Abstract
Although CDC guidelines call for universal, “opt-out” HIV testing, barriers to testing continue to exist throughout the United States, with the rural South particularly vulnerable to both HIV infection and decreased awareness of status. Therefore, the objectives of this study were to evaluate uptake of “opt-out” HIV testing and barriers to testing within the primary care setting in the South. A concurrent triangulation design guided the collection of quantitative data from patients (N=250) and qualitative data from providers (N=10) across three primary health clinics in Alabama. We found that 30% of patients had never been tested for HIV, with the highest ranked barrier among patients being perceived costs, access to specialty care, and not feeling at risk. Significant differences existed in perceived barriers between patients and providers. Increased provider-patient engagement and the routine implementation of “opt-out” HIV testing would effectively reveal and mitigate barriers to testing, thus, increasing awareness of status.
Keywords: HIV, Health Knowledge, Attitudes, Practice, Delivery of Health Care, Screening, Barriers
INTRODUCTION
More than one million men and women are living with HIV in the United States (US). Despite the public health agenda focusing on awareness of risk and screening, one in seven individuals living with HIV are estimated to be unaware of their HIV positive status1. Awareness is critical to timely linkage to care, increased quality of life, extended life expectancy, and in particular, decreased transmission of infection2. In fact, those who are unaware of their status are responsible for 45% of new transmissions3. The literature reports the primary barriers to awareness of status include: a) decreased logistical ability to access testing secondary to cost; b) not knowing where to receive specialty care; c) low risk perception; d) concern that HIV testing reflects badly on the individual; e) concern that health care providers would pass judgement; f) concern that others in the community would find out testing occurred; g) fear of the testing procedure; and h) fear of the test result itself4–9. This study adds to the literature evaluating barriers to HIV testing by comparing patients’ perceptions of barriers with those of providers. Additionally, this study addresses the sociodemographic and cultural contexts that influence barriers to testing such as race, socioeconomic status, and geographic location7.
The Southern US is disproportionately burdened with newly diagnosed HIV infection10. Fewer people living in the South are aware of their status and, consequently, individuals living with HIV in the South demonstrate a mortality rate three times higher than individuals living with HIV in the rest of the US11. Rural communities are particularly vulnerable to decreased awareness of status and linkage to care due to decreased health care infrastructure, higher levels of poverty, and higher levels of HIV-related stigma11. As a southern state, Alabama is considered highly rural with 82% of its counties meeting criteria for rurality. Alabama’s “Black Belt” region, which represents the highest poverty and unemployment rates in the state, carries 23% of the state’s HIV incidence while accounting for only 13% of Alabama’s population12.
Prior to 2006, the Centers for Disease Control and Prevention (CDC) recommended routine HIV testing for all “high-risk” populations. Specific consent for HIV testing was required and pre-test counseling was heavily encouraged13. In 2006, CDC revised the guidelines to encourage universal screening of all patients 13–64 years of age and at least annually for patients at high risk. The consent normally provided to permit routine care would now be considered encompassing of consent for HIV testing and the emphasis on pre-test counseling was revoked. The guidelines’ recommendation of routine screening without separate consent came to be known as “opt-out” testing, as HIV tests should now be routinely ordered without a separate consent being sought. Providers are encouraged to order HIV testing in the same matter-of-fact manner as they would order any routine testing, proceeding unless the patient actively opts-out14. Implementation of the guidelines would identify patients before they present with advanced disease and reduce the incidence of inadvertent transmission secondary to lack of awareness. Currently, 22% of Alabama’s population living with HIV is diagnosed late into the disease state15. Although there has been an approximate 45% increase in HIV testing from 2015 to 2016, the CDC estimates that 18% of Alabamians living with HIV are still unaware of their status12, 15. Moreover, 64% of new diagnoses are among African Americans making it important to realize cultural and/or socioeconomic-related barriers, which contribute to HIV care disparities15. Although testing in some health departments and emergency rooms are tracked, there is a general lack of evidence regarding the uptake of guidelines in primary clinics16, 17. The objective of this study was to evaluate the current uptake of the CDC’s HIV screening guidelines and to assess patient and provider perceived barriers to HIV testing amongst three primary health clinics in Alabama.
METHODS
The study was conducted in two phases, using a concurrent triangulation design, in a Federally Qualified Health Center (FQHC) in Alabama. FQHCs receive grant funding to improve the health of underserved populations and provide comprehensive, culturally sensitive care18. Specifically, FQHC clinics were selected for their emphasis on serving underserved populations at risk for HIV infection. HIV infection is a socioeconomic disease, with lower socioeconomic status being associated with an increased risk of high risk behavior and power differences that increase the risk of HIV-infection19. Three clinics within the health center were utilized for data collection, with one clinic offering HIV specialty care on site and two clinics serving as satellite clinics where referrals could be easily made. The particular FQHC was selected for their willingness to participate in research and high incidence rates for HIV and STIs within the counties served by the clinics. We used the PRECEDE/PROCEED model in both phases of this study to identify the most relevant factors influencing patient and provider testing behaviors and decisions. PRECEDE/PROCEED is a conceptual framework that examines relevant predisposing, reinforcing and enabling factors that influence health behaviors. Recognizing that patient and clinician behaviors are complex and dynamic, PRECEDE/PROCEED has been used in health care settings to assess antecedent factors to many health-related behaviors, including HIV testing behaviors20–23. The Institutional Review Board of the University of Alabama at Birmingham approved the study.
PHASE I
In phase I, quantitative data were collected from participants (N=250) across three primary care clinics in Alabama. Quota sampling was used to recruit an approximately equal number of participants from each site. Inclusion criteria included: a) being a clinic patient or family member accompanying the patient; b) being 19 to 64 years of age; and c) having the ability to speak and comprehend English. At the time this study was conducted, the legal adult age for human subjects research was 19 years of age. Within each clinic, study coordinators approached a convenience sample of all potential participants in the clinic waiting rooms. Participants were asked if they would like to participate in an iPad-administered survey that included questions about HIV testing. Potential participants were informed that neither HIV status nor prior experience with testing affected their ability to participate and individuals were assured that care as a patient would not be affected by their decision. While only a small number of eligible patients declined participation due to discomfort related to topic or concern over survey readability, it is possible that a biased sample may have been inadvertently created from individuals who were more comfortable with the topic and therefore more willing to participate. Logistical restraints prevented other recruitment methods. Participants who were willing to participate and met criteria were engaged in a written-verbal informed consent process, with 250 patients providing consent. After full informed consent was obtained, the iPad survey was administered using Qualtrics software. Surveys took participants on average 15 minutes to complete and a cash incentive of $20 was provided.
Instruments
Demographic questionnaire.
The instrument assessed participants’ basic socioeconomic information including age, gender, race, religiosity, marital status, education, employment, and geographic location.
HIV testing questionnaire.
The instrument assessed participants’ prior history of HIV testing, perceived risk for HIV infection, and willingness to be tested today.
Rating of barriers.
Participants were asked to evaluate the importance of eight barriers previously identified in the literature4–9. Barriers were rated on a scale from 0–10 with 0 indicating not important and 10 indicating extremely important. Each barrier was evaluated independently so that the perceived impact of one variable did not naturally change the rating of another.
Analysis
Participant characteristics were tabulated and descriptive statistics computed for variables of interest. The association between participant characteristics (years as patient of the clinic, gender, age, race, urban/rural residence, marital status, education, employment, and perceived risk for HIV infection) and quantitative outcomes (times tested, willingness to be tested today, if offered, and barriers to HIV testing) were explored with conditional inference tree modeling24, a non-linear, non-parametric regression approach, used to identify the characteristics most strongly associated with each outcome. SPSS v.23 and R v.3.1.3 software packages were used to conduct all statistical analyses.
PHASE II
In phase II, information was collected from primary health care providers (N=10) who worked in the same clinics where the surveys were administered. Inclusion criteria included: a) working within the facility for at least a year and b) ability to authorize HIV testing. A clinic staff member who assisted with phase I approached potential phase II providers. Names of those who were interested in participating were provided to the study coordinator to schedule individual, semi-focused interviews via telephone. Informed consent was obtained prior to initiating the interviews. Interviews lasted approximately one hour and $50 was provided as compensation for the provider’s time.
Semi-focused Interviews
Semi-focused interviews were used to collect qualitative information on attitudes towards and implementation of HIV testing guidelines in the provider’s practice as well as perception of community barriers to HIV testing. Interviews were audio-recorded and transcribed verbatim. Two researchers analyzing the qualitative data agreed that data saturation had been reached after the analysis of ten interviews. Excerpts of interview scripts can be found in Table I.
Table I.
Provider Interview-Phase II Participant’s Interview Guide
| HIV Testing Guidelines | Are you familiar with CDC’s HIV opt-out testing guidelines? If yes, do you remember what the guidelines say about HIV testing (e.g. who should be tested, how often and why)? How do you think the guidelines apply to you and your clinic? Do you think the guidelines are a good idea? If yes, how do you implement them in your practice? If no, why do you disagree and how would you change them? What barriers do you perceive with regards to implementing universal opt-out HIV testing? How would you address these barriers? |
|
| HIV Testing Practices | How much of a concern is HIV for your population? Tell me about HIV testing in your clinic. Who do you test for HIV in your clinic? Why? Can you tell me about your testing practices over the past 10 yrs? Have you made changes? Why or why not? What barriers or difficulties do you have with implementing HIV testing in your clinic? How do you address these barriers? |
|
| Referral to Specialty Care | Have you had anyone test HIV-positive in your clinic? If yes, where do you refer patients to HIV specialty care? How was that experience? If no previous encounters with managing positive HIV test results, how would you refer a newly diagnosed patient? Did you experience or do you anticipate experiencing barriers when referring a patient to HIV specialty care? How do you address these barriers? |
|
| Patients’ Perception of HIV Testing | What do you think your patients think about HIV testing? Has a patient ever asked you to be tested for HIV? If yes, can you tell us more about this experience? Did you ever have a patient refuse an HIV test offered by your clinic? If yes, tell us more about that experience and why you believe that patient refused to be tested. |
|
| Ranking of Barriers | How do you think patients would rank the importance of the following barriers for HIV testing? Rank in order of 1–8 with 1 indicating the most significant barrier. |
|
| Response to Patient Ranking | The way the barriers are listed here is the ranking that patients actually gave them. Does that surprise you at all, the order in which they ranked them compared to how you ranked them? |
|
Analysis
Transcribed recordings were downloaded onto password-protected computers used by the research team for data analyses. Interviews were transcribed verbatim by a professional transcription service. C.O. and J.W. conducted line-by-line analysis to thematically code interviews using NVivo10® by QSR International. Any discrepancies were discussed between the researchers until common codes were agreed upon.
RESULTS
PHASE I
Table II describes patient and provider demographics. The majority of participants were African American (71.2%) or Caucasian (25.2%), with 66% of participants residing in urban areas and 34% in a rural demographic. Most were female (63.2%), not living with a partner (74%), under fifty years of age (72%), and unemployed (62.8%). Although 45.6% of patients graduated from high school, only 7.2% graduated from college.
Table II.
Patient and Provider Demographics
| Demographics | Phase I (N = 250) | Phase II (N = 10) | ||
|---|---|---|---|---|
| N | N | |||
| Gender | ||||
| Male | 90 | 0 | ||
| Female | 158 | 10 | ||
| Race | ||||
| Black | 178 | 5 | ||
| White | 63 | 5 | ||
| Other | 9 | 0 | ||
| Age | ||||
| ≤40 | 120 | 5 | ||
| 40–59 | 105 | 3 | ||
| ≥60 | 24 | 2 | ||
| Rurality | ||||
| Urban | 165 | |||
| Rural | 85 | |||
| Living Situation | ||||
| Married or living with partner | 65 | |||
| Not living with partner | 185 | |||
| Education | ||||
| Did not graduate high school | 56 | |||
| High school graduate | 114 | |||
| College graduate | 14 | |||
| Employment | ||||
| Employed | 85 | |||
| Unemployed | 157 | |||
Patient ethnicity and geographic location were associated with the type of clinic serving the patients in the study. Patients in the clinic housing an internal HIV specialty care clinic (n=85) were mostly African American (95.3%) and urban (97.7%). Patients in the two satellite clinics differed in terms of geographic location and ethnicity: one clinic (n=86 patients) served mainly rural (91.9%) African Americans (96.5%), while the other (n=79 patients) served mainly urban (96.2%) Caucasians (72.2%). Neither education nor employment were substantially associated with clinic location.
Attitudes and behaviors
Overall, 30% (N=74) of participants had never been tested for HIV infection, with 26% (N=65) having been tested once, 30.4% (N=76) having been tested 2–5 times, and 13.6% (N=34) having been tested more than five times over their lifespan. Only 39% (N=98) had been offered an HIV test within the past eight years (since the implementation of the “opt out” guidelines) and 80% (N=201) were willing to be tested on the day of the survey. Participants were provided the option to write-in responses addressing reasons for refusing testing on the day of the survey. Of 37 responses, 17 were related to lack of perceived risk or desire to test.
Participants in the HIV specialty care clinic were more likely to report having ever been tested for HIV compared to those in satellite clinics (84% vs. 64%; p=.007). Additionally, racial differences existed in response to having ever been tested for HIV, with Caucasians being more likely to have never been tested (48.4% vs. 23.1%; p =.006) and less willing to get tested today (67.2% vs. 89.2%; p < .001). Most participants (N=232; 94.4%) did not consider themselves at risk for HIV infection. No other significant associations were observed between the perception of being at risk for HIV infection and patient characteristics (clinic, urban/rural status, gender, age, race, marital status, education, and employment).
Rating of barriers
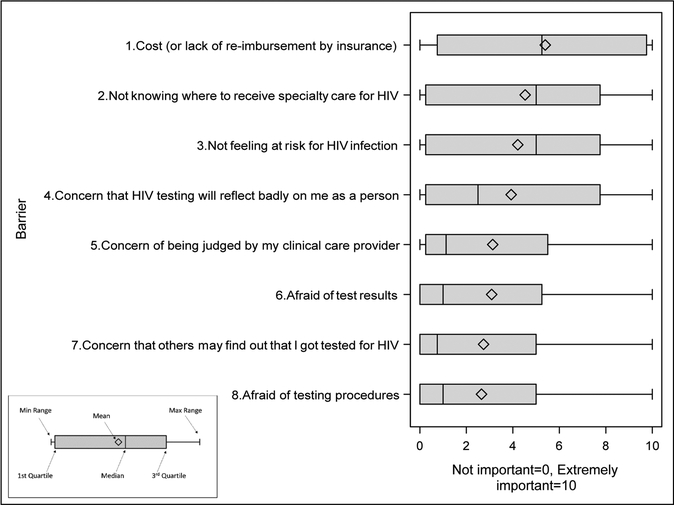
Overall, patients identified cost as the most important barrier to HIV-testing (Figure 1), followed by not knowing where to receive specialty care, not feeling at risk, and concern that testing would reflect badly on them as a person. Differences in barriers existed between clinic type in that, on average, patients in satellite clinics were more likely to rate not knowing where to receive specialty care (M=5.2 vs. 3.27, p = 0.0002) and not feeling at risk for HIV-infection (M=4.8 vs. 3.0, p = 0.0003) as more substantial barriers to HIV-testing compared to those in the specialty clinic. In addition to clinic-based differences, racial and geographic differences were found with African Americans being more likely to rate testing would reflect badly on me as a person as a greater barrier to HIV testing than Caucasians (M=4.4 vs. 2.6; p=.008). Additionally, residing in a rural location was associated with higher barrier rating for not knowing where to receive specialty care (M=6 vs. 3.8; p <.001), not feeling at risk for infection (M=5.2 vs. 3.7; p =.02), fear of test results (M=4 vs. 2.6; p =.041), and feeling judged by their health care provider (M=4.4 vs. 2.5; p<.001). No other significant associations were found between patient demographics and rating of barriers.
Figure 1.
Patient Rated Significance of Barriers to HIV Testing
*Patients were asked to rate the importance of each barrier to HIV testing, with higher scores indicating a more hindering barrier.
PHASE II
Participants in phase II worked as primary health care providers within study clinics. These providers were primarily nurse practitioners (N=8) and included a physician and a physician’s assistant. Providers were female and African American (N=5) or Caucasian (N=5). Most providers were younger than fifty years of age (N = 6).
HIV testing guidelines
Most (N=7) providers perceived the CDC HIV testing guidelines to be currently implemented at first glance. However, less than half of providers were able to clearly articulate guideline recommendations and three providers later admitted unfamiliarity with the guidelines. After being briefed on the guideline details, providers unanimously reported personal alignment with CDC policy integration into practice. There was discrepancy in the interpretation of guidelines, with some providers maintaining that opt-out testing should only be utilized among high-risk populations. One provider supported universal testing, but maintained that counseling be initiated prior to testing. An example of an appropriate implementation of opt-out testing is described in the provider quote: We don’t make a big deal out of it. Just say, ‘Don’t you wanna be tested for HIV?’ We just throw it in with their CBC, their RPR, and include HIV. Just as a way to say, ‘We wanna check you for everything, and be sure you’re [in your] best health, and that you’re well (P7).” However, other providers felt differently, expressing caution with the implementation of universal HIV-testing: “In this clinic, it’s a more rural population, so there’s stigma…. I have to approach it [offering HIV testing] with kid gloves (P1)”.
Patient barriers to HIV testing
During the in-depth interviews, providers identified barriers to implementing CDC guidelines for HIV testing, which included insurance concerns, lack of knowledge within the community, and cultural resistance to testing within the Hispanic population. Patient education, normalization of testing, and facilitating billing/insurance issues were identified as strategies to address barriers. After probing about the patients’ perception towards HIV testing, five providers identified fear and denial as patient barriers towards HIV testing. Denial was identified in two major forms: a) denial of being at risk for HIV accompanied by a feeling of invincibility; and b) denial in the form of avoidance of reality. One provider noted, “…most people think that having HIV it’s just a death sentence, so they don’t wanna know (P4).” Suggested strategies to address these barriers included education on exposure risk, importance of testing, and benefits of treatment.
When providers were asked to contemplate the rationale behind past patient refusals when HIV screening was offered, not feeling at risk and previously having been tested was commonly identified. Plausible reasons identified for decreased perception of risk included consistent safe-sex practices, monogamy, abstinence, and older age. When asked about patient-prompted testing, providers reported that patients requesting testing did so after high-risk experiences, including having casual or unprotected sex, being inadvertently involved with infidelity, using drugs, or being diagnosed with an STD. Two providers identified general health screening as a rationale for patient-requested HIV testing. One provider easily summed up the rationale for patient refusal due to lack of perceived risk: “Some of them will be hesitant or ‘I’m not unclean or anything, so no, I don’t wanna get tested.’ They don’t understand that it doesn’t matter about being clean. Anybody’s gonna be positive I think. That’s probably their [patients] biggest perspective (P4).”
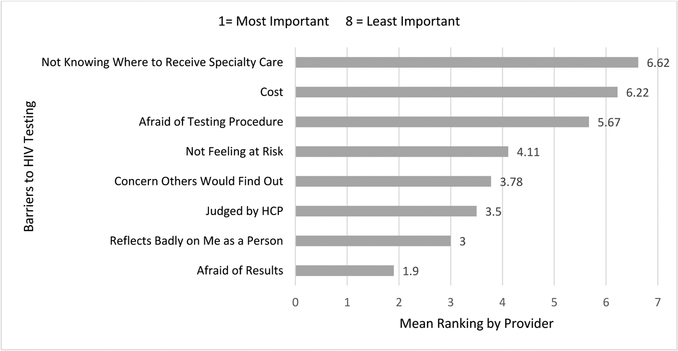
We lastly asked providers to rank the eight barriers, previously identified in the literature, by significance (See Figure 2.). Providers ranked the most significant community barriers to testing as fear of result, concern that the test would reflect badly on the person tested, and fear that their health care provider would judge them. The least significant patient barriers were cost and not knowing where to receive specialty care. Once providers had completed their ranking of perceived patient barriers to HIV testing, we revealed the results of the phase I analysis to providers. The providers responded with surprise regarding the differences in perceptions of barriers between phase I patients and phase II providers as they became aware of their lack of knowledge regarding patients’ barriers to testing. The following quote captures a provider’s response to recognizing the discrepancies between patient and provider perceived barriers: “I just wouldn’t see that [In response to patient’s ranking cost as #1 barrier].Okay. We’ll just nip that in the bud. I think that lets me know that when people come in I need to let them know that there is no cost for HIV testing, and we are going to test you. We’ll include it in your battery of tests. Yeah, okay. That means we got more education to do (P8).”
Figure 2.
Perceived Barriers to HIV Testing as Ranked by Providers
*Providers were asked to rank patient barriers to HIV testing on a scale from one to eight, with lower scores indicating the most hindering barrier.
Referral to specialty care
Because not knowing where to receive specialty care was ranked second in overall importance by patients, providers were asked about experience with the referral process to assess if the presence of system-level barriers influenced testing and linkage to care. Providers portrayed a smooth, professional referral process with patient education being shared as a common element. Providers identified the transition to specialty care to be positive for the patient and perceived that the HIV care clinic effectively alleviates patient apprehension through patient navigation efforts. The most significant barrier to receiving specialty care was identified as transportation. Strategies to promote linkage to care included patient education regarding the risks of not being treated, positive outcomes with early treatment, and availability of prescription assistance. Notably, patient awareness of easy-to-access specialty care was not mentioned.
DISCUSSION
In 2006, CDC amended their approach to HIV testing to promote earlier detection of HIV infection, identify individuals unaware of their HIV infection, and link HIV-positive individuals to clinical services necessary to improve not only the life of the infected individual, but decrease HIV transmission13. In 2014, the United Nations Program for HIV/AIDS (UNAIDS) went further, setting measurable outcomes, among them the goal that 90% of the population living with HIV are aware of their status by 202025. As 2020 rapidly approaches, we must understand the barriers to HIV testing in order to meet this goal. The purpose of this study was to explore the uptake of CDC’s HIV recommendations regarding universal “opt-out” HIV testing as well as to examine attitudes, behaviors, and beliefs affecting HIV testing from a patient and provider standpoint. The findings from this study add to the literature by comparing patients’ perceptions with those of providers. Specifically, we found that 30% of patients had never been tested for HIV—a statistic impacting the 18% of Alabamians who are unaware of their status as reported by the CDC15. Study findings suggest that estimates towards the progression of the increasing awareness of status to the current 90% UNAIDS goal lags substantially behind in pockets of the population historically most vulnerable to health disparities, including access to care. Therefore, it is increasingly critical that we understand where and why gaps in testing exist in order to bridge the gap in all populations.
In our study, we found that race, geographic location, and clinic type (specialty care vs. satellite) influenced testing attitudes and behaviors, contributing to health disparities in subsets of the population. Although African Americans reported more stigma related to HIV-testing compared to Caucasians, African Americans were also more likely to report having ever been tested and to be willing to test on the day of data collection. Although it is intuitively unexpected for Caucasians to have both lower perceived stigma and lower testing, the PRECEED/PROCEED model supports that Caucasians may not request testing or refuse testing when offered based on the perception that they are not at risk for HIV-infection21, 22, 26–28. Rurality also influenced patients’ perceptions of barriers with those in more rural locations reporting less personal risk for HIV infection, while at the same time exhibiting more fear of judgement related to testing. Clinic type adds an additional layer to race and rurality considerations. While clinic type was not significantly associated with race or residential rurality, patients seen in the satellite clinics (as opposed to the central HIV specialty care clinic) were less likely to have ever been tested, and more likely, on average, to consider “not knowing where to receive specialty care” and “not feeling at risk for HIV-infection” a more substantial barrier to testing. These results suggest that barriers to testing could potentially be mitigated or eliminated through routine education, open conversation and the consistent implementation of “opt-out” testing into clinical practice.
Specifically, our findings suggest that disparities stemming from stigma and fear of testing, or even lack of knowledge (i.e., perceived risk and where to receive specialty care), could be mitigated through routine, direct conversations between patients and providers. Providers should be sensitive to population-based differences influencing attitudes towards testing as they engage in conversations, while consistently offering universal HIV testing. For example, providers should consider that African Americans might be more sensitive to HIV-related stigma, whereas, Caucasians are more likely to refuse testing due to lack of perceived HIV-risk. Moreover, patients seen in satellite clinics may simply lack the knowledge necessary to value and prioritize HIV-testing. Although we acknowledge that a single caring conversation cannot undo deep set internalized stigma or beliefs regarding personal risk for HIV-infection, providers can strategically address common barriers in a manner that is likely to promote barrier alleviation and promote testing. Further, local advertisement targeted to address stigma and health beliefs may contribute to an overall culture shift in terms of barriers to testing6, 7, 10, 29. Through a combination of these techniques and the consistent implementation of “opt-out” HIV-testing to all patients, barriers preventing HIV testing may be sufficiently addressed, thus, facilitating awareness of status and linkage to care.
While these results provide further insight into the barriers to testing in the Deep South, they also highlight that barriers currently represented in the literature are not sufficiently comprehensive. For example, although cost and not knowing where to receive specialty care were the most substantially rated patient barriers, 35% and 45% of patients, respectively, found this barrier to be either not at all or only mildly important as a hindrance to HIV testing. Additionally, 41–52% of our sample described stigma related barriers as “not important” when making the decision to undergo HIV testing. Given these results and that 80% of our sample would be willing to undergo HIV testing the day of the survey, then what are the real barriers? Lack of knowledge regarding the importance and availability of HIV testing or lack of perceived risk may be key barriers, as evidenced by the 94% of patients who did not identify themselves to be at risk for HIV. This is particularly critical as the area in which surveys were conducted have high STD rates, indicating a high risk for HIV infection30.Without pro-action from health care providers, the HIV risk assessments by patients may be insufficient to motivate patients to request HIV testing.
The next logical question to address is what are the barriers for providers being pro-active and implementing “opt-out” testing as suggested by CDC? Namely, our study revealed that providers inaccurately identified patient barriers to testing and were not knowledgeable of the CDC’s change in recommendation for universal HIV testing. Specifically, providers ranked cost and not knowing where to receive specialty care as the least important barriers to HIV testing, whereas patients identified these as the most important barriers. Findings again suggest that although local research could reveal the unique socio-cultural barriers to testing in these communities, enhanced patient-provider communication has the potential to reveal and address these barriers-at no added cost. Within candid conversation and opt-out testing, lies the opportunity for providers to engage with their patients, addressing the needs of the communities they serve. However, our research suggests that providers may be out of touch with the communities they serve. Providers must be aware of their perceptions and concerns within their patient population, along with internalized beliefs regarding access to health care5. By recognizing these differences, providers are prepared to skillfully carry out culturally sensitive care and appropriately serve the populations they care for. While some evidence suggests that continuing education can be used as an effective venue to raise awareness of internalized provider beliefs hindering patient connection and testing, and increase culturally sensitive care, other evidence suggests that the consistent implementation of HIV-stigma policies is more effective31–33.
Overall, our study demonstrated that providers at large did not possess a sufficient understanding of the CDC’s recommendations for universal “opt-out” HIV testing. Moreover, many did not recognize that their understanding of the guidelines were insufficient. Without adequate understanding of the rationale behind the guidelines-namely to remove stigma and encourage routine testing- providers will likely continue to miss opportunities for testing while focusing on high-risk populations, hence, fueling the stigma associated with HIV testing. To counter the old notion, providers must be educated in ways that do not criticize their misunderstanding, but rather inform7, 34. Continued medical education aimed to increase provider knowledge and implementation of HIV testing guidelines within practice would serve to educate without promoting a negative connotation for their knowledge needs11, 35, 36.
We acknowledge that although this study provides valuable information regarding patient barriers to HIV testing and highlights the differences in perceived barriers amongst patients and providers, a limited sample size and geographic range across one state limits generalizability to other populations. While limited in scope, study findings are characteristic of Federally Qualified Health Centers in the Deep South, which currently represents the epicenter of the HIV epidemic in the United States. Although potential selection bias cannot be ruled out using a convenience sampling approach, only few eligible patients declined participation (estimated by our study coordinator to be five). Therefore, the results remain representative of the patients and family members served by the clinic at large. This study is also limited by the fact that we did not request disclosure of positive HIV status, which could potentially impact the attitudes and behaviors regarding HIV testing. However, participants were asked to write-in item responses if unwilling to be tested on the day of the survey, and no participants indicated HIV-positive serostatus as a rationale for their unwillingness to test. Based on this information, it is probable that our results were not biased by participants previously diagnosed with HIV-infection.
CONCLUSION
Although HIV-related stigma continues to be highly prevalent in the Southern United States11, our results indicate that the highest-ranking patient barriers were related to the cost of testing, access to specialty care, and perception of HIV risk. These results suggest that the most important patient barriers to HIV testing are not actual barriers, put rather perceived barriers, amenable to correction through education, and namely, open and direct conversation between patients and providers. Our research builds upon previous knowledge and confirms that socio-demographic factors impact attitudes and behaviors related to HIV testing, thus, serving as a reminder that cultural context matters, and that to understand context, real-person engagement must occur15, 37–40. To be effective in increasing HIV testing, providers must first accurately understand the significance of barriers affecting the willingness to be tested in their own communities. While local research can be informative and aid our efforts in addressing barriers and increasing testing and linkage to care, our research confirms that barriers differ by populations and patients cannot be viewed as a single unit. Therefore, providers must engage with each patient routinely to destigmatize, neutralize fears, and address barriers perceived by their patient population. Implications include provider-based education to ensure an adequate understanding of the current recommendations as well as adjunct education to facilitate the soft-skills necessary to have difficult, yet, always critical, conversations. Further, providers recognize their own internal biases hindering universal HIV testing in all populations. Provider based interventions, including administrative and educational strategies, will be necessary to facilitate this change. Through this combination of provider and patient-centered approaches, the realization of actual barriers can be recognized so that evidence-based and culturally relevant interventions can be implemented, with the ultimate goal of achieving 90% awareness of HIV status across populations.
AKCNOWLEDGEMENTS
Compliance with Ethical Standards:
This research was supported by the University of Alabama at Birmingham (UAB) Center For AIDS Research CFAR, an NIH funded program (P30 AI027767) that was made possible by the following institutes: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, NIDDK, NIGMS, NIMHD, FIC, NIDCR and OAR; and the National Institutes of Health (NIH), National Institute on Minority Health and Health Disparities (NIMHD), Gulf States Health Policy grant (U54MD008602).
Footnotes
All authors included on this manuscript declare that he/she has no conflict of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
References
- 1.Centers for Disease Control and Prevention. HIV in the United States: At a glance. 2017. Retrieved from https://www.cdc.gov/hiv/statistics/overview/ataglance.html
- 2.Farnham PG, Gopalappa C, Sansom SL, Hutchison AB, Brooks JT, Weidle PJ,…& Rimland D Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr,.2013; 64(2): 183–9. doi: 10.1097/QAI.0b013e3182973966 [DOI] [PubMed] [Google Scholar]
- 3.Hall HI, Holtgrave DR, Tang T, and Rhodes P HIV transmission in the United States: Considerations of viral load, risk behavior, and health disparities. AIDS Behav. 2013; 17(5): 1632–6. doi: 10.1007/s10461-013-0426-z [DOI] [PubMed] [Google Scholar]
- 4.Southern HIV/AIDS Strategy Initiative. February 2016: SASI releases: HIV/AIDS in the US Deep South: Trends from 2008–2013. 2016. Retrieved from https://southernaidsstrategy.org/research/
- 5.White BL, Walsh J, Rayasam S, Pathman DE, Adimora AA, & Golin CE. What makes me screen for HIV? Perceived barriers and facilitators to conducting recommended routine HIV testing among primary care physicians in the southeastern United States. J Int Assoc Provid AIDS Care. 2015; 14(2): 127–135. doi: 10.1177/2325957414524025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson AL, Heilemanna MV, & Rodriguez M Missed opportunities for universal HIV screening in primary care clinics. J Clin Med Res. 2012; 4(4): 242–250. doi: 10.4021/jocmr1014w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pellowski JA. Barriers to care for rural people living with HIV: A review of domestic research and health care models. J Assoc Nurses AIDS Care. 2013; 24(5): 422–437. doi: 10.1016/j.jana.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton M, Anthony M, Vila C, McLellan-Lemal E, & Weidle PJ. HIV testing and HIV/AIDS treatment services in rural counties in 10 southern states: Service provider perspectives. J Rural Health. 2010; 26: 24–247. doi: 10.1111/j.1748-0361.2010.00284.x [DOI] [PubMed] [Google Scholar]
- 9.Toth M, Messer L, & Quinlivan EB. Barriers to HIV care for women of color living in the Southeastern US are associated with physical symptoms, social environment, and self-determination. AIDS Patient Care STDs. 2013; 27(11): 613–620. doi: 10.1089/apc.2013.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. CDC fact sheet: Today’s HIV/AIDS epidemic [PDF]. 2016. Retrieved from https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/todaysepidemic-508.pdf
- 11.Centers for Disease Control and Prevention. HIV in the Southern United States [PDF]. 2016. Retrieved from https://www.cdc.gov/hiv/pdf/policies/cdc-hiv-in-the-south-issue-brief.pdf
- 12.Alabama Department of Public Health. Alabama 2012–2016 Statewide Jurisdictional HIV Prevention Plan [PDF]. n.d. Retrieved from http://www.adph.org/aids/assets/HIVplan2012-2016.pdf
- 13.Centers for Disease Control and Prevention. Revised Guidelines for HIV Counseling, Testing, and Referral. 2001. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5019a1.htm
- 14.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, & Clark J E. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings [PDF]. 2006. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm [PubMed]
- 15.AIDSVu. Alabama. n.d. Retrieved from https://aidsvu.org/state/alabama/
- 16.AIDSVu. Data methods-State/country. Retrieved from https://aidsvu.org/data-methods-statecounty/
- 17.d’Almeida KG, Kierzek G, Truchis P, Le Vu S, Pateron D, Renaud.,… & Cre´mieux A Modest public health impact of nontargeted human immunodeficiency virus screening in 29 emergency departments. Arch Intern Med. 2012; 172(1):12–20. doi: 10.1001/archinternmed.2011.535 [DOI] [PubMed] [Google Scholar]
- 18.Health Resources and Services Administration (US). Bureau of Primary Health Care. What is a health center? n.d. Retrieved from: http://bphc.hrsa.gov/about/what-is-a-health-center/index.html
- 19.Pellowski JA, Kalichman SC, Matthews KA, Adler N. A Pandemic of the Poor: Social Disadvantage and the U.S. HIV Epidemic. Am Psychol. 2013;68(4):197–209. doi: 10.1037/a0032694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jason EF, Ana MK, Katrina R, et al. Development and evaluation of a pilot nurse case management model to address multidrug-resistant tuberculosis (MDR-TB) and HIV in South Africa. PLoS ONE. 2015;9(11): e111702. doi: 10.1371/journal.pone.0111702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jane LP, John XR, Patricia MD. Developing Targeted Health Service Interventions Using the PRECEDE-PROCEED Model: Two Australian Case Studies. Nurs Res Pract. 2012; e 279431. doi: 10.1155/2012/279431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunningham N An assessment of the HIV prevention needs of injection drug users in Montana. In: Sondag KA, ed: ProQuest Dissertations Publishing; 2007. [Google Scholar]
- 23.Hackney T An HIV/AIDS intervention through churches in Mulanje District, Malawi. Carolina Papers in International Development. 2002; 3:1–38.doi: 10.1080/13691058.2014.944569 [DOI] [Google Scholar]
- 24.Hothorn THK, Zeileis A. Unbiased recursive partitioning: A conditional inference framework. J Comput Graph Stat. 2006;15(3):651–674. [Google Scholar]
- 25.Bain L, Nkoke C, Noubiap J UNAIDS 90–90–90 targets to end the AIDS epidemic by 2020 are not realistic: comment on “Can the UNAIDS 90–90–90 target be achieved? A systematic analysis of national HIV treatment cascades”. BMJ Glob Health. 2017; 2(2): e000227. doi: 10.1136/bmjgh-2016-000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebrahim SH, Anderson JE, Weidle P, Purcell DW. Race/ethnic disparities in HIV testing and knowledge about treatment for HIV/AIDS: United States, 2001. AIDS Patient Care STDS. January 2004;18(1):27–33. [DOI] [PubMed] [Google Scholar]
- 27.Lo CC, Runnels RC, Cheng TC. Racial/ethnic differences in HIV testing: An application of the health services utilization model. SAGE Open Med. 2018;6. doi: 10.1177/2050312118783414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenstock IM. Historical Origins of the Health Belief Model. Health Education & Behavior. 1974;2(4):328–335. doi: 10.1177/109019817400200403 [DOI] [Google Scholar]
- 29.Gignon M, Idris H, Manaouil C, Ganry O. The waiting room: vector for health education? The general practitioner’s point of view. BMC Res Notes. 2012; 5:511. doi: 10.1186/1756-0500-5-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. NCHHSTP AtlasPlus. 2017. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5019a1.htm
- 31.Betancourt J, Green A, Carrillo J, Park E. Cultural Competence and Health Care Disparities: Key Perspectives and Trends. Health Affairs. 2005;24(2):499–505. doi: 10.1377/hlthaff.24.2.499 [DOI] [PubMed] [Google Scholar]
- 32.Marla BH, Jeffrey JG, Mckyer ELJ, Corliss O, Danny B. Continuing Education Effects on Cultural Competence Knowledge and Skills Building among Health Professionals. Online Journal of Health & Allied Sciences. 2013;12(2). [Google Scholar]
- 33.Batey DS, Whitfield S, Mulla M, et al. Adaptation and Implementation of an Intervention to Reduce HIV-Related Stigma Among Healthcare Workers in the United States: Piloting of the FRESH Workshop. AIDS Patient Care STDs. 2016; 30(11):519–527. doi: 10.1089/apc.2016.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larson KE & Bradhsaw CP. Cultural competence and social desirability among practitioners: A systematic review of the literature. Child Youth Serv Rev. 2017; 76:100–111. 10.1016/j.childyouth.2017.02.034 [DOI] [Google Scholar]
- 35.Edmunds JM, Beidas RS, & Kendall PC. Dissemination and implementation of evidence-based practice: Training and consultation as implementation strategies. Clin Psychol. 2013; 20(2): 152–165. doi: 10.1111/cpsp.12031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders GD, Anaya HD, Asch S, et al. Cost-effectiveness of strategies to improve HIV testing and receipt of results: economic analysis of a randomized controlled trial. J Gen Intern Med. Jun 2010; 25(6):556–563. doi: 10.1007/s11606-010-1265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reif S, Pence BW, Hall I, Hu X, Whetten K, & Wilson E HIV diagnoses, prevalence and outcomes in nine southern states. J Community Health. 2015; 40(4): 642–651. 10.1007/s10900-014-9979-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godlonton S, and Rebecca L. Thornton. Learning from Others’ HIV Testing: Updating Beliefs and Responding to Risk. Am Econ Rev. 2013; 103(3):439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henkel KE, Brown K, & Kalichman SC AIDS-Related Stigma in Individuals with Other Stigmatized Identities in the USA: A Review of Layered Stigmas. Soc Personal Psychol Compass. 2008; 2(4):1586–1599. doi: 10.1111/j.1751-9004.2008.00116.x [DOI] [Google Scholar]
- 40.Kempf M-C, McLeod J, Boehme AK, et al. A qualitative study of the barriers and facilitators to retention-in-care among HIV-positive women in the rural southeastern United States: implications for targeted interventions. AIDS Patient Care STDs. 2010; 24(8):515. doi: 10.1089/apc.2010.0065 [DOI] [PubMed] [Google Scholar]