Abstract
Meningiomas present as intracranial extra-axial lesions with dural attachment, which are primarily managed surgically. The extent of resection (EOR) may vary depending on patient- and tumor-related factors. The aim of this study is to identify preoperative predictive factors of EOR and to propose an estimation of the predicted gross total resection (GTR) based of patient- and tumor-characteristics. 1469 patients from a retrospectively (1990 to 2002) and prospectively managed (2003 to 2010) databank of Oslo University Hospital, Norway, totalling 11,414 patient-years of follow-up were included. Patients had a mean age at surgery of 64 ± 20.1 years with a female-to-male ratio was 2.4:1 and a mean KPS of 81.2 ± 12.1. Skull-base meningiomas represented 47% of all cases. WHO grades were I in 92.3%, II in 5.2%, and III in 2.2%. Bone infiltration was described in 18.7% of cases. 39.3% of patients had Simpson I resection, 34.3% had Simpson II, 5.4% had Simpson III, 20.6% had Simpson IV, and 0.5% had Simpson V. The risk factors for incomplete resection were: symptomatic presentation (OR 0.56 [0.43–0.72]), skull-base location (OR 0.79 [0.70–0.88]), and bone invasion (OR 0.85 [0.73–0.99]). Using a recursive partitioning analysis, we propose a classification-tree for the prediction of GTR rate based on preoperatively determinable patient- and tumor characteristics. The identification of preoperative predictors of poor GTR rate may aid clinicians managing meningioma patients. In selected cases were the predicted GTR rate is low, staged treatment with surgical debulking followed by adjuvant therapy may be favored in order to minimize postoperative morbidity and mortality.
Introduction
Meningiomas are generally considered histologically benign tumors that typically present as an intracranial extracerebral dural lesion with homogenous contrast enhancement on magnetic resonance imaging (MRI). Owned to an aging population and the increasing availability of imaging diagnostics, more incidental meningioma are detected, thus leading to a higher overall incidence1,2.
Besides surveillance, the therapeutic management of meningiomas is primarily surgical and aims at maximal tumor removal as the complete removal of tumor and its dural tail, which is important for later disease control, and to obtain a pathological diagnosis3–5. Depending on the size, location, and anatomical relationship of the tumor with the surrounding structures, achieving a complete resection can be challenging. The extent of resection (EOR) is quantified using the Simpson scale6. Several studies assessed EOR as a prognostic factor of overall and progression-free survival (OS and PFS)7–11, but none addressed specifically preoperative factors determining EOR. However, identification of predictive factors of surgical resection might be helpful for “personalized surgery”, i.e. in tailoring surgical resection on a case-by-case basis.
The aim of this study is to identify predictive factors of EOR and to propose an estimation of the predicted EOR based on patient’s and tumor’s characteristics.
Results
Patients characteristics
1469 patients (1033 females & 436 males) surgically treated for a meningioma were identified. The mean age at surgery was 64 ± 20.1 years. The female-to-male ratio was 2.4:1. The mean Karnofsky performance status (KPS) was 81.2 ± 12.1. Neurologic deficit was the most common presenting symptom (60.2%), followed by signs of intracranial hypertension (ICH) (31.7%) and seizures (29.6%). 5.4% of patients were asymptomatic. The mean follow-up was 7.8 ± 5.5 years (Table 1). One patient moved abroad and was lost to follow-up.
Table 1.
Characteristics of a surgical population of patients with meningiomas (n = 1469).
| n | % | |
|---|---|---|
| Age | 64 ± 20.1 | — |
| Sex | 1033 F/436 M | — |
| Preoperative KPS | 81.18 ± 12.1 | — |
| Presenting symptoms | ||
| Asymptomatic | 79 | 5.4% |
| Seizures | 435 | 29.6% |
| ICH | 466 | 31.7% |
| Neurological deficit | 855 | 60.2% |
| Skull base meningioma | 690 | 47% |
| WHO grade | ||
| I | 1352 | 92.3% |
| II | 77 | 5.2% |
| III | 32 | 2.2% |
| Bone invasion | 274 | 18.7% |
| Simpson grade | ||
| I | 575 | 39.2% |
| II | 503 | 34.2% |
| III | 79 | 5.4% |
| IV | 302 | 20.6% |
| V | 8 | 0.6% |
| GTR | 1159 | 78.9% |
| Follow-up (years) | 7.8 ± 5.5 | — |
GTR: Gross total resection; ICH: intracranial hypertension; KPS: Karnofsky performance score; WHO: World Health Organization.
Tumors characteristics
47% of the meningiomas were skull base meningiomas. World Health Organization (WHO) grades were I for n = 1352 (92.3%), II in n = 77 (5.2%), and III for n = 32 patients (2.2%). Bone infiltration was described in n = 274 (18.7%) of the cases (Table 1).
Extent of resection
Regarding EOR, n = 575 patients (39.3%) had Simpson I resection, n = 503 (34.3%) Simpson II, n = 79 (5.4%) Simpson III, n = 302 (20.6%) Simpson IV, and n = 8 (0.5%) Simpson V. GTR defined as a Simpson grade I, II or III resection12, was achieved in n = 1075 (79.3%) of surgeries (Table 1).
Skull base meningiomas were associated with higher Simpson grades than non skull-base meningiomas. This held true especially for anatomically difficult locations such as the orbit, the petroclival region or the cavernous sinus. As expected, GTR was more often achieved in convexity (96.7%) and lateral sphenoid wing (87.2%) meningiomas.
Predictive factors of EOR
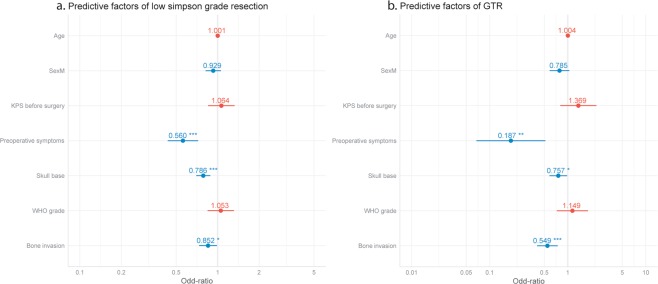
Three independent risk factors for incomplete resection were identified: symptoms at presentation (seizure, intracranial hypertension and/or a neurological deficit) (OR 0.56 [0.43–0.72]), a skull base meningioma location (OR 0.79 [0.70–0.88]), and associated bone invasion (OR 0.85 [0.73–0.99]) (Table 2, Fig. 1). When considering the different modes of clinical presentation, GTR was 25% less likely in patients who had a preoperative neurological deficit and 33% more likely in patients presenting with preoperative seizures (Table 2). Age, sex, preoperative Karnofsky, WHO grade and preoperative intracranial hypertension did not have a significant impact on the EOR.
Table 2.
Predictive factors of meningioma surgical extent of resection.
| Low Simpson grade | Gross Total Resection | |||
|---|---|---|---|---|
| OR | p-value | OR | p-value | |
| Age | 1.00 [0.99;1.01] | 0.67 | 1.00 [0.99;1.01] | 0.38 |
| Sex (Male) | 0.93 [0.82;1.06] | 0.26 | 0.79 [0.58;1.05] | 0.10 |
| Preoperative KPS ≥ 70 | 1.06 [0.85;1.33] | 0.58 | 1.37 [0.82;2.40] | 0.25 |
| Preoperative symptoms | 0.56 [0.43;0.72] | <0.001 | 0.19 [0.06;0.46] | <0.001 |
| Skull base meningioma | 0.79 [0.70;0.88] | <0.001 | 0.76 [0.58;0.98] | 0.03 |
| WHO tumor grade | 1.05 [0.84;1.31] | 0.64 | 1.15 [0.84;1.31] | 0.55 |
| Bone invasion | 0.85 [0.73;0.99] | 0.03 | 0.55 [0.73;0.99] | <0.001 |
KPS = Karnofsky Performance Score. OR = Odds ratio. WHO = World Health Organization.
Odd-ratios (OR) represent the factor association with gross total resection (GTR: defined as Simpson 1, 2 or 3).
Figure 1.
Forrest plots of predictive factors for meningioma surgical extent of resection. (a) Predictive factors of a good resection based on the Simpson grade. (b) Predictive factors of gross total resection (GTR).
Classification tree of EOR’s probability
The identified preoperative predictors of meningioma EOR were put in hierarchical order using the recursive partitioning analysis with: the existence of symptoms at presentation, a tumor bone invasion, a skull-base located tumor, the patient’s sex and the preoperative KPS (Fig. 2). The GTR varies greatly between the groups from 95% for patients without a preoperative deficit to 60.8% for female patients with a preoperative deficit, a KPS < 70 and a skull base tumor with a bony infiltration, for example (Fig. 2).
Figure 2.
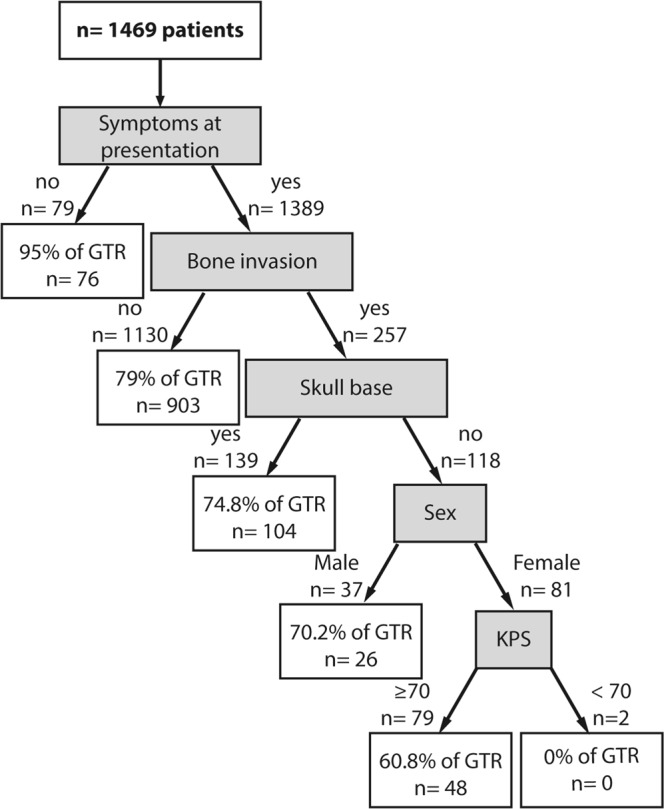
Classification tree of the preoperative predictors of meningioma’s extent of resection (EOR).
Discussion
The presence of symptoms at presentation, a skull-base location and/or a bone invasion were all negative predictive factors for GTR. To the best of our knowledge, this is the largest study analyzing predictive factors of the surgical EOR in meningiomas9–11,13. The preoperative KPS was not a significant predictive factor of EOR despite being predictive for postoperative neurological outcome, OS and PFS14,15. Age, preoperative KPS, and female-to-male ratio in our cohort were comparable to meningioma patients characteristics in the literature5,11,13,16–18. Similar to other series11,19, a Simpson grade I resection was achieved in 39.4% and a GTR in 78.9% of the cases.
Besides the presence of symptoms at presentation, the patients’ baseline characteristics were not predictive for EOR. The clinical presentation (seizure, ICH and/or a neurological deficit) was the sole patient-dependent factor identified as a significant factor of low EOR. This never previously described association may be explained by the surgeon’s apprehensiveness to aim for GTR in an already neurologically impaired patient. Increased GTR rates in patients with preoperative seizures may be linked to the meningioma’s location. Convexity and anterior skull base meningiomas are notoriously more epileptogenic, while also being more surgically accessible20,21.
Patient’s age was not a significant prognostic factor of EOR. Simpson I resection was generally attempted even in older patients, which is in line with previous studies22. Patients’ gender was not a significant independent prognostic factor in multivariate analysis, despite the increased incidence skull base meningiomas in women (female-to-male ratio 3.13:1, p < 0.001)11,16. In our study, the preoperative KPS was not a significant predictive factor of surgical resection’s quality despite being an important predictor of postoperative neurological outcome in several publications14,15.
Meningiomas with skull-base location and bone invasion were less often completely resected. This is unsurprising as these locations can be technically more challenging due to their restricted surgical access and vicinity to vascular and/or neurological structures11,18,23,24. This difference was particularly stark between convexity and cavernous or petroclival meningiomas, where a Simpson grade I or even grade II resection was rarely possible.
Bone invasion was another significant independent factor of poor resection quality. This infiltration requires additional drilling of the bone close to the dural insertion, often on the skull flap. In certain cases, bone invasion management represents a major part of the surgical procedure, for instance in spheno-orbital meningiomas where the extent of bone invasion and cavernous sinus involvement may not allow complete removal25.
This study is the first to propose a classification-tree of the predictors of EOR (Fig. 2). As can be seen from the first level of the tree, the EOR is very high for patients without a symptoms at presentation. Possibly, meningioma surgery for asymptomatic patients is usually more carefully planned and more likely proposed almost only if a complete resection can be performed. Going further down the tree, the presence of a bone invasion represents the second main predictor of low EOR, followed by the tumor location. This may be explained by the fact that these tumors are harder to remove completely while removing the bony infiltration. Note that the preoperative factors play only a minor role in predicting the EOR. However, this classification tree did not consider the meningioma’s radiological features, that are important for the preoperative planning. For example, the size of the lesion and the mass effect on the surrounding brain structures has an impact on the planned and observed EOR, even in asymptomatic patients.
Considering these factors may be helpful in the decision-making process and the planning process of the surgical resection. The proposed classification tree that allows for a rapid estimation of the estimated EOR in consideration of the patient and tumor preoperative characteristics. Although complete surgical resection while preserving the patient’s neurological status is the goal, a Simpson I resection is neither always attainable nor does it guarantee increased OS or PFS9,11. This holds especially true in view of other available adjuvant therapeutic options such as radiotherapy26–28, second surgery29–31, or a watchful wait-and-scan follow-up.
Recently, there has been a trend towards more conservative treatment for meningiomas, mainly because it has been shown that the Simpson grade is not universally applicable to all meningiomas9,26,32. Hence, a staged treatment with debulking followed by adjuvant treatment might be preferable in order to minimize postoperative morbidity and mortality, especially in skull-base meningiomas. The predictive factors of low EOR identified in this study may help neurosurgeons to identify those patients who may fare better with staged treatment9,33.
The main strengths of this clinical study are the clinical setting, the number of patients as well as the length of follow-up up to 21 years. Loss of follow-up was minimal since all patients with a postoperative complication or recurrence were systematically re-referred to our tertiary center. Only one patient was lost to follow-up, moving abroad. The data stem from one neurosurgical center with mostly homogenous surgical practices. This can make the generalization of our finding to all patients difficult. All patients with a histologically proven meningioma were included, which reduces selection bias. The retrospective data collection before 2003 is a limitation. Also, due to the long period of data collecting starting in 1990, radiological data, especially MRI, tumor size and molecular biomarkers such as Mib-1 or Ki67 were not available for all patients and were included in the statistical analysis.
The choice to regroup all meningiomas together for the statistical analysis and the data interpretation may also be subject to question as meningioma is not a homogenous pathology, with different pattern of evolution and therapeutic management for specific subgroups, especially depending of the location and WHO grade. The global results may not be representative of these specific categories of meningioma.
Conclusions
Clinical symptoms at presentation, skull-base location and bone invasion were significant predictors of a poor EOR for meningiomas. The identification of these factors may aid clinicians managing patients with meningiomas.
Methods
Patient cohort
Data were acquired from a retrospectively (1990 to 2002) and prospectively managed (2003 to 2010) databank of Oslo University Hospital (OUH). OUH is the main Norwegian tertiary referral center and has two neurosurgical units (Rikshospitalet and Ullevaal), which covers an area of approximately three million inhabitants, i.e. 56% of the Norwegian population.
All patient operated for a meningioma during the study period at OUH were included. Preoperative imaging studies were reviewed to confirm tumor location and size, contrast enhancement, and presence of calcification. The WHO grading system was used to classify the histology of meningiomas. The WHO criteria changed during the study period. From 1990 to 2001, the tumors were classified as benign, atypical or anaplastic. The present WHO-grading system for meningioma was implemented in 2001, which divides the tumors into grade I, II and III. For this study, we reclassified the tumors operated before 2001 to the present WHO classification; benign = WHO grade I, atypical = WHO grade II and anaplastic = WHO grade III. The definition of skull base meningioma was based on Al-Mefty et al.34. All patients were operated on by the neurosurgical teams of OUH. The EOR was assessed using the Simpson grade scale, based on the surgical report in conjunction with post-operative imaging. Gross total resection (GTR) was defined as a Simpson grade I, II or III resection12. The histopathological diagnosis of meningioma was confirmed by experienced neuropathologists.
Ethics
The study was regulated by the Personal Data Act/Personal Health Data Filing System Act and approved by the Data Protection Official at OUH (2017/5204). Informed consent was not required according to the Personal Data Act/Personal Health Data Filing System Act.
Statistical analysis
Statistical analysis was performed using R v3.5.1 (https://www.r-project.org). The significant p-value threshold was defined at 0.05. Multivariate analysis was performed using a linear generalized model approach. The variable considered for the multivariate analysis were patient’s age, sex, preoperative Karnofsky prognostic scale (KPS) and clinical status, as well as the tumor location, WHO histopathological grade and the presence of a bone invasion.
The above-mentioned factors were used to build a classification of predictors of EOR using the classification and regression tree (CART) recursive partitioning analysis. The variables considered were patient’s age, sex, preoperative KPS and preoperative status, as well as tumor location and bone invasion. The generated CART tree was pruned by adjusting the complexity variable to minimize the estimated error in order to avoid data overfitting.
Acknowledgements
The authors would like to thank Bernt Filip Hasseleid MD, Andreas Mathisen MD, Andreas Hessen Schei MD, and Kristina M Ødegaard MD for their valuable contributions in collecting data for this manuscript and the neurosurgeons of Rikshospitalet and Ullevaal for their dedicated patient care.
Author Contributions
Data collection: T.R.M., E.H. and D.S. Statistical analysis: J.M.L. Manuscript drafting: J.M.L., M.D.B., M.V.C., H.J. and T.R.M. Critical revision: All authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ostrom QT, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro-Oncol. 2016;18:v1–v75. doi: 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solheim O, Torsteinsen M, Johannesen TB, Jakola AS. Effects of cerebral magnetic resonance imaging in outpatients on observed incidence of intracranial tumors and patient survival: a national observational study. J. Neurosurg. 2014;120:827–832. doi: 10.3171/2013.12.JNS131312. [DOI] [PubMed] [Google Scholar]
- 3.Adegbite AB, Khan MI, Paine KW, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J. Neurosurg. 1983;58:51–56. doi: 10.3171/jns.1983.58.1.0051. [DOI] [PubMed] [Google Scholar]
- 4.Ildan F, et al. Predicting the probability of meningioma recurrence in the preoperative and early postoperative period: a multivariate analysis in the midterm follow-up. Skull Base Off. J. North Am. Skull Base Soc. Al. 2007;17:157–171. doi: 10.1055/s-2007-970554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasseleid BF, Meling TR, Rønning P, Scheie D, Helseth E. Surgery for convexity meningioma: Simpson Grade I resection as the goal: clinical article. J. Neurosurg. 2012;117:999–1006. doi: 10.3171/2012.9.JNS12294. [DOI] [PubMed] [Google Scholar]
- 6.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rydzewski NR, et al. Gross total resection and adjuvant radiotherapy most significant predictors of improved survival in patients with atypical meningioma. Cancer. 2018;124:734–742. doi: 10.1002/cncr.31088. [DOI] [PubMed] [Google Scholar]
- 8.Ehresman JS, et al. The Relevance of Simpson Grade Resections in Modern Neurosurgical Treatment of World Health Organization Grade I, II, and III Meningiomas. World Neurosurg. 2018;109:e588–e593. doi: 10.1016/j.wneu.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Gousias K, Schramm J, Simon M. The Simpson grading revisited: aggressive surgery and its place in modern meningioma management. J. Neurosurg. 2016;125:551–560. doi: 10.3171/2015.9.JNS15754. [DOI] [PubMed] [Google Scholar]
- 10.Gallagher MJ, Jenkinson MD, Brodbelt AR, Mills SJ, Chavredakis E. WHO grade 1 meningioma recurrence: Are location and Simpson grade still relevant? Clin. Neurol. Neurosurg. 2016;141:117–121. doi: 10.1016/j.clineuro.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Voß KM, et al. The Simpson grading in meningioma surgery: does the tumor location influence the prognostic value? J. Neurooncol. 2017;133:641–651. doi: 10.1007/s11060-017-2481-1. [DOI] [PubMed] [Google Scholar]
- 12.Goldbrunner R, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17:e383–391. doi: 10.1016/S1470-2045(16)30321-7. [DOI] [PubMed] [Google Scholar]
- 13.Sicking J, et al. The evolution of cranial meningioma surgery-a single-center 25-year experience. Acta Neurochir. (Wien) 2018;160:1801–1812. doi: 10.1007/s00701-018-3617-6. [DOI] [PubMed] [Google Scholar]
- 14.Kreßner M, Arlt F, Riepl W, Meixensberger J. Prognostic factors of microsurgical treatment of intracranial meningiomas - A multivariate analysis. PloS One. 2018;13:e0202520. doi: 10.1371/journal.pone.0202520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanda A, et al. Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization Grade I meningioma. J. Neurosurg. 2017;126:201–211. doi: 10.3171/2016.1.JNS151842. [DOI] [PubMed] [Google Scholar]
- 16.Zouaoui S, et al. Descriptive epidemiology of 13,038 newly diagnosed and histologically confirmed meningiomas in France: 2006–2010. Neurochirurgie. 2018;64:15–21. doi: 10.1016/j.neuchi.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 17.da Silva CE, Peixoto de Freitas PE. Recurrence of Skull Base Meningiomas: The Role of Aggressive Removal in Surgical Treatment. J. Neurol. Surg. Part B Skull Base. 2016;77:219–225. doi: 10.1055/s-0035-1566251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schipmann S, et al. Is the Simpson Grading System Applicable to Estimate the Risk of Tumor Progression After Microsurgery for Recurrent Intracranial Meningioma? World Neurosurg. 2018;119:e589–e597. doi: 10.1016/j.wneu.2018.07.215. [DOI] [PubMed] [Google Scholar]
- 19.Shakir, S. I. et al. Prognostic factors for progression in atypical meningioma. J. Neurosurg. 1–9, 10.3171/2017.6.JNS17120 (2018). [DOI] [PubMed]
- 20.Baumgarten P, et al. Focused review on seizures caused by meningiomas. Epilepsy Behav. EB. 2018;88:146–151. doi: 10.1016/j.yebeh.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Chen WC, et al. Factors Associated With Pre- and Postoperative Seizures in 1033 Patients Undergoing Supratentorial Meningioma Resection. Neurosurgery. 2017;81:297–306. doi: 10.1093/neuros/nyx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meling TR, Da Broi M, Scheie D, Helseth E. Skull base versus non-skull base meningioma surgery in the elderly. Neurosurg. Rev. 2018 doi: 10.1007/s10143-018-1005-6. [DOI] [PubMed] [Google Scholar]
- 23.Otero-Rodriguez A, et al. Re-Evaluating Simpson Grade I, II, and III Resections in Neurosurgical Treatment of World Health Organization Grade I Meningiomas. World Neurosurg. 2016;96:483–488. doi: 10.1016/j.wneu.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Meling, T. R., Da Broi, M., Scheie, D. & Helseth, E. Meningiomas - skull-base vs non-skull base. Neurosurg. Rev. (in press) (2018). [DOI] [PubMed]
- 25.Terrier L-M, et al. Spheno-Orbital Meningiomas Surgery: Multicenter Management Study for Complex Extensive Tumors. World Neurosurg. 2018;112:e145–e156. doi: 10.1016/j.wneu.2017.12.182. [DOI] [PubMed] [Google Scholar]
- 26.Aboukais R, et al. Surgery followed by radiosurgery: a deliberate valuable strategy in the treatment of intracranial meningioma. Clin. Neurol. Neurosurg. 2014;124:123–126. doi: 10.1016/j.clineuro.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 27.Davidson L, et al. Postoperative Gamma Knife surgery for benign meningiomas of the cranial base. Neurosurg. Focus. 2007;23:E6. doi: 10.3171/FOC-07/10/E6. [DOI] [PubMed] [Google Scholar]
- 28.Adeberg S, et al. Long-term outcome after radiotherapy in patients with atypical and malignant meningiomas–clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:859–864. doi: 10.1016/j.ijrobp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Sanna M, Bacciu A, Falcioni M, Taibah A, Piazza P. Surgical management of jugular foramen meningiomas: a series of 13 cases and review of the literature. The Laryngoscope. 2007;117:1710–1719. doi: 10.1097/MLG.0b013e3180cc20a3. [DOI] [PubMed] [Google Scholar]
- 30.Takase H, et al. Characteristics and surgical strategies for posterior clinoid process meningioma: two case reports and review of the literature. Neurosurg. Rev. 2017;40:163–169. doi: 10.1007/s10143-016-0774-z. [DOI] [PubMed] [Google Scholar]
- 31.Aras Y, Akcakaya MO, Aydoseli A, Izgi N. Staged surgery for sylvian fissure meningiomas without dural attachment: report of two cases. Clin. Neurol. Neurosurg. 2013;115:1527–1529. doi: 10.1016/j.clineuro.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Combs SE, Ganswindt U, Foote RL, Kondziolka D, Tonn J-C. State-of-the-art treatment alternatives for base of skull meningiomas: complementing and controversial indications for neurosurgery, stereotactic and robotic based radiosurgery or modern fractionated radiation techniques. Radiat. Oncol. Lond. Engl. 2012;7:226. doi: 10.1186/1748-717X-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zentner J, Meyer B, Vieweg U, Herberhold C, Schramm J. Petroclival meningiomas: is radical resection always the best option? J. Neurol. Neurosurg. Psychiatry. 1997;62:341–345. doi: 10.1136/jnnp.62.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeMonte, F., W.McDermott, M. & Al-Mefty, O. Al-Mefty’s Meningiomas. May 17th (2011).