Abstract
Objectives:
To characterize the short-term prognosis of a clinical population of pediatric and young adult patients with migraine and explore predictors of clinical worsening while in care.
Methods:
This was a retrospective study of all migraine patients seen at the Cincinnati Children’s Hospital Headache Center from 09/01/2006 to 12/31/2017, who had at least one follow-up visit within 1–3 months of the index visit analyzed. Included data were: age, sex, race, primary ICHD diagnosis, chronic migraine, medication overuse, history of status migrainosus, BMI percentile, headache frequency, headache severity, PedMIDAS score, allodynia, preventive treatment type, lifestyle habits, disease duration, depressive and anxiety symptoms. Clinical worsening was defined as an increase of 4 or more headache days per month between the index visit and the follow-up visit.
Results:
Data for 13,160 visit pairs (index and follow-up), from 5,316 patients were analyzed. Clinical worsening occurred in only 14.5% (1,908/13,160), whereas a reduction in headache frequency was observed in 56.8% of visit intervals (7,475/13,160), with 34.8% of the intervals (4,580/13,160) showing a reduction of 50% or greater. The change in headache frequency was minimal (increase in 0–3 headaches/month) in 28.7% of intervals (3,737/13,160). In the multivariable model, the odds of worsening were significantly higher with increasing age, female sex, chronic migraine, status migrainosus, depressive symptoms, higher PedMIDAS scores, and use of nutraceuticals, whereas the odds of worsening were lower for summer visits, caffeine drinkers, higher headache frequencies and use of pharmaceuticals.
Conclusions:
The majority of pediatric patients who receive multimodal interdisciplinary care for migraine improve over time. Our findings highlight a set of clinical features that may help in identifying specific factors that may contribute to an unfavorable short-term prognosis.
Keywords: pediatric migraine, prognosis, pediatric headache
INTRODUCTION
Headache is a common complaint in children and adolescents1 and is among the top three reasons for referral to a neurologist2–4. Migraine accounts for a significant proportion of childhood and adolescent headaches, and in child neurology practice, it is the most common diagnosis made3. Children and adolescents with migraine experience significant migraine-related disability that affects their functioning at school, at home and in extra-curricular activities5–8. At a population level, migraine ranks among the top two most prevalent causes of neurological disability in children and adolescents worldwide9.
The majority of children and adolescents who seek and receive care for migraine appear to have favorable outcomes10,11 that are likely sustained over the long term12,13. To date, little is known about what factors may help differentiate patients who will respond to migraine care compared to those who worsen and may have a more refractory course. To our knowledge, no study to date has aimed to determine predictors of short-term clinical worsening while receiving multimodal migraine treatment. Identifying predictors of worsening would be beneficial for clinical providers and researchers alike, as it may allow for the study and development of more efficient stratified care models. In this study, we aim: 1) to characterize the short-term prognosis of a large sample of children, adolescents and young adults presenting for outpatient headache care and 2) to identify predictors of clinical worsening while in headache care for the subset of patients that have this occur.
PATIENTS AND METHODS
Design and Setting
This was a retrospective observational study of patients with migraine seen at the Cincinnati Children’s Headache Center with one or more short-term follow-up intervals (1–3 months). This interval was chosen as this time period is the expected time period for follow-up evaluation and is based on the trajectory response time observed in our cognitive behavioral therapy – amitriptyline study11. The Cincinnati Children’s Headache Center provides multimodal interdisciplinary headache care to children, adolescents and young adults with headache through outpatient clinics, an infusion center and an inpatient service. At the initial outpatient visit, patients are seen by a board certified neurologist with UCNS specialization in Headache Medicine, a pediatric psychologist with training in pain management and a registered nurse. At follow-up visits, patients are seen by either a board certified neurologists or a nurse practitioner experienced in Headache Medicine. The infusion center is an outpatient facility whereby patients can receive interventions for acute migraine. It is staffed by the nurse practitioners. Inpatients are seen by the neurologists. After an initial visit, all patients are offered follow-up in the 6–8 week range. The treating care provider determines the timing of subsequent follow-up visits based on the current level of headache control, the interventions prescribed and patient preference. Management of migraine involves an interdisciplinary multimodal approach including education about migraines, education about self-management strategies including healthy lifestyle habits, acute migraine interventions, and multimodal preventive migraine interventions with nutraceuticals, psychological interventions (biofeedback or pain-focused cognitive behavioral therapy) and pharmacologic interventions. Of note, nutraceutical prescriptions are only recommended to patients with deficiencies: blood levels are drawn at the initial consultation visit for Vitamin D, riboflavin, coenzyme Q10 and folate and thereafter patients with deficiencies are sent a letter recommending the corresponding nutraceutical supplement(s) along with specific dosing recommendations. At each visit, patients are asked to complete a standardized questionnaire that is matched to their visit type and providers complete standardized questionnaires to document the treatment plan, investigations and follow-up plan. Following completion, the patient questionnaires are reviewed in a semi-structured interview with the care provider.
Participants
All patients with a diagnosis of migraine who were seen from 09/01/2006 to 12/31/2017, who had one or more short-term follow-up intervals, were included in this study. A short-term follow-up interval was defined as having at least one follow-up visit (visit B) within 1–3 months (30–93 days inclusive) of a prior visit (visit A – index visit). Visit As included any visit type: new, follow-up, infusion center, or inpatient visit. Similarly, visit Bs comprised follow-up, infusion center, or inpatient visits. All patients included in this study had a diagnosis of migraine with or without aura made by a board certified neurologist with UCNS specialization in Headache Medicine using the International Classification of Headache Disorders (ICHD) criteria14.
Standard Protocol Approvals, Registrations and Patient Consents
Patients and their guardians signed a Health Information Privacy and Accountability (HIPAA) and Informed Consent for data collection and analysis. Those who consent and sign the form have their data entered into a password secured research database, as has been approved by the Cincinnati Children’s Hospital Medical Center institutional review board.
Data Collected
Data were extracted from a database and chart reviews were completed for missing data or data outside the standard ranges. The primary determination of clinical worsening of headaches between two consecutive follow-up visits was defined as an increase of 4 or more headache days per month from visit A to B. Headache days were measured using patient recall at each visit, and were validated by reviewing the headache calendars where available. The rationale for setting the threshold for clinical worsening at 4 or more headache days per month was based on clinical experience in our practice and consensus from our practitioners. In our experience, patients tend to view a change of one headache day per week as clinically meaningful, and this threshold often guides clinical decision-making in our practice model. The following data were included as potential predictors of the outcome: age, sex, race (White, Black, Other), primary ICHD diagnoses (migraine without aura and/or migraine with aura), presence of chronic migraine diagnosis at visit A, medication overuse headache (MOH) diagnosis at visit A, history of status migrainosus, BMI category at visit A, headache frequency at visit A, average headache severity at visit A, PedMIDAS or MIDAS score at visit A, history of allodynia, preventive treatment type at visit A (none, pharmaceutical, nutraceutical, or pharmaceutical plus nutraceutical), months with headache at initial consultation visit, feeling depressed at initial consultation visit, anxiety symptoms at initial consultation visit (endorsing any of the following: feeling anxious, feelings of low self-esteem, worrying a lot or shyness) and the following lifestyle habits at visit A: caffeine intake, history of skipping meals, hours of sleep, amount of exercise and daily fluid intake. The ICHD criteria14 were used to make the diagnoses of migraine, chronic migraine, MOH and status migrainosus. While cognitive behavioral therapy (CBT) and biofeedback-assisted relaxation training are often provided to our patients, we were not able to reliably document the use of these evidence-based strategies13,15 in the current analysis. Although we did record which patients were recommended a trial of CBT or biofeedback, we did not have access to information regarding which patients actually accessed those services and received the prescribed care.
Statistical Analysis
Patient demographic and baseline characteristics were summarized using descriptive statistics, either means with standard deviations, medians with interquartile ranges, or frequencies with percentages as applicable. For patients with multiple follow-up visits (visit Bs) within one 30–93 day interval, the first chronological visit B was selected for analysis. A dichotomous variable was generated for the primary outcome using monthly headache frequency (measured in headache days) at visits A and B, whereby clinical worsening was present if monthly headache frequency increased by 4 or more headaches per month between visits. The BMI percentile was trichotomized into: normal or underweight (<85th percentile), overweight (≥85th and ≤95th percentile) or obese (>95th percentile) as per the Centers for Disease Control and Prevention guidelines16. Hours of sleep were dichotomized into less than 8 hours per night or greater than 8 hours per night. Amount of exercise was dichotomized into less than 3 days per week or greater than 3 days per week. Finally, daily fluid intake was dichotomized into either less than 48 ounces per day or greater than 48 ounces per day. The rationale for dichotomizing these variables was to create categories that are roughly divided according to the recommendations for healthy lifestyle habits made in our clinic (eg. at least 8 hours of sleep per night, at least 3 days of exercise per week, etc).
A generalized linear model with a logit link function, i.e., logistic regression, was analyzed where the response was whether or not a subject met the definition for clinical worsening between visits A and B. Since patients could have more than one distinct visit A (ie. multiple follow-up intervals), repeated measures were incorporated into this model using the GLIMMIX procedure in SAS®. For the independent variables of interest, logistic regression models with repeated measures were analyzed individually and their odds ratios (OR) and 95% confidence intervals (CI) were derived. In order to account for multiple hypothesis testing, the p-values for all of these ORs were adjusted using the False Discovery Rate with a family-wise error rate set at 0.05. Finally, all of the independent variables were included in a single logistic model with repeated measures and a backward elimination variable selection procedure was used such that only variables with two-sided p-values < 0.05 were remaining.
Finally, in a post-hoc analysis designed to assess for selection bias due to differential follow-up, the visit A demographic and clinical characteristics of the study sample were compared to those of the sample of patients who did not have a follow-up visit within 12 months of a visit A. All visits for patients with a diagnosis of migraine with or without aura that were not associated with a follow-up visit within 12 months were included in this comparison group. The groups were compared with Fisher’s exact tests for discrete dichotomous variables, Chi-square tests for polychotomous variables and with t-tests for continuous variables. The threshold for statistical significance was set at p-values < 0.05. All statistical analyses were conducted using the SAS® statistical software version 9.3 (SAS Institute Inc., Cary, NC).
Data Availability Policy
Anonymized data will be shared by request from the qualified investigators.
RESULTS
Data for 13,160 visit pairs, from 5,316 unique patients were included in the analyses. Patient demographic and clinical characteristics are summarized in Table 1. On average, patients improved within their follow-up intervals, with the mean change in headache frequency between visits A and B equal to a decrease in 5.0 ± 9.3 headaches per month. The median change in headache frequency was 1.0 headache per month (interquartile range = 7.0). In 56.8% of visit intervals (7,475/13,160), a reduction in headache frequency was observed, and in 34.8% of the intervals (4,580/13,160), this reduction had a magnitude of 50% or greater. In 28.7% of intervals (3,737/13,160), the change in headache frequency was not deemed to be clinically significant (ie. an increase in 0–3 headaches per month). Overall, our definition of clinical worsening was only met in a very small proportion of visit intervals: in 14.5% of visit intervals (1,908/13,160) an increase in 4 or more headache days per month was observed. Only 26.1% (1,387) of the 5,316 patients had ever experienced an episode of worsening over a 1–3 month interval. Detailed data on the change in headache frequency between visits A and B is displayed in Figure 1.
Table 1.
Demographic and descriptive characteristics of the sample and pairwise comparisons with the no follow-up group
| Characteristic | Study sample | No follow-up group | P Value |
|---|---|---|---|
| Number of patients | 5,316 | 5,341 | N/A |
| Sex | <0.0001* | ||
| Male | 1,730 (32.5%) | 2,153 (40.3%) | |
| Female | 3,586 (67.5%) | 3,188 (59.7%) | |
| Mean age (years) | 13.4 ± 3.7 | 12.9 ± 4.0 | <0.0001* |
| Race | <0.0001* | ||
| White | 4,366 (82.2%) | 4,146 (78.3%) | |
| Black | 607 (11.4%) | 846 (16.0%) | |
| Other | 336 (6.3%) | 304 (5.7%) | |
| Migraine with aura | 0.423 | ||
| Yes | 1,073 (20.2%) | 1,044 (19.5%) | |
| No | 4,243 (79.8%) | 4,297 (80.5%) | |
| Chronic migraine diagnosis at visit A | <0.0001* | ||
| Yes | 2,808 (52.8%) | 2,019 (37.8%) | |
| No | 2,508 (47.2%) | 3,322 (62.2%) | |
| Medication overuse diagnosis at visit A | <0.0001* | ||
| Yes | 804 (15.1%) | 574 (10.7%) | |
| No | 4,512 (84.9%) | 4,767 (89.3%) | |
| Status migrainosus diagnosis | <0.0001* | ||
| Yes | 559 (10.5%) | 402 (7.5%) | |
| No | 4,757 (89.5%) | 4,939 (92.5%) | |
| Mean headache frequency at visit A (headache days/month) | 15.2 ± 9.6 | 11.4 ± 9.6 | <0.0001* |
| Mean headache severity at visit A (on 0–10 numeric pain rating scale) | 5.9 ± 1.9 | 5.8 ± 2.1 | 0.021* |
| Mean PedMIDAS score at visit A | 37.8 ± 42.1 | 30.7 ± 42.6 | <0.0001* |
| Mean MIDAS score at visit A | 47.7 ± 58.2 | 42.2 ± 53.8 | 0.303 |
| PedMIDAS/MIDAS grade at visit A | <0.0001* | ||
| Grade I | 1,419 (26.9%) | 2,104 (39.7%) | |
| Grade II | 1,599 (30.4%) | 1,500 (28.3%) | |
| Grade III | 895 (17.0%) | 697 (13.1%) | |
| Grade IV | 1,356 (25.7%) | 1,003 (18.9%) | |
| Mean number of months with headaches at initial consultation visit | 44.2 ± 36.2 (0–228) | 41.5 ± 35.3 (0–228) | 0.0002 |
| BMI category at visit A | 0.750 | ||
| Normal or underweight (<85th percentile) | 3,299 (63.0%) | 3,281 (62.8%) | |
| Overweight (>85th and <95th percentile) | 905 (17.3%) | 888 (17.0%) | |
| Obese (≥95th percentile) | 1,030 (19.7%) | 1,058 (20.2%) | |
| Recommended preventive treatment type at visit A | <0.0001* | ||
| None | 923 (17.4%) | 2,176 (40.7%) | |
| Nutraceutical alone | 99 (1.9%) | 366 (6.9%) | |
| Pharmaceutical alone | 3,490 (65.7%) | 2,283 (42.7%) | |
| Nutraceutical plus pharmaceutical | 804 (15.1%) | 516 (9.7%) | |
| Allodynia | 0.010* | ||
| Yes | 2,297 (48.7%) | 2,105 (45.6%) | |
| No | 2,225 (47.2%) | 2,316 (50.2%) | |
| Maybe | 193 (4.1%) | 194 (4.2%) | |
| Anxiety symptoms at consultation visit | 0.400 | ||
| Yes | 2,400 (45.8%) | 2,378 (44.9%) | |
| No | 2,844 (54.2%) | 2,914 (55.1%) | |
| Feeling depressed at consultation visit | 0.387 | ||
| Yes | 885 (16.9%) | 860 (16.3%) | |
| No | 4,351 (83.1%) | 4,428 (83.7%) | |
| Quantity of exercise reported at visit A | 0.119 | ||
| < 3 days/week | 3,631 (74.1%) | 3,692 (75.5%) | |
| ≥ 3 days/week | 1,267 (25.9%) | 1,197 (24.5%) | |
| Quantity of liquid intake reported at visit A | 0.001* | ||
| <48 ounces/day | 1,267 (25.9%) | 2,563 (49.5%) | |
| >48 ounces/day | 3,631 (74.1%) | 2,611 (50.5%) | |
| Quantity of sleep reported at visit A | 0.179 | ||
| < 8 hours/day | 1,604 (31.0%) | 1,553 (29.8%) | |
| ≥ 8 hours/day | 3,565 (69.0%) | 3,656 (70.2%) | |
| Reporting skipping meals at visit A | 0.087 | ||
| Yes | 2,026 (38.5%) | 2,121 (40.1%) | |
| No | 3,241 (61.5%) | 3,167 (59.1%) | |
| Caffeine consumption at visit A | 0.668 | ||
| Yes | 2,529 (48.0%) | 2,515 (47.6%) | |
| No | 2,738 (52.0%) | 2,769 (52.4%) | |
Means are expressed as mean ± standard deviation (range)
Frequencies are expressed as n (percentage)
Figure 1. Change in headache frequency between visits A and B.
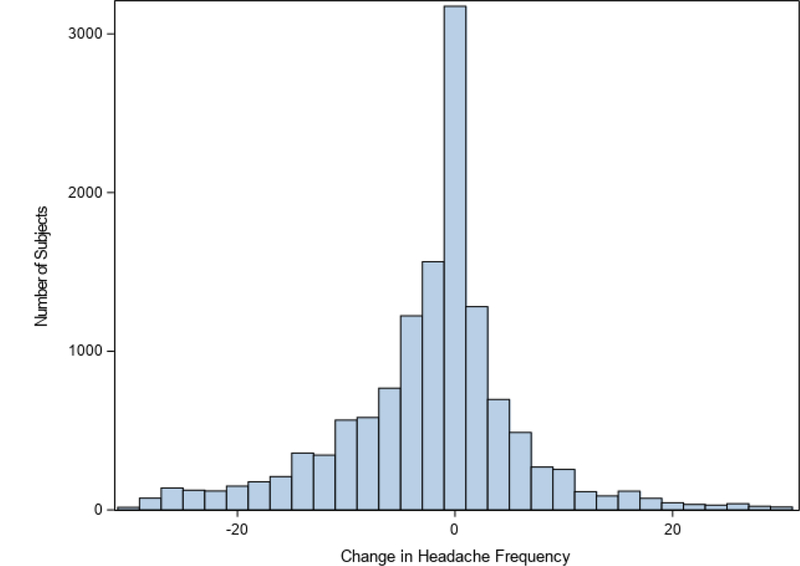
This histogram illustrates the distribution of the change in headache frequency between visits A and B. Values below zero represent a decrease in headache frequency between visit B and visit A.
Table 2 summarizes the results of the univariate models. In the multivariable model, several variables remained statistically significant in predicting the odds of clinical worsening during follow-up intervals (Table 3). Females had 27% higher odds of worsening (OR=1.27, 95% CI=1.11–1.44, p=0.0003). Older patients were more likely to experience clinical worsening: each additional year of age increased the odds of worsening by 4% (OR=1.04, 95% CI=1.02–1.06, p<0.0001). Patients with a history of chronic migraine at visit A had 57% higher odds of worsening (OR=1.57, 95% CI=1.35–1.83, p<0.0001), and patients with a history of status migrainosus had 46% higher odds of worsening (OR=1.46, 95% CI=1.26–1.69, p<0.0001). Headache frequency was inversely related to the odds of clinical worsening: for each additional headache day per month, the odds of worsening decreased by 9% (OR=0.91, 95% CI=0.90–0.92, p<0.0001). Patients with higher disability scores were more likely to worsen: those with a PedMIDAS or MIDAS grade 3 score had 24% higher odds of worsening (OR=1.24, 95% CI=1.03–1.48, p=0.020) and those with a grade 4 score had 54% higher odds of worsening (OR=1.54, 95% CI=1.29–1.84, p<0.0001) as compared to those with a grade 1 score. There was a 31% higher odds of worsening between visits for patients who endorsed depressive symptoms at their consultation visit (OR=1.31, 95% CI=1.13–1.51, p=0.0003). Visit intervals during the summer months were associated with a 35% lower odds of worsening (OR=0.65, 95% CI=0.57–0.74, p<0.0001). Patients who reported caffeine consumption at visit A had a 13% lower odds of worsening as compared to those who did not consume caffeine (OR=0.87, 95% CI=0.78–0.97, p=0.010). Finally, patients who were prescribed pharmaceuticals at visit A had a 22% lower odds of worsening (OR=0.78, 95% CI=0.68–0.88, p<0.0001), whereas patients who were prescribed nutraceuticals at visit A had a 23% higher odds of worsening (OR=1.23, 95% CI=1.10–1.37, p=0.0002).
Table 2.
Significant predictors of clinical worsening during follow-up intervals in univariate repeated measures logistic regression model
| Positive Predictors of Clinical Worsening | OR (95% CI) | P Value |
|---|---|---|
| Female | 1.27 (1.13–1.43)* | 0.0002* |
| Age | 1.03 (1.02–1.05)* | <0.0001* |
| Race | ||
| Black vs. White | 0.95 (0.80–1.13) | 0.242 |
| Other vs. White | 0.81 (0.64–1.02) | |
| Black vs. Other | 1.18 (0.89–1.56) | |
| Migraine with aura | 1.01 (0.88–1.16) | 0.951 |
| Status migrainosus at visit A | 1.47 (1.28–1.68)* | <0.0001* |
| Number of months with headaches at initial visit | 1.00 (0.99–1.00) | 0.175 |
| BMI category at visit A | 0.951 | |
| Overweight vs. Normal/underweight | 0.97 (0.85–1.12) | |
| Obese vs. Normal/underweight | 1.01 (0.88–1.15) | |
| Obese vs. Overweight | 1.03 (0.87–1.22) | |
| Recommended preventive treatment type at visit A | <0.0001* | |
| Nutraceutical alone vs. None | 1.22 (0.98–1.52) | |
| Pharmaceutical alone vs. None | 0.58 (0.50–0.68)* | |
| Nutraceutical plus Pharmaceutical vs. None | 0.84 (0.72–0.97)* | |
| Pharmaceutical alone vs. Nutraceutical alone | 0.48 (0.39–0.58)* | |
| Nutraceutical plus pharmaceutical vs Nutraceutical alone | 0.69 (0.57–0.83)* | |
| Nutraceutical plus pharmaceutical vs Pharmaceutical alone | 1.45 (1.28–1.61)* | |
| Allodynia | 0.0984 | |
| Yes vs. no | 1.06 (0.95–1.19) | |
| Maybe vs. No | 0.68 (0.45–1.02) | |
| Yes vs. maybe | 1.57 (1.04–2.36)* | |
| Anxiety symptoms at consultation visit | 1.17 (1.05–1.30)* | 0.0066* |
| Feeling depressed at consultation visit | 1.28 (1.13–1.47)* | 0.0004* |
| Quantity of liquid intake reported as at least 48 ounces/day at visit A | 1.13 (1.01–1.26)* | 0.0439* |
| Reporting skipping meals at visit A | 1.05 (0.94–1.17) | 0.456 |
| Negative Predictors of Clinical Worsening | OR (95% CI) | P Value |
| Chronic migraine at visit A | 0.62 (0.55–0.69)* | <0.0001* |
| Medication overuse at visit A | 0.50 (0.39–0.65)* | <0.0001* |
| Visit B in the summer | 0.69 (0.60–0.78)* | <0.0001* |
| Headache frequency at visit A | 0.94 (0.93–0.94)* | <0.0001* |
| Headache severity at visit A | 0.96 (0.94–0.98)* | 0.0018* |
| PedMIDAS/MIDAS grade at visit A | 0.0049* | |
| Grade 2 vs. 1 | 0.90 (0.80–1.01) | |
| Grade 3 vs. 1 | 0.82 (0.69–0.96)* | |
| Grade 4 vs. 1 | 0.77 (0.66–0.89)* | |
| Grade 3 vs. 2 | 0.91 (0.76–1.09) | |
| Grade 4 vs. 2 | 0.85 (0.73–1.01) | |
| Grade 4 vs. 3 | 0.94 (0.78–1.15) | |
| Exercise reported as at least 3 times/week at visit A | 0.95 (0.84–1.07) | 0.494 |
| Quantity of sleep reported as at least 8 hours/day at visit A | 0.98 (0.87–1.10) | 0.828 |
| History of caffeine intake at visit A | 0.90 (0.81–1.00) | 0.0654 |
Indicates statistical significance at adjusted p<0.05 level (adjusted p value after correcting for multiple hypothesis testing)
Table 3.
Significant predictors of clinical worsening during follow-up intervals in multivariate repeated measures logistic regression model
| Positive Predictors of Clinical Worsening | OR (95% CI) | P Value |
|---|---|---|
| Female | 1.27 (1.11–1.44) | 0.0003 |
| Age | 1.04 (1.02–1.06) | <0.0001 |
| Chronic migraine at visit A | 1.57 (1.35–1.83) | <0.0001 |
| History of status migrainosus | 1.46 (1.26–1.69) | <0.0001 |
| PedMIDAS/MIDAS grade at visit A | ||
| Grade 3 vs. 1 | 1.24 (1.03–1.48) | 0.020 |
| Grade 4 vs. 1 | 1.54 (1.29–1.84) | <0.0001 |
| Nutraceutical intervention recommended at visit A | 1.23 (1.10–1.37) | 0.0002 |
| Feeling depressed at consultation visit | 1.31 (1.13–1.51) | 0.0003 |
| Negative Predictors of Clinical Worsening | OR (95% CI) | P Value |
| Visit B in the summer | 0.65 (0.57–0.74) | <0.0001 |
| Headache frequency at visit A | 0.91 (0.90–0.92) | <0.0001 |
| Pharmaceutical intervention recommended at visit A | 0.78 (0.68–0.88) | 0.0001 |
| History of caffeine intake at visit A | 0.87 (0.78–0.97) | 0.010 |
In order to assess the impact on the results of patients who fail to follow-up in a timely manner, the study sample characteristics were compared to those of the group of patients who did not have a follow-up visit within 12 months of a visit A (Table 1). The no follow-up group had a higher proportion of males (p<0.0001), was younger (p<0.0001) and had a different racial composition (p<0.0001), comprised of a higher proportion of Black patients and lower proportions of White patients and patients of other races. The no follow-up group overall appeared to have characteristics consistent with a less severe baseline disease as compared to the study sample: they were less likely to have chronic migraine (p<0.0001), MOH (p<0.0001), status migrainosus (p<0.0001) or allodynia (p=0.010) and had a lower baseline headache frequency (p<0.0001), lower mean headache severity (p=0.021) and lower PedMIDAS/MIDAS scores (p<0.0001). The no follow-up group were recommended different treatment types at visit A as compared to the study sample (p=0.010).
DISCUSSION
In this study, we found that the majority of children and adolescents receiving multimodal interdisciplinary care for migraine sustained a reduction in headache frequency over time, which mirrors prior observations in this population10–13. In fact, consistent with recent clinical trials10,11, the average reduction in headache frequency observed between visits was approximately 5 days per month.
Only a minority, that is, approximately 15% of the short-term follow-up intervals and 26% of the patients followed ever met the criteria for clinical worsening. We identified that several factors were associated with differential odds of experiencing clinical worsening in headache frequency within a short-term follow-up interval for this small subset.
The observation that older patients are more likely to worsen as compared to younger patients replicates findings from two published pediatric studies17,18. It is unclear why older patients are more refractory to treatment than younger patients, but considering that after puberty the incidence of migraine becomes significantly higher in females19,20 and considering that we also found that females were more likely to worsen than males, it could be due to a hormonal effect. Fluctuations in hormone levels throughout the menstrual cycle are associated with headache parameters in females with migraine21–23 and recent pilot data in girls suggests that the stage of pubertal development may impact the effect of sex steroids on headache parameters22. Therefore, part of the age effect observed in the present study could be mediated by the hormonal changes that occur with puberty. It is also possible that older patients are exposed to higher levels of stress as their responsibilities and developmental tasks grow. Given that stress is one of the most commonly reported triggers for migraine in children and adolescents24, it is plausible that stress could play a role in the effect of age on clinical worsening.
Several of the associations observed in this study support the hypothesis that a more severe baseline headache disorder increases the risk of experiencing short-term clinical worsening: those with higher disability scores, those with chronic migraine and those with a history of status migrainosus were more likely to worsen while in care. The finding that higher disability scores predict higher odds of clinical worsening mirrors data from the adult population that have shown that those with the highest baseline disability scores have the highest risk of developing chronic migraine25.
To our knowledge, this is the first study to link a history of status migrainosus with prognosis in migraine. However, recently, a group proposed a new migraine subtype: episodic status migrainosus. In this small case series, 18 adult patients were identified who exclusively had migraines with duration over 72 hours and who had less than 15 headache days per month at baseline. The majority of these patients with episodic status migrainosus progressed to develop chronic migraine26. It is therefore possible that status migrainosus portends a poor prognosis in patients with migraine. The observation that clinical worsening is more likely in those with chronic migraine and in those with a history of status migrainosus may also support the concept that “pain begets pain”. Exposure to more frequent and severe pain is thought to increase the risk of disease progression through an increase in and/or lack of recovery of central sensitization between attacks27. Surprisingly, we also found that patients with a higher baseline headache frequency had lower odds of clinical worsening, which contradicts the theory that more frequent pain exposure portends a worse prognosis. This finding could be a result of regression towards the mean observed between visits, a hypothesis that aligns well with findings from the Chronic Migraine Epidemiology and Outcomes Study (CaMEO), which supported the concept that headache frequency in adults with migraine fluctuates in a cyclic manner over short-term follow-up intervals28. Additionally, it is possible that patients with lower baseline headache frequencies either did not choose preventive interventions, were less adherent to interventions or received less aggressive multimodal interventions at visit A, and were therefore more likely to worsen in a follow-up interval. Another plausible explanation for this finding is sampling bias due to differential follow-up. A comparison of our study sample to patients who did not return to our clinic for follow-up within 1 year of a visit A (Table 1) showed that the no follow-up group were younger, had lower baseline headache frequency, headache severity and disability scores and were also less likely to have chronic migraine, MOH, status migrainosus and allodynia. Therefore, it is likely that at least part of the observed effect of headache frequency was due to the fact that patients at the lower end of the headache frequency spectrum who were clinically stable or improving within a regular follow-up interval were less likely to return for follow-up than those at the lower end of the frequency spectrum who were clinically worsening.
Patients who endorsed feeling depressive symptoms at their initial consultation visit were more likely to experience clinical worsening in subsequent follow-up intervals as compared to peers who did not endorse feeling these types of mood symptoms. This finding corroborates prior work that has identified depression as a risk factor for the development29 and persistence30 of chronic daily headache in adolescents and in adults27.
Having a visit B during the summer months was associated with a lower risk of experiencing clinical worsening. A seasonal pattern for pediatric primary headaches has been noted in prior studies carried out in the emergency department setting, where the prevalence of visits for primary headaches has been found to be higher during the academic year as compared to the summer months31,32. The reason for the seasonal pattern observed is unclear. The stress provoked by the academic and social demands experienced during the academic year could be a significant factor in this association. Given that sleep and exercise patterns show seasonal changes in youth33,34, it is also possible that migraine could improve during the summer months due to more optimal sleep and exercise regimens. Although we did not observe a significant effect of sleep or exercise in the present study, this could be due to how we defined and measured the lifestyle variables.
Caffeine consumption was associated with lower odds of experiencing clinical worsening. Prior work has shown that adolescents with migraine are more likely to consume caffeine than their peers35,36, though it is unclear why this association exists and whether or not caffeine consumption has any impact on migraine prognosis, aside from the well described phenomenon of caffeine-withdrawal headaches14. It is possible that regular caffeine consumption results in beneficial effects on headache control, particularly through its role in aborting headaches37. However, the concept that regular caffeine consumption could portend a more favorable prognosis in youth with migraine does contradict what many experts believe. It is also possible that the observed association between caffeine intake and prognosis in this study was due to unmeasured confounders.
Our final observation was that the type of preventive intervention recommended at visit A was associated with differential odds of experiencing clinical worsening. Patients who were asked to take pharmaceutical interventions were less likely to worsen, whereas patients who were asked to take nutraceutical interventions were more likely to worsen between visits. However, given the design of this study, conclusions about the efficacy of interventions cannot be drawn because patients and providers were unblinded, interventions were not randomized and analyses were carried out retrospectively. There is a known strong placebo effect that is associated with taking a preventive oral medication in youth with migraine10,38, and this likely largely explains the relationship observed between taking a preventive pharmaceutical intervention and the lower odds of clinical worsening.
The reason for the observed inverse association between clinical worsening and the recommendation to take a nutraceutical intervention is unclear. However, unmeasured confounders, as opposed to a true negative effect of nutraceuticals, are likely to explain this association. In our practice, nutraceuticals are only recommended to patients who were deficient in the corresponding vitamin. Therefore, this subpopulation of patients likely systematically differs from the non-vitamin deficient patients in a variety of ways that could negatively influence migraine prognosis (eg. socioeconomic status, diet quality, genetic factors, etc). In addition, patient and provider perceptions and communications about interventions are known to significantly impact the placebo response, as are intervention factors such as cost, pill size and invasiveness39. These factors are likely playing a role in the observed association between the intervention type and the odds of clinical worsening observed in our study. Although it is not known how patient and provider perceptions and communications differ between nutraceutical and pharmaceutical interventions for migraine, it is highly likely that there are significant differences that could impact treatment response and bias against a strong placebo response with nutraceutical interventions.
While MOH27,30, obesity27,41 and allodynia27 have been associated with migraine prognosis in prior studies, there was no observed association between any of these variables and the odds of short-term clinical worsening in this study. Most of the published studies assessing clinical predictors of migraine prognosis have assessed outcomes over a longer term than our interval of 30–93 days. It is therefore possible that the short-term prognosis of pediatric migraine is not significantly impacted by MOH, weight nor allodynia and that rather these factors may be more important over the longer term. Given that all of our patients with MOH are advised to complete an acute medication washout period for 6 weeks, it is possible that adherence to these recommendations resulted in a better prognosis than was observed in the community-based pediatric study30. Although an association between obesity and migraine in children and adolescents has been found, it is not consistently replicated in the pediatric literature41. It therefore remains unclear whether or not weight status has a significant impact on the course of migraine throughout childhood and adolescence. While there is a reported association between allodynia and migraine prognosis in the adult migraine literature27, three other studies carried out in the pediatric migraine population have failed to find an association between allodynia and migraine frequency42–44 or chronic migraine42. The lack of an association may be explained by shorter disease duration in our study and the other published pediatric studies42–44 or could be due to measurement error given that there is no validated questionnaire to assess allodynia in the pediatric population.
The present study has several strengths and limitations. To our knowledge, this is the largest study to date to examine migraine prognosis in the pediatric population and the sample size allowed us to assess for associations between our outcome and a more comprehensive number of potential prognostic factors as compared to prior similar studies29,30. It is the first study of its kind, in that no prior work has specifically aimed to assess the short-term outcome of pediatric patients in care for migraine and potential clinical predictors of short-term prognosis. Our study is limited by its retrospective observational design. Included patients were those who were referred to and followed up at a tertiary care headache center, and thus results lack external validity and cannot be applied to the general population of children and adolescents with migraine. Drawing conclusions about intervention efficacy based on retrospective data is not possible given that patients and providers were unblinded, care within particular interventions was not standardized as it would be in a clinical trial and patients were not randomized to interventions. Our measures of anxiety, depressive symptoms and allodynia were limited by a lack of prior validation studies. Selection bias was present in that we were only able to include patients who presented for follow-up to clinic, and we were able to show that they differed systematically from those who did not follow up (Table 1). In addition, we were unable to ascertain the reasons for which patients were lost to follow-up and this limits contextualization of our results. Finally, while CBT and biofeedback-assisted relaxation training are often provided to our patients, we were not able to reliably document the use of these evidence-based strategies13,15 in the current analysis. As a result, the role of these non-pharmacological treatments was not assessed in these analyses.
CONCLUSIONS
The vast majority of pediatric patients who receive multimodal interdisciplinary care for migraine improve over time. For the few who have an unfavorable short-term prognosis, it is important to consider predictors of clinical worsening in their headache frequency. Specifically, it appears that a particular subset of patients may be at higher risk of a poor short-term prognosis: older, female patients with chronic migraine, status migrainosus, a history of depressive symptoms, high disability scores and caffeine abstinence were more likely to worsen between visits. Given that this minority of patients poses the greatest clinical challenge to care providers and the health care system, it is incumbent upon the scientific and clinical community to develop evidence-based care models for this small subset of patients.
Acknowledgments
Study funding: This project did not receive any specific funding.
Abbreviations:
- BMI
body mass index
- CBT
cognitive behavioral therapy
- ICHD
International Classification of Headache Disorders 3rd Edition
- MOH
medication overuse headache
- PedMIDAS
Pediatric Migraine Disability Assessment Scale
- UCNS
United Council for Neurologic Subspecialties
Footnotes
Conflict of Interest Statements: The authors have no conflicts of interest relevant to this article to disclose.
Financial Disclosures:
Serena L. Orr: Dr. Orr receives royalties from Cambridge University Press.
Abigail Turner: Nothing to disclose
Marielle A. Kabbouche: Nothing to disclose.
Paul S. Horn: Dr. Horn has received funding from the NIH and receives book royalties from the American Association of Clinical Chemistry.
Hope L. O’Brien: Dr. O’Brien is a committee member of the American Academy of Neurology. She is also on the speaker bureau for Avanair and Amgen Pharmaceuticals and is on the advisory board for Allergan. She receives royalties for written contributions published in Up-to-Date, an on-line medical reference.
Joanne Kacperski: Nothing to disclose
Susan LeCates: Nothing to disclose
Shannon White: Nothing to disclose
Jessica Weberding: Nothing to disclose
Mimi Miller: Nothing to disclose.
Scott W. Powers: Dr. Powers has received funding from the NIH.
Andrew D. Hershey: Dr. Hershey has received support or been an advisor to Alder, Allergan, Amgen, Avanir, Curelator, Depomed, Dr. Reddy, Impax, Lilly, Migraine Research Foundation, and NIH
REFERENCES
- 1.Abu-Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: a systematic review of population-based studies. Dev Med Child Neurol 2010;52(12):1088–97. [DOI] [PubMed] [Google Scholar]
- 2.Doja A, Orr SL, McMillan HJ, et al. Canadian paediatric neurology workforce survey and consensus statement. Can J Neurol Sci 2016;43(3):402–9. [DOI] [PubMed] [Google Scholar]
- 3.Curless RG. Diagnostic problems in three pediatric neurology practice plans. Pediatr Neurol 1998;19(4):272–4. [DOI] [PubMed] [Google Scholar]
- 4.Martínez Menéndez B, Escolar Escamilla E, Pinel González A, Cerezo García M, Martínez Sarries FJ, Morlán Gracia L. Has clinical activity in paediatric neurology changed in the past 11 years? Neurología 2016;31(9):606–12. [DOI] [PubMed] [Google Scholar]
- 5.Kröner-Herwig B, Heinrich M, Vath N. The assessment of disability in children and adolescents with headache: adopting PedMIDAS in an epidemiological study. Eur J Pain 2010;14(9):951–8. [DOI] [PubMed] [Google Scholar]
- 6.Orr SL, Christie SN, Akiki S, McMillan HJ. Disability, quality of life, and pain coping in pediatric migraine: an observational study. J Child Neurol 2017;32(8):717–24. [DOI] [PubMed] [Google Scholar]
- 7.Hershey AD, Powers SW, Lecates S, Kabbouche MA, Maynard MK. PedMIDAS - Development of a questionnaire to assess disability of migraines in children. Neurology 2001;57:2034–9. [DOI] [PubMed] [Google Scholar]
- 8.Hershey A, Powers SW, Vockell A-LB, LeCates SL, Segers A, Kabbouche M. Development of a patient-based grading scale for PedMIDAS. Cephalalgia 2004;24(10):844–9. [DOI] [PubMed] [Google Scholar]
- 9.Feigin VL, Abajobir AA, Abate KH, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017;16:877–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powers SW, Coffey CS, Chamberlin LA, et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med 2017;376(2):115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers SW, Kashikar-Zuck SM, Allen JR, et al. Cognitive behavioral therapy plus amitriptyline for chronic migraine in children and adolescents: a randomized clinical trial. JAMA 2013;310(24):2622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabbouche MA, Powers SW, Vockell A-LB, et al. Outcome of a multidisciplinary approach to pediatric migraine at 1, 2, and 5 years. Headache 2005;45(10):1298–303. [DOI] [PubMed] [Google Scholar]
- 13.Kroner JW, Hershey AD, Kashikar-Zuck SM, et al. Cognitive behavioral therapy plus amitriptyline for children and adolescents with chronic migraine reduces headache days to ≤4 per month. Headache 2016;56(4):711–6. [DOI] [PubMed] [Google Scholar]
- 14.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38(1):1–211. [DOI] [PubMed] [Google Scholar]
- 15.Eccleston C, Palermo T, Williams A, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents (Review). Cochrane Database Syst Rev 2014;5(5):1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Division of Nutrition, Physical Activity, and Obesity National Center for Chronic Disease Prevention and Health Promotion. Defining childhood obesity [Internet]. Centers for Disease Control and Preventrion; 2016;Available from: https://www.cdc.gov/obesity/childhood/defining.html [Google Scholar]
- 17.Markus TE, Moad B, Haimi-Cohen Y, Zeharia A. Factors influencing response to pharmacologic treatment of migraine in a pediatric headache clinic. Headache 2016;56(7):1120–31. [DOI] [PubMed] [Google Scholar]
- 18.Eidlitz-Markus T, Haimi-Cohen Y, Steier D, Zeharia A. Effectiveness of nonpharmacologic treatment for migraine in young children: Research submission. Headache 2010;50(2):219–23. [DOI] [PubMed] [Google Scholar]
- 19.Merikangas KR. Contributions of epidemiology to our understanding of migraine. Headache 2013;53(2):230–46. [DOI] [PubMed] [Google Scholar]
- 20.Lipton RB, Bigal ME. Migraine: epidemiology, impact, and risk factors for progression. Headache 2005;45(S1):S2–13. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Diao X, Chen C, Li C, Zhang Y, Li Y. Changes in hormones of the hypothalamic-pituitary-gonadal axis in migraine patients. J Clin Neurosci 2018;50:165–71. [DOI] [PubMed] [Google Scholar]
- 22.Martin VT, Allen JR, Houle TT, et al. Ovarian hormones, age and pubertal development and their association with days of headache onset in girls with migraine: An observational cohort study. Cephalalgia 2018;38(4):707–17. [DOI] [PubMed] [Google Scholar]
- 23.Kibler JL, Rhudy JL, Penzien DB, et al. Hormones, menstrual distress, and migraine across the phases of the menstrual cycle. Headache 2005;45(9):1181–9. [DOI] [PubMed] [Google Scholar]
- 24.Solotareff L, Cuvellier J-C, Duhamel A, Vallée L, Nguyen The Tich S. Trigger factors in childhood migraine: a prospective clinic-based study from north of france. J Child Neurol 2017;32(8):754–8. [DOI] [PubMed] [Google Scholar]
- 25.Lipton RB, Serrano D, Pavlovic JM, et al. Improving the classification of migraine subtypes: An empirical approach based on factor mixture models in the american migraine prevalence and prevention (AMPP) study. Headache 2014;54(5):830–49. [DOI] [PubMed] [Google Scholar]
- 26.Singh TD, Cutrer FM, Smith JH. Episodic status migrainosus: A novel migraine subtype. Cephalalgia 2018;38(2):304–11. [DOI] [PubMed] [Google Scholar]
- 27.May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol 2016;12(8):455–64. [DOI] [PubMed] [Google Scholar]
- 28.Serrano D, Lipton RB, Scher AI, et al. Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J Headache Pain 2017;18(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu SR, Fuh JL, Wang SJ, et al. Incidence and risk factors of chronic daily headache in young adolescents: a school cohort study. Pediatrics 2013;132(1):e9–16. [DOI] [PubMed] [Google Scholar]
- 30.Wang SJ, Fuh JL, Lu SR, Juang KD. Outcomes and predictors of chronic daily headache in adolescents: a 2-year longitudinal study. Neurology 2007;68(8):591–6. [DOI] [PubMed] [Google Scholar]
- 31.Kedia S, Ginde A a, Grubenhoff J a, Kempe A, Hershey AD, Powers SW Monthly variation of United States pediatric headache emergency department visits. Cephalalgia 2014;34(6):473–8. [DOI] [PubMed] [Google Scholar]
- 32.Caperell K, Pitetti R. Seasonal variation of presentation for headache in a pediatric emergency department. Pediatr Emerg Care 2014;30(3):174–6. [DOI] [PubMed] [Google Scholar]
- 33.Quante M, Wang R, Weng J, et al. Seasonal and weather variation of sleep and physical activity in 12–14-year-old children. Behav Sleep Med 2017;18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira S, Borges A, Gomes TN, et al. Correlates of children’s compliance with moderate-to-vigorous physical activity recommendations: a multilevel analysis. Scand J Med Sci Sport 2017;27(8):842–51. [DOI] [PubMed] [Google Scholar]
- 35.Bektaş O, Uğur C, Gençtürk ZB, Aysev A, Sireli O, Deda G. Relationship of childhood headaches with preferences in leisure time activities, depression, anxiety and eating habits: A population-based, cross-sectional study. Cephalalgia 2015;35(6):527–37. [DOI] [PubMed] [Google Scholar]
- 36.Milde-Busch A, Blaschek A, Borggräfe I, Heinen F, Straube A, von Kries R. Associations of diet and lifestyle with headache in high-school students: results from a cross-sectional study. Headache 2010;50(7):1104–14. [DOI] [PubMed] [Google Scholar]
- 37.Lipton RB, Diener HC, Robbins MS, Garas SY, Patel K. Caffeine in the management of patients with headache. J Headache Pain 2017;18(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evers S, Marziniak M, Frese A, Gralow I. Placebo efficacy in childhood and adolescence migraine: An analysis of double-blind and placebo-controlled studies. Cephalalgia 2009;29(4):436–44. [DOI] [PubMed] [Google Scholar]
- 39.Weeks RE, Newman E. Behavioral factors in the placebo response. Neurol Sci 2011;32(SUPPL. 1):3–8. [DOI] [PubMed] [Google Scholar]
- 40.Snyder FJ, Dundas ML, Kirkpatrick C, Neill KS. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nurt Elder 2009;28(1):81–95. [DOI] [PubMed] [Google Scholar]
- 41.Farello G, Ferrara P, Antenucci A, Basti C, Verrotti A. The link between obesity and migraine in childhood: A systematic review. Ital J Pediatr 2017;43(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levinsky Y, Zeharia A, Eidlitz-Markus T. Cephalic cutaneous allodynia in children and adolescents with migraine of short duration: A retrospective cohort study. Cephalalgia 2018;[epub ahead of print]:1–7. [DOI] [PubMed] [Google Scholar]
- 43.Eidlitz-Markus T, Shuper A, Gorali O, Zeharia A. Migraine and cephalic cutaneous allodynia in pediatric patients. Headache 2007;47(8):1219–23. [DOI] [PubMed] [Google Scholar]
- 44.Raieli V, Trapolino D, Giordano G, et al. Juvenile migraine and allodynia: Results of a retrospective study. Headache 2015;55(3):413–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from the qualified investigators.


