (Lenti-enSM22αgc-ZsGreen-IRES-shPKCδ) (Figure 2A). The chimeric promoter has been reported in the literature to direct SMC-specific gene expression39. The G/C-rich cis-element mediates injury-induced downregulation of SM22α promoter, therefore the removal of this repressive element renders higher promoter activity in de-differentiated SMCs 40. The specificity of the lentivirus was tested in cultured SMCs, ECs and adventitial cells (Adv). Contrasting to the ubiquitous activity of cytomegalovirus (CMV)-promoter, enSM22αgc promoter selectively drove gene expression in SMCs (Figure 2B-D). Furthermore, the enSM22αgc promoter produced a comparable PKCδ knockdown as the CMV promoter (Figure 2E).
Figure 2. enSM22αgc promoter mediates SMC-specific gene expression.
(A) Schematic design of SMC-specific PKCδ shRNA expressing vector. ZsGreen cDNA and PKCδ shRNA are driven by a chimeric promoter (enSM22αgc) that contains a smooth muscle-myosin heavy chain (SM-MHC) enhancer and a modified SM22α promoter whose G/C-rich cis-element is deleted. (B-D) enSM22αgc mediates gene expression specifically in SMCs (C), but not ECs (B) and adventitial fibroblasts (Adv) (D). Cells were infected with either the Lenti-CMV-ZsGreen-IRES-shPKCδ or the Lenti-enSM22αgc-ZsGreen-IRES-shPKCδ lentiviral particles. Bright-field (upper panel) and fluorescence (bottom panel) images of cells were taken 72h after transduction. Scale bar = 50 μm. (E) SMCs were transduced with lentivirus expressing non-targeting shRNA (shNT) or PKCδ shRNA (shPKCδ) under the indicated promoter. After 72h transduction, cells were harvested and the whole-cell lysates were subjected to immunoblot analysis.
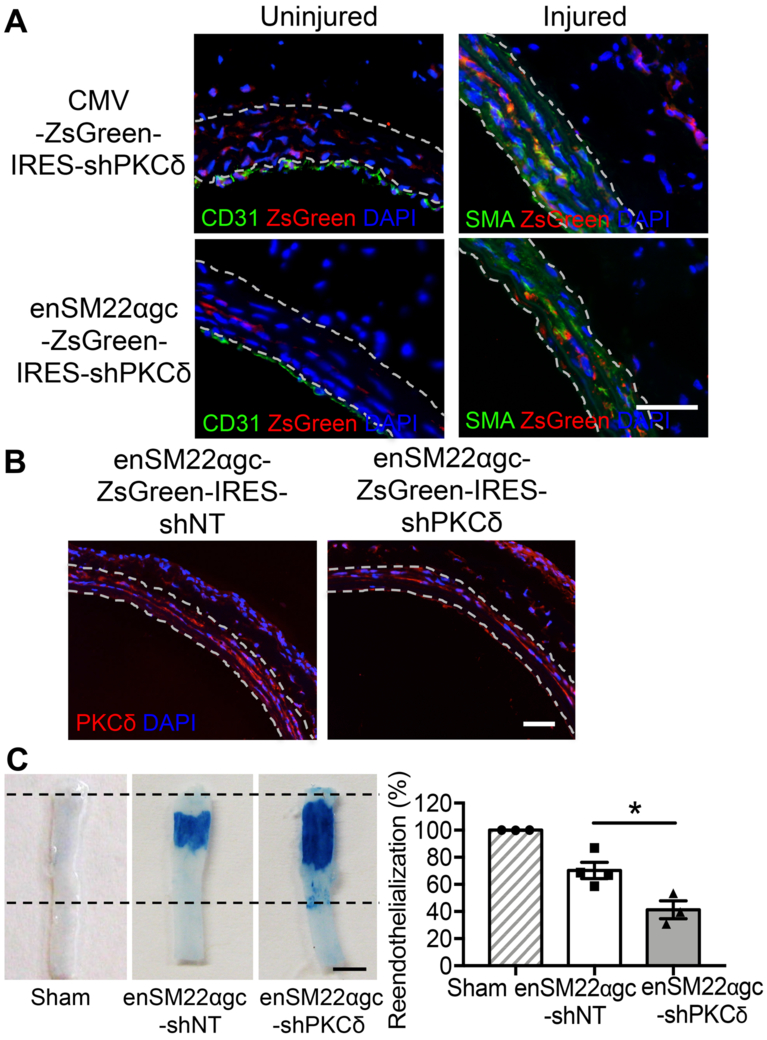
The lentiviruses were delivered to intact or balloon-injured carotid arteries through intraluminal perfusion (Online Figure I A). It is believed that the intact endothelial cells, as well as base membrane, prevent viruses from penetrating deep into the vascular wall 41–43. Therefore, viral vectors delivered intraluminally primarily transduced intima and consequently produced weak transgene expression in medial and adventitia region. As expected, CMV-promoter drove transgene expression in ECs, SMCs, and adventitial cells (Figure 3A). In contrast, the enSM22αgc promoter was active in SMCs but not in ECs or adventitial cells (Figure 3A). Furthermore, enSM22αgc-driven shPKCδ produced downregulation of PKCδ in tunica media of injured arteries (Figure 3B). When evaluated 21 days post-injury, a late stage of reendothelialization after angioplasty in rats 44, arteries treated with Prkcd shRNA had a significantly smaller dye-free area than the non-targeting shRNA treated arteries (41.31±6.54% vs. 70.31±5.97%) (Figure 3C), indicating an impairment in endothelial recovery.
Figure 3. SMC-specific knockdown of PKCδ delays re-endothelialization.
(A) Representative images of rat carotid cross-sections harvested 2 days after intraluminal perfusion with indicated lentiviral vectors. Sections were co-immunostained for CD31 (green) and ZsGreen (red) or SMA (green) and ZsGreen (red). Nuclei were stained with DAPI (blue). The locations of the internal and external elastic lamina defining boundaries of the media are shown as white dashed lines. Scale bar = 50 μm. (B) Representative images of rat carotid cross-sections harvested 3 days after balloon-injury followed by intraluminal perfusion with indicated lentiviral vectors. Sections were immunostained for PKCδ (red). Nuclei were stained with DAPI (blue). The locations of the internal and external elastic lamina defining boundaries of the media are shown as white dashed lines. Scale bar = 50 μm. (C) Representative Evans blue dye-stained carotid arteries harvested 21 days after angioplasty from sham, injured and Lenti-enSM22αgc-non-targeting shRNA-treated, or injured and Lenti-enSM22αgc-shPKCδ-treated rats. Boundaries of the injured areas are indicated by dashed lines. Reendothelialization was quantitatively expressed by the percentage of Evans blue negative area over the total injured area using ImageJ software. Scale bar = 3 mm. Results are expressed as mean±SEM. n=3-4, *p<0.05, One-way ANOVA.
Gene transfer of PKCδ to SMCs promotes endothelial regeneration.
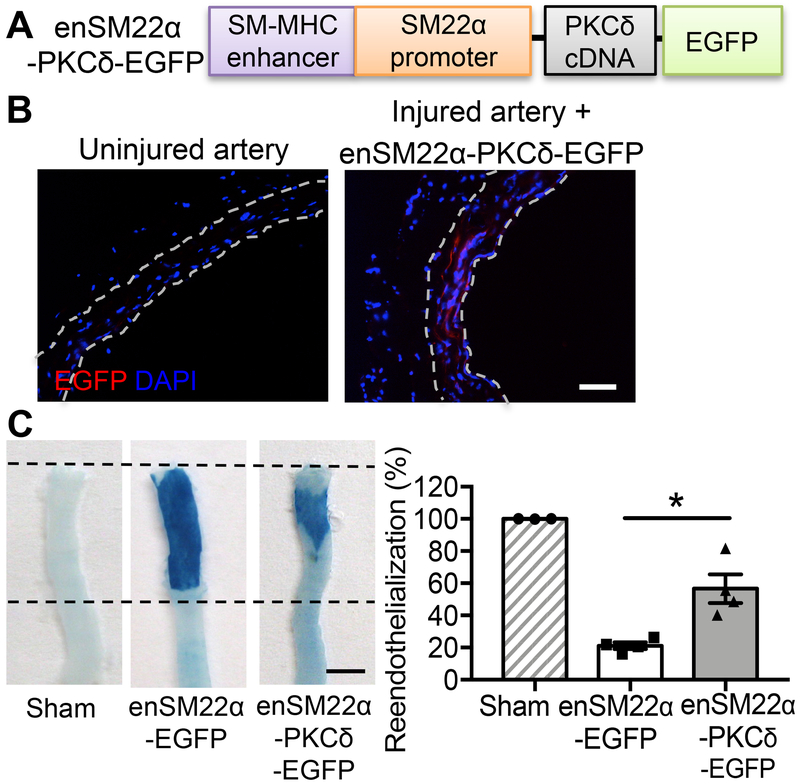
Using a CMV-driven adenovirus we constructed previously 25, we replicated the previous finding that Prkcd gene transfer to a balloon-injured rat carotid artery significantly reduced the neointimal formation (Online Figure I B). Comparing to AdNull (empty vector), intraluminal delivery of AdPKCδ (adenoviral vector expressing Prkcd gene) increased the intimal area positive of vWF and VE-cadherin from 7.92±0.71% to 40.97±4.03% and 16.73±1.01% to 31.05±3.04%, respectively (Online Figure I C&D). Since viral vectors delivered intraluminal predominately infect the medial cells of injured vessels 23, 32, we postulated that AdPKCδ affects endothelial repair indirectly, likely through a mechanism mediated by SMCs. To test this hypothesis more rigorously, we constructed a new adenoviral vector that expresses PKCδ under the chimeric enSM22α promoter (Figure 4A). Similar to what we showed with the lentivirus, enSM22α-containing adenoviruses drove EGFP expression in SMCs but not in ECs or Adv (Figure 4B & Online Figure II). The en face Evens blue assay showed that AdenSM22α-PKCδ accelerated the endothelium restoration from 25.38±7.52% to 59.60±5.01% 14 days post-injury (Figure 4C).
Figure 4. SMC-specific overexpression of PKCδ accelerates re-endothelialization.
(A) Schematic design of SMC-specific PKCδ expression vector. Expression of PKCδ cDNA and EGFP cDNA is driven by a chimeric promoter (enSM22α) that contains a smooth muscle-myosin heavy chain (SM-MHC) enhancer and the SM22α promoter. (B) Representative images of uninjured carotid arteries or AdenSM22α-PKCδ-EGFP infected arteries. Sections were immunostained for EGFP. Nuclei were stained with DAPI (blue). The locations of the internal and external elastic lamina defining boundaries of the media are shown as white dashed lines. Scale bar = 50 μm. (C) Representative Evans blue-stained carotid arteries harvested 14 days after angioplasty from sham, injured AdenSM22α-EGFP-treated, or injured AdenSM22α-PKCδ-treated rats. Boundaries of the injured areas are indicated by the dashed lines. Reendothelialization was determined by the percentage of Evans blue negative area over the total injured area using ImageJ software. Scale bar = 3 mm. Results are expressed as mean±SEM. n=3-4, *p<0.05, One-way ANOVA.
SMCs influence endothelial functions through a PKCδ-mediated paracrine mechanism.
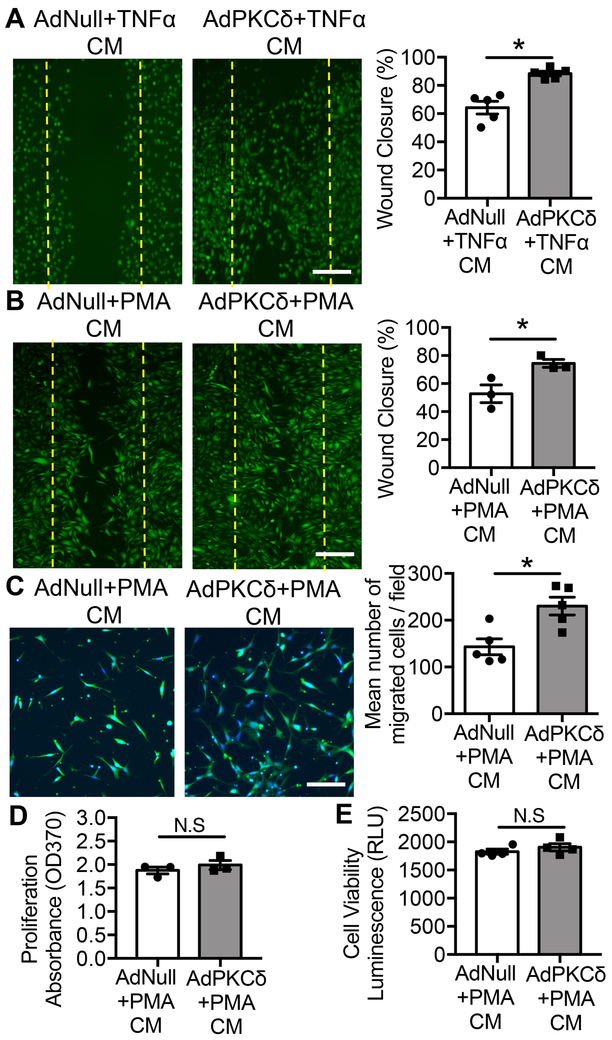
Regeneration of the endothelium after denudation involves migration and proliferation of ECs residing at the border zones adjacent to injured area 14, 16. Using an in vitro endothelial wound closure model, we tested whether paracrine signals from SMCs influence EC proliferation or migration. To mimic the pathological environment, we titrated the dosages of PKCδ-expressing adenovirus (AdPKCδ) to upregulate PKCδ protein to a level comparable to the injury-associated elevation of PKCδ and incubated infected SMCs with TNFα (1 ng/ml), a critical IH-associated physiological stimulus 45–48. Medium conditioned by AdPKCδ-infected SMCs significantly promoted endothelial wound closure compared to medium conditioned by AdNull (empty vector)-infected SMCs (Figure 5A). Similar outcomes were produced with a low concentration of PKC activator PMA (1 nM) (Figure 5B), which alone has minimal effect on chemokine expression in SMCs 22. To further characterize the paracrine function of PKCδ, we used the transwell assay and showed that medium conditioned by AdPKCδ-infected SMCs significantly induced migration of endothelial cells (Figure 5C). EC proliferation, as well as viability, appeared to be similar regardless whether they were cultured in the medium conditioned by AdNull- or AdPKCδ-infected SMCs (Figure 5D&E).
Figure 5. SMCs regulate endothelial cell migration through a PKCδ-mediated paracrine mechanism.
(A&B) Wound closure of endothelial monolayers grown in medium condition by AdNull- or AdPKCδ-infected and TNFα- (1ng/ml) or PMA- (1nM) treated SMCs. ECs were labeled with CMFDA green fluorescence dye for visualization. Dashed lines mark the wound edges generated by scratching. Wound closure was determined by the percentage of the recovered area over the total injured area using ImageJ software. Scale bar = 50 μm. (C) EC migration toward medium condition by AdNull-, or AdPKCδ-infected and PMA- (1nM) treated SMCs. ECs were labeled with CMFDA green fluorescence dye and counterstained with DAPI for visualization. Representative images of migrated cells are shown. Data are expressed as the mean number of migrated cells/fields±SEM. Scale bar = 50 μm. (D&E) Proliferation and viability of endothelial cells grew in medium conditioned by AdNull- and AdPKCδ-infected and PMA- (1nM) treated SMCs were measured by BrdU incorporation and CellTiter-Glo® luminescent cell viability assay, respectively. Results are expressed as mean±SEM. n=3-5, *p<0.05, Two-tailed Student’s t-test.
CXCR2 ligands mediate crosstalk between SMCs and ECs.
To search for a paracrine factor(s) that may mediate the crosstalk between SMCs and ECs, we conducted PCR arrays to identify PKCδ-dependent expression of cytokines and chemokines. SMCs were infected with AdNull or AdPKCδ followed by incubation with PMA (1 nM). Compared to AdNull control, activation of PKCδ for 6 h increased expression of Ccl2, Ccl7, Cxcl16, and Cx3cl1 (Online Figure III A) in AdPKCδ-infected SMCs, which is consistent with our prior study 22. However, the 48h activation resulted in a different expression profile. Since reendothelialization trails SMC apoptosis and other injury responses, we focused on factors that were uniquely upregulated at 48h in AdPKCδ-infected SMCs, particularly the two CXCR2 ligands (CXCL1 and CXCL7) (Online Figure III B). Consistent with the array results, PKCδ significantly up-regulated Cxcl7 and to a lesser extent Cxcl1. (Online Figure III C&D). In injured arteries, CXCL7 was readily detectable and its levels were decreased by shPKCδ and increased by AdPKCδ, respectively (Figure 6A&B). Similar observations were made with CXCL1 (Online Figure III E).
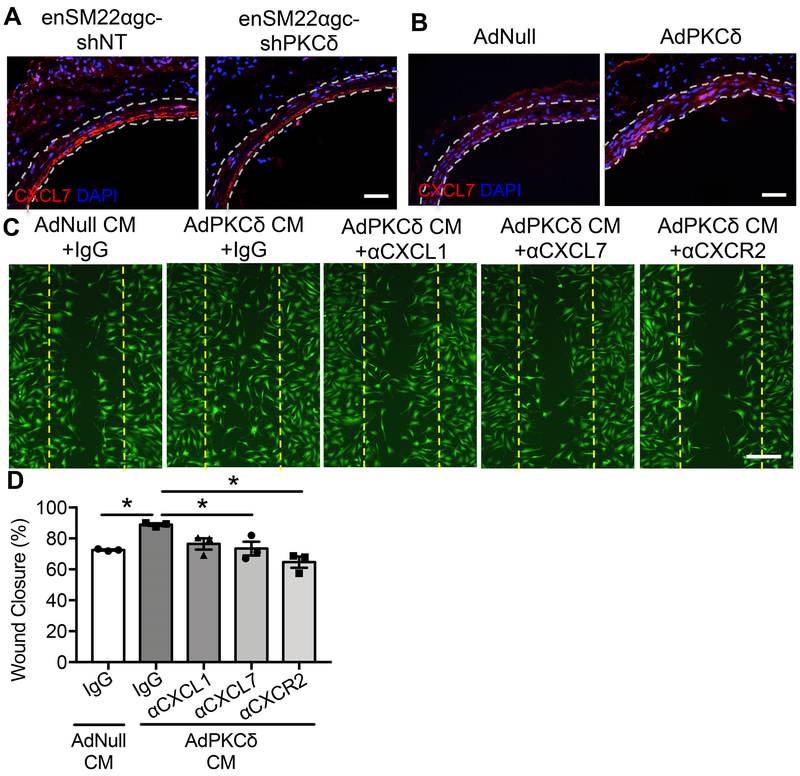
Figure 6. CXCR2 ligands function downstream from PKCδ in the recruitment of ECs.
(A) Representative images of CXCL7 stained rat carotid cross-sections harvested from injured Lenti-enSM22αgc-non-targeting shRNA-treated or injured Lenti-enSM22αgc-shPKCδ-treated rats (7 days post-angioplasty). Nuclei were indicated by positive stains with DAPI (blue). The locations of the internal and external elastic lamina defining boundaries of the media are shown as white dashed lines. Scale bar = 50 μm. (B) Representative images of CXCL7 stained rat carotid cross-sections harvested 3 days after angioplasty from injured AdNull-treated or injured AdPKCδ-infected rats. Nuclei were indicated by positive stains with DAPI (blue). The locations of the internal and external elastic lamina defining boundaries of the media are shown as white dashed lines. Scale bar = 50 μm. (C) Wound closure of mouse ECs cultured in medium conditioned by AdNull- or AdPKCδ-infected and PMA- (1nM) treated SMCs in the presence of IgG, neutralizing antibody anti-CXCL1, anti-CXCL7 or anti-CXCR2. ECs were labeled with CMFDA green fluorescence dye for visualization. Dashed lines indicate the cell-free gap right after the scratch. Scale bar = 50 μm (D) Quantification of endothelial wound closure, determined by the percentage of the recovered area over the total injured area using ImageJ software. Results are expressed as mean±SEM. n=3, *p<0.05, One-way ANOVA.
To determine the role of CXCR2 ligands in the SMC-EC crosstalk, we added neutralizing antibodies against CXCL1 or CXCL7 to the EC wound healing assay. Neutralizing CXCL7 significantly attenuated the stimulatory effect of medium conditioned by AdPKCδ-infected SMCs on EC wound closure (Figure 6C&D). In addition, pre-incubating ECs with an anti-CXCR2 antibody diminished the ability of EC to respond to AdPKCδ-infected SMCs conditioned medium (Figure 6C&D). Neutralizing CXCL1 produced a moderate reduction in EC wound closure, but this trend did not reach statistical significance (Figure 6C&D). Taken together, these results support that SMCs recruit ECs predominantly through a PKCδ-dependent release of CXCL7.
PKCδ regulates CXCL7 expression in SMCs through STAT3.
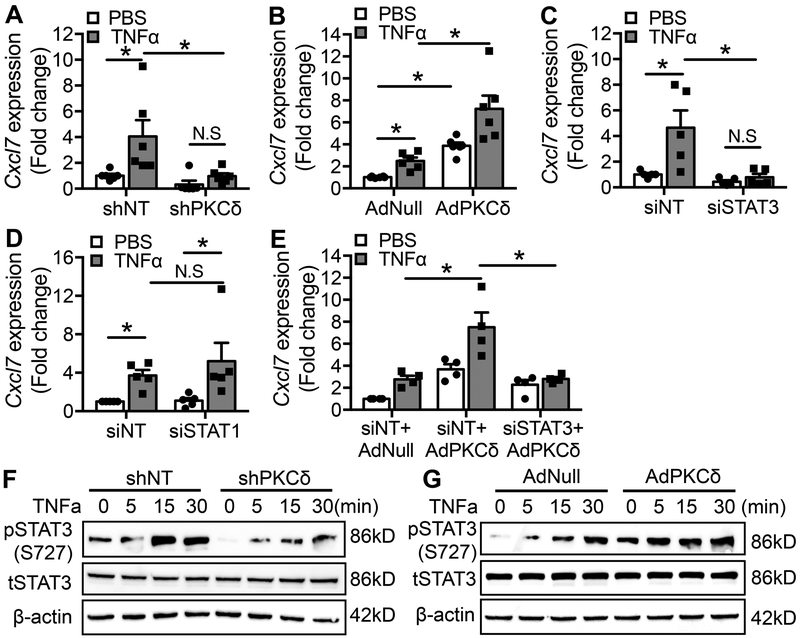
Next, we sought to determine the mechanism underlying upregulation of CXCL7 by PKCδ. In cultured rat carotid SMCs, TNFα increased Cxcl7 mRNA levels in a PKCδ-dependent manner; the TNFα effect was diminished by shPKCδ but enhanced by AdPKCδ (Figure 7A&B). Since signal transducer and activator of transcription-1 (STAT1) and STAT3 play a critical role in regulating TNFα-mediated signaling 49, 50, we tested whether siRNA-mediated knocking down of STAT1 and STAT3 affects Cxcl7 expression in SMCs. Knockdown of STAT3 completely eliminated the effect of TNFα on Cxcl7 mRNA expression (Figure 7C), whereas knockdown of STAT1 had minimal effects (Figure 7D). Furthermore, knockdown of STAT3 abolished the effect of AdPKCδ on Cxcl7 expression (Figure 7E), suggesting PKCδ regulates Cxcl7 through STAT3. The relationship between PKCδ and STAT3 was further demonstrated by examining STAT3 phosphorylation at Ser727, which regulates its transcriptional activation 51, 52. Treatment of SMCs with TNFα did not change the total level of STAT3, but rapidly increased STAT3 phosphorylation at Ser727 (Figure 7F&G). Ser727 phosphorylation was attenuated by knockdown of PKCδ but enhanced by overexpression of PKCδ (Figure 7F&G). Collectively, these results indicate the PKCδ regulates Cxcl7 expression via STAT3 in SMCs, likely through modulating its phosphorylation at Ser727.
Figure 7. PKCδ regulates Cxcl7 expression through STAT3.
. (A&B) SMCs were infected with Lenti-non-targeting shRNA (shNT) or Lenti-PKCδ shRNA (shPKCδ) (A), AdNull or AdPKCδ (B) followed by incubation with PBS or TNFα for 12h. Levels of Cxcl7 mRNA were analyzed using qPCR. Results are expressed as mean±SEM. n=6, *p<0.05, One-way ANOVA. (C&D) SMCs were transduced with non-targeting siRNA (siNT), STAT3 siRNA (siSTAT3) (C), or STAT1 siRNA (siSTAT1) followed by incubation with PBS or TNFα for 12h. Levels of Cxcl7 mRNA expression were analyzed using qPCR. Results are expressed as mean±SEM. n=5, *p<0.05, One-way ANOVA. (E) SMCs were transduced with indicated siRNA and adenoviral vectors followed by incubation with PBS or TNFα for 12h, Cxcl7 mRNA expression was analyzed using qPCR. Results are expressed as mean±SEM. n=4, *p<0.05, One-way ANOVA. (F&G) TNFα induced STAT3 S727 phosphorylation in SMCs infected with lentivirus shNT or shPKCδ (F) and in SMCs infected with adenovirus AdNull or AdPKCδ (G). Cells were harvested at the indicated time and whole-cell lysates were subjected to immunoblot analysis. n=3.
Restoring CXCL7 expression in SMCs rescues reendothelialization.
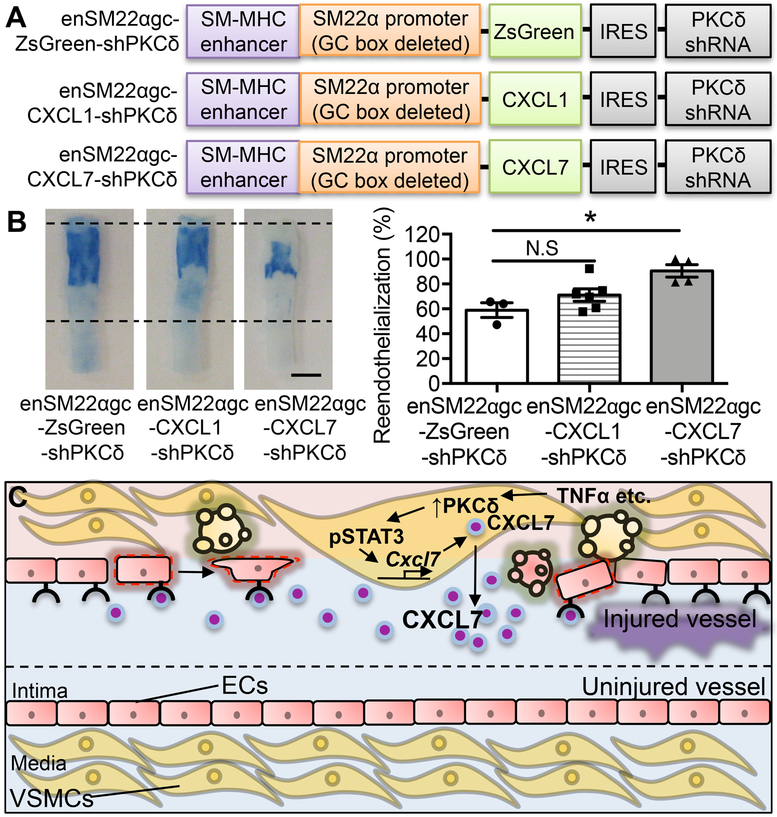
We reasoned that if SMCs facilitate endothelial repair through CXCL7, inserting the Cxcl7 cDNA to the Prkcd shRNA lentiviral vector would overcome the adverse effect of Prkcd knockdown on endothelial regeneration (Figure 8A). Using the Evans blue dye exclusion assay, we demonstrated that the Cxcl7 cDNA rescued reendothelialization (Evans blue negative area: Prkcd shRNA: 58.97±5.90% vs. Prkcd shRNA+ Cxcl7 cDNA 90.45±5.03%) (Figure 8B). In contrast, insertion of the Cxcl1 cDNA produced a moderate effect on reendothelialization, which did not reach statistical significance (Figure 8B). Although restoration of CXCL7 in shPKCδ-treated arteries rescued endothlialization, it did not fully counter the adverse effect of PKCδ on I/M ratio (Online Figure IV A&B). We speculate that CXCL7 is not responsible for all functions of PKCδ in injury response. Indeed, knocking down PKCδ caused apoptosis resistance in SMCs. However, the apoptosis resistance to H2O2 was similar between of the shPKCδ vectors with or without the Cxcl7 cDNA (Online Figure IV C). Taken together, these results coupled with our previous findings suggest that PKCδ plays multiple roles in vascular injury response – acceleration of reendothelialization likely in part through upregulating CXCL7 and inhibition of intimal thickening through promoting SMC apoptosis 25
Figure 8. Restoration of CXCL7 rescues reendothelialization.
(A) Schematics of SMC-specific expression vectors expressing both PKCδ shRNA and CXCR2 ligand. (B) Representative Evans blue dye-stained carotid arteries harvested 21 days after angioplasty from Lenti-enSM22αgc-ZsGreen-shPKCδ-, Lenti-enSM22αgc-CXCL1-shPKCδ-, and Lenti-enSM22αgc-CXCL7-shPKCδ-treated rats. Boundaries of the injured areas are indicated by the dashed lines. Reendothelialization was determined by the percentage of Evans blue negative area over the total injured area using ImageJ software. Scale bar = 3 mm. Results are expressed as mean±SEM. n=3-6, *p<0.05, One-way ANOVA. (C) Proposed mechanisms through which SMCs facilitate endothelial regeneration. Vascular injury, which leads to endothelial denudation, increases PKCδ (↑ PKCδ). Activation of PKCδ causes SMC apoptosis and stimulates the production of paracrine factors including STAT3 activation-mediated CXCL7, which in turn triggers migration of neighboring ECs to the denuded region.
DISCUSSION
The rate of restoring damaged endothelium inversely correlates with neointima formation in atherosclerosis and restenosis 8–10. Our current study provides new mechanistic insights by demonstrating injured vascular SMCs play an active role in the reendothelialization process. Using a combination of gene therapy and conditional knockout approach, we demonstrated in rodent arterial injury models that SMCs facilitate endothelial regeneration in a PKCδ-dependent manner, likely in part through the release of CXCL7 which recruits ECs (Figure 8C). This novel SMC-to-EC communication, along with the established EC-to-SMC and SMC-fibroblast crosstalks, underscores the concept that different vascular cell types work in a coordinated fashion when responding to injury.
Our data further emphasize the complex roles of PKCδ in vascular injury response. Prior studies from our group and others established PKCδ as a central stress mediator in SMCs whose expression is markedly upregulated in human restenotic lesions 25. Global deletion of Prkcd gene causes apoptosis resistance in the injured vessel wall thus increases the size of neointimal lesion 25, 26. The exacerbated intimal hyperplasia in Prkcd−/− mice is accompanied by delayed reendothelialization as shown by Bai et al. in a mouse wire injury model 29. They attributed this phenotype to a defective migratory property of Prkcd−/− ECs 29. While our findings are consistent with Bai’s study regarding the impeded endothelial repair, we provide a novel explanation that is different but not necessarily mutually exclusive from Bai’s. Through SMC-specific knockdown and knockout of Prkcd gene in rats and mouse arteries, respectively, we explicitly illustrated inhibition of PKCδ in medial SMCs alone is sufficient to delay reendothelialization. However, due to technical difficulties associated with intraluminal delivery, we did not test whether restoring Prkcd expression rescues the endothelial phenotype of SMC-specific Prkcd knockout mice. In addition, no available assays allowing us to monitor EC migration in injured arteries. However, ectopic expression of PKCδ in medial SMCs of injured rat carotid arteries support the role of PKCδ in SMC-EC communication. This notion is further illustrated by in vitro studies in which medium conditioned by AdPKCδ-infected SMCs promoted migration of ECs.
The plasticity of vascular SMCs has been demonstrated in atherosclerosis, restenosis, hypertension as well as abdominal aortic aneurysm 6, 7, 53. In response to numerous stimuli, vascular SMCs switch from a quiescent contractile state to more migratory, proliferative, synthetic, endocytic, phagocytic, or even osteoblastic phenotypes. The current work coupled with our previous studies highlights SMCs as an important source of chemokines 22. Using conditioned medium as well as gene transfer approaches, we demonstrated both in vitro and in vivo that SMCs employ a PKCδ-dependent mechanism to produce chemokines. The observation that restoration of Cxcl7 expression in Prkcd knockdown arteries rescued reendothelialization suggests the central role of this particular CXCR2 ligand. As such, the current study provides the first evidence that PKCδ upregulates CXCR2 ligands. CXCR2 plays an essential role in mediating migration of microvascular ECs in response to IL-8 54, 55. Liehn et al. showed that antibody blockade of CXCL1 inhibits endothelial recovery and enhances plaque formation in ApoE-deficient mice 56. Here, immunodepletion or genetic knockdown of CXCL7, but not CXCL1, markedly diminished EC wound closure, suggesting CXCL7 plays a predominant role in the PKCδ-mediated SMC-EC crosstalk. It is widely accepted that the regeneration of the endothelial lining is largely attributed to the migration and proliferation of neighboring cells. However, our data do not exclude the possibility that SMCs may influence endothelial repair through paracrine effects on circulating progenitors or progenitor-like cells.
Despite the important role of CXCL7 in vascular diseases 57, the mechanism underlying CXCL7 regulation after arterial injury is rather poorly understood. Our results suggest the SMCs produce CXCL7 in a PKCδ-dependent manner in injured arteries. Vascular injury, caused by overstretching of an artery during vascular interventions, produces apoptotic bodies, necrotic cell debris, and increased expression of cytokines 20. Among the injury-induced cytokines, TNFα has been implicated in vascular injury in patients who underwent percutaneous coronary intervention (PCI) as well as in preclinical models of restenosis 45–47. Therefore, we use TNFα to activate PKCδ in vitro, although PKCδ is likely to be activated by multiple factors associated with injured vessels. We demonstrated that PKCδ regulates Cxcl7 mRNA expression in cultured rat carotid SMCs. Interestingly, the regulation of Cxcl7 by PKCδ appears to depend on STAT3, but not STAT1 although both have been implicated in PKCδ-mediated signaling 58, 59. Phosphorylation of STAT3 is linked to its transcriptional activation and function 51, 52. Upon TNFα stimulation, we observed that STAT3 was phosphorylated at Ser727, which is required for maximal activation by diverse stimuli 60, 61. Deficiency in PKCδ significantly attenuated Ser727 phosphorylation whereas overexpression of PKCδ enhanced TNFα-induced STAT3 Ser727 phosphorylation. Taken together, our results suggest PKCδ regulates CXCL7 through STAT3 in SMCs, likely by increasing STAT3 phosphorylation at Ser727.
In summary, the current study reveals a novel function of medial SMCs in vascular injury repair. Specifically, high expression of PKCδ in SMCs is required for rapid regeneration of denuded endothelium, likely in part through CXCR2 ligands-mediated EC migration.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Timely endothelial regeneration or reendothelialization after vascular injury prevents thrombotic events and restrains the development of intimal hyperplasia (IH). IH contributes to the re-narrowing of treated vessels and therefore hampers the long-term success of vascular interventions.
Smooth muscle cells (SMCs) respond to injury with functional changes including proliferation, migration, and secretion of chemokines and cytokines.
Protein kinase C-delta (PKCδ) is a crucial stress-response gene that is up-regulated and activated following injury. PKCδ plays a critical role in the regulation of SMC apoptosis and chemokine production.
What New Information Does This Article Contribute?
SMCs can regulate reendothelialization through a paracrine mechanism that requires PKCδ.
Mice with SMC-specific Prkcd deficiency display impaired reendothelialization and develop greater IH after femoral artery wire injury. Conversely, SMC-specific gene delivery of PKCδ accelerates reendothelialization.
Paracrine signals from high PKCδ expressing SMCs promote endothelial wound closure but have minimal effect on endothelial cell (EC) proliferation.
PKCδ activation in SMCs increases the expression of CXCR2 ligands CXCL1 and CXCL7 through signal transducer and activator of transcription 3 (STAT3), likely by increasing STAT3 phosphorylation at Serine 727.
In the context of SMC-specific Prkcd deficiency, restoration of Cxcl7 expression rescues the defective reendothelialization.
Injury to the endothelium occurs during vascular interventions, including balloon angioplasty and stenting. Current therapies to prevent restenosis primarily focus on inhibiting SMCs. It is generally believed that SMCs undergo a phenotypic change in response to injury associated with vascular surgical procedures. Great advances have been made in identifying the molecular mechanisms involved in modulating the SMC phenotypic switch. Less is known about the crosstalk between SMCs and other types of cells. In this paper, we report that injured SMCs promote reendothelialization by attracting ECs to the site of injury through a PKCδ-dependent paracrine mechanism. Mechanistically, activation of PKCδ upregulates the expression of the CXCR2 ligands CXCL1 and CXCL7 in SMCs. Regulation of Cxcl7 by PKCδ in SMCs depends on STAT3, but not STAT1, likely through phosphorylation of STAT3 at Serine 727. The delayed reendothelialization in Prkcd deficient arteries can be rescued by restoration of CXCL7. These results, along with our previous findings, demonstrate the ability of SMCs to communicate with other cell types in the vascular wall as a part of a coordinated vascular injury response. In-depth understanding of cell-cell cross-talk expands our knowledge of the vascular injury response and promotes therapeutic development.
ACKNOWLEDGMENTS
The authors are grateful to Dr. C. Ronald Kahn (Harvard Medical School) for providing the Prkcdfl/fl mice, and Dr. Mary Reyland (University of Colorado Denver) for providing pEGFP-PKCδ plasmid.
SOURCES OF FUNDING
This study was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute [Grant R01HL122562–01 to B.L.], and the American Heart Association [Grant 15PRE25670074 to J.R, 14PRE18560035 to Q.W, 17POST33680095 to T.Z, and 17PRE33670082 to K.G]. The N.S. lab was supported by an unrestricted award from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences, Retina Research Foundation, P30 EY016665, P30 CA014520, EPA 83573701, EY022883, and EY026078. NS is a recipient of RPB Stein Innovation Award.
Nonstandard Abbreviations and Acronyms:
- ADAMTS7
a disintegrin and metalloproteinase with thrombospondin motifs 7
- Adv
adventitial fibroblasts
- BM
bone marrow
- BSA
bovine serum albumin
- CXCL1
chemokine (C-X-C motif) ligand 1
- CXCL7
chemokine (C-X-C motif) ligand 7
- CXCR2
chemokine (C-X-C Motif) receptor 2
- CMV
cytomegalovirus
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethylsulfoxide
- ECs
endothelial cells
- EGFP
Enhanced green fluorescent protein
- FBS
fetal bovine serum
- HRP
horseradish peroxidase
- IH
intimal hyperplasia
- NO
nitric oxide
- PKCδ
Protein kinase C-delta
- PMA
phorbol-12-myristate-13-acetate
- PFA
paraformaldehyde
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- SMCs
smooth muscle cells
- SMMHC
smooth muscle myosin heavy chain
- STAT1
signal transducer and activator of transcription 1
- STAT3
signal transducer and activator of transcription 3
- SV
simian virus
- TNFα
tumor necrosis factor alpha
- TBS
trisbuffered saline
- TM
tamoxifen
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Esper RJ, Nordaby RA, Vilarino JO, Paragano A, Cacharron JL, Machado RA. Endothelial dysfunction: A comprehensive appraisal. Cardiovascular diabetology. 2006;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochimica et biophysica acta. 2008;1778:794–809 [DOI] [PubMed] [Google Scholar]
- 3.Ahanchi SS, Tsihlis ND, Kibbe MR. The role of nitric oxide in the pathophysiology of intimal hyperplasia. Journal of vascular surgery. 2007;45 Suppl A:A64–73 [DOI] [PubMed] [Google Scholar]
- 4.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32 [DOI] [PubMed] [Google Scholar]
- 5.Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nature medicine. 2011;17:1410–1422 [DOI] [PubMed] [Google Scholar]
- 6.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovascular research. 2012;95:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marx SO, Totary-Jain H, Marks AR. Vascular smooth muscle cell proliferation in restenosis. Circulation. Cardiovascular interventions. 2011;4:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Versari D, Lerman LO, Lerman A. The importance of reendothelialization after arterial injury. Current pharmaceutical design. 2007;13:1811–1824 [DOI] [PubMed] [Google Scholar]
- 9.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-eluting stent and coronary thrombosis: Biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058 [DOI] [PubMed] [Google Scholar]
- 10.Hutter R, Sauter BV, Reis ED, Roque M, Vorchheimer D, Carrick FE, Fallon JT, Fuster V, Badimon JJ. Decreased reendothelialization and increased neointima formation with endostatin overexpression in a mouse model of arterial injury. Circulation. 2003;107:1658–1663 [DOI] [PubMed] [Google Scholar]
- 11.Newby AC. An overview of the vascular response to injury: A tribute to the late russell ross. Toxicology letters. 2000;112–113:519–529 [DOI] [PubMed] [Google Scholar]
- 12.Van Belle E, Bauters C, Asahara T, Isner JM. Endothelial regrowth after arterial injury: From vascular repair to therapeutics. Cardiovascular research. 1998;38:54–68 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz SM, Haudenschild CC, Eddy EM. Endothelial regneration. I. Quantitative analysis of initial stages of endothelial regeneration in rat aortic intima. Laboratory investigation; a journal of technical methods and pathology. 1978;38:568–580 [PubMed] [Google Scholar]
- 14.Fishman JA, Ryan GB, Karnovsky MJ. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Laboratory investigation; a journal of technical methods and pathology. 1975;32:339–351 [PubMed] [Google Scholar]
- 15.Clopath P, Muller K, Staubli W, Burk RR. In vivo and in vitro studies on endothelial regeneration. Haemostasis. 1979;8:149–157 [DOI] [PubMed] [Google Scholar]
- 16.Itoh Y, Toriumi H, Yamada S, Hoshino H, Suzuki N. Resident endothelial cells surrounding damaged arterial endothelium reendothelialize the lesion. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1725–1732 [DOI] [PubMed] [Google Scholar]
- 17.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C. Microrna-126–5p promotes endothelial proliferation and limits atherosclerosis by suppressing dlk1. Nature medicine. 2014;20:368–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler T, Zhang L, Liu Z, Yin X, Huang Y, Wang Y, Fu Y, Mayr M, Ge Q, Xu Q, Zhu Y, Wang X, Schmidt K, de Wit C, Erdmann J, Schunkert H, Aherrahrou Z, Kong W. Adamts-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1. Circulation. 2015;131:1191–1201 [DOI] [PubMed] [Google Scholar]
- 19.Libby P Inflammation in atherosclerosis. Nature. 2002;420:868–874 [DOI] [PubMed] [Google Scholar]
- 20.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochemical pharmacology. 2009;78:539–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu B, Wong MM, Potter CM, Simpson RM, Karamariti E, Zhang Z, Zeng L, Warren D, Hu Y, Wang W, Xu Q. Vascular stem/progenitor cell migration induced by smooth muscle cell-derived chemokine (c-c motif) ligand 2 and chemokine (c-x-c motif) ligand 1 contributes to neointima formation. Stem cells. 2016;34:2368–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren J, Wang Q, Morgan S, Si Y, Ravichander A, Dou C, Kent KC, Liu B. Protein kinase c-delta (pkcdelta) regulates proinflammatory chemokine expression through cytosolic interaction with the nf-kappab subunit p65 in vascular smooth muscle cells. The Journal of biological chemistry. 2014;289:9013–9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Si Y, Ren J, Wang P, Rateri DL, Daugherty A, Shi XD, Kent KC, Liu B. Protein kinase c-delta mediates adventitial cell migration through regulation of monocyte chemoattractant protein-1 expression in a rat angioplasty model. Arterioscler Thromb Vasc Biol. 2012;32:943–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryer EJ, Sakakibara K, Wang C, Sarkar D, Fisher PB, Faries PL, Kent KC, Liu B. Protein kinase c delta induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. The Journal of biological chemistry. 2005;280:35310–35317 [DOI] [PubMed] [Google Scholar]
- 25.Yamanouchi D, Kato K, Ryer EJ, Zhang F, Liu B. Protein kinase c delta mediates arterial injury responses through regulation of vascular smooth muscle cell apoptosis. Cardiovascular research. 2010;85:434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, Ghaffari-Tabrizi N, Baier G, Hu Y, Xu Q. Exacerbated vein graft arteriosclerosis in protein kinase cdelta-null mice. The Journal of clinical investigation. 2001;108:1505–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Ryer EJ, Kundi R, Kamiya K, Itoh H, Faries PL, Sakakibara K, Kent KC. Protein kinase c-[delta] regulates migration and proliferation of vascular smooth muscle cells through the extracellular signal-regulated kinase 1/2. Journal of Vascular Surgery. 2007;45:160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato K, Yamanouchi D, Esbona K, Kamiya K, Zhang F, Kent KC, Liu B. Caspase-mediated protein kinase c-{delta} cleavage is necessary for apoptosis of vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2009;297:H2253–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai X, Margariti A, Hu Y, Sato Y, Zeng L, Ivetic A, Habi O, Mason JC, Wang X, Xu Q. Protein kinase c{delta} deficiency accelerates neointimal lesions of mouse injured artery involving delayed reendothelialization and vasohibin-1 accumulation. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:2467–2474 [DOI] [PubMed] [Google Scholar]
- 30.Bezy O, Tran TT, Pihlajamaki J, Suzuki R, Emanuelli B, Winnay J, Mori MA, Haas J, Biddinger SB, Leitges M, Goldfine AB, Patti ME, King GL, Kahn CR. Pkcdelta regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. The Journal of clinical investigation. 2011;121:2504–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sata M, Maejima Y, Adachi F, Fukino K, Saiura A, Sugiura S, Aoyagi T, Imai Y, Kurihara H, Kimura K, Omata M, Makuuchi M, Hirata Y, Nagai R. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. Journal of molecular and cellular cardiology. 2000;32:2097–2104 [DOI] [PubMed] [Google Scholar]
- 32.Kundi R, Hollenbeck ST, Yamanouchi D, Herman BC, Edlin R, Ryer EJ, Wang C, Tsai S, Liu B, Kent KC. Arterial gene transfer of the tgf-beta signalling protein smad3 induces adaptive remodelling following angioplasty: A role for ctgf. Cardiovascular research. 2009;84:326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Laboratory investigation; a journal of technical methods and pathology. 1983;49:208–215 [PubMed] [Google Scholar]
- 34.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Molecular vision. 2003;9:171–178 [PubMed] [Google Scholar]
- 35.Wara AK, Manica A, Marchini JF, Sun X, Icli B, Tesmenitsky Y, Croce K, Feinberg MW. Bone marrow-derived kruppel-like factor 10 controls reendothelialization in response to arterial injury. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JM, Wang Y, Miao YJ, Zhang Y, Wu YN, Jia LX, Qi YF, Du J. Knockout of cd8 delays reendothelialization and accelerates neointima formation in injured arteries of mouse via tnf-alpha inhibiting the endothelial cells migration. PloS one. 2013;8:e62001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yue TL, Vickery-Clark L, Louden CS, Gu JL, Ma XL, Narayanan PK, Li X, Chen J, Storer B, Willette R, Gossett KA, Ohlstein EH. Selective estrogen receptor modulator idoxifene inhibits smooth muscle cell proliferation, enhances reendothelialization, and inhibits neointimal formation in vivo after vascular injury. Circulation. 2000;102:III281–288 [DOI] [PubMed] [Google Scholar]
- 38.Kawabe-Yako R, Ii M, Masuo O, Asahara T, Itakura T. Cilostazol activates function of bone marrow-derived endothelial progenitor cell for re-endothelialization in a carotid balloon injury model. PloS one. 2011;6:e24646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribault S, Neuville P, Mechine-Neuville A, Auge F, Parlakian A, Gabbiani G, Paulin D, Calenda V. Chimeric smooth muscle-specific enhancer/promoters: Valuable tools for adenovirus-mediated cardiovascular gene therapy. Circulation research. 2001;88:468–475 [DOI] [PubMed] [Google Scholar]
- 40.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. The Journal of clinical investigation. 2000;106:1139–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cefai D, Simeoni E, Ludunge KM, Driscoll R, von Segesser LK, Kappenberger L, Vassalli G. Multiply attenuated, self-inactivating lentiviral vectors efficiently transduce human coronary artery cells in vitro and rat arteries in vivo. Journal of molecular and cellular cardiology. 2005;38:333–344 [DOI] [PubMed] [Google Scholar]
- 42.Rome JJ, Shayani V, Flugelman MY, Newman KD, Farb A, Virmani R, Dichek DA. Anatomic barriers influence the distribution of in vivo gene transfer into the arterial wall. Modeling with microscopic tracer particles and verification with a recombinant adenoviral vector. Arteriosclerosis and thrombosis : a journal of vascular biology. 1994;14:148–161 [DOI] [PubMed] [Google Scholar]
- 43.Fischer L, Preis M, Weisz A, Koren B, Lewis BS, Flugelman MY. Viral vectors for vascular gene therapy. Experimental and clinical cardiology. 2002;7:106–112 [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Y, Gao Y, Qin J, Kuang CY, Song MB, Yu SY, Cui B, Chen JF, Huang L. Ccn1 promotes the differentiation of endothelial progenitor cells and reendothelialization in the early phase after vascular injury. Basic research in cardiology. 2010;105:713–724 [DOI] [PubMed] [Google Scholar]
- 45.Monraats PS, Pires NM, Schepers A, Agema WR, Boesten LS, de Vries MR, Zwinderman AH, de Maat MP, Doevendans PA, de Winter RJ, Tio RA, Waltenberger J, t Hart LM, Frants RR, Quax PH, van Vlijmen BJ, Havekes LM, van der Laarse A, van der Wall EE, Jukema JW. Tumor necrosis factor-alpha plays an important role in restenosis development. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1998–2004 [DOI] [PubMed] [Google Scholar]
- 46.Rakhit RD, Seiler C, Wustmann K, Zbinden S, Windecker S, Meier B, Eberli FR. Tumour necrosis factor-alpha and interleukin-6 release during primary percutaneous coronary intervention for acute myocardial infarction is related to coronary collateral flow. Coronary artery disease. 2005;16:147–152 [DOI] [PubMed] [Google Scholar]
- 47.Bonz AW, Lengenfelder B, Jacobs M, Strotmann J, Held S, Ertl G, Voelker W. Cytokine response after percutaneous coronary intervention in stable angina: Effect of selective glycoprotein iib/iiia receptor antagonism. American heart journal. 2003;145:693–699 [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger KC, Zhang C. Role of tnf-alpha in vascular dysfunction. Clinical science. 2009;116:219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miscia S, Marchisio M, Grilli A, Di Valerio V, Centurione L, Sabatino G, Garaci F, Zauli G, Bonvini E, Di Baldassarre A. Tumor necrosis factor alpha (tnf-alpha) activates jak1/stat3-stat5b signaling through tnfr-1 in human b cells. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 2002;13:13–18 [PubMed] [Google Scholar]
- 50.Wesemann DR, Benveniste EN. Stat-1 alpha and ifn-gamma as modulators of tnf-alpha signaling in macrophages: Regulation and functional implications of the tnf receptor 1:Stat-1 alpha complex. J Immunol. 2003;171:5313–5319 [DOI] [PubMed] [Google Scholar]
- 51.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of stat3 during cntf signaling is mediated by the rapamycin target mtor. Current biology : CB. 2000;10:47–50 [DOI] [PubMed] [Google Scholar]
- 52.Wen Z, Zhong Z, Darnell JE Jr., Maximal activation of transcription by stat1 and stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250 [DOI] [PubMed] [Google Scholar]
- 53.Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, Lau CL, Owens GK. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. The Journal of thoracic and cardiovascular surgery. 2009;138:1392–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schraufstatter IU, Chung J, Burger M. Il-8 activates endothelial cell cxcr1 and cxcr2 through rho and rac signaling pathways. American journal of physiology. Lung cellular and molecular physiology. 2001;280:L1094–1103 [DOI] [PubMed] [Google Scholar]
- 55.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The cxc chemokine receptor 2, cxcr2, is the putative receptor for elr+ cxc chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277 [DOI] [PubMed] [Google Scholar]
- 56.Liehn EA, Schober A, Weber C. Blockade of keratinocyte-derived chemokine inhibits endothelial recovery and enhances plaque formation after arterial injury in apoe-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1891–1896 [DOI] [PubMed] [Google Scholar]
- 57.Hristov M, Zernecke A, Bidzhekov K, Liehn EA, Shagdarsuren E, Ludwig A, Weber C. Importance of cxc chemokine receptor 2 in the homing of human peripheral blood endothelial progenitor cells to sites of arterial injury. Circulation research. 2007;100:590–597 [DOI] [PubMed] [Google Scholar]
- 58.Jain N, Zhang T, Kee WH, Li W, Cao X. Protein kinase c delta associates with and phosphorylates stat3 in an interleukin-6-dependent manner. The Journal of biological chemistry. 1999;274:24392–24400 [DOI] [PubMed] [Google Scholar]
- 59.Uddin S, Sassano A, Deb DK, Verma A, Majchrzak B, Rahman A, Malik AB, Fish EN, Platanias LC. Protein kinase c-delta (pkc-delta) is activated by type i interferons and mediates phosphorylation of stat1 on serine 727. The Journal of biological chemistry. 2002;277:14408–14416 [DOI] [PubMed] [Google Scholar]
- 60.Sakaguchi M, Oka M, Iwasaki T, Fukami Y, Nishigori C. Role and regulation of stat3 phosphorylation at ser727 in melanocytes and melanoma cells. The Journal of investigative dermatology. 2012;132:1877–1885 [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Liu G, Dong Z. Msk1 and jnks mediate phosphorylation of stat3 in uva-irradiated mouse epidermal jb6 cells. The Journal of biological chemistry. 2001;276:42534–42542 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.