Summary
Interleukin‐35 (IL‐35) is a recently identified heterodimeric cytokine in the IL‐12 family. It consists of an IL‐12 subunit α chain (P35) and IL‐27 subunit Epstein–Barr virus‐induced gene 3 (EBI3) β chain. Unlike the other IL‐12 family members, it signals through four unconventional receptors: IL‐12Rβ2–IL‐27Rα, IL‐12Rβ2–IL‐12Rβ2, IL‐12Rβ2–GP130, and GP130–GP130. Interleukin‐35 signaling is mainly carried out through the signal transducer and activator of transcription family of proteins. It is secreted not only by regulatory T (Treg) cells, but also by CD8+ Treg cells, activated dendritic cells and regulatory B cells. It exhibits immunosuppressive functions distinct from those of other members of the IL‐12 family; these are mediated primarily by the inhibition of T helper type 17 cell differentiation and promotion of Treg cell proliferation. Interleukin‐35 plays a critical role in several immune‐associated diseases, such as autoimmune diseases and viral and bacterial infections, as well as in tumors. In this review, we summarize the structure and function of IL‐35, describe its role in immune‐related disorders, and discuss the mechanisms by which it regulates the development and progression of diseases, including inflammatory bowel disease, collagen‐induced arthritis, allergic airway disease, hepatitis, and tumors. The recent research on IL‐35, combined with improved techniques of studying receptors and signal transduction pathways, allows for consideration of IL‐35 as a novel immunotherapy target.
Keywords: immune‐related diseases, immunosuppression, interleukin‐35, regulatory T cells
Abbreviations
- AHR
airway hyperresponsiveness
- CD
cluster of differentiation
- CFA/I
colonization factor of enterotoxigenic Escherichia coli
- CIA
collagen‐induced arthritis
- DC
dendritic cell
- EBI3
Epstein–Barr virus‐induced gene 3
- FOXP3
forkhead box 3
- GP130
glycoprotein 130
- IBD
inflammatory bowel disease
- IL‐12Rβ1
interleukin 12 receptor, β1 subunit
- IL‐12Rβ2
interleukin 12 receptor, β2 subunit
- IL‐27Rα
interleukin 27 receptor, α (also known as WSX‐1)
- IL
interleukin
- JAK2
Janus‐related kinase 2
- STAT
signal transducer and activator of transcription
- Teff
effector T
- Th
T helper
- Treg
regulatory T
- TYK2
tyrosine kinase 2
Introduction
Cytokines are a group of small proteins synthesized and secreted by a broad range of cells including immune cells such as monocytes, macrophages, T cells and B cells and certain non‐immune cells such as endothelial cells, epidermal cells and fibroblasts.1 Recent studies have uncovered a wealth of information on the role of cytokines in the regulation of immunomodulatory processes.2, 3 Endogenous and exogenous cytokines play a variety of roles in maintaining the delicate balance of the immune system, and disruption of this equilibrium may lead to debilitating immune‐related diseases.4 In 2007, Collison et al.5 identified interleukin‐35 (IL‐35) as a novel inhibitory cytokine, consisting of Epstein–Barr virus‐induced gene 3 (EBI3) and IL‐12 subunit α chain (P35) subunits and belonging to the IL‐12 cytokine family. Unlike other IL‐12 family members, which are known to be primarily secreted by activated antigen‐presenting cells,6, 7 IL‐35 is primarily expressed in unstimulated regulatory T (Treg) cells in mice, but is not detected in the unstimulated human Treg cells.5, 8, 9 Recently, it was shown to be secreted in a wide range of tissues and by different cell types, including regulatory B cells,10, 11 dendritic cells (DCs),12 endothelial cells, smooth muscle cells, and monocytes.13 Interleukin‐35 has also been reported to play an important regulatory role in several autoimmune diseases, inflammatory diseases, bacterial and viral infectious diseases, and tumors.
Discovery and composition of IL‐35
In 1997, Devergne et al. first reported that specific co‐immune precipitates of P35 and EBI3 constitute a large portion of the nourishing component extract of the normal human full‐term placenta, indicating that the two components heterodimerize in vivo.14 In 2007, the heterodimer was formally named IL‐35 at the 13th Immunology Conference. The same year, researchers demonstrated that the immunosuppressive cytokine IL‐35 is a member of the IL‐12 family of cytokines and that it regulates the function of Treg cells and inhibits the proliferation of CD4+ CD25− effector T (Teff) cells.5, 15
There are four members of the IL‐12 family, namely, IL‐12, IL‐23, IL‐27 and IL‐35. All four are heterodimers: P35 and P40 subunits heterodimerize to form IL‐12, P40 and P19 form IL‐23, EBI3 and P28 form IL‐27, and EBI3 and P35 form IL‐35 (Fig. 1).5, 16, 17, 18, 19 EBI3 is widely expressed in B lymphocytes and tissues, including the spleen and tonsils transformed by the Epstein–Barr virus.14 EBI3 plays a critical role in the immune response by down‐regulating the expression of transcription factor RORγt in T helper type 17 (Th17) cells and inhibiting inflammatory response.20 On the other hand, P35 can regulate inflammation, but has also been shown to lead to herpes simplex keratitis in mice.21 Either subunit of IL‐35 can independently regulate the immune response, and these functions are augmented in the IL‐35 heterodimeric form.22
Figure 1.
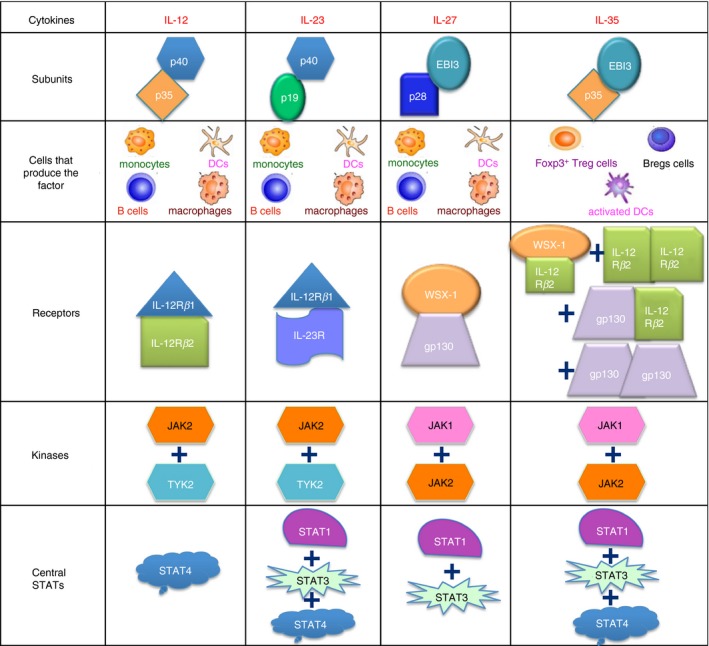
Four members of the interleukin‐12 (IL‐12) cytokine family and their downstream signaling pathways: IL‐12 (p35/p40) signals through IL‐12Rβ1 and IL‐12Rβ2, IL‐23 (p19/p40) signals through IL‐23R and IL‐12Rβ1, IL‐27 (p28/Ebi3) signals through gp130 and WSX‐1, while IL‐35 (p35/Ebi3) uses IL‐12Rβ2 and WSX‐1 heterodimers, IL‐12Rβ2 and IL‐12Rβ2 homodimers, IL‐12Rβ2 and gp130 heterodimers, and gp130 and gp130 homodimers.
Studies suggest that except for IL‐35, the other members of the IL‐12 family are primarily secreted by antigen‐presenting cells, including DCs, macrophages, and monocytes.23, 24 Initial studies reported that only FOXP3+ Treg cells, but not Teff cells, can secrete active IL‐35.5, 25 Moreover, Treg cells participate in a variety of inhibitory mechanisms. Wei et al.26 reported that IL‐35‐producing Treg cells were distinct from the IL‐10‐producing subset. Furthermore, Seyerl et al. observed that human rhinovirus can activate DCs, and the activated DCs can secrete and release IL‐35 into human peripheral blood.27 In 2014, Shen et al.10 further revealed that B cells can also secrete IL‐35 and have an immunosuppressive function in autoimmune diseases and bacterial infectious diseases (Fig. 1). These results suggest that IL‐35 production may be affected by multiple stimulants and immune microenvironments.
IL‐35 receptors and signaling pathways
The receptors of IL‐12 family cytokines are dimers that signal through the Janus kinase–signal transducer and activator of transcription (JAK–STAT) pathway.11, 28, 29, 30, 31 The IL‐12 receptor is composed of IL‐12Rβ1 and IL‐12Rβ2, both of which are members of the GP130 cytokine receptor family. Interaction between IL‐12 and its receptor causes the mutual phosphorylation of Janus‐related kinase 2 (JAK2) and tyrosine kinase 2 (TYK2), which further induces tyrosine phosphorylation of the receptors, and recruits STAT1, STAT3, STAT4, and STAT5.32, 33 Together, IL‐23R and IL‐12Rβ1 comprise the IL‐23 receptor complex on IL‐23‐responsive cells.29 After binding to the receptor, IL‐23 too induces the phosphorylation of JAK2 and TYK2, which, in turn, phosphorylate STAT1, STAT3, STAT4, and STAT5.29, 34 The IL‐27 receptor is a heterodimer formed by IL‐27Rα and GP130.35 After binding to the receptor, STAT1, STAT3, STAT4, and STAT5 are activated through phosphorylation by JAK1 and JAK2.36, 37 Unlike other IL‐12 family members, the receptors of IL‐35 are: GP130–GP130, IL‐12Rβ2–IL‐12Rβ2, IL‐12Rβ2–GP130, and IL‐12Rβ2–IL‐27Rα (Fig. 1).31 Following the binding of IL‐35 to its receptors, its downstream signal transduction occurs through a unique heterodimer formed by STAT1 and STAT4, leading to the expression of target genes, including EBI3 and P35. These target genes lead to a feedback loop promoting the expression of IL‐35.31 The IL‐35 signaling pathway consists of STAT1, STAT3, STAT4, JAK1, and JAK2 molecules (Fig. 1).38, 39 However, the binding of IL‐35 to different receptor subunits depends on the cell‐type. In T cells, IL‐35 signaling uses three receptor subunits, namely, GP130–GP130, IL‐12Rβ2–IL‐12Rβ2, and IL‐12Rβ2–GP130, which activate STAT1 and STAT4.40, 41 Regulatory B cells have four receptor subunits: GP130, IL‐27Rα, IL‐12Rβ1, and IL‐12Rβ2. Interestingly, a study employing small interfering RNA to silence each subunit individually showed that IL‐35 signaling in B cells occurs through IL‐27Rα and IL‐12Rβ2, which activate STAT1 and STAT3, rather than through GP130 and IL‐12Rβ1.11, 42
IL‐35 and immune‐related diseases
The last decade has seen remarkable progress in IL‐35 research. Studies have shown that it has two major biological effects in a variety of disease models: inhibition of T‐cell proliferation43, 44 and inhibition of the development and differentiation of Th17 cells.45, 46 It has been shown to play an important role in the development and progression of inflammatory and autoimmune diseases. Recent results suggest that the plasma concentration of IL‐35 is lower in psoriasis patients than in healthy individuals.47 Furthermore, it was found that IL‐35 could alleviate the pathological characteristics of severe psoriatic lesions in K14‐VEGF‐A transgenic mice by reducing the ratio of the total number of macrophages to M1/M2 macrophages.48, 49
IL‐35 and inflammatory bowel disease
Currently, the pathogenesis of inflammatory bowel disease (IBD) is not clear. It is widely believed that intestinal flora and environmental factors play an important role in the etiology of IBD.50 In 2007, Collison et al. first demonstrated in an IBD model that the inhibitory effect of Treg cells on Teff cells, and thereby the therapeutic effect on the IBD model, is greatly reduced by the deletion of the two subunits EBI3 and P35. The researchers also observed that CD4+ CD25− iTR35 cells stimulated by exogenous IL‐35 could have an immunosuppressive effect in IBD model mice.51, 52 Wirtz et al. reported that enteritis symptoms in the IBD mouse model were significantly alleviated by vector‐mediated IL‐35 overexpression. At the same time, the authors showed that, after the establishment of the IBD model in EBI3 −/− and P28 −/− mice, the EBI3 −/− animals suffered more severe inflammation, indicating that IL‐35, rather than IL‐27 regulates IBD inflammation.43 Wang et al. demonstrated that IL‐35 recombinant protein could regulate IBD inflammation by promoting the secretion of IL‐10 and inhibiting the expression of pro‐inflammatory cytokines such as IL‐6, tumor necrosis factor‐α, and IL‐17 in an acute model of colitis.49 When IL‐35 was integrated into mesenchymal stem cells, the cells could ameliorate ulcerative colitis by down‐regulating the expression of pro‐inflammatory cytokines.53 The above studies suggest that IL‐35 plays a significant biological role in inhibiting IBD.
IL‐35 and collagen‐induced arthritis
The collagen‐induced arthritis (CIA) mouse model of rheumatoid arthritis is induced by type II collagen immunization.54 IL‐35 was first reported to have a therapeutic effect on the collagen‐induced arthritis model in 2007. This study showed that IL‐35 could promote the proliferation of CD4+ CD25− and CD4+ CD25+ T cells in vitro and increase the secretion of interferon‐γ. Simultaneously, IL‐35 inhibited the aggravation of the CIA model by inhibiting the differentiation of CD4+ T cells into Th17 cells.15 In previous studies, the colonization factor of enterotoxigenic Escherichia coli (CFA/I) could improve the CIA model by inducing the proliferation of IL‐10‐secreting CD4+ CD39+ Foxp3+ T cells.55, 56 Kochetkova et al. reported that, in the CIA model, CD4+ CD39+ Foxp3+ T cells could secrete IL‐35 after CFA/I treatment. The therapeutic effect of CFA/I on the model was greatly reduced and IL‐35 was blocked in vivo.57 Other studies have shown that exogenous IL‐35 could inhibit the proliferation of Th1 and Th17 cells and promote CD4+ T cells to express CD39 in the CIA model. Meanwhile, the authors have also shown that CD4+ CD39+ CD25− T cells isolated from mice in the IL‐35‐treated group could express IL‐10 strongly, but not induce CD4+ CD39+ T cells when IL‐35 was used to treat Il10 −/− mice.44 The study reveals that when IL‐35 treatment is applied in the CIA model, it can induce the production of IL‐10 in CD4+ CD39+ Treg cells.
IL‐35 and allergic airway disease
Clinically defined allergic asthma is characterized by reversible airway obstruction and airway hyper‐responsiveness. The main feature of allergic airway disease is that the hyperproliferative Th2 cells promote the secretion of IL‐4, IL‐5, and IL‐13, which increases IgE levels in the respiratory tract, leading to increased numbers of eosinophils and the secretion of mucus into the airway.58, 59 In recent years, studies have shown that IL‐17 produced by macrophages or Th17 cells plays an important role in allergic airway disease.60, 61 Whitehead et al. observed that the allergen‐induced allergic airway disease is more severe when EBI3 or P35 are absent in CD278+ Treg cells. However, in IL‐10‐ or transforming growth factor‐β‐deficient mice, the disease model did not show any sign of exacerbation. The p28 subunit was expressed at relatively low levels in CD278+ Treg cells; however, EBI3 can pair with p28 as well to form IL‐27. Thus IL‐35, but not IL‐27, is selectively expressed by murine CD278+ Treg cells. This study also showed that endogenous IL‐35 expressed by CD278+ Treg cells had a protective effect in the allergic airway disease model.62 In ovalbumin‐sensitized allergic airway disease mouse models, IL‐35 alleviated the symptoms of the disease by inhibiting the recruitment of inflammatory DCs at the inflammatory sites and draining lymph nodes.63 Interleukin‐35 vectors can effectively treat allergic airway inflammation induced by memory/effector Th2 cells reactive to allergen mites. Furthermore, this therapeutic effect is mainly achieved by regulating the inflammatory factors IL‐4, IL‐5, and IL‐13 and the chemokines CCL2, CXCL1, and CXCL5.64
Increased eosinophils could be pathogenic in various diseases, such as eosinophilic pneumonias, allergic asthma, and eosinophil‐associated gastrointestinal disorders.65, 66 Recent findings suggest that IL‐35 decreases airway eosinophilia by reducing the production of the eosinophil‐attracting chemokines CCL11 and CCL24, which demonstrates that IL‐35 may provide a new therapeutic strategy to reduce tissue recruitment of eosinophils in such diseases.67 In addition, studies have shown that the expression of IL‐35 is down‐regulated in chronic obstructive pulmonary disease, a chronic bronchitis and emphysema characterized by airflow obstruction.68 Moreover, decreased IL‐35 levels were negatively correlated with the smoking status, indicating that IL‐35 can serve as a biomarker to estimate progression of chronic obstructive pulmonary disease.69 The above results suggest that IL‐35 may be a good indicator of allergic inflammation and can be used as a biomarker.
IL‐35 and type I diabetes
Type I diabetes is a chronic autoimmune disease, which is primarily caused by the destruction of the β‐cells that secrete insulin, resulting in significantly elevated blood glucose levels.25, 70 Currently, intramuscular injection of insulin is the primary treatment for type I diabetes, which only temporarily relieves the symptoms and does not cure the disease; cell therapy involving islet or insulin‐secreting cell transplantation presents a more effective strategy.71 In recent years, researchers have established IL‐35 transgenic mice, in which IL‐35 is only expressed on islet β‐cells, and found that IL‐35 can inhibit the occurrence of primary and secondary type I diabetes. The inhibitory effect of IL‐35 occurs primarily through inhibition of the proliferation and infiltration of CD4+ and CD8+ T cells.72 Similarly, transgenic IL‐35 expression selectively targeted against β‐cells in NOD mice can reduce the number of islet‐resident conventional CD4+ and CD8+ T cells, DCs, and islet Foxp3+ Treg cells.73 Although IL‐35 administration did not increase the number of Treg cells, it decreased the number of Th1 and Th17 cells, as well as interferon‐γ‐ or IL‐17A‐expressing CD8+ T cells, and reduced the infiltration of mononuclear cells in the islets.74 At the same time, clinical research studies showed that the expression of IL‐35 in serum was markedly lower in C‐peptide‐negative patients, and this was associated with a simultaneous decrease in the proportion of IL‐35+ Treg cells, IL‐35+ regulatory B cells, and IL‐35‐producing CD8+ FOXP3+ cells.45 These results indicate that high expression of IL‐35 can affect autoimmune diabetes. Moreover, the expression of IL‐35 in local islet β‐cells can control the immune response of CD4+ and CD8+ T cells, which suggests that IL‐35 plays an important role in the regulation of the immune response in type I diabetes.
IL‐35 and hepatitis
Hepatitis virus causes destruction of liver parenchymal cells, leading to severe liver disorders, such as cirrhosis and hepatocellular carcinoma.75 Hepatitis is a common and frequently occurring disease in the clinic, its incidence being highest in Asia.76 The T‐cell‐mediated immune response plays an important role in the pathogenesis of the disease.77 Studies suggest that unstimulated human Treg cells do not express IL‐35;8 however, substantial up‐regulation of EBI3 and P35, not IL‐10 and transforming growth factor‐β, was observed in activated human Treg cells compared with conventional T cells.9 On the other hand, IL‐35 expression has been detected in peripheral blood CD4+ T lymphocytic cells of chronic hepatitis B patients.78 However, whether IL‐35 is involved in the development of hepatitis has not yet been confirmed. Researchers have found that IL‐35 is highly expressed in the peripheral blood of patients with hepatitis B, and the immunomodulatory function of Teff cells activated by hepatitis virus antigens is significantly inhibited after IL‐35 treatment, with a concomitant decrease in the ability to secrete interferon‐γ.79 Conversely, a study showed that the level of IL‐35 was significantly decreased in patients with chronic HBV compared with healthy control individuals.80 These studies further suggest that IL‐35 plays an important immunomodulatory role in the occurrence and development of hepatitis.
IL‐35 and tumors
Inflammation is a well‐established factor in the regulation of cancer progression, and recent studies have suggested that IL‐35 might influence tumor‐related inflammation.81 In advanced gastric cancer, the frequency of IL‐35‐producing B cells is significantly increased, suggesting that these cells may participate in the progression of the disease.82 Elevated expression of IL‐35 can be detected in the peripheral blood of adult patients with acute myeloid leukemia, and the expression levels depend on the severity of the disease. In leukemia patients, IL‐35 can aggravate the disease condition by promoting the proliferation of Treg cells and by inhibiting CD4+ CD25− Teff cells.83 On the other hand, IL‐35 was not detected in the peripheral blood of patients with non‐small cell lung cancer, but its expression was significantly increased at the tumor site.84 Similarly, high expression of IL‐35 can be detected in tumor tissues of human nasopharyngeal carcinoma, melanoma, and malignant B‐cell lymphoma. The IL‐35 produced by these tumors can activate CD11b+ GR1+ bone‐marrow‐derived immunosuppressive cells in the tumor microenvironment to help the immune escape of tumor cells.85 Turnis et al. suggested that the presence of IL‐35 in the tumor microenvironment leads to reduced lymphocytic infiltration, decreases effector cell proliferation, increases tumor burden, and decreases survival of the immunocompetent host. Furthermore, Treg‐derived IL‐35 promotes the expression of multiple inhibitory receptors (PD1, TIM3, and LAG3), thereby facilitating intratumoral T‐cell exhaustion.86 Another study showed that IL‐35 promoted the growth of pancreatic cancer cells in vitro by inhibiting their apoptosis through increasing the expression of CDK2, CDK4, cyclin B, and cyclin D.87 Overexpression of IL‐35 in hepatoma cells (HepG2) enhanced apoptotic sensitivity and induced cell cycle arrest of hepatocellular carcinoma through the regulation of genes related to the cell cycle and apoptosis, including an increase in FAS expression and down‐regulation of the expression of cyclin D1, survivin, and Bcl‐2.88 Similar clinical test results indicate that the mean serum concentrations of IL‐35 were significantly higher in patients with prostate cancer than in the healthy control group. These findings indicate that IL‐35 is possibly involved in tumor progression.89 Recent results suggest that IL‐35 can promote tumor progression by functioning as an upstream cytokine to promote cancer‐associated inflammation and control neutrophil polarization.90 Research results suggest that IL‐35 can promote tumor progression by functioning as an upstream cytokine for promoting cancer‐associated inflammation and controlling neutrophil polarization.90 Studies also demonstrated that IL‐35 expression was elevated in both serum and tumors in patients with colorectal cancer. These elevated IL‐35 can suppress T‐cell proliferation and may participate in tumor immunotolerance.91 However, Zhang et al. demonstrated that IL‐35 levels are decreased in human colon cancer and that IL‐35 is capable of exerting anti‐tumor activity by suppressing β‐catenin expression. Interleukin‐35 inhibits cell migration, invasion, proliferation, and colony formation when it is highly expressed in colon cancer cells.92 A study has found that IL‐35 could inhibit angiogenesis and inflammation in rheumatoid arthritis by down‐regulating the expression of vascular endothelial growth factor‐induced ANG2 and disturbing ANG2/TIE2 signaling.93 Conversely, IL‐35 produced by tumor cells can promote tumor growth by promoting the recruitment of CD11b+ GR1+ myeloid‐derived suppressor cells and increase angiogenesis in the tumor microenvironment.85 In conclusion, IL‐35 has significant immunomodulatory effects in the process of tumor formation and development, and the molecular mechanism of IL‐35‐mediated immune regulation varies based on tumor type. It has been confirmed that IL‐35 can not only promote tumor proliferation and progression by a variety of mechanisms such as angiogenesis promotion, but also inhibit tumor cell apoptosis by regulating apoptosis genes (Fig. 2). Furthermore, IL‐35 can also promote tumor growth through a variety of mechanisms, such as by inhibiting Teff cells and promoting the proliferation of Treg cells and the accumulation of bone‐marrow‐derived immunosuppressive CD11b+ GR1+ cells (Fig. 2). Immunoregulation by IL‐35 is also achieved by collecting and modulating the secretion of multiple immune‐related inflammatory factors (Fig. 2).
Figure 2.
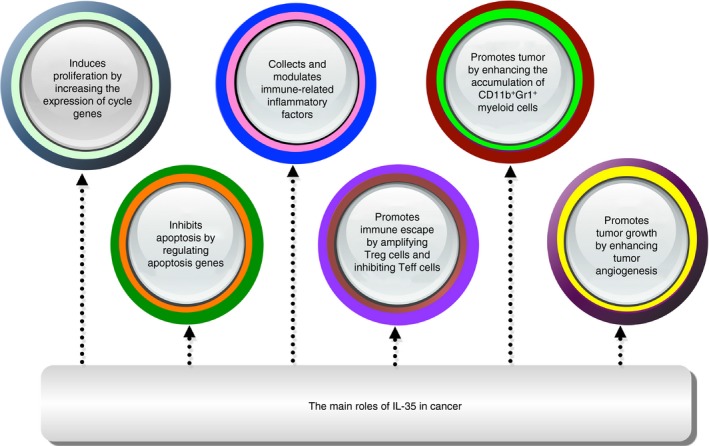
The main roles of interleukin‐35 (IL‐35) in tumors are shown.
Concluding remarks
In summary, IL‐35 shares numerous similarities in structure and composition with other members of the IL‐12 cytokine family. However, it differs considerably in function, to the extent of having effects opposite to those of the other members. In this review, we have discussed data that suggest that targeting IL‐35‐producing Treg cells has significant therapeutic potential. Furthermore, IL‐35 plays an important role in the immune system as an inhibitory cytokine and can modulate dysfunctional T cells in immune dysfunctions, activate bone‐marrow‐derived immunosuppressive cells, and regulate multiple immune‐related inflammatory factors. Therefore, the regulation of IL‐35 is of great significance in immune disorders. Advances in research on IL‐35, combined with improved techniques of studying receptors and signal‐transduction pathways, allow for consideration of IL‐35 as a novel therapeutic target for immune regulation. The possibility of incorporating IL‐35 in novel therapeutic strategies to treat deadly immune diseases promise a greater clinical significance of IL‐35 in the near future. According to the different immunomodulatory effects of IL‐35 in different diseases, IL‐35 can be considered as a treatment strategy for immune‐related diseases. For example, if the expression of IL‐35 is reduced in a certain disease, then treat with IL‐35 recombinant protein. If the expression of IL‐35 is significantly elevated in a certain disease, then IL‐35 can be blocked.
Author contributions
HBX and CPS conceptualized the review and finalized the manuscript preparation. YSZ, QPW, CLL, and HXD modified the grammar of this review. JFZ performed the literature search and drafted the manuscript.
Disclosures
The authors have no financial conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81801557), the Shandong Provincial Natural Science Foundation of China (no. ZR2017BH016), the Doctoral Startup Fund of Jining Medical University (no. JY2016QD030), the Supporting Fund for Teachers’ research of Jining Medical University (no. JYFC2018KJ066).
Contributor Information
Chuanping Si, Email: chpsi@163.com.
Huabao Xiong, Email: huabao.xiong@mssm.edu.
References
- 1. Gajiyev JN, Tagiyev EG, Gadjiyev NJ. Impact of roncoleukin on balance of cytokins in complex treatment of obturation jaundice of nontumoral genesis. Klin Khir 2016; 2:24–7. [PubMed] [Google Scholar]
- 2. Banchereau J, Pascual V, O'Garra A. From IL‐2 to IL‐37: the expanding spectrum of anti‐inflammatory cytokines. Nat Immunol 2012; 13:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017; 542:177–85. [DOI] [PubMed] [Google Scholar]
- 4. Oyesola OO, Fruh SP, Webb LM, Tait Wojno ED. Cytokines and beyond: regulation of innate immune responses during helminth infection. Cytokine 2018; 18:30359–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM et al The inhibitory cytokine IL‐35 contributes to regulatory T‐cell function. Nature 2007; 450:566–9. [DOI] [PubMed] [Google Scholar]
- 6. Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL‐23 and IL‐27: related but functionally distinct regulators of inflammation. Annu Rev Immunol 2007; 25:221–42. [DOI] [PubMed] [Google Scholar]
- 7. Ma X, Trinchieri G. Regulation of interleukin‐12 production in antigen‐presenting cells. Adv Immunol 2001; 79:55–92. [DOI] [PubMed] [Google Scholar]
- 8. Bardel E, Larousserie F, Charlot‐Rabiega P, Coulomb‐L'Hermine A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL‐35. J Immunol 2008; 181:6898–905. [DOI] [PubMed] [Google Scholar]
- 9. Chaturvedi V, Collison LW, Guy CS, Workman CJ, Vignali DA. Cutting edge: human regulatory T cells require IL‐35 to mediate suppression and infectious tolerance. J Immunol 2011; 186:6661–6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E et al IL‐35‐producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014; 507:366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV et al Interleukin‐35 induces regulatory B cells that suppress autoimmune disease. Nat Med 2014; 20:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dixon KO, van der Kooij SW, Vignali DA, van Kooten C. Human tolerogenic dendritic cells produce IL‐35 in the absence of other IL‐12 family members. Eur J Immunol 2015; 45:1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sha X, Meng S, Li X, Xi H, Maddaloni M, Pascual DW et al Interleukin‐35 inhibits endothelial cell activation by suppressing MAPK‐AP‐1 pathway. J Biol Chem 2015; 290:19307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devergne O, Birkenbach M, Kieff E. Epstein–Barr virus‐induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U S A 1997; 94:12041–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB et al IL‐35 is a novel cytokine with therapeutic effects against collagen‐induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 2007; 37:3021–9. [DOI] [PubMed] [Google Scholar]
- 16. Ringkowski S, Thomas PS, Herbert C. Interleukin‐12 family cytokines and sarcoidosis. Front Pharmacol 2014; 5:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gately MK, Desai BB, Wolitzky AG, Quinn PM, Dwyer CM, Podlaski FJ et al Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL‐12 (cytotoxic lymphocyte maturation factor). J Immunol 1991; 147:874–82. [PubMed] [Google Scholar]
- 18. Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B et al Novel p19 protein engages IL‐12p40 to form a cytokine, IL‐23, with biological activities similar as well as distinct from IL‐12. Immunity 2000; 13:715–25. [DOI] [PubMed] [Google Scholar]
- 19. Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J et al IL‐27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002; 16:779–90. [DOI] [PubMed] [Google Scholar]
- 20. Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X et al Epstein–Barr virus‐induced gene 3 negatively regulates IL‐17, IL‐22 and RORγt. Eur J Immunol 2008; 38:1204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frank GM, Divito SJ, Maker DM, Xu M, Hendricks RL. A novel p40‐independent function of IL‐12p35 is required for progression and maintenance of herpes stromal keratitis. Invest Ophthalmol Vis Sci 2010; 51:3591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Fang P, Yang WY, Wang H, Yang X. IL‐35, as a newly proposed homeostasis‐associated molecular pattern, plays three major functions including anti‐inflammatory initiator, effector, and blocker in cardiovascular diseases. Cytokine 2017; 17:30169–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL‐27 and IFN‐α signal via Stat1 and Stat3 and induce T‐Bet and IL‐12Rβ2 in naive T cells. J Interferon Cytokine Res 2003; 23:513–22. [DOI] [PubMed] [Google Scholar]
- 24. Collison LW, Vignali DA. Interleukin‐35: odd one out or part of the family? Immunol Rev 2008; 226:248–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol 2009; 21:612–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wei X, Zhang J, Gu Q, Huang M, Zhang W, Guo J et al Reciprocal expression of IL‐35 and IL‐10 defines two distinct effector Treg subsets that are required for maintenance of immune tolerance. Cell Rep 2017; 21:1853–69. [DOI] [PubMed] [Google Scholar]
- 27. Seyerl M, Kirchberger S, Majdic O, Seipelt J, Jindra C, Schrauf C et al Human rhinoviruses induce IL‐35‐producing Treg via induction of B7‐H1 (CD274) and sialoadhesin (CD169) on DC. Eur J Immunol 2010; 40:321–9. [DOI] [PubMed] [Google Scholar]
- 28. Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY et al A functional interleukin 12 receptor complex is composed of two β‐type cytokine receptor subunits. Proc Natl Acad Sci USA 1996; 93:14002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J et al A receptor for the heterodimeric cytokine IL‐23 is composed of IL‐12Rβ1 and a novel cytokine receptor subunit, IL‐23R. J Immunol 2002; 168:5699–708. [DOI] [PubMed] [Google Scholar]
- 30. Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF et al WSX‐1 and glycoprotein 130 constitute a signal‐transducing receptor for IL‐27. J Immunol 2004; 172:2225–31. [DOI] [PubMed] [Google Scholar]
- 31. Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D et al The composition and signaling of the IL‐35 receptor are unconventional. Nat Immunol 2012; 13:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mikus LD, Rosenthal LA, Sorkness RL, Lemanske RF Jr. Reduced interferon‐γ secretion by natural killer cells from rats susceptible to postviral chronic airway dysfunction. Am J Respir Cell Mol Biol 2001; 24:74–82. [DOI] [PubMed] [Google Scholar]
- 33. Gollob JA, Murphy EA, Mahajan S, Schnipper CP, Ritz J, Frank DA. Altered interleukin‐12 responsiveness in Th1 and Th2 cells is associated with the differential activation of STAT5 and STAT1. Blood 1998; 91:1341–54. [PubMed] [Google Scholar]
- 34. Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS et al STAT3 regulates cytokine‐mediated generation of inflammatory helper T cells. J Biol Chem 2007; 282:9358–63. [DOI] [PubMed] [Google Scholar]
- 35. Liu S, Han J, Gong H, Li Y, Bao X, Qi J et al Soluble interleukin‐27 receptor α is a valuable prognostic biomarker for acute graft‐versus‐host disease after allogeneic haematopoietic stem cell transplantation. Sci Rep 2018; 8:10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T et al The IL‐27 receptor chain WSX‐1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol 2005; 174:3534–44. [DOI] [PubMed] [Google Scholar]
- 37. Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T et al Two‐sided roles of IL‐27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL‐23‐induced IL‐17 on activated CD4+ T cells partially through STAT3‐dependent mechanism. J Immunol 2006; 177:5377–85. [DOI] [PubMed] [Google Scholar]
- 38. Floss DM, Schonberg M, Franke M, Horstmeier FC, Engelowski E, Schneider A et al IL‐6/IL‐12 cytokine receptor shuffling of extra‐ and intracellular domains reveals canonical STAT activation via synthetic IL‐35 and IL‐39 signaling. Sci Rep 2017; 7:15172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu S, Li Y, Yao L, Li Y, Jiang S, Gu W et al Interleukin‐35 inhibits angiogenesis through STAT1 signalling in rheumatoid synoviocytes. Clin Exp Rheumatol 2017; 36:223–7. [PubMed] [Google Scholar]
- 40. Liu H, Zhang W, Tian FF, Kun A, Zhou WB, Xiao B et al IL‐35 is involved in the pathogenesis of guillain‐barre syndrome through its influence on the function of CD4+ T cells. Immunol Invest 2015; 44:566–77. [DOI] [PubMed] [Google Scholar]
- 41. Garbers C, Aparicio‐Siegmund S, Rose‐John S. The IL‐6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol 2015; 34:75–82. [DOI] [PubMed] [Google Scholar]
- 42. Olson BM, Sullivan JA, Burlingham WJ. Interleukin 35: a key mediator of suppression and the propagation of infectious tolerance. Front Immunol 2013; 4:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wirtz S, Billmeier U, Mchedlidze T, Blumberg RS, Neurath MF. Interleukin‐35 mediates mucosal immune responses that protect against T‐cell‐dependent colitis. Gastroenterology 2011; 141:1875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. IL‐35 stimulation of CD39+ regulatory T cells confers protection against collagen II‐induced arthritis via the production of IL‐10. J Immunol 2010; 184:7144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Espes D, Singh K, Sandler S, Carlsson PO. Increased interleukin‐35 levels in patients with type 1 diabetes with remaining C‐peptide. Diabetes Care 2017; 40:1090–5. [DOI] [PubMed] [Google Scholar]
- 46. Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL‐35‐ and IL‐10‐dependent manner. J Immunol 2009; 182:6121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li T, Gu M, Liu P, Liu Y, Guo J, Zhang W et al Clinical significance of decreased interleukin‐35 expression in patients with psoriasis. Microbiol Immunol 2018; 62:454–61. [DOI] [PubMed] [Google Scholar]
- 48. Zhang J, Lin Y, Li C, Zhang X, Cheng L, Dai L et al IL‐35 decelerates the inflammatory process by regulating inflammatory cytokine secretion and M1/M2 macrophage ratio in psoriasis. J Immunol 2016; 197:2131–44. [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Mao Y, Zhang J, Shi G, Cheng L, Lin Y et al IL‐35 recombinant protein reverses inflammatory bowel disease and psoriasis through regulation of inflammatory cytokines and immune cells. J Cell Mol Med 2018; 22:1014–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barnett MP, Dommels YE, Butts CA, Zhu S, McNabb WC, Roy NC. Inoculation with enterococci does not affect colon inflammation in the multi‐drug resistance 1a‐deficient mouse model of IBD. BMC Gastroenterol 2016; 16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J et al IL‐35‐mediated induction of a potent regulatory T cell population. Nat Immunol 2010; 11:1093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McLean MH, Neurath MF, Durum SK. Targeting interleukins for the treatment of inflammatory bowel disease‐what lies beyond anti‐TNF therapy? Inflamm Bowel Dis 2014; 20:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yan Y, Zhao N, He X, Guo H, Zhang Z, Liu T. Mesenchymal stem cell expression of interleukin‐35 protects against ulcerative colitis by suppressing mucosal immune responses. Cytotherapy 2018; 20:911–18. [DOI] [PubMed] [Google Scholar]
- 54. Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature 1980; 283:666–8. [DOI] [PubMed] [Google Scholar]
- 55. Kochetkova I, Thornburg T, Callis G, Pascual DW. Segregated regulatory CD39+CD4+ T cell function: TGF‐β‐producing Foxp3‐ and IL‐10‐producing Foxp3+ cells are interdependent for protection against collagen‐induced arthritis. J Immunol 2011; 187:4654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kochetkova I, Trunkle T, Callis G, Pascual DW. Vaccination without autoantigen protects against collagen II‐induced arthritis via immune deviation and regulatory T cells. J Immunol 2008; 181:2741–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kochetkova I, Thornburg T, Callis G, Holderness K, Maddaloni M, Pascual DW. Oral Escherichia coli colonization factor antigen I fimbriae ameliorate arthritis via IL‐35, not IL‐27. J Immunol 2014; 192:804–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol 2003; 3:405–12. [DOI] [PubMed] [Google Scholar]
- 59. Robinson DS. The role of regulatory T lymphocytes in asthma pathogenesis. Curr Allergy Asthma Rep 2005; 5:136–41. [DOI] [PubMed] [Google Scholar]
- 60. Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R et al Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen‐specific T regulatory 1 and T helper 2 cells. J Exp Med 2004; 199:1567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hansen JJ. Immune responses to intestinal microbes in inflammatory bowel diseases. Curr Allergy Asthma Rep 2015; 15:61. [DOI] [PubMed] [Google Scholar]
- 62. Whitehead GS, Wilson RH, Nakano K, Burch LH, Nakano H, Cook DN. IL‐35 production by inducible costimulator (ICOS)‐positive regulatory T cells reverses established IL‐17‐dependent allergic airways disease. J Allergy Clin Immunol 2012; 129:207–15.e1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dong J, Wong CK, Cai Z, Jiao D, Chu M, Lam CW. Amelioration of allergic airway inflammation in mice by regulatory IL‐35 through dampening inflammatory dendritic cells. Allergy 2015; 70:921–32. [DOI] [PubMed] [Google Scholar]
- 64. Huang CH, Loo EX, Kuo IC, Soh GH, Goh DL, Lee BW et al Airway inflammation and IgE production induced by dust mite allergen‐specific memory/effector Th2 cell line can be effectively attenuated by IL‐35. J Immunol 2011; 187:462–71. [DOI] [PubMed] [Google Scholar]
- 65. Giovannini‐Chami L, Blanc S, Hadchouel A, Baruchel A, Boukari R, Dubus JC et al Eosinophilic pneumonias in children: a review of the epidemiology, diagnosis, and treatment. Pediatr Pulmonol 2016; 51:203–16. [DOI] [PubMed] [Google Scholar]
- 66. George L, Brightling CE. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther Adv Chronic Dis 2016; 7:34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kanai K, Park AM, Yoshida H, Tsunoda I, Yoshie O. IL‐35 suppresses lipopolysaccharide‐induced airway eosinophilia in EBI3‐deficient mice. J Immunol 2017; 198:119–27. [DOI] [PubMed] [Google Scholar]
- 68. Himani G, Badini A, Nanji K. Depression and its associated factors among patients with chronic obstructive pulmonary disease in Karachi, Pakistan. Cureus 2018; 10:e2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jiang S, Shan F, Zhang Y, Jiang L, Cheng Z. Increased serum IL‐17 and decreased serum IL‐10 and IL‐35 levels correlate with the progression of COPD. Int J Chron Obstruct Pulmon Dis 2018; 13:2483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001; 358:221–9. [DOI] [PubMed] [Google Scholar]
- 71. Luo X, Herold KC, Miller SD. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity 2010; 32:488–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. Prevention of autoimmune diabetes by ectopic pancreatic β‐cell expression of interleukin‐35. Diabetes 2012; 61:1519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Manzoor F, Johnson MC, Li C, Samulski RJ, Wang B, Tisch R. β‐cell‐specific IL‐35 therapy suppresses ongoing autoimmune diabetes in NOD mice. Eur J Immunol 2017; 47:144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Singh K, Kadesjo E, Lindroos J, Hjort M, Lundberg M, Espes D et al Interleukin‐35 administration counteracts established murine type 1 diabetes – possible involvement of regulatory T cells. Sci Rep 2015; 5:12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009; 373:582–92. [DOI] [PubMed] [Google Scholar]
- 76. Yarygin KN, Lupatov AY, Kholodenko IV. Cell‐based therapies of liver diseases: age‐related challenges. Clin Interv Aging 2015; 10:1909–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yoshio S, Kanto T. Immunopathogenesis of hepatitis B virus infection. Nihon Rinsho 2015; 73(Suppl 9):361–5. [PubMed] [Google Scholar]
- 78. Liu F, Tong F, He Y, Liu H. Detectable expression of IL‐35 in CD4+ T cells from peripheral blood of chronic hepatitis B patients. Clin Immunol 2011; 139:1–5. [DOI] [PubMed] [Google Scholar]
- 79. Li X, Tian L, Dong Y, Zhu Q, Wang Y, Han W et al IL‐35 inhibits HBV antigen‐specific IFN‐γ‐producing CTLs in vitro . Clin Sci (Lond) 2015; 129:395–404. [DOI] [PubMed] [Google Scholar]
- 80. Cheng ST, Yuan D, Liu Y, Huang Y, Chen X, Yu HB et al Interleukin‐35 level is elevated in patients with chronic hepatitis B virus infection. Int J Med Sci 2018; 15:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74. [DOI] [PubMed] [Google Scholar]
- 82. Wang K, Liu J, Li J. IL‐35‐producing B cells in gastric cancer patients. Medicine (Baltimore) 2018; 97:e0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tao Q, Pan Y, Wang Y, Wang H, Xiong S, Li Q et al Regulatory T cells‐derived IL‐35 promotes the growth of adult acute myeloid leukemia blasts. Int J Cancer 2015; 137:2384–93. [DOI] [PubMed] [Google Scholar]
- 84. Wang HM, Zhang XH, Feng MM, Qiao YJ, Ye LQ, Chen J et al Interleukin‐35 suppresses the antitumor activity of T cells in patients with non‐small cell lung cancer. Cell Physiol Biochem 2018; 47:2407–19. [DOI] [PubMed] [Google Scholar]
- 85. Wang Z, Liu JQ, Liu Z, Shen R, Zhang G, Xu J et al Tumor‐derived IL‐35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol 2013; 190:2415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Turnis ME, Sawant DV, Szymczak‐Workman AL, Andrews LP, Delgoffe GM, Yano H et al Interleukin‐35 limits anti‐tumor immunity. Immunity 2016; 44:316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nicholl MB, Ledgewood CL, Chen X, Bai Q, Qin C, Cook KM et al IL‐35 promotes pancreas cancer growth through enhancement of proliferation and inhibition of apoptosis: evidence for a role as an autocrine growth factor. Cytokine 2014; 70:126–33. [DOI] [PubMed] [Google Scholar]
- 88. Long J, Zhang X, Wen M, Kong Q, Lv Z, An Y et al IL‐35 over‐expression increases apoptosis sensitivity and suppresses cell growth in human cancer cells. Biochem Biophys Res Commun 2013; 430:364–9. [DOI] [PubMed] [Google Scholar]
- 89. Chatrabnous N, Ghaderi A, Ariafar A, Razeghinia MS, Nemati M, Jafarzadeh A. Serum concentration of interleukin‐35 and its association with tumor stages and FOXP3 gene polymorphism in patients with prostate cancer. Cytokine 2018; 13:221–27. [DOI] [PubMed] [Google Scholar]
- 90. Zou JM, Qin J, Li YC, Wang Y, Li D, Shu Y et al IL‐35 induces N2 phenotype of neutrophils to promote tumor growth. Oncotarget 2017; 8:33501–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ma Y, Chen L, Xie G, Zhou Y, Yue C, Yuan X et al Elevated level of interleukin‐35 in colorectal cancer induces conversion of T cells into iTr35 by activating STAT1/STAT3. Oncotarget 2016; 7:73003–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhang J, Mao T, Wang S, Wang D, Niu Z, Sun Z et al Interleukin‐35 expression is associated with colon cancer progression. Oncotarget 2017; 8:71563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jiang S, Li Y, Lin T, Yuan L, Li Y, Wu S et al IL‐35 inhibits angiogenesis through VEGF/Ang2/Tie2 pathway in rheumatoid arthritis. Cell Physiol Biochem 2016; 40:1105–16. [DOI] [PubMed] [Google Scholar]


