Abstract
Handedness and language lateralization are the most investigated phenotypes among functional hemispheric asymmetries, i.e. differences in function between the left and the right half of the human brain. Both phenotypes are left hemisphere-dominant, while investigations of the molecular factors underlying right hemisphere-dominant phenotypes are less prominent. In the classical line bisection task, healthy subjects typically show a leftward attentional bias due to a relative dominance of the right hemisphere for visuospatial attention. Based on findings of variations in dopamine-related genes affecting performance in the line bisection task, we first tested whether DNA methylation in non-neuronal tissue in the promoter regions of DBH, SLC6A3, and DRD2 are associated with line bisection deviation. We replicated the typical behavioral pattern and found an effect of DNA methylation in the DBH promoter region on line bisection deviation in right-aligned trials. A second exploratory analysis indicated that an overall DNA methylation profile of genes involved in dopamine function predicts line bisection performance in right-aligned trials. Genetic variation in dopamine-related genes has been linked to attention deficit hyperactivity disorder (ADHD), a neurodevelopmental trait associated with rightward attentional bias. Overall, our findings point towards epigenetic markers for functional hemispheric asymmetries in non-neuronal tissue not only for left hemisphere-dominant, but also for right hemisphere-dominant phenotypes.
Introduction
Like most of our inner organs, human brain and behavior are asymmetrically organized1. However, in contrast to visceral asymmetry, hemispheric specialization for different cognitive and motor functions shows more interindividual variability, reflecting the immense complexity of brain asymmetries2. The ontogenesis of structural3 and functional hemispheric asymmetries such as handedness4,5 and language lateralization6,7 is partly influenced by genetic variation. However, in line with findings on environmental factors also playing a role, recent research has also suggested epigenetic regulation to contribute to the development of functional hemispheric asymmetries8–10. Epigenetic mechanisms summarize several chemical modifications to the DNA itself or proteins involved in DNA packaging that regulate the accessibility of transcription factors to the DNA, thus enhancing or repressing gene expression11. Empirical research on the epigenetics of hemispheric asymmetries so far mainly focused on left hemisphere-dominant phenotypes. For handedness, two studies have identified epigenetic markers in non-neuronal tissue10,12. For language lateralization, our recent work has revealed an effect of DNA methylation in the KIAA0319 promoter region on attentional modulation of language lateralization, but not on language lateralization per se13. However, in order to come closer to a full understanding of hemispheric asymmetries, right hemisphere-dominant phenotypes also need to be considered.
One of the most investigated right hemisphere-dominant processes is visuospatial attention14. In the classical line bisection task (see Fig. 1), subjects are instructed to determine the center of a horizontal line15,16. While patients with right-hemispheric lesions tend to show a rightward bias due to neglect of the left hemifield17, healthy subjects typically show a leftward bias, a phenomenon that has been called pseudoneglect18. As the right hemisphere is typically dominant for visuospatial attention, the left hemifield is thought to be overrepresented, leading to a leftward bias19. In a meta-analysis of 73 studies in healthy subjects, a significant leftward bisection error was confirmed. The analysis also revealed that left-hand use results in stronger pseudoneglect than right-hand use (independent of handedness). Moreover, pseudoneglect is more pronounced in right-handers compared to left-handers (independent of hand use) and in men compared to women20.
Figure 1.

Illustration of the line bisection task.
Performance in the line bisection task is linked to brain structure and function. In line with lesion studies, fMRI studies in healthy subjects revealed activation in right-hemispheric posterior parietal areas during visuospatial attention tasks14,21. Correspondingly, rTMS over the right posterior parietal cortex induced a rightward shift in healthy subjects22. Using electrical stimulation during brain surgery, Thiebaut de Schotten et al.23 could show that the bisection error in the line bisection task depends on stimulation of the right-hemispheric inferior parietal lobule or the right-hemispheric caudal superior temporal gyrus. Due to an involvement of the superior occipitofrontal fasciculus, it was concluded that not only the parietal cortex, but also communication between the parietal and frontal cortex is crucial for visuospatial attention and neglect23. This conclusion was confirmed in a subsequent DTI study24.
Brain function likely acts as an intermediate phenotype between molecular determinants of functional hemispheric asymmetries and the observable phenotype8. Among genetic variations affecting attentional bias, several candidate genes affecting dopaminergic pathways have been investigated. This selection of candidate genes is based on a direct role of dopamine and noradrenaline in lateralized visuospatial attention that has been concluded from research in animals and humans. For example, unilateral injections of 6-hydroxydopamine induce lesions of the dopaminergic pathway, thereby causing spatial neglect in monkeys25 and rats26. In humans, dopamine agonists have shown beneficial effects on hemispatial neglect after right-hemispheric stroke27. Recently, it has been shown that other motivational factors such as monetary reward, which are also mediated by dopaminergic pathways, are also effective in reducing neglect after right-hemispheric stroke. Dopaminergic stimulation, however, is only effective in the absence of other motivational factors28. In healthy subjects, asymmetric binding of dopamine D2 receptors in striatal and cortical areas predicts visuospatial attention bias in the grayscales task29. Suppression or reversal of pseudoneglect has also been reported in normal aging and associated with age-related loss of dopamine30.
The dopamine beta-hydroxylase gene (DBH) encodes for the protein converting dopamine to norepinephrine31. In healthy adults, homozygous T allele carriers for the C-1021T polymorphism (linked to increased dopamine availability) have displayed an enhanced visuospatial attention bias towards the right side32. Similarly, homozygous carriers of the 9-repeat allele located in the 3′ untranslated region (3′UTR) of the dopamine transporter gene (SLC6A3; DAT1), also leading to increased availability of dopamine, resulted in greater rightward spatial bias32. In contrast, the 10-repeat allele has been linked to rightward bias in children affected by ADHD33,34. Moreover, homozygous carriers of the dopamine receptor D2 (DRD2) gene A2 allele showed significantly less rightward bias in a visuospatial attention task35
Overall, these findings suggest a genetic component in the ontogenesis of visuospatial attention bias. However, pseudoneglect has been shown to be modulated by different environmental factors. For example, reduced pseudoneglect has been reported in experienced videogame players36 or in urbanized compared to remote people37. Our first aim was to investigate potential peripheral epigenetic markers in the promoter regions of DBH, SLC6A3, and DRD2 on the line bisection task in healthy adults. As genetic variation in these genes has been directly associated with this task, we chose a hypothesis-driven approach by only selecting these genes for analysis. We hypothesized that DNA methylation in the corresponding promoter regions predicts deviation from the midline in the line bisection task. In a second exploratory analysis, we assessed all genes involved in dopamine function to evaluate if a general DNA methylation profile predicts line bisection performance. As DNA methylation is tissue-specific, findings are interpreted as epigenetic signatures of visuospatial attention bias in non-neuronal tissue38,39.
Results
For the line bisection task, the repeated measures ANOVA revealed a significant main effect of condition (F(2,94) = 34.18, p = 0.000, partial η2 = 0.42) and a significant main effect of hand (F(1,47) = 6.04, p = 0.018, partial η2 = 0.11). No interaction with handedness or sex reached statistical significance. Bonferroni-corrected post-hoc tests revealed that subjects showed less pseudoneglect (a rightward bias) when lines were right-aligned, in contrast to the left-aligned (+3.16%, 95%-CI[1.95, 4.37], p = 0.000) and central trials (+2.68%, 95%-CI[1.79, 3.57], p = 0.000), which were not significantly different from each other (−0.48%, 95%-CI[−1.42, 0.46], p = 0.636). The main effect of hand was due to less pseudoneglect (a rightward bias) in right-handed compared to left-handed trials (+0.99%, 95%-CI[0.18, 1.80], p = 0.018). We performed one-sample t-tests against zero for each condition with Bonferroni correction for 6 comparisons (α = 0.011) to test for pseudoneglect. In line with previous studies40, we found significant pseudoneglect in left-aligned and central trials when using the left hand (left-aligned: t(50) = −4.10, p = 0.000, central: t(50) = −3.25, p = 0.002). However, t-tests were only nominally significant for left-aligned (t(50) = −2.07, p = 0.043) and non-significant for central trials performed with the right hand (t(50) = −0.98, p = 0.331). For right-aligned trials, there was significant rightward bias in right-handed trials (t(50) = 4.17, p = 0.000), but only nominally significant in left-handed trials (t(50) = 2.43, p = 0.019) (see Fig. 2). In order to test whether there was a significant difference between left-and right-handed trials in each condition, paired t-tests were performed with Bonferroni correction for 3 comparisons (α = 0.017). For left-aligned trials, pseudoneglect did not significantly differ between left- and right-handed trials (t(50) = −1.34, p = 0.186). For the other two conditions, there was a nominally significant difference with left-hand trials leading to more leftward bias than right-hand trials (central: t(50) = −2.10, p = 0.041; right-aligned: t(50) = −2.14, p = 0.037) (see Fig. 2).
Figure 2.

Bisection error in percent for left-aligned, central, and right-aligned trials performed with either the left hand (LH) or the right hand (RH). *p < 0.011, (*) nominal significance. Error bars indicate standard errors.
In the hypothesis-driven analysis, DNA methylation in the DRD2 and SLC6A3 promoter regions did not significantly predict the percentage of line bisection deviation in any of the six conditions (all p > 0.0028). For DBH, the regression reached significance for the right-aligned trials performed with the left hand (F(1,49) = 14.88, p = 0.000, R2 = 0.22). The beta weight for one individual predictor reached significance (cg11619181: β = 0.48, t = 3.86, p = 0.000) (see Fig. 3). The regression was non-significant for the other conditions (all p > 0.0028).
Figure 3.
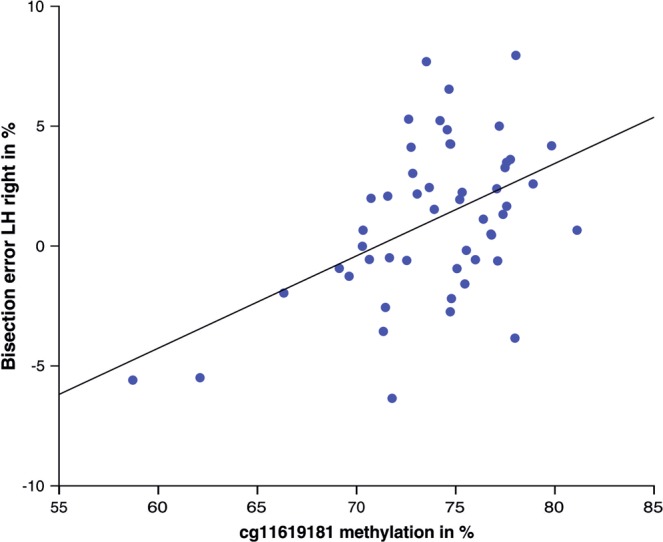
Scatterplot of DNA methylation of DBH cg11619181 in % and corresponding bisection error in the LH right condition in %.
In the exploratory analysis of DNA methylation, the regression did not reach significance for left-aligned and central trials performed with the left or right hand (all p > 0.0083). For right-aligned trials performed with the left hand, the regression model reached nominal significance (F(1,49) = 6.57, p = 0.014, R2 = 0.12). The individual beta weight reached nominal significance for PC1 (β = −0.34, t = −2.56, p = 0.014). For right-aligned trials performed with the right hand, the regression model reached significance (F(2,48) = 6.41, p = 0.003, R2 = 0.21). Individual beta weights reached nominal significance for sex (β = 0.33, t = 2.60, p = 0.012) and PC1 (β = −0.30, t = −2.30, p = 0.026).
Discussion
The first aim of the present study was to investigate the effect of DNA methylation in the promoter regions of three dopamine candidate genes on attentional bias in the line bisection task. In previous studies conducted on buccal cells, we have shown an association of DNA methylation in promoter regions of respective candidate genes with handedness12 and attentional modulation of language lateralization13. Here, we aimed to test whether such an association could also be shown for a right hemisphere-dominant phenotype such as attentional bias. The second aim was to evaluate if an overall DNA methylation pattern in dopamine-related genes can predict deviation from the midline in the line bisection task.
The behavioral data are very much in line with what has been reported in studies with much larger sample sizes20,40, with a leftward bias in left-aligned and central trials, but a rightward bias in right-aligned trials. By trend, leftward bias was larger in trials performed with the left hand compared to the right. In the hypothesis-driven analysis, DNA methylation in the DBH promoter region significantly predicted line bisection deviation in right-aligned trials performed with the left hand. This effect was based on a single CpG site and independent from sex or handedness.
The DBH gene encodes for a protein converting dopamine to norepinephrine, thus strongly affecting dopamine availability. It has been linked to emotion processing, addiction41 and several neuropsychiatric disorders such as bipolar disorder42 and Parkinson’s disease43. Moreover, DBH might modulate psychotic symptoms44 and cognitive functioning in patients affected by schizophrenia45. In healthy subjects, the DBH genotype has been linked to cognition, especially attention46 and attentional bias for facial expression47. This is in line with genotype-associated corticostriatal-limbic activity revealed by fMRI41. To the best of our knowledge, only one study has been published on behavioral epigenetics of DBH. In an epigenome-wide DNA methylation study comparing alcohol dependent and control subjects, hypomethylation of CpG sites within DBH was associated alcohol dependence48. Alcoholism has also been linked to the development of hemispheric asymmetries as alcohol dependent patients seem to be more often non-right-handed than controls49,50. Moreover, alcoholism was associated to impairment of right hemisphere function in cued detection51 and visuospatial tasks52,53. In contrast, acute alcohol consumption leads to a more pronounced leftward bias in the line bisection task54. In search of potential environmental factors influencing DNA methylation of the DBH promoter, the only study in rats hints towards altered transcription levels of DBH through stress induced by sleep deprivation55.
In the exploratory analysis, DNA methylation in promoter regions of dopamine-related genes was associated with performance in the line bisection task in right-aligned trials performed with the right hand and by trend with the left hand. This analysis is in line with dopamine-related genes playing a role in visuospatial attentional bias, not only at the level of genetic variation. The balance of dopamine and noradrenaline plays an important role in the etiology of attention deficit hyperactivity disorder (ADHD), making DBH56 and other dopamine-relevant genes57 important influencing factors in the development of ADHD. In rats, 6-hydroxydopamine injection not only disrupts the dopaminergic pathway and induces neglect26, but also ADHD-like behavior58. Interestingly, several studies have revealed a rightward bias in the line bisection task in ADHD patients59,60 and healthy subjects with ADHD-like behavior61. This is in line with the idea that ADHD is associated with right hemisphere inefficiency and dysfunction62 that is also reflected in atypical asymmetry in the frontostriatal network63,64. There are several prenatal risk factors to the development of ADHD that might be mediated by epigenetic regulation65. Using cord blood at birth, an epigenome-wide association study on ADHD symptoms recently revealed 13 CpG sites showing differential DNA methylation between different trajectories of ADHD symptoms66. Peripheral epigenetic markers of ADHD have also been reported in an epigenome-wide association study on salivary DNA67.
In laterality research, the most investigated phenotypes are handedness and language lateralization, which are both left hemisphere-dominant in the majority of individuals68. The present study suggests peripheral epigenetic markers of visuospatial attentional bias, which is usually right hemisphere-dominant69, leading to a complementary specialization for language and visuospatial attention in the majority of individuals. Moreover, subjects showing right hemisphere dominance for language have left hemisphere dominance for visuospatial attention, suggesting a causal relationship with hemispheric specialization of one function determining the other70. This finding mostly contradicts early single gene theories71 which assumed that lateralization of different functions is independent from each other70. However, molecular factors associated with both left and right hemisphere-dominant functions remain to be uncovered.
In conclusion, our data suggest that DNA methylation in the promoter region of the DBH gene and other dopamine-related genes is associated with attentional bias in the line bisection task, a right hemisphere-dominant phenotype. The obvious limitation of this study is that DNA was extracted from buccal cells instead of brain tissue. However, it has been suggested that interindividual variation of cerebral DNA methylation is reflected in peripheral tissue72. Thus, the findings from the present study should be interpreted cautiously as peripheral epigenetic markers of visuospatial attention bias. Midline deviation in the line bisection task is likely influenced by multiple genetic, epigenetic, and environmental factors that are not uncovered in the present study and will be determined in future large-scale studies. Future research should also integrate epigenetic markers on laterality phenotypes with brain structure and function. Moreover, replication in samples selected for ADHD or other traitsassociated with a rightward bias of attention such as obsessive compulsive disorders (OCD)73 or personality disorders74 might be worthwhile.
Methods
Subjects
Fifty-one (25 female) healthy German university students between 19 and 33 years of age (mean 24.41 years, SD 3.01 years) participated in the study. Handedness was determined using the Edinburgh handedness inventory (EHI)75. Since sex and handedness might have an effect on line bisection performance20, we balanced men and women as well as left- and right-handers in our sample. Among all subjects, 24 (11 females) were consistently left-handed as indicated by an EHI LQ < −60 and 27 (14 females) were consistently right-handed as indicated by an EHI LQ > 60. All subjects had normal or corrected to normal vision and had no history of neurological or psychiatric disease. All subjects gave written informed consent and were treated in accordance with the declaration of Helsinki. The study was approved by the ethics committee of the Psychological Faculty at Ruhr University Bochum.
Behavioral assessment
The line bisection task was used to determine laterality of visuospatial attention similar to previous studies40,76. Seventeen horizontal lines of 10.0 to 26.0 cm in length and 1 mm in width were presented on a white sheet of paper with 21.0 cm × 29.7 cm in size that was centered on the table in front of the subject. Five horizontal lines were positioned left-aligned, seven were positioned centrally and five were positioned right-aligned (see Fig. 1). Subjects were asked to bisect each line at the midpoint with a fine pencil. The task was completed with the left and the right hand each with the order balanced across subjects. Overall, this procedure resulted in six conditions depending on the position of the line (left, central, right) and the hand used to complete the task (left, right).
DNA methylation
DNA was isolated from buccal cells that were brushed from the subjects’ oral mucosa using buccal swabs. DNA isolation was conducted with the blackPREP Swab DNA Kit (Analytik Jena, Germany). The isolated gDNA was stored at −20 °C. The EpiTect Kit (Qiagen, Germany) was used for bisulfite conversion of 500 ng gDNA. After elution of bisulfite-converted DNA in 10 µl elution buffer, 4 µl were used for analysis on the MethylationEPIC array (Illumina, CA, USA).
Bioinformatics
Preprocessing and processing of the data was performed using RStudio77 version 0.99.903 and the Bioconductor packages implemented in the RnBeads workflow78. Overall, 867,926 probes were imported from signal intensity data (idat). During quality control, we excluded potential sample mix-ups or duplications, unspecific probe hybridization and problems with bisulfite conversion. Probes were removed in cases of high detection p values (>0.01) or overlap with known SNPs. Moreover, probes showing unreliable measurements (using the Greedycut algorithm), non-CpG (CpH) probes and gonosomal probes were removed from the dataset. The methylation ß values of the remaining probes were normalized using the β-mixture quantile (BMIQ) method79 and annotated using the reference genome GRCh37 (hg19). Promoter regions were defined as 1500 bp upstream and 500 bp downstream of transcription start sites.
Table 1 shows the location of promoter regions and number of included CpG sites for the candidate genes DBH (chr9: 136501482-136524466), SLC6A3 (chr5: 1392909-1445545), and DRD2 (chr11: 113284586-113346413). No effect of genetic imprinting was found in the current literature for DRD2 or DBH. However, there is evidence for paternal expression of SLC6A380,81.
Table 1.
Promoter regions of examined genes with chromosomal locations and number of tested CpG sites.
| Gene | Chromosome | Start of promoter region | End of promoter region | Number of CpG sites tested within the promoter region |
|---|---|---|---|---|
| DBH | 9 | 136499982 | 136501981 | 8 |
| SLC6A3 | 5 | 1445046 | 1447045 | 9 |
| DRD2 | 11 | 113345914 | 113347913 | 10 |
In order to analyze a possible association between line bisection performance and DNA methylation in promoter regions of dopamine-related genes, we performed a search for all genes involved in dopamine function using the Gene Ontology website using the term “dopamine” (http://geneontology.org/). After filtering for homo sapiens, this tool revealed 186 dopamine-related genes, 171 of which were also available on the MethylationEPIC array after preprocessing. The genes as well as chromosomal positions of the corresponding promoter regions are found in Supplementary Table S1.
Statistical analysis
For the line bisection task, the percentage of deviation from the midline of each horizontal line was determined using the formula [(measured left half − true half) / true half] × 100, which resulted in negative values indicating a leftward bias and positive values indicating a rightward bias. A mean score was calculated for each condition and each hand used. The effect of condition and hand was calculated using a 3 × 2 repeated measures ANOVA with the within-subject factor condition (left-aligned, centered, right-aligned) and hand (left, right) and the between-subject factors sex and handedness. For the ANOVA, partial η2 is reported as a measure of effect size. Bonferroni-adjusted post hoc tests were performed for significant main and interaction effects.
Hypothesis-driven analysis of DNA methylation
The hypothesis-driven analysis was aimed at evaluating if DNA methylation in promoter regions of candidate genes are associated with line bisection performance at single CpG site level. For each of the 6 conditions and each of the 3 genes, we conducted a linear step-wise regression analysis with individual % DNA methylation levels of all CpG sites within the respective promoter region, sex and handedness as predictors and the percentage of line bisection deviation as dependent variable. To correct for multiple comparisons, the alpha level was set to 0.0028 after Bonferroni correction (0.05 / 3 genes / 6 conditions = 0.0028). R2 is reported as a measure of effect size.
Exploratory analysis of DNA methylation
The exploratory analysis was aimed at evaluating if an overall DNA methylation profile predicts line bisection performance at the level of promoter regions. As DNA methylation levels are intercorrelated and to reduce the number of predictors, principal component analysis (PCA) was performed on mean % DNA methylation levels of the promoter regions of the 171 dopamine-related genes10. PCA revealed 28 principal components (PCs) with an eigenvalue >1. The first four PCs (PC1, PC2, PC3 and PC4) were considered for further analysis as determined per screeplot. Factor loadings are shown in Supplementary Table S2. DRD4 showed no factor loading >0.2 with any of the PCs and was excluded from analysis. PCA was repeated without DRD4. PC1, PC2, PC3, PC4, sex and handedness were used as predictors in a linear step-wise regression analysis using the percentage of line bisection deviation as dependent variable. This analysis was performed for each of the 6 conditions. To correct for multiple comparisons, the alpha level was set to 0.0083 after Bonferroni correction (0.05 / 6 conditions = 0.0083). R2 is reported as a measure of effect size.
ANOVAs, PCA and linear regression analyses were calculated using IBM SPSS Statistics 20 (IBM, United States).
Supplementary information
Acknowledgements
This work was supported by the Mercator Research Center Ruhr (Project number GZ: An-2015-0061). We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum.
Author Contributions
J.S. collected and analyzed the data and wrote the manuscript, R.K. and D.M. analyzed the data, O.G. and S.O. conceptualized the study. All authors discussed and reviewed the manuscript.
Data Availability
The dataset generated during the current study is available from the corresponding author on request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-42553-8.
References
- 1.Ocklenburg, S. & Güntürkün, O. The lateralized brain: The neuroscience and evolution of hemispheric asymmetries (Academic Press, London, 2018). [DOI] [PubMed]
- 2.Corballis MC, Häberling IS. The Many Sides of Hemispheric Asymmetry: A Selective Review and Outlook. J Int Neuropsychol Soc. 2017;23:710–718. doi: 10.1017/S1355617717000376. [DOI] [PubMed] [Google Scholar]
- 3.Guadalupe T, et al. Asymmetry within and around the human planum temporale is sexually dimorphic and influenced by genes involved in steroid hormone receptor activity. Cortex. 2015;62:41–55. doi: 10.1016/j.cortex.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Ocklenburg S, Beste C, Güntürkün O. Handedness: A neurogenetic shift of perspective. Neurosci Biobehav Rev. 2013;37:2788–2793. doi: 10.1016/j.neubiorev.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Brandler WM, Paracchini S. The genetic relationship between handedness and neurodevelopmental disorders. Trends Mol Med. 2014;20:83–90. doi: 10.1016/j.molmed.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ocklenburg S, Beste C, Arning L, Peterburs J, Güntürkün O. The ontogenesis of language lateralization and its relation to handedness. Neurosci Biobehav Rev. 2014;43:191–198. doi: 10.1016/j.neubiorev.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Somers M, et al. Linkage analysis in a Dutch population isolate shows no major gene for left-handedness or atypical language lateralization. J Neurosci. 2015;35:8730–8736. doi: 10.1523/JNEUROSCI.3287-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz J, Metz GAS, Güntürkün O, Ocklenburg S. Beyond the genome-Towards an epigenetic understanding of handedness ontogenesis. Prog Neurobiol. 2017;159:69–89. doi: 10.1016/j.pneurobio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Ocklenburg, S. et al. Epigenetic regulation of lateralized fetal spinal gene expression underlies hemispheric asymmetries. Elife6 (2017). [DOI] [PMC free article] [PubMed]
- 10.Leach EL, Prefontaine G, Hurd PL, Crespi BJ. The imprinted gene LRRTM1 mediates schizotypy and handedness in a nonclinical population. J Hum Genet. 2014;59:332–336. doi: 10.1038/jhg.2014.30. [DOI] [PubMed] [Google Scholar]
- 11.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz J, Kumsta R, Moser D, Güntürkün O, Ocklenburg S. DNA methylation in candidate genes for handedness predicts handedness direction. Laterality. 2018;23:441–461. doi: 10.1080/1357650X.2017.1377726. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz J, Kumsta R, Moser D, Güntürkün O, Ocklenburg S. KIAA0319 promoter DNA methylation predicts dichotic listening performance in forced-attention conditions. Behav Brain Res. 2018;337:1–7. doi: 10.1016/j.bbr.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Zago L, et al. Pseudoneglect in line bisection judgement is associated with a modulation of right hemispheric spatial attention dominance in right-handers. Neuropsychologia. 2017;94:75–83. doi: 10.1016/j.neuropsychologia.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Hausmann M, Waldie KE, Corballis MC. Developmental changes in line bisection: A result of callosal maturation? Neuropsychology. 2003;17:155–160. doi: 10.1037/0894-4105.17.1.155. [DOI] [PubMed] [Google Scholar]
- 16.Hausmann M. Hemispheric asymmetry in spatial attention across the menstrual cycle. Neuropsychologia. 2005;43:1559–1567. doi: 10.1016/j.neuropsychologia.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Bisiach E, Luzzatti C. Unilateral neglect of representational space. Cortex. 1978;14:129–133. doi: 10.1016/S0010-9452(78)80016-1. [DOI] [PubMed] [Google Scholar]
- 18.Bowers D, Heilman KM. Pseudoneglect: effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- 19.Kerkhoff G. Spatial hemineglect in humans. Prog Neurobiol. 2001;63:1–27. doi: 10.1016/S0301-0082(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 20.Jewell G, McCourt ME. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38:93–110. doi: 10.1016/S0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 21.Fink GR, et al. Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology. 2000;54:1324–1331. doi: 10.1212/WNL.54.6.1324. [DOI] [PubMed] [Google Scholar]
- 22.Göbel SM, Calabria M, Farnè A, Rossetti Y. Parietal rTMS distorts the mental number line: simulating ‘spatial’ neglect in healthy subjects. Neuropsychologia. 2006;44:860–868. doi: 10.1016/j.neuropsychologia.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Thiebaut de Schotten M, et al. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309:2226–2228. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- 24.Urbanski M, et al. Brain networks of spatial awareness: Evidence from diffusion tensor imaging tractography. J Neurol Neurosurg Psychiatry. 2008;79:598–601. doi: 10.1136/jnnp.2007.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apicella P, Legallet E, Nieoullon A, Trouche E. Neglect of contralateral visual stimuli in monkeys with unilateral striatal dopamine depletion. Behav Brain Res. 1991;46:187–195. doi: 10.1016/S0166-4328(05)80112-3. [DOI] [PubMed] [Google Scholar]
- 26.Heuer A, Dunnett SB. Characterisation of spatial neglect induced by unilateral 6-OHDA lesions on a choice reaction time task in rats. Behav Brain Res. 2013;237:215–222. doi: 10.1016/j.bbr.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Gorgoraptis N, et al. The effects of the dopamine agonist rotigotine on hemispatial neglect following stroke. Brain. 2012;135:2478–2491. doi: 10.1093/brain/aws154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, K. et al. Reward sensitivity predicts dopaminergic response in spatial neglect. Cortex (2018). [DOI] [PubMed]
- 29.Tomer R, et al. Dopamine asymmetries predict orienting bias in healthy individuals. Cereb Cortex. 2013;23:2899–2904. doi: 10.1093/cercor/bhs277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz R, Peigneux P. Age-related changes in visual pseudoneglect. Brain Cogn. 2011;76:382–389. doi: 10.1016/j.bandc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Bourdélat-Parks BN, et al. Effects of dopamine beta-hydroxylase genotype and disulfiram inhibition on catecholamine homeostasis in mice. Psychopharmacology (Berl) 2005;183:72–80. doi: 10.1007/s00213-005-0139-8. [DOI] [PubMed] [Google Scholar]
- 32.Greene CM, Robertson IH, Gill M, Bellgrove MA. Dopaminergic genotype influences spatial bias in healthy adults. Neuropsychologia. 2010;48:2458–2464. doi: 10.1016/j.neuropsychologia.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Bellgrove MA, et al. Association between dopamine transporter (DAT1) genotype, left-sided inattention, and an enhanced response to methylphenidate in attention-deficit hyperactivity disorder. Neuropsychopharmacology. 2005;30:2290–2297. doi: 10.1038/sj.npp.1300839. [DOI] [PubMed] [Google Scholar]
- 35.Zozulinsky P, et al. Dopamine system genes are associated with orienting bias among healthy individuals. Neuropsychologia. 2014;62:48–54. doi: 10.1016/j.neuropsychologia.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Latham AJ, Patston LLM, Tippett LJ. The precision of experienced action video-game players: Line bisection reveals reduced leftward response bias. Atten Percept Psychophys. 2014;76:2193–2198. doi: 10.3758/s13414-014-0789-x. [DOI] [PubMed] [Google Scholar]
- 37.Linnell KJ, Caparos S, Davidoff J. Urbanization increases left-bias in line-bisection: An expression of elevated levels of intrinsic alertness? Front Psychol. 2014;5:1127. doi: 10.3389/fpsyg.2014.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freytag V, et al. A peripheral epigenetic signature of immune system genes is linked to neocortical thickness and memory. Nat Comms. 2017;8:15193. doi: 10.1038/ncomms15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klengel T, Pape J, Binder EB, Mehta D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology. 2014;80:115–132. doi: 10.1016/j.neuropharm.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Ocklenburg S, et al. PLP1 Gene Variation Modulates Leftward and Rightward Functional Hemispheric Asymmetries. Mol Neurobiol. 2018;55:7691–7700. doi: 10.1007/s12035-018-0941-z. [DOI] [PubMed] [Google Scholar]
- 41.Yang B-Z, Balodis IM, Lacadie CM, Xu J, Potenza MN. A Preliminary Study of DBH (Encoding Dopamine Beta-Hydroxylase) Genetic Variation and Neural Correlates of Emotional and Motivational Processing in Individuals With and Without Pathological Gambling. J Behav Addict. 2016;5:282–292. doi: 10.1556/2006.5.2016.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ates O, Celikel FC, Taycan SE, Sezer S, Karakus N. Association between 1603CT polymorphism of DBH gene and bipolar disorder in a Turkish population. Gene. 2013;519:356–359. doi: 10.1016/j.gene.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 43.Shao P, Yu Y-X, Bao J-X. Association of Dopamine Beta-Hydroxylase (DBH) Polymorphisms with Susceptibility to Parkinson’s Disease. Med Sci Monit. 2016;22:1617–1622. doi: 10.12659/MSM.895798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto K, et al. Dopamine beta-hydroxylase (DBH) gene and schizophrenia phenotypic variability: A genetic association study. Am J Med Genet B Neuropsychiatr Genet. 2003;117B:33–38. doi: 10.1002/ajmg.b.10011. [DOI] [PubMed] [Google Scholar]
- 45.Hui L, et al. Association between DBH 19bp insertion/deletion polymorphism and cognition in schizophrenia with and without tardive dyskinesia. Schizophr Res. 2017;182:104–109. doi: 10.1016/j.schres.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 46.Voelker P, Sheese BE, Rothbart MK, Posner MI. Methylation polymorphism influences practice effects in children during attention tasks. Cogn Neurosci. 2017;8:72–84. doi: 10.1080/17588928.2016.1170006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong P, et al. The effects of DBH, MAOA, and MAOB on attentional biases for facial expressions. J Mol Neurosci. 2013;49:606–613. doi: 10.1007/s12031-012-9894-2. [DOI] [PubMed] [Google Scholar]
- 48.Zhao R, et al. Genome-wide DNA methylation patterns in discordant sib pairs with alcohol dependence. Asia Pac Psychiatry. 2013;5:39–50. doi: 10.1111/appy.12010. [DOI] [PubMed] [Google Scholar]
- 49.Sperling W, et al. Non-right-handedness and free serum testosterone levels in detoxified patients with alcohol dependence. Alcohol Alcohol. 2010;45:237–240. doi: 10.1093/alcalc/agq014. [DOI] [PubMed] [Google Scholar]
- 50.Denny K. Handedness and drinking behaviour. Br J Health Psychol. 2011;16:386–395. doi: 10.1348/135910710X515705. [DOI] [PubMed] [Google Scholar]
- 51.Evert DL, Oscar-Berman M. Selective attentional processing and the right hemisphere. Effects of aging and alcoholism. Neuropsychology. 2001;15:452–461. [PubMed] [Google Scholar]
- 52.Bertera JH, Parsons OA. Impaired visual search in alcoholics. Alcohol Clin Exp Res. 1978;2:9–14. doi: 10.1111/j.1530-0277.1978.tb04685.x. [DOI] [PubMed] [Google Scholar]
- 53.Drake AI, Hannay HJ, Gam J. Effects of chronic alcoholism on hemispheric functioning: An examination of gender differences for cognitive and dichotic listening tasks. J Clin Exp Neuropsychol. 1990;12:781–797. doi: 10.1080/01688639008401019. [DOI] [PubMed] [Google Scholar]
- 54.Leone L, McCourt ME. The effect of acute ethanol challenge on global visuospatial attention: Exaggeration of leftward bias in line bisection. Laterality. 2010;15:327–342. doi: 10.1080/13576500902781745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narwade SC, Mallick BN, Deobagkar DD. Transcriptome Analysis Reveals Altered Expression of Memory and Neurotransmission Associated Genes in the REM Sleep Deprived Rat Brain. Front Mol Neurosci. 2017;10:67. doi: 10.3389/fnmol.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kopecková M, Paclt I, Goetz P. Polymorphisms and low plasma activity of dopamine-beta-hydroxylase in ADHD children. Neuro Endocrinol Lett. 2006;27:748–754. [PubMed] [Google Scholar]
- 57.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: A meta-analytic review. Hum Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 58.Bouchatta O, et al. Neonatal 6-OHDA lesion model in mouse induces Attention-Deficit/ Hyperactivity Disorder (ADHD)-like behaviour. Sci Rep. 2018;8:15349. doi: 10.1038/s41598-018-33778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheppard DM, Bradshaw JL, Mattingley JB, Lee P. Effects of stimulant medication on the lateralisation of line bisection judgements of children with attention deficit hyperactivity disorder. J Neurol Neurosurg Psychiatry. 1999;66:57–63. doi: 10.1136/jnnp.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waldie KE, Hausmann M. Right fronto-parietal dysfunction in children with ADHD and developmental dyslexia as determined by line bisection judgements. Neuropsychologia. 2010;48:3650–3656. doi: 10.1016/j.neuropsychologia.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 61.Manly T, Cornish K, Grant C, Dobler V, Hollis C. Examining the relationship between rightward visuo-spatial bias and poor attention within the normal child population using a brief screening task. J Child Psychol Psychiatry. 2005;46:1337–1344. doi: 10.1111/j.1469-7610.2005.01432.x. [DOI] [PubMed] [Google Scholar]
- 62.Sandson TA, Bachna KJ, Morin MD. Right hemisphere dysfunction in ADHD: Visual hemispatial inattention and clinical subtype. J Learn Disabil. 2000;33:83–90. doi: 10.1177/002221940003300111. [DOI] [PubMed] [Google Scholar]
- 63.Silk TJ, et al. Abnormal asymmetry in frontostriatal white matter in children with attention deficit hyperactivity disorder. Brain Imaging Behav. 2016;10:1080–1089. doi: 10.1007/s11682-015-9470-9. [DOI] [PubMed] [Google Scholar]
- 64.Casey BJ, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 65.Hamza, M. et al. Epigenetics and ADHD: Toward an Integrative Approach of the Disorder Pathogenesis. J Atten Disord, 1087054717696769 (2017). [DOI] [PubMed]
- 66.Walton E, et al. Epigenetic profiling of ADHD symptoms trajectories: A prospective, methylome-wide study. Mol Psychiatry. 2017;22:250–256. doi: 10.1038/mp.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilmot B, et al. Methylomic analysis of salivary DNA in childhood ADHD identifies altered DNA methylation in VIPR2. J Child Psychol Psychiatry. 2016;57:152–160. doi: 10.1111/jcpp.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Somers M, et al. On the relationship between degree of hand-preference and degree of language lateralization. Brain Lang. 2015;144:10–15. doi: 10.1016/j.bandl.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Longo MR, Trippier S, Vagnoni E, Lourenco SF. Right hemisphere control of visuospatial attention in near space. Neuropsychologia. 2015;70:350–357. doi: 10.1016/j.neuropsychologia.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 70.Cai Q, van der Haegen L, Brysbaert M. Complementary hemispheric specialization for language production and visuospatial attention. Proc Natl Acad Sci USA. 2013;110:E322–30. doi: 10.1073/pnas.1212956110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McManus IC. Handedness, language dominance and aphasia: A genetic model. Psychol Med Monogr Suppl. 1985;8:1–40. doi: 10.1017/S0264180100001879. [DOI] [PubMed] [Google Scholar]
- 72.Hannon E, Lunnon K, Schalkwyk L, Mill J. Interindividual methylomic variation across blood, cortex, and cerebellum: Implications for epigenetic studies of neurological and neuropsychiatric phenotypes. Epigenetics. 2015;10:1024–1032. doi: 10.1080/15592294.2015.1100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rao NP, et al. abnormalities in obsessive-compulsive disorder: A line bisection study. Acta Neuropsychiatr. 2015;27:242–247. doi: 10.1017/neu.2015.23. [DOI] [PubMed] [Google Scholar]
- 74.Wang W, et al. Line bisection performance in patients with personality disorders. Cogn Neuropsychiatry. 2003;8:273–285. doi: 10.1080/13546800344000048. [DOI] [PubMed] [Google Scholar]
- 75.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 76.Hausmann M, Ergun G, Yazgan Y, Güntürkün O. Sex differences in line bisection as a function of hand. Neuropsychologia. 2002;40:235–240. doi: 10.1016/S0028-3932(01)00112-9. [DOI] [PubMed] [Google Scholar]
- 77.Racine JS. RStudio: A Platform-Independent IDE for R and Sweave. J. Appl. Econ. 2012;27:167–172. doi: 10.1002/jae.1278. [DOI] [Google Scholar]
- 78.Assenov Y, et al. Comprehensive analysis of DNA methylation data with RnBeads. Nat Methods. 2014;11:1138–1140. doi: 10.1038/nmeth.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teschendorff AE, et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics. 2013;29:189–196. doi: 10.1093/bioinformatics/bts680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hawi Z, et al. ADHD and DAT1: Further evidence of paternal over-transmission of risk alleles and haplotype. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:97–102. doi: 10.1002/ajmg.b.30960. [DOI] [PubMed] [Google Scholar]
- 81.Hawi Z, et al. Preferential transmission of paternal alleles at risk genes in attention-deficit/hyperactivity disorder. Am J Hum Genet. 2005;77:958–965. doi: 10.1086/498174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated during the current study is available from the corresponding author on request.


