ABSTRACT
Objective:
To determine the frequency of active smoking among patients with asthma and individuals without asthma by self-report and urinary cotinine measurement.
Methods:
This was a cross-sectional study conducted in the city of Salvador, Brazil, and involving 1,341 individuals: 498 patients with severe asthma, 417 patients with mild-to-moderate asthma, and 426 individuals without asthma. Smoking status was determined by self-report (with the use of standardized questionnaires) and urinary cotinine measurement. The study variables were compared with the chi-square test and the Kruskal-Wallis test.
Results:
Of the sample as a whole, 55 (4.1%) reported being current smokers. Of those, 5 had severe asthma, 17 had mild-to-moderate asthma, and 33 had no asthma diagnosis. Of the 55 smokers, 32 (58.2%) were daily smokers and 23 (41.8%) were occasional smokers. Urinary cotinine levels were found to be high in self-reported nonsmokers and former smokers, especially among severe asthma patients, a finding that suggests patient nondisclosure of smoking status. Among smokers, a longer smoking history was found in patients with severe asthma when compared with those with mild-to-moderate asthma. In addition, the proportion of former smokers was higher among patients with severe asthma than among those with mild-to-moderate asthma.
Conclusions:
Former smoking is associated with severe asthma. Current smoking is observed in patients with severe asthma, and patient nondisclosure of smoking status occurs in some cases. Patients with severe asthma should be thoroughly screened for smoking, and findings should be complemented by objective testing.
Keywords: Asthma, Smoking, Cotinine
RESUMO
Objetivo:
Descrever a frequência de tabagismo ativo entre pacientes com asma e indivíduos sem asma, usando questionários padronizados e dosagem da cotinina urinária.
Métodos:
Estudo transversal realizado em Salvador (BA), com 1.341 indivíduos, sendo 498 com asma grave, 417 com asma leve/moderada e 426 sem asma. O tabagismo foi identificado por meio de autorrelato utilizando questionários e por mensuração da cotinina urinária. Para a comparação das variáveis estudadas, utilizaram-se os testes do qui-quadrado e de Kruskal-Wallis.
Resultados:
Dos 55 participantes (4,1%) que se declararam tabagistas atuais, 5, 17 e 33 eram dos grupos asma grave, asma leve/moderada e sem asma, respectivamente. Desses 55, 32 (58,2%) eram tabagistas diários e 23 (41,8%) eram tabagistas ocasionais. Observaram-se níveis elevados de cotinina urinária entre não fumantes autodeclarados e tabagistas pregressos, especialmente no grupo asma grave, o que sugere omissão do hábito atual de fumar. A carga tabágica entre os fumantes e a proporção de ex-tabagistas foram maiores no grupo asma grave do que no grupo asma leve/moderada.
Conclusões:
O tabagismo pregresso esteve associado à asma grave. Tabagismo atual também foi observado em alguns pacientes com asma grave e detectou-se omissão em alguns casos. A investigação de tabagismo deve ser meticulosa em pacientes com asma grave e a entrevista desses deve ser complementada por uma avaliação objetiva.
Descritores: Asma, Fumar, Cotinina
INTRODUCTION
Smoking is recognized worldwide as a chronic disease resulting from nicotine dependence and as a risk factor for the development and worsening of chronic respiratory diseases such as asthma and COPD. 1 Smoking is a major cause of preventable death and is associated with increased health care costs, morbidity, and mortality, accounting for more than 6 million deaths per year. 2
Asthma is a chronic disease that has a high worldwide prevalence (i.e., 1-16%). 3 , 4 In the city of Salvador, Brazil, 13.4% of all adolescents and 5.1% of all adults have asthma. 5 , 6 Smoking is directly related to uncontrolled asthma and increased asthma severity, increasing the risk of exacerbations, decreased lung function, persistent dyspnea, 7 and limited response to treatment with corticosteroids. 8 Nevertheless, smoking remains prevalent among patients with asthma. In a study conducted in the city of São Paulo, Brazil, the prevalence of self-reported smoking among asthma patients was 3%, and the prevalence of self-reported former smoking was 33%. 9
Exposure to smoking can be determined by patient self-report and measurement of biological markers such as exhaled carbon monoxide and carboxyhemoglobin, as well as thiocyanate, nicotine, and cotinine levels, which can be measured in saliva, plasma, and urine. 10 Patient self-report is commonly used because it is an easy-to-use and inexpensive method for assessing smoking; however, inaccurate self-reporting (patient nondisclosure of smoking status) constitutes a disadvantage. 11
Cotinine is a byproduct of nicotine metabolism, and measurement of cotinine levels is the most widely recommended method for quantifying exposure to tobacco smoking because it is not influenced by other exposures. 12 The technique used in order to measure cotinine levels is reliable 12 - 16 and allows detection of smoking occurring 19-40 h prior to urine sample collection. 17 - 19 This is due to the fact that the renal excretion of cotinine is low, meaning that cotinine can be easily detected by laboratory monitoring and has the prerequisites of specificity that make it the analyte of choice for quantifying exposures. 20
The objective of the present study was to determine the frequency of active smoking among patients with varying degrees of asthma severity and individuals without asthma in the city of Salvador by self-report (with the use of standardized questionnaires) and urinary cotinine measurement.
METHODS
Study design
This was a cross-sectional study of patients diagnosed with asthma. The study was conducted between 2013 and 2015 at the Federal University of Bahia Center of Excellence in Asthma, which is located in the city of Salvador and is a research center affiliated with the Programa para o Controle da Asma na Bahia (ProAR, Bahia State Program for the Control of Asthma and Allergic Rhinitis).
The present study is part of a larger study entitled “Fatores de risco, biomarcadores e endofenótipos da asma grave” (Severe asthma: risk factors, biomarkers, and endophenotypes), which is a case-control study investigating patients with severe asthma and involving two control groups: participants with mild-to-moderate asthma and participants without asthma.
Selection and sampling
A total of 1,341 individuals were studied. Of those, 915 had been diagnosed with asthma. Of those, 417 had mild-to-moderate asthma and 498 had severe asthma (and were followed in the ProAR). The study also included 426 individuals without asthma.
The severe asthma patients participating in the study had not been under regular treatment prior to admission to the ProAR, when they were diagnosed with severe asthma (having been followed ever since). The study participants with mild-to-moderate asthma and those without asthma were recruited through advertisements in the media, in public transportation, and in public places, as well as through peer referral. Asthma severity was determined on the basis of the 2012 Global Initiative for Asthma criteria. 21
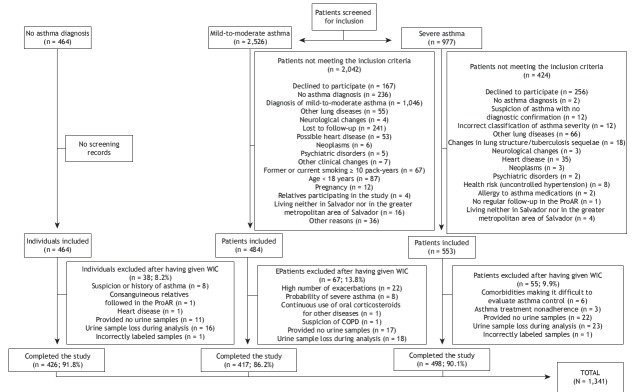
Individuals ≥ 18 years of age living in Salvador (or in the greater metropolitan area of Salvador) and treated via the Brazilian Unified Health Care System were included in the study. All of the severe asthma patients included in the study had been under regular treatment for at least six months. Patients presenting with comorbidities that made it difficult to evaluate asthma control (including congestive heart failure, stroke, myopathies, advanced neoplasia, psychiatric disorders, and lung diseases other than asthma) were excluded, as were those with a smoking history of more than 10 pack-years, because of the difficulty in making a differential diagnosis between asthma and COPD. At the end of the study period, some of the participants were excluded for various reasons, including problems with the urine sample, treatment abandonment, and exacerbation of comorbidities that made patient evaluation difficult (Figure 1).
Figure 1. Flow chart of patient recruitment. ProAR: Programa para o Controle da Asma na Bahia (Bahia State Program for the Control of Asthma and Allergic Rhinitis); and WIC: written informed consent.
Participants with severe asthma
Severe asthma was diagnosed in accordance with the Global Initiative for Asthma criteria 21 by two specialists, who reviewed patient medical records during the selection phase. Disagreements between the two specialists regarding asthma diagnosis or severity were resolved by a third specialist. At the end of this phase, 949 patients meeting the inclusion criteria were contacted by telephone and invited to visit the Federal University of Bahia Center of Excellence in Asthma. Of those, only 553 visited the Federal University of Bahia Center of Excellence in Asthma, where they underwent clinical evaluation and spirometry. A total of 55 individuals were excluded, the total sample of patients with severe asthma therefore consisting of 498 individuals (Figure 1).
Participants with mild-to-moderate asthma or without asthma
A total of 2,526 patients with mild-to-moderate asthma and individuals without asthma were contacted for prescreening. Of those, 484 patients with mild-to-moderate asthma were included in the study. However, only 417 completed all tests. For comparison purposes, 464 individuals without asthma were included in the study. However, only 426 completed all tests (Figure 1).
Study procedures and data collection
During appointment scheduling, participants were instructed to collect first morning urine samples following basic safety and hygiene procedures. After delivery, the samples were labeled and stored in a freezer at −70°C. Patients were then referred for a clinical evaluation in order to confirm the diagnosis and determine the severity of asthma. In addition, they answered questions regarding exposure to smoking and use of medications. None of the participants were on nicotine replacement therapy.
Self-reported smoking status
The participants who reported smoking cigarettes daily or occasionally were considered to be current smokers. The participants who reported being former smokers and having quit smoking at least six months before their interview were considered to be former smokers.
Data regarding exposure to smoking were collected by asking participants questions regarding smoking history (the questions being part of the Brazilian Telephone-based System for the Surveillance of Risk and Protective Factors for Chronic Noncommunicable Diseases questionnaire) 22 and exposure to secondhand smoke at home, school, and work, as well as questions regarding exposure to smoking in public transportation and in public places (the questions being part of a questionnaire used by the Brazilian Institute of Geography and Statistics in the 2010 Census). 23
Urinary cotinine measurement
Urinary cotinine was measured in accordance with the procedures described by Cattaneo et al. 24 A high-performance liquid chromatograph (1290 Infinity; Agilent®, Santa Clara, CA, USA) equipped with a Zorbax Eclipse XDB-C8 (4.6 mm × 150 mm × 5 µm) column and a UV-Vis (λ = 260 nm) detector (Agilent®) was used, with an injection volume of 20 µL and an isocratic mobile phase flow rate of 0.4 mL/min. The methodology was validated by using the parameters set forth in Brazilian National Health Oversight Agency Resolution no. 899. 25 Because of its sensitivity and specificity, high-performance liquid chromatography is recommended for measuring cotinine; in addition to being less expensive than other methods, high-performance liquid chromatography allows determination of low concentrations of cotinine. 26 The limits of detection and quantification were 6.46 µg/L and 19.59 µg/L, respectively.
Urinary cotinine levels are directly related to biological factors such as renal function, urine flow, and urine pH. For increased accuracy, urinary cotinine levels were adjusted for urinary creatinine levels (urinary cotinine/creatinine ratio, in µg/g). 27
Urinary creatinine was measured with a creatinine assay kit and a spectrophotometer with a thermostated cuvette at 37°C (readings at 30 s and 90 s; wavelength, 510 ηm). An automated chemistry analyzer (BT 3000 PLUS; Wiener lab Group, Rosario, Argentina) was used.
Statistical analysis
All severe asthma patients followed in the ProAR until study initiation were included. Therefore, there was no sample size calculation. The numbers of participants with mild-to-moderate asthma and without asthma were established in order to guarantee the comparability of the groups.
The collected data were processed with the Statistical Package for the Social Sciences, version 17.0 (SPSS Inc., Chicago, IL, USA) and are presented as graphs and tables. The Shapiro-Wilk test and the Kolmogorov-Smirnov test were used in order to determine the nature of the distribution of the variables. Continuous variables were expressed as mean and standard deviation if distribution was Gaussian or as median and interquartile range (IR) if distribution was non-Gaussian. Categorical variables were expressed as absolute frequency and valid proportion. The chi-square test was used in order to compare proportions, and the Kruskal-Wallis test was used in order to compare continuous variables, given that most of the data had non-normal distribution.
Ethical considerations
The study was approved by the Research Ethics Committee of the Federal University of Bahia Climério de Oliveira Maternity Hospital (Ruling no. 099/2009; addendum no. 032/2014), as well as by the Brazilian National Health Council (Ruling no. 450/10). All of the study participants gave written informed consent.
RESULTS
A total of 1,341 patients were evaluated. Of those, 55 (4.1%) reported being current smokers, 273 (20.4%) reported being former smokers, and 1,013 (75.5%) reported being nonsmokers. The characteristics of the study participants are described in Table 1. Participants were divided into three groups on the basis of asthma status and severity: severe asthma (n = 498), mild-to-moderate asthma (n = 417), and no asthma diagnosis (n = 426).
Table 1. Sociodemographic characteristics of the study sample, by self-reported smoking status.a .
| Characteristic | Group | ||||
|---|---|---|---|---|---|
| Smokers | Former smokers | Nonsmokers | |||
| Sample | 55 (4.1) | 273 (20.4) | 1,013 (75.5) | ||
| Classification | |||||
| No asthma diagnosis | 33 (60.0) | 84 (30.8) | 309 (30.5) | ||
| Mild-to-moderate asthma | 17 (39.9) | 56 (20.5) | 344 (34.0) | ||
| Severe asthma | 5 (0.1) | 133 (48.7) | 360 (35.5) | ||
| Female sex | 40 (72.7) | 199 (72.9) | 862 (85.1) | ||
| Age, years | 41.2 ± 13.1 | 51.5 ± 12.2 | 43.1 ± 14.4 | ||
| Family income, Brazilian reals | 850.00 [678.00-1,500.00] | 830.00 [700.00-1,400.00] | 1,000.00 [720.00-1,500.00] | ||
| Marital status | |||||
| Single Married/SP Divorced Widowed |
38 (69.1) 10 (18.2) 6 (10.9) 1 (1.8) |
108 (39.6) 111 (40.7) 37 (13.5) 17 (6.2) |
439 (43.3) 429 (42.3) 79 (7.8) 66 (6.5) |
||
| Level of education | |||||
| No schooling 5 years of schooling 9 years of schooling High school College |
3 (5.5) 6 (10.9) 15 (27.3) 24 (43.6) 7 (12.7) |
16 (5.9) 71 (26.0) 67 (24.5) 99 (36.3) 20 (7.3) |
25 (2.5) 110 (10.9) 189 (18.7) 521 (51.4) 168 (16.6) |
||
| Self-reported skin color | |||||
| Black Brown Otherb |
23 (41.8) 31 (56.4) 1 (1.8) |
90 (33.0) 156 (57.1) |
27 (9.9) | 436 (43.0) 486 (48.0) 91 (9.0) |
|
SP: steady partner. aValues expressed as n (%), mean ± SD, or median [interquartile range]. bWhite, red, or yellow.
Of the 55 participants who reported being active smokers, 32 (58.2%) reported smoking cigarettes daily and 23 (41.8%) reported smoking occasionally (Table 1). Table 2 provides detailed information on smoking in each study group. Among current smokers, smoking duration was longer in patients with severe asthma and individuals without asthma than in patients with mild-to-moderate asthma.
Table 2. Exposure to smokinga and creatinine-corrected urinary cotinine levels (in µg/g) in the study groups.b .
| Characteristic | Group | p* | |
|---|---|---|---|
| Current smokers | Former smokers | ||
| (n = 55) | (n = 273) | ||
| Age at smoking initiation, years Severe asthma Mild-to-moderate asthma No asthma diagnosis |
20.0 [13.5-23.5] 18.0 [16.5-20.5] 17.0 [15.0-19.8] |
15.0 [13.0-18.0] 18.0 [15.0-20.8] 16.0 [14.0-18.0] |
0.20 0.25 0.20 |
| Age at smoking cessation, years Severe asthma Mild-to-moderate asthma No asthma diagnosis |
31.5 [23.0-40.0] 30.0 [24.0-59.0] 32.0 [25.0-40.0] |
||
| Attempted to quit smoking Severe asthma Mild-to-moderate asthma No asthma diagnosis |
3 (60.0) 6 (35.3) 11 (34.4) |
||
| Duration of smoking, years Severe asthma Mild-to-moderate asthma No asthma diagnosis |
33.0 [8.5-43.5] 10.0 [6.0-18.0] 27.5 [16.3-37.0] |
15.0 [5.3-24.0] 11.3 [3.0-14.5] 10.2 [7.0-25.0] |
0.14 0.07 < 0.01 |
| Number of cigarettes/day Severe asthma Mild-to-moderate asthma No asthma diagnosis |
2.0 [1.5-12.5] 2.0 [1.0-4.0] 5.0 [3.0-9.5] |
6.0 [3.0-20.0] 5.0 [3.0-10.0] 10.0 [3.0-20.0] |
0.18 < 0.01 0.03 |
| Smoking history, pack-years Severe asthma Mild-to-moderate asthma No asthma diagnosis |
25.5 [0.4-36.9] 1.3 [0.2-4.0] 7.7 [2.5-18.4] |
4.4 [1.2-16.8] 1.2 [0.8-7.0] 8.0 [1.3-19.8] |
0.52 0.25 0.89 |
| Urinary cotinine, µg/gc
Severe asthma Mild-to-moderate asthma No asthma diagnosis |
807.8 [49.1-3.239.3] 41.1 [4.1-201.6] 598.3 [219.8-2.027.8] |
62.5 [19.2-409.5] 30.3 [13.0-110.1] 40.9 [9.9-129.1] |
0.03 0.27 < 0.01 |
| Exposure to secondhand tobacco smoke in the last 24 h Severe asthma Mild-to-moderate asthma No asthma diagnosis |
4 (80.0) 10 (58.8) 24 (72.7) |
59 (44.4) 24 (42.9) 29 (34.5) |
0.12 0.25 < 0.01 |
On the basis of references 22 and 23. bValues expressed as n (%) or median [interquartile range]. cResults below the limit of detection are not included. *Chi-square test for categorical variables and Kruskal-Wallis test for continuous variables.
Smoking initiation was found to have occurred at an early age (i.e., during adolescence). Among smokers and former smokers, the mean age at smoking initiation was significantly lower in the group of patients with severe asthma (15.9 ± 5.3 years) than in that of patients with mild-to-moderate asthma (18.8 ± 5.7 years) and that of individuals without asthma (16.8 ± 4.2 years; p = 0.02).
Among current smokers, a longer smoking history was found in the group of patients with severe asthma (25.5 pack-years) when compared with that of those with mild-to-moderate asthma (1.3 pack-years) and that of those without asthma (7.7 pack-years). Among former smokers, patients with severe asthma had a smoking history of 4.4 pack-years, patients with mild-to-moderate asthma had a smoking history of 1.2 pack-years, and individuals without asthma had a smoking history of 8.0 pack-years.
All of the study participants who reported smoking daily were positive for urinary cotinine. Of the study participants who reported smoking occasionally, 8 had urinary cotinine levels below the limit of detection. Median urinary cotinine levels were higher among daily smokers (758.2 µg/g; IR: 433.2-2,066.8) than among occasional smokers (97.1 µg/g; IR: 30.7-1.036.9; Table 3). Among daily and occasional smokers, urinary cotinine levels were highest in the group of patients with severe asthma.
Table 3. Creatinine-corrected urinary cotinine (in µg/g) in the study participants (n = 1,341), by self-reported smoking status.a .
| Smoking status | Number of participants | Urinary cotinine, µg/gb | p* | |
|---|---|---|---|---|
| n/N | % | |||
| Daily smoker Severe asthma Mild-to-moderate asthma No asthma diagnosis TOTAL |
2/498 7/417 23/426 32/1,341 |
0.4 1.7 5.4 2.4 |
930.4 (807.8-1,053.1) 140.4 (11.9-2,189.7) 710.8 (499.1-2,357.7) 758.2 (433.2-2,066.8) |
0.35 |
| Occasional smoker Severe asthma Mild-to-moderate asthma No asthma diagnosis TOTAL |
3/498 10/417 10/426 23/1,341 |
0.6 2.4 2.3 1.7 |
2,761.3 (97.1-5,425.5) 41.1 (16.2-129.1) 635.1 (32.3-3,945.0) 97.1 (30.7-1,036.9) |
0.17 |
| Former smoker Severe asthma Mild-to-moderate asthma No asthma diagnosis TOTAL |
133/498 56/417 84/426 273/1,341 |
26.7 13.4 19.7 20.4 |
62.5 (19.2-409.5) 30.3 (13.0-110.3) 40.9 (9.9-129.0) 44.9 (17.4-147.9) |
0.17 |
| Nonsmoker Severe asthma Mild-to-moderate asthma No asthma diagnosis TOTAL |
360/498 344/417 309/426 1,013/1,341 |
72.3 82.5 72.5 75.5 |
27.7 (14.3-69.5) 14.3 (6.8-39.9) 28.2 (11.4-67.3) 24.2 (10.9-58.5) |
< 0.01 |
Values expressed as median (interquartile range). bIndividuals presenting with results below the limit of detection are not included. *Kruskal-Wallis test.
Of the study participants who reported being nonsmokers (n = 1,286), 273 (21.3%) were former smokers. Median urinary cotinine levels were higher among former smokers (44.9 µg/g; IR: 17.4-147.9) than among individuals who reported never having smoked a cigarette (24.2 µg/g; IR: 10.9-58.5). Median urinary cotinine levels were higher among former smokers in the severe asthma group than among those in the remaining groups (Table 3). Figure 2 shows median urinary cotinine levels in smokers, former smokers, and nonsmokers, by asthma status.
Figure 2. Creatinine-corrected urinary cotinine levels, by self-reported smoking status. Results expressed as (log) per µg/g of creatinine.
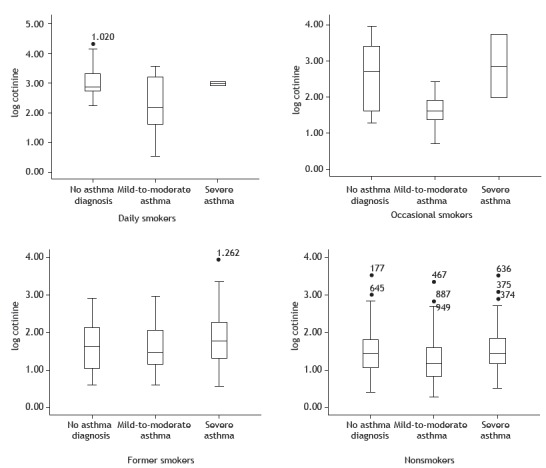
Among former smokers, median urinary cotinine levels were highest in those with severe asthma. Among former smokers, median urinary cotinine levels were higher in those with severe asthma (62.5 µg/g; IR: 19.2-409.5) than in those with mild-to-moderate asthma (30.3 µg/g; IR: 13.0-110.3) and those without asthma (40.9 µg/g; IR: 9.9-129.1; p > 0.05).
Of the study participants who reported being nonsmokers, 440 (34.3%) reported having been exposed to secondhand smoke (at home, at work, in public transportation, in public places, or any combination of the four) in the last 24 h. Of the nonsmokers who reported having been exposed to secondhand smoke in the last 24 h, 36.7% were patients with severe asthma, 34.6% were patients with mild-to-moderate asthma, and 30.8% were individuals without asthma.
DISCUSSION
In the present study, 4.1% of the participants reported being active smokers, a proportion that is lower than the mean proportion of smokers in the Brazilian population but similar to the proportion of smokers among adults in the city of Salvador. 5
As expected, urinary cotinine levels were higher among daily smokers than among occasional smokers, former smokers, and nonsmokers. In addition, urinary cotinine levels were found to be higher in former smokers than in nonsmokers, a finding that suggests patient nondisclosure of smoking status. Urinary cotinine levels were higher in severe asthma patients who reported being former smokers than in former smokers with mild-to-moderate asthma and no asthma diagnosis, a finding that suggests that the issue of patient nondisclosure of smoking status is even more problematic in patients with severe asthma. The proportion of former smokers was highest among patients with severe asthma and lowest among patients with mild-to-moderate asthma.
The prevalence of current smoking was found to be higher in individuals without asthma than in patients with asthma, being lower in severe asthma patients than in mild-to-moderate asthma patients. The low rates of self-reported smoking among asthma patients in the present study are similar to those found in the literature 9 and might be due to the fact that smoking has a negative impact on the clinical status and quality of life of asthma patients, who therefore avoid cigarettes. Because of their disease, patients with asthma are less likely to continue smoking. Another factor that can play an important role in reducing smoking among patients with asthma is being followed at health care clinics that provide education on the harmful effects of smoking. However, the possibility of patient nondisclosure of smoking status should be taken into account. 9 , 11
The fact that the proportion of former smokers in the present study was highest among severe asthma patients suggests that smoking is a risk factor for the development of severe asthma in asthma patients who smoke despite feeling discomfort and despite warnings about the effects of smoking. In asthma patients with an increased smoking history, increased asthma severity might be due to asthma-COPD overlap syndrome.
Among smokers and former smokers in the present study, smoking initiation was found to have occurred during adolescence, a finding that is consistent with those of Malcon et al. 28 and Abreu et al. 29 In the present study, smoking initiation was found to have occurred earlier in the group of patients with severe asthma than in that of those with mild-to-moderate asthma (15.9 years vs. 18.8 years), a finding that is consistent with the possibility that exposure to smoking is a risk factor for the development of severe asthma. 30
Among smokers and former smokers, the median duration of smoking was shorter in those with mild-to-moderate asthma than in those with severe asthma. This suggests that smoking is associated with asthma severity. In the present study, mild-to-moderate asthma patients smoked less than did severe asthma patients and individuals without asthma. It is possible that the discomfort associated with cigarette smoke inhalation led mild-to-moderate asthma patients to quit smoking, whereas those who continued to smoke developed asthma that is more severe.
In the present study, patients with severe asthma were found to have a longer smoking history (in pack-years) than that of those with mild-to-moderate asthma, a finding that suggests an association between smoking and increased asthma severity.
In the present study, urinary cotinine levels varied among the groups, differences being found between urinary cotinine measurements and self-reported smoking status. Median urinary cotinine levels were found to be higher in self-reported daily smokers than in self-reported occasional smokers, except in the group of patients with severe asthma, a finding that suggests patient nondisclosure of smoking behavior. Cotinine levels are typically lower in individuals who do not smoke daily than in those who do, being high in those who smoke more cigarettes daily, 31 a single measurement of cotinine being sufficient to show that. 32
Other studies have shown discrepancies between self-reported smoking status and cotinine measurements, 33 , 34 suggesting patient nondisclosure of smoking status. In a study conducted in the city of São Paulo, Brazil, urinary cotinine levels were found to be high in severe asthma patients who reported being former smokers, a finding that alerts us to the possibility of inaccurate self-reporting. 11
Smoking is known to be associated with a poor asthma prognosis, reducing patient response to inhaled corticosteroids, increasing asthma symptoms, increasing the need for emergency room visits, increasing the need for hospitalization, and increasing treatment costs, as well as having a negative impact on quality of life. Cessation of smoking and smoke exposure can improve the clinical status of patients with asthma. 8 , 35
Although our sample was large, the present study has limitations that should be taken into account. Urinary cotinine measurements might have been affected by passive exposure to tobacco smoke, ethnicity, and consumption of nicotine-containing foods, such as tomatoes, potatoes, and black tea. 36 , 37 However, there were no differences in cotinine levels among nonsmokers or self-reported ethnicities exposed to secondhand smoke (exposure being expressed as number of hours). The influence of dietary habits on urinary cotinine levels was not investigated in the present study. The low frequency of current smokers in our sample reduced the power of subgroup analyses. During patient recruitment, asthma patients who reported a smoking history ≥ 10 pack-years were excluded in order to avoid mistaking COPD for asthma and ensure that the inclusion criteria were similar for patients with severe asthma and those with mild-to-moderate asthma. This might have introduced a bias in the comparison with the individuals without asthma. However, the bias would have favored a shorter smoking history among asthma patients; the fact that this was not observed in the severe asthma group reinforces the internal validity of our study. The proportion of former smokers was considerably higher in the severe asthma group (i.e., 27%) than in the remaining groups.
In conclusion, the prevalence of self-reported smoking was low among patients with varying degrees of asthma severity, being particularly low among those with severe asthma. However, among patients with severe asthma, findings of an increased proportion of self-reported former smokers, an increased smoking history, and increased urinary cotinine levels suggest patient nondisclosure of smoking status and an association between exposure to active smoking and severe asthma. Patients with severe asthma should be thoroughly screened for smoking via interviews and objective assessment.
Financial support: This study received financial support from the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico/Programa de Apoio a Núcleos de Excelência (CNPq/PRONEX, National Council for Scientific and Technological Development/Program for the Support of Centers of Excellence; Grant no. 020/2009), the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB, Foundation for the Support of Research in the State of Bahia; Grant no. 6353 PNX 0018/2009), and GlaxoSmithKline’s Trust in Science program investigator-initiated grant (2012-2015).
Study carried out under the auspices of the Programa para o Controle da Asma na Bahia - ProAR - Universidade Federal da Bahia - UFBA - Salvador (BA) Brasil.
REFERENCES
- 1.World Health Organization . WHO strategy for prevention and control of chronic respiratory diseases. Geneva: WHO; 2002. [Google Scholar]
- 2.World Health Organization . WHO global report on trends in prevalence of tobacco smoking. Geneva: WHO; 2015. [Google Scholar]
- 3.World Health Organization . WHO Report on the Global Tobacco Epidemic 2015: raising taxes on tobacco. Geneva: WHO; 2015. [Google Scholar]
- 4.Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention: online appendix. Bethesda: GINA; 2016. [Google Scholar]
- 5.Brasil . Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância de Doenças e Agravos não Transmissíveis e Promoção da Saúde. Vigitel Brasil 2016: vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico: estimativas sobre frequência e distribuição sociodemográfica de fatores de risco e proteção para doenças crônicas nas capitais dos 26 estados brasileiros e no Distrito Federal em 2016. Brasília: Ministério da Saúde; 2017. [Google Scholar]
- 6.Barreto ML, Ribeiro-Silva RC, Malta DC, Oliveira-Campos M, Andreazzi MA, Cruz AA. Prevalence of asthma symptoms among adolescents in Brazil National Adolescent School-based Health Survey (PeNSE 2012) Rev Bras Epidemiol. 2014;17(1):106–115. doi: 10.1590/1809-4503201400050009. [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. Bethesda: GINA; 2016. [Google Scholar]
- 8.Polosa R, Thomson NC. Smoking and asthma dangerous liaisons. Eur Respir J. 2013;41(3):716–726. doi: 10.1183/09031936.00073312. [DOI] [PubMed] [Google Scholar]
- 9.Dias-Júnior AS, Pinto RC, Angelini L, Fernandes FL, Cukier A, Stelmach R. Prevalence of active and passive smoking in a population of patients with asthma. J Bras Pneumol. 2009;35(3):261–265. doi: 10.1590/S1806-37132009000300011. [DOI] [PubMed] [Google Scholar]
- 10.Haufroid V, Lison D. Urinary cotinine as a tobacco-smoke exposure index a minireview. Int Arch Occup Environ Health. 1998;71(3):162–168. doi: 10.1007/s004200050266. [DOI] [PubMed] [Google Scholar]
- 11.Stelmach R, Fernandes FL, Carvalho-Pinto RM, Athanazio RA, Rached SZ, Prado GF. Comparison between objective measures of smoking and self-reported smoking status in patients with asthma or COPD are our patients telling us the truth? J Bras Pneumol. 2015;41(2):124–132. doi: 10.1590/S1806-37132015000004526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H, Lim Y, Lee S, Park S, Kim C, Hong C. Relationship between environmental tobacco smoke and urinary cotinine levels in passive smokers at their residence. J Expo Anal Environ Epidemiol. 2004;14(1):S65–S70. doi: 10.1038/sj.jea.7500360. [DOI] [PubMed] [Google Scholar]
- 13.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 14.Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke focus on developmental toxicology. Ther Drug Monit. 2009;31(1):14–30. doi: 10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto M, Inaba Y, Yamaguchi I, Endo O, Hammond D, Uchiyama S. Smoking topography and biomarkers of exposure among Japanese smokers associations with cigarette emissions obtained using machine smoking protocols. Environ Health Prev Med. 2013;18(2):95–103. doi: 10.1007/s12199-012-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machado Jde B, Plínio VM, Filho, Petersen GO, Chatkin JM. Quantitative effects of tobacco smoking exposure on the maternal-fetal circulation. BMC Pregnancy and Childbirth. 2011;11:24–24. doi: 10.1186/1471-2393-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benowitz NL, Kuyt F, Jacob 3rd P, Jones RT, Osman AL. Cotinine disposition and effects. Clin Pharmacol Ther. 1983;34(5):604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- 18.Etzel RA, Greenberg RA, Haley NJ, Loda FA. Urine cotinine excretion in neonates exposed to tobacco smoke products in utero. J Pediatr. 1985;107(1):146–148. doi: 10.1016/S0022-3476(85)80637-5. [DOI] [PubMed] [Google Scholar]
- 19.Jacob 3rd P, Benowitz NL, Shulgin AT. Recent studies of nicotine metabolism in humans. Pharmacol Biochem Behav. 1988;30(1):249–253. doi: 10.1016/0091-3057(88)90453-4. [DOI] [PubMed] [Google Scholar]
- 20.Malafatti L, Martins I. Analytical aspects of the continine's determination in biological matrices. Rev Bras Toxicol. 2009;22(1-2):9–20. [Google Scholar]
- 21.Global Initiative for Asthma . Global Strategy for Asthma Management and Prevention. Bethesda: GINA; 2012. [Google Scholar]
- 22.Brasil . Ministério da Saúde. Departamento de Vigilância de Doenças e Agravos não Transmissíveis e Promoção da Saúde. Vigitel Brasil 2010. Vigilância de fatores de risco e proteção de doenças crônicas por inquérito telefônico: estimativas sobre frequência e distribuição sociodemográfica de fatores de risco e proteção para doenças crônicas nas capitais dos 26 estados brasileiros e no distrito federal em 2010. Brasília: Ministério da Saúde; 2011. [Google Scholar]
- 23.Brasil . Ministério da Saúde. Instituto Nacional do Câncer; Organização Pan-Americana da Saúde. Pesquisa especial de tabagismo (PETab): relatório Brasil. Rio de Janeiro: Instituto Nacional do Câncer; 2011. [Google Scholar]
- 24.Cattaneo R, Alegretti AP, Sagebin FR, Abreu CM, Petersen GO, Chatkin JM. Validação do método para determinação de cotinina em urina por cromatografia líquida de alta eficiência. Rev Bras Toxicol. 2006;19(1):25–31. [Google Scholar]
- 25.Brasil . Ministério da Saúde. Agência Nacional de Vigilância Sanitária (ANVISA). Guia para validação de métodos analíticos e bioanalíticos. Resolução RE nº 899, de 29 de maio de 2003. Brasília: ANVISA; 2003. [Google Scholar]
- 26.Petersen GO, Leite CE, Chatkin JM, Thiesen FV. Cotinine as a biomarker of tobacco exposure development of a HPLC method and comparison of matrices. J Sep Sci. 2010;33(4-5):516–521. doi: 10.1002/jssc.200900575. [DOI] [PubMed] [Google Scholar]
- 27.Watts RR, Langone JJ, Knight GJ, Lewtas J. Cotinine analytical workshop report consideration of analytical methods for determining cotinine in human body fluids as a measure of passive exposure to tobacco smoke. Environ Health Perspect. 1990;84:173–182. doi: 10.1289/ehp.9084173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malcon MC, Menezes AB, Chatkin M. Prevalence and risk factors for smoking among adolescents. Rev Saude Publica. 2003;37(1):1–7. doi: 10.1590/S0034-89102003000100003. [DOI] [PubMed] [Google Scholar]
- 29.Abreu MN, Souza CF, Caiaffa WT. Smoking among adolescents and young adults in Belo Horizonte, Minas Gerais State, Brazil the influence of family setting and social group. Cad Saude Publica. 2011;27(5):935–943. doi: 10.1590/S0102-311X2011000500011. [DOI] [PubMed] [Google Scholar]
- 30.Annesi-Maesano I, Oryszczyn MP, Raherison C, Kopferschmitt C, Pauli G, Taytard A. Increased prevalence of asthma and allied diseases among active adolescent tobacco smokers after controlling for passive smoking exposure A cause for concern? Clin Exp Allergy. 2004;34(7):1017–1023. doi: 10.1111/j.1365-2222.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- 31.Caraballo RS, Giovino GA, Pechacek TF, Mowery PD. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2001;153(8):807–814. doi: 10.1093/aje/153.8.807. [DOI] [PubMed] [Google Scholar]
- 32.Lee K, Lim S, Bartell S, Hong YC. Interpersonal and temporal variability of urinary cotinine in elderly subjects. Int J Hyg Environ Health. 2011;215(1):46–50. doi: 10.1016/j.ijheh.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Boyd NR, Windsor RA, Perkins LL, Lowe JB. Quality of measurement of smoking status by self-report and saliva cotinine among pregnant women. Matern Child Health J. 1998;2(2):77–83. doi: 10.1023/A:1022936705438. [DOI] [PubMed] [Google Scholar]
- 34.Man CN, Fathelrahman AI, Harn GL, Lajis R, Samin AS, Omar M. Correlation between urinary nicotine, cotinine and self-reported smoking status among educated young adults. Environ Toxicol Pharmacol. 2009;28(1):92–96. doi: 10.1016/j.etap.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. Eur Respir J. 2004;24(5):822–833. doi: 10.1183/09031936.04.00039004. [DOI] [PubMed] [Google Scholar]
- 36.Bramer SL, Kallungal BA. Clinical considerations in study designs that use cotinine as a biomarker. Biomarkers. 2003;8(3-4):187–203. doi: 10.1080/13547500310012545. [DOI] [PubMed] [Google Scholar]
- 37.Siegmund B, Leitner E, Pfannhauser W. Determination of the nicotine content of various edible nightshades (Solanaceae) and their products and estimation of the associated dietary nicotine intake. J Agric Food Chem. 1999;47(8):3113–3120. doi: 10.1021/jf990089w. [DOI] [PubMed] [Google Scholar]