Abstract
Purpose:
Rural cancer survivors (RCS) have poorer health outcomes, and face multiple challenges—older age, and limited transportation, education, income, and healthcare access. Yet, RCS are understudied. The Reach-out to ENhancE Wellness(RENEW) trial, a home-based, diet and exercise intervention among 641 breast, prostate, and colorectal cancer survivors addressed many of these challenges.
Methods:
We examined whether rural and urban participants differed in their response to the RENEW intervention (e.g., physical functioning, quality-of-life, intakes of fruits and vegetables(F&V) and saturated fat, body mass index(BMI), physical activity, and adverse events).
Results:
Rural versus urban survivors report significantly more favorable mean(SE) changes in physical functioning [−0.66(1.47) v −1.71(1.00] and physical health [+0.14(0.71) v −0.74(0.50)], and fewer adverse events[1.58(0.08) v 1.64(0.06)]. Rural versus urban survivors reported smaller increases in F&Vs[+1.47(0.23) v +1.56(0.16);p=0.018], and lower percentages achieved goal behavior for endurance exercise and intakes of F&Vs and saturated fat.
Conclusions:
The RENEW intervention reduced declines in physical health and functioning among RCS to a significantly greater extent than for urban cancer survivors. All survivors significantly improved intakes of F&V and saturated fat, and endurance exercise; however, lower percentages of rural versus urban survivors met goal suggesting that more intensive interventions may be needed for RCS.
Keywords: rural, cancer survivors, health outcomes, lifestyle interventions, health disparities
Introduction
Rural cancer survivors are at increased risk for poorer health outcomes [1]. Compared to urban residents, rural residents are less likely to have access to medical care, support services, and healthcare facilities [2, 3]. Furthermore, rural residents are more likely to experience transportation issues, face financial challenges, and be less educated, older, and uninsured [4, 5]. These factors can increase stress and exacerbate poor health.
Research suggests that rural residents are more likely to be obese and physically inactive as compared to their urban counterparts [6–9], and less likely to follow recommendations for healthful lifestyle changes from their healthcare providers [10–13]. Additionally, rural cancer survivors are more likely to report fair-to-poor health as compared to good-to-excellent health [14]. A study of 117 cancer survivors in Kentucky found poorer mental health among rural, as compared to urban cancer survivors [15]. Rural cancer survivors also have been shown to have lower physical functioning [16]; this is a key concern among those ages ≥65, since previous research suggests that older individuals diagnosed with cancer do not recover their functional ability post-treatment as patients who are younger [17]. Thus, older cancer survivors often chart a steep trajectory of decline that threatens their independence, further jeopardizing their quality of life (QOL), and that of their family members on whom they must depend [18].
Physical functioning was the primary outcome of the Reach-out to ENhancE Wellness (RENEW) trial that was conducted among 641 older survivors of breast, prostate, and colorectal cancer. The tailored mailed print and telephone counseling, diet and exercise intervention resulted in a significant reduction in self-reported functional decline [19]. Because RENEW enrolled significant proportions of both rural and urban cancer survivors, it provides a unique opportunity to examine rural-urban differences in health outcomes among elderly, cancer survivors.
Methods
Study Design and Participants:
The RENEW randomized controlled trial conducted baseline interviews from July 1, 2005 through May 17, 2007, and follow-up data were collected at both 1-(July 2006 to May 2008) and 2-year (July 2007 to May 2009) follow-up on elderly colorectal, breast, and prostate cancer survivors, who were recruited from the North Carolina Central and Duke cancer registries, and physician referrals. The Duke Institutional Review Board approved this study and all participants provided written informed consent.
Inclusion criteria for RENEW participants included: ≥ 65 years, ≥ 5 years post-diagnosis of a loco-regionally staged, breast, prostate, or colorectal cancer with no subsequent malignancies (exception: non-melanoma skin cancer), and oncologist approved for contact. Participants were English speakers/writers, had a body mass index [BMI]: 25–40 kg/m2), and reported <150 minutes of weekly moderate-to-vigorous physical activity (MVPA). Survivors were excluded if contraindications to unsupervised exercise existed (e.g., angina, recent myocardial infarction, congestive heart failure, chronic obstructive pulmonary disease, scheduled hip or knee replacement, walker or wheelchair use, hemiparesis resulting from recent stroke), or if they were on warfarin or dialysis (contraindications to increased consumption of fruits and vegetables [F&V]) [20].
The initial study included cancer survivors residing outside of the U.S.; for this analysis, two cancer survivors living in Canada and the United Kingdom were excluded. Identical dropout rates of 24% (total number of dropouts=153) were observed among both rural and urban cancer survivors. The analytic sample included 487 cancer survivors: 160 classified as rural and 327 classified as urban.
Intervention:
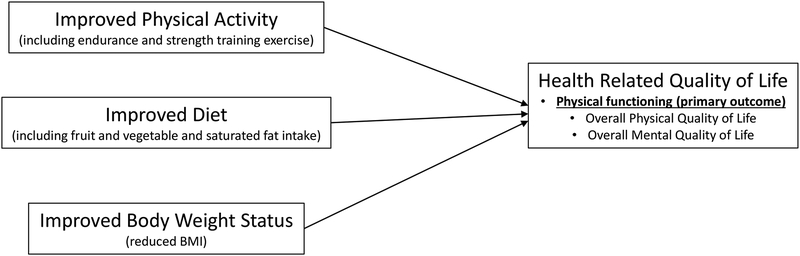
RENEW was a yearlong, iteratively-tailored behavioral intervention for which details are published [20,21]. Briefly, the goals of the intervention were to promote ≥30 minutes of MVPA/day, 15 minutes of strength training every other day, 7–9 daily servings of F&Vs, limited consumption of saturated fat (<10% of total Calories), and modest weight loss of up to one pound/week in an effort to improve the primary outcome of physical function (see Fig 1: Conceptual Model). The intervention was grounded by Social Cognitive Theory [22], and was delivered via mailed print materials (initial workbook followed by four quarterly newsletters), eight telephone prompts, and weekly-tapered-to monthly telephone counseling. The CONSORT diagram has been published previously [21].
Figure 1.
Conceptual model
Data Collection:
Socio-demographic (gender, age, race) and medical information (cancer type, stage, date of diagnosis) were collected from the cancer registry and oncologists. Address information also was collected from these sources or self-reported by participants. Rural-Urban Commuting Area (RUCA) Codes (version 2.0, Rural Health Research Center) were used to establish rural versus urban residence via a census tract-based classification scheme using the US Bureau of Census Urbanized Area and Urban Cluster definitions and work-related commuting data to characterize ZIP codes [22]. RUCA codes for urban residence were 1.0, 1.1, 2.0, 2.1, 3.0, 4.1, 5.1, 7.1, 8.1, 10.1; whereas rural RUCA codes included large rural (4.0, 4.2, 5.0, 5.2, 6.0, 6.1), small rural (7.0, 7.2–7.4, 8.0, 8.2–8.4, 9.0–9.2), and isolated regions (10.0, 10.2–10.6), as per a previous analysis.[16] Income, education, smoking status, cancer treatment, and adverse events were self-reported. Adverse events were classified into those that were “serious” (i.e., life-threatening, permanently disabling, or resulting in overnight hospitalization [e.g., aortic rupture, severe falls, and cancer recurrence]) or “non-serious” (e.g., minor falls, shortness of breath, and back pain). The Medical Outcomes Study Short-Form 36 (SF-36) questionnaire was used to assess health-related QOL [23]. The physical function subscale served as the primary outcome and has been widely used to assess the health impact on physical performance, which includes basic self-care to vigorous physical activity; both physical and mental component scores of the SF36 were investigated. Physical activity and diet served as secondary outcomes. The 41-item Community Health Activities Model program for Seniors (CHAMPS) questionnaire was used to assess physical activity given its broad use in older adults to assess leisure-time, transport, household, and self-care activities. CHAMPS has demonstrated reliability (intraclass correlations: 0.66–0.76), construct validity (significant correlations of 0.22–0.54 with physical performance) and sensitivity to change [20, 24]. The Nutrition Data System for Research software (Version 2, Nutrition Coordinating Center, Minneapolis, Minnesota) was used to assess dietary intake from two unannounced dietary recalls (one weekday and one weekend day) at baseline and 12-month follow-up. Height and weight were self-reported and used to estimate BMI and changes in weight status [19].
Data Analysis:
To assess baseline characteristics between rural and urban residents, chi-square for categorical variables, t-tests for normally distributed continuous variables, and Wilcoxon ranked tests for non-normally distributed continuous variables were used, as appropriate. The dependent variables were changes in physical functioning, as well as other outcomes of interest, i.e., physical and mental component summary scores, F&V intake, and total saturated fat, BMI, duration of endurance, and strength training exercise, and adverse events that occurred during the period of active intervention. Because RENEW trial participants were randomized to receive the intervention either immediately or to a wait-list control arm who received the identical intervention after a 12-month delay, baseline to 12-month physical function data from the immediate intervention arm and 12-month to 24-month data from the wait-listed arm were pooled in order to assess the influence of urban-rural status on response to the intervention. These analyses were adjusted for the baseline level of the outcome of interest.
Since continuous variable outcomes were not all normally distributed, ordinary least squares (OLS) regression was performed on ranks of outcomes to assess unadjusted and adjusted model p-values. The adjusted model included the covariates of age, race, sex, education, comorbidity and symptom count, intervention/waitlist group, and baseline physical function, which are variables of clinical significance and commonly adjusted for in other reported studies [1, 10, 11, 14–16, 19]. Rural group means and standard errors (SE) at baseline and follow-up are presented along with rural group mean differences and 95% confidence intervals (CIs). Unadjusted and adjusted covariate model’s p-values are presented. In addition, percentages of participants meeting goals for lifestyle behaviors (e.g., saturated fat intake <10% of total Calories, F&V intake ≥ 5 daily servings,[25] endurance exercise ≥150 minutes/week, and strength training ≥30 minutes/week) and weight status (BMI <25 kg/m2 or a 10% weight loss) at study completion were compared using Chi-square testing. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
The study sample was comprised largely of breast and prostate cancer survivors of whom the majority received cancer surgery, and less than half received either radiation or adjuvant chemotherapy (Table 1). Most were non-Hispanic white, non-smokers with mean age of 73 years, who reported roughly two comorbid conditions. No rural-urban differences were detected in any of these factors; however, rural cancer survivors were significantly more likely to be female and less educated, with lower incomes and fewer years since diagnosis compared to urban counterparts. At baseline, mean levels of overall physical function were similar for both urban and rural survivors, as was mental and physical component summary scores, BMI, saturated fat intake, and duration of endurance exercise; however, rural cancer survivors had significantly lower F&V consumption and strength training activity.
Table 1.
Baseline characteristics’ comparison by rural vs. urban residenc for RENEW participants
| Variables | Urban Residence (n=327) | Rural Residence (n=160) | P – value |
|---|---|---|---|
| Age, mean (SD) † | 73 (5) | 73 (5) | 0.980 |
| Gender, n (%) | |||
| Male | 149 (45.6) | 69 (43.1) | 0.611 |
| Female | 178 (54.4) | 91 (56.9) | |
| Race, n (%) | |||
| Black | 28 (8.6) | 21 (13.1) | 0.116 |
| White | 299 (91.4) | 139 (86.9) | |
| Annual Income,* n (%) | |||
| <$12,500 | 15 (3.5) | 12 (5.7) | 0.003 |
| $12,500–30,000 | 102 (23.8) | 72 (34.3) | |
| >$30,000 to $50,000 | 116 (27.1) | 57 (27.1) | |
| >$50,000 | 160 (37.4) | 49 (23.3) | |
| Education,* n (%) | 0.015 | ||
| Some high school | 23 (5.4) | 25 (11.9) | |
| HS Graduate/GED | 108 (25.4) | 55 (26.2) | |
| Vocational | 21 (4.9) | 11 (5.2) | |
| Some college | 101 (23.7) | 58 (27.6) | |
| College Degree | 108 (25.4) | 41 (19.5) | |
| Advanced degree | 65 (15.3) | 20 (9.5) | |
| Current Smoker, n (%) | 15 (4.6) | 9 (5.6) | 0.619 |
| Co-morbidity (OARS) counts, mean (SD) † | 1.9 (1.3) | 2.1 (1.2) | 0.212 |
| Cancer Type,* n (%) | |||
| Colorectal | 48 (14.7) | 26 (16.3) | 0.675 |
| Breast | 145 (44.3) | 75 (46.9) | |
| Prostate | 134 (41.0) | 59 (36.9) | |
| Years since cancer diagnosis, mean (SD) † | 8.8 (2.8) | 8.3 (2.6) | 0.030 |
| Cancer treatment, n (%) | |||
| Surgical | 294 (89.9) | 143 (89.4) | 0.856 |
| Chemotherapy | 81 (24.8) | 46 (28.8) | 0.348 |
| Radiation | 143 (43.7) | 76 (47.5) | 0.432 |
| Hormonal | 140 (42.8) | 68 (42.5) | 0.948 |
| Other | 54 (16.5) | 21 (13.1) | 0.331 |
| Physical Function, mean (SD) † | 73.6 (21.3) | 72.5 (18.9) | 0.213 |
| Mental Component Score, mean (SD) † | 56.5 (6.7) | 56.7 (7.3) | 0.349 |
| Physical Component Score, mean (SD) † | 44.7 (10.1) | 43.7 (8.7) | 0.073 |
| Daily F&V Servings,, mean (SD) † | 3.7 (2.0) | 3.1 (2.0) | 0.001 |
| Total Saturated Fat Intake (g/day), mean (SD) † | 19.6 (9.3) | 18.8 (9.8) | 0.304 |
| BMI (kg/m2), mean (SD) † | 28.8 (3.4) | 29.1 (3.5) | 0.448 |
| Minutes/week of Endurance Exercise, mean (SD) † | 43.4 (91.4) | 38.6 (68.1) | 0.835 |
| Minutes/week of Strength Training Exercise, mean (SD) † | 11.5 (36.4) | 5.3 (20.5) | 0.039 |
Chi-Square ratios and likelihood Chi-Square Ratios (categorical variables) used
Wilcoxon rank sum test used (non-normally distributed continuous variables)
Change in Physical Function:
Rural residents had mean declines in physical function of −0.66 [95% CI, −3.56 to 2.25], which were roughly half that reported by urban survivors −1.71 [95% CI, −3.56 to 2.25](Table 2). Statistically significant differences were observed in crude and adjusted models.
Table 2:
Group means and group differences for outcomes from baseline to follow-up
| Mean (SE) | |||||||
|---|---|---|---|---|---|---|---|
| Urban Residence (n=327) | Rural Residence (n=160) | ||||||
| Outcomes | Baseline | Change in Follow-up | Baseline | Change in Follow-up | Mean Group Difference (95% CI) | Group Effect p-value | |
| Unadjusted | Adjusteda | ||||||
| SF 36 Physical Function | 73.7 (1.1) | −1.71 (1.00)b | 72.6 (1.4) | −0.66 (1.47)b | 1.06 (−2.42, 4.53) | 0.003 | 0.015 |
| Quality of life | |||||||
| Mental Component | 56.4 (0.4) | 0.50 (0.35)c | 56.4 (0.6) | −0.47 (0.64)c | −0.97 (−2.40, 0.46) | 0.975 | 0.052 |
| Physical Component | 44.9 (0.5) | −0.74 (0.50)d | 43.7 (0.7) | 0.14 (0.71)d | 0.88 (−0.84, 2.59) | 0.975 | 0.044 |
| Lifestyle Factors | |||||||
| F&V daily servings | 3.7 (0.1) | 1.56 (0.16)e | 3.1 (2.0) | 1.47 (0.23)e | −0.10 (−0.64, 0.45) | 1.000 | 0.018 |
| Saturated Fat intake (g/d) | 19.3 (0.5) | −2.91 (0.54)f | 18.3 (0.7) | −3.33 (0.75)f | −0.42 (−2.25, 1.42) | 0.975 | 0.578 |
| BMI (kg/m2) | 28.7 (0.2) | −0.70 (0.07)g | 29.1 (0.3) | −0.64 (0.12)g | 0.06 (−0.22, 0.34) | 0.822 | 0.639 |
| Endurance Exercise (min/week) | 42.8 (5.0) | 39.62 (6.95)h | 38.3 (5.0) | 27.12 (7.98)h | −12.50 (−33.31, 8.30) | 0.084 | 0.272 |
| Strength Training Exercise (min/week) | 11.4 (1.8) | 20.40 (2.84)i | 7.0 (1.9) | 22.71 (3.94)i | 2.31 (−7.32, 11.9) | 0.557 | 0.242 |
| Adverse Eventsj | |||||||
| Total Events | X | 1.64 (0.06) | X | 1.58 (0.08) | X | 0.638 | 0.009 |
| Serious Events | X | 0.56 (0.05) | X | 0.46 (0.06) | X | 0.830 | 0.079 |
Wilcoxon rank test and OLS regression on ranks was used for non-normal data.
Adjusted for age, race, sex, education, comorbidity, baseline physical function, intervention/waitlist group, and years since diagnosis
Contrasting to the control group decrease of −4.84(0.9).[19]
Contrasting to the control group decrease of −2.04(0.74).[19]
Contrasting to the control group decrease of −1.94(0.8).[19]
Contrasting to the control group increase of 0.13(0.11) servings/day.[19]
Contrasting to the control group decrease of −1.07(0.49) g/day.[19]
Contrasting to the control group decrease of −0.31(0.08) kg/m2.[19]
Contrasting to the control group increase of 23.4(5.6) minutes/week.[19]
Contrasting to the control group increase of 0.2(0.1) minutes/week.[19]
The control group had similar numbers of adverse events. [19]
Change in Health-related Quality-of-Life:
Rural survivors reported greater improvements in physical component summary scores (0.14 [95% CI, −1.26 to 1.54]) than urban survivors (−0.74 [95% CI −1.72 to 0.25], P-value=0.044, adjusting for model covariates). However, there were no statistically significant between-group differences for mental component summary scores.
Change in Lifestyle behaviors:
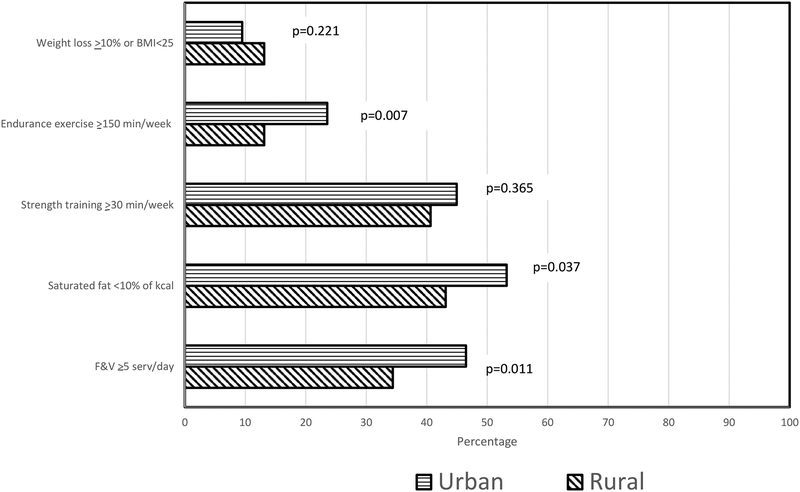
Both rural and urban cancer survivors reported increases in servings/day of F&Vs [+1.47(0.23) v +1.56(0.16)]; however, the magnitude of improvement among rural cancer survivors was significantly less than for urban residents (P=0.018). As shown in Figure 2, the percentage of rural, as compared to urban, participants who achieved an intake of at least five servings of F&Vs/day also was significant lower. While no statistically significant between-group differences were detected for the magnitude of change for saturated fat intake and weekly minutes of endurance exercise (Table 2), the percentage of rural as compared to urban participants meeting goal behavior was significantly lower (Fig. 2). No urban-rural differences were detected either in the magnitude of change or in the percentage of participants achieving goals for BMI or strength training.
Figure 2.
Percentage of urban and rural participants meeting goals upon completing the RENEW intervention
Adverse Events:
Rural, as compared to urban survivors, reported significantly fewer total adverse events during the intervention (P-value=0.009). However, there were no statistically significant between-group differences for serious adverse events.
Discussion
Declines in physical functioning are a key concern for cancer survivors, especially older survivors [17, 18]. Therefore, five [19, 26–29] of the seven[19, 26–31] diet and exercise intervention trials among cancer survivors ages 65+ have assessed physical function as an outcome. Only two interventions, i.e., RENEW and a home-based diet and exercise intervention conducted among 94 patients receiving androgen deprivation therapy for prostate cancer of mean age 69.8 years [19, 29], showed significantly improved scores in comparison to a control arm (p-values<.05). However, only RENEW, perhaps because of its larger sample size or longer period of intervention (i.e., 12 months vs. 6 months) showed differences that were both statistically and clinically significant. Ward et al.[32] suggests that differences of 3 points (for SF-36 subscales) and 2.5 points (for SF-36 component summary scores) must be exceeded for results to be considered clinically meaningful. In this study, the mean decline in the physical function subscale score among controls was −4.84 [19]; however, declines were at least 3.13 points less among urban cancer survivors and 4.18 points less among rural cancer survivors.. While it is debatable if the difference between rural and urban survivors is clinically significant, given that the difference in change scores did not exceed the 3-point threshold, it nonetheless is notable that rural cancer survivors responded at least as well as did urban cancer survivors, especially given the challenges (e.g., lower health literacy and attitudinal barriers toward health seeking) associated with engaging rural people in health care interventions. Additionally, findings suggest that the improvement observed among rural cancer survivors in physical function also extended to the physical component summary score – a more global outcome, though while these differences are statistically significant, they do not meet the 2.5-point threshold of clinical significance upheld for component summary scores.
Four diet and exercise interventions among older cancer survivors have assessed mental health-related QOL among their outcomes [19, 27–29], of these only RENEW observed improved scores of statistical significance in relation to the control arm (i.e., +0.5 vs. −2.04).[25] In the current rural-urban comparison, there is some suggestion (though it only reached borderline statistical significance; p=0.052) that urban cancer survivors experienced greater benefit (net gain of 2.54 points) as compared to rural cancer survivors (who only experienced a net gain of 1.57 points). Previous research indicates that rural, as compared to non-rural cancer survivors, report higher levels of anxiety, distress, emotional problems, cognitive dysfunction, depressive symptomology, and decreased life satisfaction [15], suggesting that these issues may need to be addressed via additional behavioral and psychological interventions (e.g., cognitive behavioral training, meditation) or pharmacologic means.
As in the CanChange home-based multiple behavior intervention [28], RENEW also resulted in both clinically and statistically significant improvements in physical activity [19]. Given that we observed relatively greater improvements on physical health among rural as compared to urban cancer survivors, we also expected to observe rural-urban differences in physical activity, but found none. In fact, there was a non-significant trend toward greater changes in physical activity among urban cancer survivors than among those who were rural. Moreover, proportionately fewer rural as compared to urban participants met the goal of at least 150 minutes of endurance exercise per week. These data could be an artifact of the uneven gender distribution between our rural-urban groups (since older women are documented to have lower activity levels than males) [33], or it may call for further development of physical activity interventions for rural cancer survivors.
In contrast, statistically significant rural-urban differences were found for F&V consumption, though these only amount to a tenth of a serving/day difference. However, more important is the fact that rural survivors had smaller increases coupled with significantly lower F&V intakes at baseline. Thus, urban cancer survivors more often achieved the goal of consuming ≥5 daily servings of F&Vs whereas rural cancer survivors continued to fall short of this goal at the conclusion of the intervention, as also was found for their intake of saturated fat. Research reveals that rural elders, especially those who are homebound, have limited access to full service grocery stores that routinely stock fresh produce, and reduced fat dairy products [9, 33, 34]. Also, government programs like Meals on Wheels have limited access to rural areas secondary to lower tax bases and an increased demand for transportation requirements among community volunteers [35]. Our findings point to a need to better understand the barriers to F&V consumption in rural populations to order to increase F&V intake among rural elders. While there have been other dietary interventions that have focused on older cancer survivors, two that produced non-significant increases in F&V consumption [27, 30], one that produced a 0.6 serving/day change [28], and another that produced increases of >1 serving/day [29], none of these studies investigated subgroup differences by rural-urban status.
To date, most lifestyle interventions among cancer survivors have been conducted among those who live primarily in urban centers. However, Frensham et al. [36] conducted a web- and pedometer-based walking intervention among nine rural Australians and found that it was feasible and improved the motivation to walk and quality of life. Befort and colleagues also successfully completed two weight loss interventions among rural breast cancer survivors in the Midwestern U.S. Both studies implemented a 6-month intervention of weekly conference calls and mailed print materials. The first, among 34 breast cancer survivors, resulted in a mean weight loss of 12.8%, as well as significant decreases in total fat intake and increases in F&V consumption and physical activity (p-values <0.01) [37]. The subsequent trial among 172 breast cancer survivors resulted in a 14.0% weight loss, of which a net loss of >5% was maintained in majority of participants a year later [38]. Thus, while the body work of represented by these three studies and RENEW is relatively small, it nonetheless provides support of the potential benefits of home-based diet and exercise interventions among rural cancer survivors.
In addition to improving diet and physical activity behaviors and quality of life [19,27–29,36–38], these home-based lifestyle interventions among cancer survivors also appear safe. In fact, findings of the current analysis show comparatively fewer adverse events reported by rural, as compared to urban residents. Since safety is a large concern for populations for whom health care access may be an issue, this finding is significant.
This study has some limitations to consider that may affect the generalizability of our findings. First, the majority of our health outcomes are self-report and subject to bias. Secondly, >80% of our study sample was Caucasian; therefore, it is unknown if these results would apply to a more racially-diverse population. This concern is somewhat minimized by the comparison of the RENEW sample to elders participating in the National Health Interview Survey (NHIS) and finding roughly the same racial-diversity (>75% Caucasian) [36], an identical number of reported comorbidities (mean of 2) [37],40], and similar BMI’s (29.1 in RENEW and 28.6 in the NHIS) [36], though cancer survivors participating in RENEW had lower baseline F&V consumption than elders participating in the NHIS (i.e., mean of 3.5 servings/day vs. and estimated 4.9 servings/day) [38],41]. Another factor to consider is that the RENEW trial was completed in 2009 and therefore the data may be somewhat dated; however, it is a trial that stands as one of the few lifestyle interventions conducted among older cancer survivors and is definitely the largest. Moreover, to our knowledge, it is one of the only trials among cancer survivors in which an urban-rural comparison of responses to a lifestyle intervention could be conducted.
Overall, the RENEW intervention serves as an example of an effective intervention that lessened physical function decline in older cancer survivors overall and appeared to promote slightly better, if not comparable effects in both rural and urban populations. Given poorer baseline intakes of F&V among rural cancer survivors, coupled with poorer increases in response to the intervention, more study is warranted to assist in overcoming the shortage of low cost, high quality food that may exist in rural areas in an effort to decrease rural and urban disparities. Furthermore, since rural cancer survivors experience financial challenges and are more likely to be older [4–6], it is important to consider interventions that reduce travel to obviate these barriers. Home-based interventions constitute a means to overcome the lack of access to services that exists in rural populations. As reviewed in this paper, while model home-based interventions exist with proven efficacy in improving lifestyle behaviors among cancer survivors, a need still exists to optimize these interventions so that they work just as well in rural areas. In addition to these lifestyle interventions, other programs aimed at early detection, treatment and general survivorship are needed in rural populations, in order to effectively reduce the disparities in survival and address the much poorer overall outcomes experienced by rural cancer survivors.
ACKNOWLEDGEMENTS:
The authors acknowledge the contributions of UAB Cancer Research Experiences for Students (CaRES) director, Dr. John Waterbor, and CaRES faculty and staff for their pioneering efforts to increase opportunities for students in cancer research. Most of all, we thank all of the RENEW study participants, who make these studies possible. Funding was provided by grants from the National Cancer Institute (R01 CA81191 and R25CA 076023).
Funding: This study was funded by grants from the National Cancer Institute (R01CA81191 and R25CA076023.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- [1].Weaver KE, Geiger AM, Lu L, Case LD (2013) Rural-urban disparities in health status among US cancer survivors Cancer 119:1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arcury TA, Preisser JS, Gesler WM, Powers JM (2005) Access to transportation and health care utilization in a rural region, J Rural Health 21: 31–38. [DOI] [PubMed] [Google Scholar]
- [3].Beck SL, Towsley GL, Caserta MS, Lindau K, Dudley WN (2009) Symptom experiences and quality of life of rural and urban older adult cancer survivors, Cancer Nurs, 32: 359–369. [DOI] [PubMed] [Google Scholar]
- [4].Glover S, Moore CG, Samuels ME, Probst JC (2004) Disparities in access to care among rural working-age adults, J Rural Health 20: 193–205. [DOI] [PubMed] [Google Scholar]
- [5].Muskie School of Public Services (2004) Kaiser Commission on Medicaid and the Uninsured, Health Insurance Coverage in Rural America, Washington, DC: Kaiser Family Foundation. [Google Scholar]
- [6].Bennett KOB, Probst JC (2008) Health Disparities: A Rural - Urban Chartbook. [Google Scholar]
- [7].Martin SL, Kirkner GJ, Mayo K, Matthews CE, Durstine JL, Hebert JR (2005) Urban, rural, and regional variations in physical activity, J Rural Health 21: 239–244. [DOI] [PubMed] [Google Scholar]
- [8].Reis JP, Bowles HR, Ainsworth BE, Dubose KD, Smith S, Laditka JN (2004) Nonoccupational physical activity by degree of urbanization and U.S. geographic region, Med Science Sports Exerc 36: 2093–2098. [DOI] [PubMed] [Google Scholar]
- [9].Sharkey JR, Johnson CM, Dean WR (2011) Less-healthy eating behaviors have a greater association with a high level of sugar-sweetened beverage consumption among rural adults than among urban adults, Food Nutr Res, 55: 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bellizzi KM, Rowland JH, Jeffery DD, McNeel T (2005) Health behaviors of cancer survivors: examining opportunities for cancer control intervention, J Clin Oncol 23: 8884–8893. [DOI] [PubMed] [Google Scholar]
- [11].Blanchard CM, Courneya KS, Stein K (2008) Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II, J Clin Oncol 26: 2198–2204. [DOI] [PubMed] [Google Scholar]
- [12].Rausch SM, Millay S, Scott C, Pruthi S, Clark MM, Patten C, Stan D, Sellers T, Vachon C (2012) Health behaviors among cancer survivors receiving screening mammography, Am J Clin Oncol 35: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Underwood JM, Townsend JS, Stewart SL, Buchannan N, Ekwueme DU, Hawkins NA, Li J, Peaker B, Pollack LA, Richards TB, Rim SH, Rohan EA, Sabatino SA, Smith JL, Tai E, Townsend GA, White A, Fairley TL (2012) Surveillance of demographic characteristics and health behaviors among adult cancer survivors--Behavioral Risk Factor Surveillance System, United States, 2009, MMWR 61: 1–23. [PubMed] [Google Scholar]
- [14].Weaver KE, Palmer N, Lu L, Case LD, Geiger AM (2013) Rural-urban differences in health behaviors and implications for health status among US cancer survivors, Cancer Causes Contr 24: 1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Burris JL, Andrykowski M (2010) Disparities in mental health between rural and nonrural cancer survivors: A preliminary study. Psycho-oncol 19: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Miller PE, Morey MC, Hartman TJ, Snyder DC, Sloane R, Cohen HJ, Demark-Wahnefried W (2012) Dietary patterns differ between urban and rural older, long-term survivors of breast, prostate, and colorectal cancer and are associated with body mass index, J Acad Nutr Diet 112: 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Silliman RA, Prout MN, Field T, Kalish SC, Colton T (1999) Risk factors for a decline in upper body function following treatment for early stage breast cancer, Breast Cancer Res Treat 54: 25–30. [DOI] [PubMed] [Google Scholar]
- [18].Chirikos TN, Russell-Jacobs A, Cantor AB (2002) Indirect economic effects of long-term breast cancer survival, Cancer Pract 10: 248–255. [DOI] [PubMed] [Google Scholar]
- [19].Morey MC, Snyder DC, Sloane RC, Cohen HJ, Peterson B, Hartman TJ, Miller P, Mitchell DC, Demark-Wahnefried W (2009) Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial JAMA 301: 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Snyder DC, Morey MC, Sloane RC, Stull V, Cohen HJ, Peterson B, Pieper C, Hartman TJ, Miller PE, Mitchell DC, Demark-Wahnefried W (2009) Reach out to ENhancE Wellness in Older Cancer Survivors (RENEW): Design, methods and recruitment challenges of a home-based exercise and diet intervention to improve physical function among long-term survivors of breast, prostate, and colorectal cancer, Psycho-oncol, 18: 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, Cohen HJ (2012) Reach Out to Enhance Wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol 30: 2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rural Health Research Center (2006) Rural-urban commuting area codes (version 2.0). [Google Scholar]
- [23].Ware JE, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection, Med Care 30: 473–483. [PubMed] [Google Scholar]
- [24].Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL (2001) CHAMPS physical activity questionnaire for older adults: outcomes for interventions, Med Science Sports Exerc 33: 1126–1141. [DOI] [PubMed] [Google Scholar]
- [25].Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T (2012) Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 62: 243–274. [DOI] [PubMed] [Google Scholar]
- [26].Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, Robb KA, Saxton JM, Taylor SJC (2014) Interventions to improve exercise behaviour in sedentary people living with and beyond cancer: a systematic review, Br J Cancer 110: 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Demark-Wahnefried W, Clipp EC, Morey MC, Pieper CF, Sloane R, Snyder DC, Cohen HJ (2006) Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from Project LEAD. J Clin Oncol 24: 3465–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hawkes AL, Chambers SK, Pakenham KI, Patrao TA, Baade PD, Lynch BM, Aitken JF, Meng X, Courneya KS (2013) Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. J Clin Oncol 31: 2313–2321. [DOI] [PubMed] [Google Scholar]
- [29].O’Neill RF, Haseen F, Murray LJ, O’Sullivan JM, Cantwell MM (2015) A randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer, J Cancer Surv 9: 431–440. [DOI] [PubMed] [Google Scholar]
- [30].Campbell MK, Carr C, Devellis B, Switzer B, Biddle A, Amamoo MA, Walsh J, Zhou B, Sandler R (2009) A randomized trial of tailoring and motivational interviewing to promote fruit and vegetable consumption for cancer prevention and control, Ann Behav Med 38: 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Golsteijn RHJ, Bolman C, Peels DA, Volders E, de Vries H, Lechner L (2017) Web-based and print-based computer-tailored physical activity intervention for prostate and colorectal cancer survivors: A comparison of user characteristics and intervention use. J Med Internet Res 19: e298. doi: 10.1186/s12966-018-0734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ward MM, Guthrie LC, Alba MI (2014) Clinically important changes in Short Form-36 scales for use in rheumatoid arthritis clinical trials: The impact of low responsiveness. Arthritis Care Res 66: 1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Locher JL, Ritchie CS, Roth DL, Baker PS, Bodner EV, Allman RM (2005) Social isolation, support, and capital and nutritional risk in an older sample: ethnic and gender differences. Soc Science Med 60: 747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rimkus L, Isgor Z, Ohri-Vachaspati P, Zenk SN, Powell LM, Barker DC, Chaloupka FJ (2015) Disparities in the availability and price of low-fat and higher-fat milk in US food stores by community characteristics. J Acad Nutr Diet 115: 1975–1985. [DOI] [PubMed] [Google Scholar]
- [35].Krout JA (1994) An overview of older rural populations and community-based services, Providing community-based services to the rural elderly In Glasgow N & Berry EH Rural Aging in 21st Century America. NY: Springer, pp 3–18. [Google Scholar]
- [36].Frensham LJ, Zarnowiecki DM, Parfitt G, King S, Dollman J. (2014) The experiences of participants in an innovative online resource designed to increase regular walking among rural cancer survivors: a qualitative pilot feasibility study. Support Care Cancer 22: 1923–9. [DOI] [PubMed] [Google Scholar]
- [37].Befort CA, Klemp JR, Austin HL, Perri MG, Schmitz KH, Sullivan DK, Fabian CJ. (2012) Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 132: 631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Befort CA, Klemp JR, Sullivan DK, Shireman T, Diaz FJ, Schmitz K, Perri MG, Fabian C. (2016) Weight loss maintenance strategies among rural breast cancer survivors: The rural women connecting for better health trial. Obesity (Silver Spring). 24: 2070–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nelson DE, Powell-Griner E, Town M, Kovar MG (2003) A comparison of national estimates from the National Health Interview Survey and the Behavioral Risk Factor Surveillance System. Am J Public Health 93:1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Falci L, Shi Z, Greenlee H (2016) Multiple chronic conditions and use of complementary and alternative medicine among US adults: Results from the 2012 National Health Interview Survey. Prev Chron Dis 13: E61. doi: 10.5888/pcd13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thompson FE, Midthune D, Subar AF, McNeel T, Berrigan D, Kipnis V (2005) Dietary intake estimates in the National Health Interview Survey, 2000: Methodology, results, and interpretation. J Am Diet Assoc 105: 352–363. [DOI] [PubMed] [Google Scholar]