Abstract
Host and environmental factors contribute to variation in human immune responses, yet the genetic and evolutionary drivers of alternative splicing in response to infection remain largely uncharacterised. Leveraging 970 RNA-sequencing profiles of resting and stimulated monocytes from 200 individuals of African- and European-descent, we show that immune activation elicits a marked remodelling of the isoform repertoire, while increasing the levels of erroneous splicing. We identify 1,464 loci associated with variation in isoform usage (sQTLs), 9% of them being stimulation-specific, which are enriched in disease-related loci. Furthermore, we detect a longstanding increased plasticity of immune gene splicing, and show that positive selection and Neanderthal introgression have both contributed to diversify the splicing landscape of human populations. Together, these findings suggest that differential isoform usage has been an important substrate of innovation in the long-term evolution of immune responses and a more recent vehicle of population local adaptation.
Genetic ancestry might influence immunological response to infection at different regulatory levels. Here, the authors use RNA-Seq to investigate the variability of alternative splicing patterns in resting and stimulated monocytes of African- and European-descent.
Introduction
Alternative splicing (AS) is an essential mechanism for generating functional diversity, as it allows individual genes to express multiple mRNAs and encode numerous proteins, through rearrangement of existing domains1. It is estimated that nearly 95% of mammalian genes undergo AS, with strong impact on essential regulatory processes such as chromatin modification and signal transduction1,2. Because it allows to increase protein diversity at a minimal cost for the organism, AS has undergone rapid evolution across vertebrates3–5 and is thought to play a key role in primate adaptation, including humans6,7. One function where innovation is essential is immunity, as the constant arms race against invading pathogens requires the ability of the host to rapidly adapt to new pathogenic cues, while maintaining homeostasis8–10. Thus, if we are to fully understand the regulation of immune function in humans, we need to dissect the degree of population-level variation of AS and define its genetic and evolutionary determinants in a cellular setting relevant to immunity to infection.
Previous studies, fuelled by the advent of RNA sequencing, have defined the splicing landscape of a large variety of human cells and tissues11–14. The contribution of AS in the context of immunity is increasingly recognised13–16, and a few recent studies have reported widespread post-transcriptional modifications in response to environmental cues including infection17–21. However, there is increasing evidence to suggest that a large fraction of isoform diversity results from the usage of cryptic splice sites, leading to the formation of non-functional transcripts, a phenomenon known as noisy splicing22–24. Yet, the degree of such a stochastic noise in immune cells and how cellular perturbation with external stimuli, such as infection, generates non-functional transcripts remain to be determined.
The genetic determinants of AS are increasingly well-defined by studies of splicing quantitative trait loci (sQTLs)19,20,25–32 and massively parallel splicing assays33,34, which indicate that splicing is under strong genetic control in humans and, in most cases, has direct effects on protein sequences. Furthermore, besides the known role of splice-altering variants in Mendelian disorders35,36, there is increasing support to the notion that they can also contribute to common disease risk, broadening our understanding of the mechanistic links between genetic variation, gene regulation and ultimate phenotypes19,20,27,35,37. However, the genetic determinants of differential isoform usage in response to immune stimulation, which inform gene–environment (G × E) interactions, and their contribution to complex immune-related traits are far from clear.
Recent studies have also shown that the intensity of immune responses, defined through transcriptional profiling of macrophages and monocytes exposed to various bacterial and viral challenges, differs substantially across human populations, owing to past adaptation to pathogen pressure29,38. Similarly, variation in AS has been reported across populations from different ethnic backgrounds28,39,40, and signals of selection have been detected in splicing regulatory elements7,41. Yet, the contribution of differential isoform usage to ancestry-related differences in immune responses and the role of splicing as a vehicle of recent population adaptation, through different evolutionary mechanisms, are largely unknown.
In this study, we leverage RNA-sequencing data of human primary monocytes both at the basal state and after stimulation with different ligands, in 200 healthy individuals of African and European descent. We characterise the splicing landscape of the innate immune response and explore both long-term and recent evolution of immune gene splicing. We show that immune stimulation has a pervasive impact on AS, leading to increased isoform diversity but also elevated levels of noisy splicing. We map the genetic determinants of AS, including those that manifest in a context-specific manner, and uncover their contribution to chronic immune-related disorders. Finally, using population genetics tools, we show that positive selection and admixture with Neanderthals have both contributed to shape the current population variability in the splicing landscape of the human immune response.
Results
Characterising the landscape of splicing in human monocytes
To characterise the diversity of splicing in immune responses, we extracted ~7.2 billion spliced reads from 970 RNA-sequencing profiles obtained from resting and activated monocytes, originating from 200 individuals of African and European ancestry38. Monocytes were activated with various Toll-like receptor (TLR) ligands lipopolysaccharides (LPS), Pam3CSK4 and R848 activating TLR4, TLR1/2 and TLR7/8 pathways, respectively), or a live strain of influenza A virus (IAV). After excluding reads that did not match known exon–exon junctions (<0.4% of all spliced reads, Supplementary Fig. 1a–c), we identified ~1.9 million unique junctions, defining over 1.7 million acceptor and donor sites. Among the 1.1 million splice sites that could be unambiguously assigned to a gene expressed with more than 1 fragment per kilobase per million (FPKM > 1), 80% presented weak activity across conditions (i.e., <1 supporting read per sample or the reads supporting the splice site account for <5% of all reads, referred as weak splice sites). The remaining 20%, corresponding to 217,792 high-activity splice sites, were composed of 56,049 sites that were active in a subset of transcripts (i.e., reads supporting the splice site account for 5–95% of all reads, alternative splice sites), and 161,743 sites that were constitutively active in our dataset (>95% of supporting reads, constitutive splice site). These observations, which set the bases of our study, indicate pervasive, AS in resting and activated monocytes.
Reduced long-term conservation of immune gene splicing
We first investigated the evolutionary pressures characterising the various types of splice sites (weak, alternative and constitutive) in the whole dataset, using the Genomic Evolutionary Rate Profiling (Gerp) statistical framework42. We observed a bimodal distribution of Gerp Rejected Substitutions (RS) scores (Fig. 1a), with 60% of splice sites showing little to no conservation (GerpRS < 2). The percentage of conserved sites increased with splice site usage, across all levels of gene expression (Supplementary Fig. 1d–f). Thus, 98 and 71% of constitutive and alternative splice sites, respectively, showed moderate to high conservation (GerpRS > 2), whereas only 25% of weak splice sites were conserved (Fig. 1b). Among weak splice sites that were not conserved, 95% were absent from Ensembl annotations and were thus considered as cryptic splice sites. Interestingly, we found that active, non-conserved splice sites are preferentially observed in genes with functions related to immune response (likelihood-ratio test; odds ratio (OR) > 1.2, p < 3.2 × 10−11, Supplementary Data 1). Furthermore, we found that genes whose expression was induced upon stimulation in our setting (log2FC > 1) were also more likely to harbour non-conserved splice sites, relative to non-induced genes that were expressed at similar levels, with the strongest enrichment being found in response to IAV stimulation (likelihood-ratio test; OR > 2.3, p < 4.2 × 10−24, Supplementary Data 1).
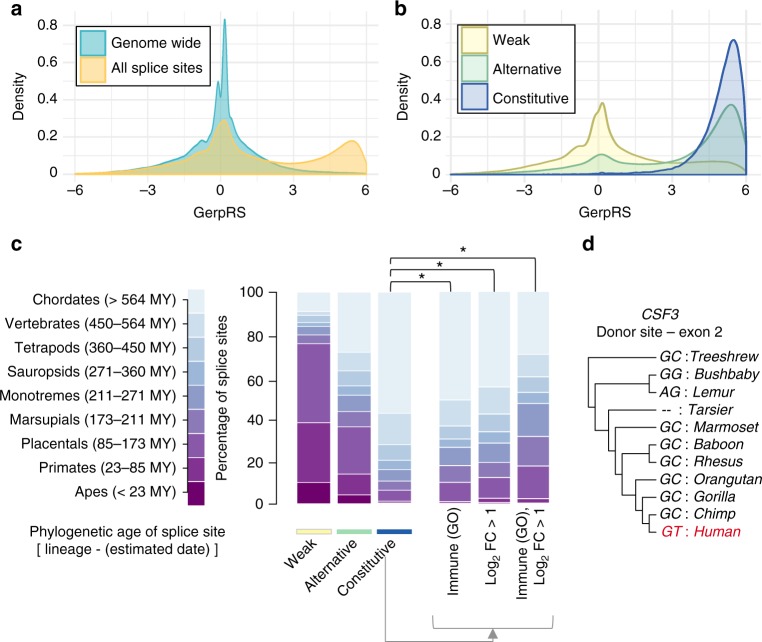
Fig. 1.
Splice sites of immune genes show decreased inter-species conservation. a Conservation of splice sites shows a strong bimodal distribution (orange). One-third of splice sites show strong conservation (GerpRS > 2), whereas the remaining splice sites match genome-wide expectations (turquoise). b Breakdown of GerpRS scores according to splice site activity (weak, alternative or constitutive). c Phylogenetic age of donor/acceptor sites according to splice site activity (weak, alternative or constitutive), and breakdown of the age of constitutive splice sites, across various categories of genes. These include all genes, immune response genes (GO:0006955), stimulation-induced genes and the intersection of the latter two categories. Significance was obtained by resampling, matching for the phylogenetic age of the gene and its expression. *Resampling p < 0.001. d Example of a human-specific splice site at the CSF3 gene. The phylogenetic tree is drawn according to the established phylogeny, and aligned sequences are shown at the two-base pairs matching the human splice site. Dashes (–) indicate alignment gap. Source data are provided as a Source Data file
We next determined whether such a decreased conservation reflects higher redundancy of ancient splice sites or increased rate of recent splice sites among immune genes, and dated human splice sites based on their occurrence in the vertebrate phylogeny (Supplementary Note 1). Immune genes were defined based on both Gene Ontology (GO:0006955, immune response) and their fold-change of expression in response to stimulation (log2FC > 1 in at least one stimulation condition). Overall, the age of splice sites was found to be strongly correlated to their activity; for instance, 94% of constitutive splice sites appeared before the split with marsupials more than 173 MY ago (Fig. 1c). We then assessed the age of splice sites of immune genes, and found that their constitutive splice sites were 8–12% younger than those of random genes with similar expression levels and phylogenetic ages (overall: 452.9 MY, immune response GO: 417.5 MY, stimulation induced: 397.9 MY, resampling p < 1.5 × 10−6, Fig. 1c). Similar trends were observed for alternative splice sites (overall: 292.5 MY, immune response GO: 247.7 MY, stimulation induced: 221.5 MY, resampling p < 2.1 × 10−4).
We subsequently searched for splice sites that differ between human and non-human primates, focusing on the 174,138 splice sites of coding exons that could be aligned in >80% of primate lineages. We identified 28 high-confidence human-specific splice sites (Supplementary Data 1), several of which involved immune genes such as the interferon-inducible gene NUB1, the leukocyte immunoglobulin-like receptor LILRB4, or the cytokine CSF3 that is responsible for the regulation of granulocyte survival and proliferation (Fig. 1d). Altogether, these results support a more recent emergence of splice sites at immune genes, with respect to non-immune genes, and suggest that AS has been an important substrate of innovation in the evolution of human immune responses.
Widespread impact of immune activation on isoform usage
To characterise the variability of splice site usage in humans, we next quantified inter-individual differences in AS at the basal state and following immune stimulation. Focusing on highly expressed genes (FPKM > 10), we quantified 39,030 AS events and calculated the percent-spliced-in (PSI) index, which reflects the frequency at which a specific splicing event occurs. After quality checks and filtering of redundant AS events (Methods; Supplementary Fig. 2a), we obtained a final set of 16,173 independent AS events that affect a total of 4,739 genes (Fig. 2a; Supplementary Data 2, Supplementary Fig. 2b–d). Among AS events, 56% were associated with a switch between two functional protein-coding isoforms (N = 9,098, referred to as modified protein), 37% with a switch between a protein-coding and a non-coding isoform (N = 6,067, gain/loss-of function), and 6% with a switch between two non-coding isoforms (N = 1,008, non-coding). Among cases of gain/loss-of-function events, the non-coding isoform was lowly expressed in most cases (Supplementary Fig. 2e), suggesting strong constraints towards the expression of functional isoforms and/or a high rate of degradation of non-functional transcripts.
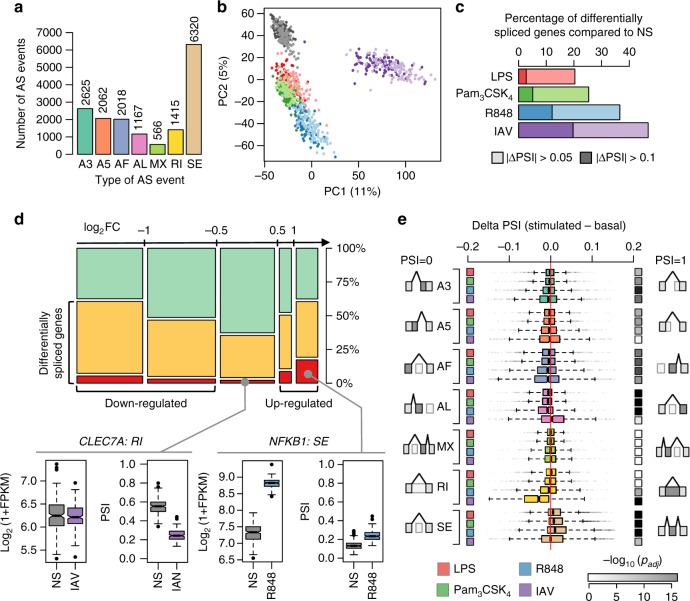
Fig. 2.
Immune stimulation induces major shifts in alternative splicing (AS) patterns. a Number of detected AS events by type: A3, alternative 3′ splice site; A5, alternative 5′ splice site; AF, alternative first exon; AL, alternative last exon; MX, mutually exclusive exons; RI, retained intron; SE, skipped exon. Each type of AS event is presented in a different colour that is consistently used across all figures. b Principal components analysis of AS events in different conditions: non-stimulated (grey), and activated with LPS (red), Pam3CSK4 (green), R848 (blue) and IAV (purple). Light and dark shades represent European and African individuals, respectively. The same colour code is used for conditions, across all figures. c Percentage of differentially spliced genes across conditions. d Percentage of genes differentially spliced after stimulation (in at least one condition), according to maximal fold-change in gene expression upon stimulation (log2FC). Non-differentially spliced genes are shown in green, and differentially spliced genes in orange. Among the latter, immune response genes, as defined by GO, are highlighted in red. Levels of genes expression—log2(1 + FPKM)—and alternative splicing—PSI—are shown for two immune genes, CLEC7A and NFKB1, at the basal state and in the stimulation condition where the changes in isoform usage are the most pronounced (centre line, median; box limits, upper and lower quartiles; whiskers, 1.5× interquartile range; points, outliers). Boxplots are coloured according to the condition of stimulation. e Boxplots showing the shifts in PSI values, for each type of AS event and stimulus (boxplots drawn as previously). The nature of the stimuli is indicated on the left-hand side by coloured squares and significance is indicated on the right-hand side by grey squares (darker for increased significance). Boxplots are coloured according to the type of AS event considered. Source data are provided as a Source Data file
Principal component (PC) analysis, after adjusting PSI values for batch effects and technical variability (Methods; Supplementary Note 2; Supplementary Fig. 2f), revealed immune activation as the main source of splicing variability, with PC1 and PC2 reflecting the effects of IAV infection and TLR activation, respectively (Fig. 2b). Focusing on genes that change their isoform levels upon stimulation, we found 1,919 genes that were differentially spliced in at least one condition, with respect to the basal state (5% false discovery rate (FDR), |ΔPSI| > 0.05, Supplementary Data 2). A large number of differentially spliced genes (59%) displayed differential isoform usage in a stimuli-specific manner, with 789 being differentially spliced in a single condition (Supplementary Fig. 3a). The changes were markedly stronger following treatment with viral ligands (R848 and IAV), irrespectively of the |ΔPSI| threshold considered (Fisher’s exact test for equal proportions; |ΔPSI| > 0.05; p < 2.6 × 10−23, |ΔPSI| > 0.1; p < 6.2 × 10−25, Fig. 2c).
We found that genes that are differentially expressed upon stimulation (i.e., |log2FC| > 0.5) are more likely to change their isoform ratios (Fisher’s exact test; OR = 2.2, p < 4.34 × 10−26, Fig. 2d). This involved key transcription factors controlling inflammatory and antiviral responses, such as NFKB1 (change of protein isoform upon all TLR stimulations, Wilcoxon p < 2.5 × 10−26, |ΔPSI| > 0.06, max |ΔPSI| = 0.11, log2FC > 1.2, Fig. 2d) and STAT2 (increased percentage of protein-coding transcripts upon R848 and IAV stimulations, Wilcoxon p < 6.9 × 10−41, |ΔPSI| > 0.1, max |ΔPSI| = 0.19, log2FC > 0.8). Notably, we identified 460 genes presenting differential isoform usage after stimulation that were missed when searching for expression changes at the gene level (|log2FC| < 0.2, Supplementary Data 2). An interesting example is provided by CLEC7A, a C-type lectin sensing β-glucans, which is differentially spliced across all stimulations (Wilcoxon p < 2.9 × 10−19, |ΔPSI| > 0.08), but does not change its expression in the IAV stimulation where the changes in splicing are the strongest (increased percentage of protein-coding transcripts, max |ΔPSI| = 0.28, Fig. 2d). These results reveal a wide array of AS modifications upon monocyte exposure to immune challenges, and highlight the importance of considering isoform modifications in the context of immune function.
Understanding the nature of isoform changes upon stimulation
We next explored the qualitative nature of isoform changes in response to immune or infectious challenges. We found systematic shifts in splicing patterns upon stimulation (Fig. 2e), including increased inclusion of cassette exons (skipped exon class, SE, Wilcoxon p < 2.9 × 10−124) and usage of upstream transcript termination sites (alternative last exon class, AL, Wilcoxon p < 5.4 × 10−23) as observed in response to bacterial infections18. In addition, we detected a decrease in intron retention (retained intron class, RI, Wilcoxon p < 5.9 × 10−67) that was specific to IAV stimulation. Consistent with previous findings17–20, these qualitative changes were associated to an increased usage of the minor isoforms upon stimulation, leading to higher isoform diversity upon immune challenge for 70% of the genes.
Focusing on gain/loss-of-function AS events, we observed a strong shift towards an increase in non-coding isoforms upon all stimulations (Wilcoxon p < 1.1 × 10−29, Supplementary Fig. 3b). However, such an increase was found to be less common among upregulated genes (Fisher’s exact test; OR = 0.68, p < 0.008) and more frequent among downregulated genes (Fisher’s exact test; OR = 1.8, p < 1.2 × 10−9) (Supplementary Fig. 3c). This observation suggests a contribution of AS-induced nonsense-mediated decay (AS-NMD) to the regulation of gene expression. To assess the extent to which AS-NMD may play an active role to downregulate gene expression or simply result from splicing errors, we focused on exons whose inclusion leads to a nonsense isoform. We reasoned that splice sites that are used to actively regulate gene expression through NMD are likely to be evolutionary conserved, whereas those that disrupt essential proteins will tend to be removed by natural selection. We found that 35% of the exons examined have at least one conserved splice site, consistent with an active role in downregulating gene expression (Supplementary Fig. 3d). Together, these findings increase our understanding of the nature of isoform changes in response to immune stimuli, and support a role of AS as an additional layer of immune response regulation through AS-NMD.
Increased noisy splicing upon immune activation
The increase in non-coding isoforms detected in response to immune stimulation could also reflect noisy splicing (i.e., splicing leading to non-functional transcripts)22–24. To explore this possibility, we quantified the frequency at which constitutive donor or acceptor sites are joined with cryptic (unannotated) splice sites, as a measure of mis-splicing events. We then defined the rate of noisy splicing of a gene as the average of these frequencies across all its constitutive splice sites (Fig. 3a). At the basal state, ~0.29% of splicing events on average across genes corresponded to noisy splicing (Fig. 3b; Supplementary Data 2), a figure that ranged from <0.001% (6% of multi-exonic genes) to >10% (0.2% of multi-exonic genes). Indeed, noisy splicing increased with mean intron length (Spearman’s ρ = 0.33, Student’s p < 2.8 × 10−231), and decreased with gene expression levels (Spearman’s ρ = −0.23, Student’s p < 4.6 × 10−109). Interestingly, noisy splicing also decreased with levels of nascent mRNAs (measured from intronic reads, Spearman’s ρ = −0.19, Student’s p < 1.8 × 10−74), suggesting coupling between transcription rate and splicing efficiency. However, gene-to-gene differences in transcription rate could not fully account for the correlation between noisy splicing and gene expression levels (Spearman’s ρ = −0.16, Student’s p < 3.2 × 10−50 after adjusting on mean intronic coverage), consistent with the degradation by NMD of non-functional transcripts produced through noisy splicing.
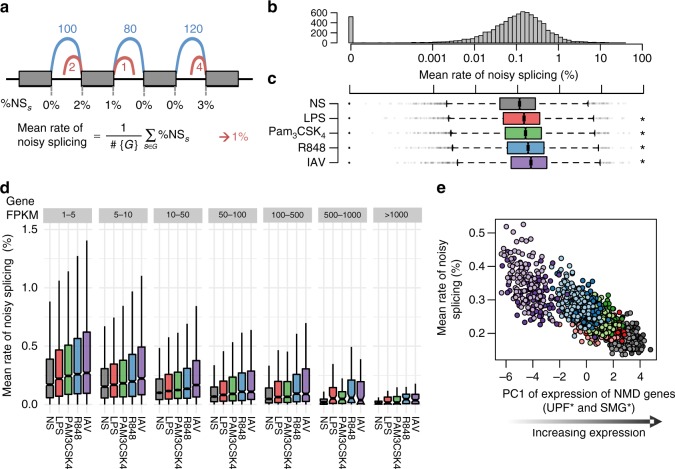
Fig. 3.
Increase in noisy splicing upon immune stimulation. a For each constitutive splice site neighbouring a coding exon, functional splicing events (blue) that join two exons are distinguished from non-functional splicing events (red) where the splice site is joined with a cryptic, non-conserved splice site (GerpRS < 2). For each gene, the mean rate of noisy splicing was computed as the average across all constitutive splice sites of the frequency at which non-functional splicing events occur. b Distribution of the rate of noisy splicing at the basal state. c Distribution of the levels of noisy splicing per gene according to the condition of stimulation (centre line, median; box limits, upper and lower quartiles; whiskers, 1.5× interquartile range; points, outliers). The significance of differences between noisy splicing in each stimulation condition and the basal state was assessed by a Wilcoxon rank test (*p < 10−20). d Distribution of the rate of noisy splicing per gene as a function of gene expression. For each bin of expression, the rate of noisy splicing is shown across all conditions of stimulation (boxplots drawn as previously). e Average rate of noisy splicing per sample correlates with expression of nonsense-mediated decay genes (first PC). For each sample, the colour reflects the condition of stimulation (grey: NS, red: LPS, green: Pam3CSK4, blue: R848, purple: IAV). Light and dark shades indicate European and African individuals respectively. Source data are provided as a Source Data file
Upon stimulation, we found that noisy splicing increased by 15–67% on average (Wilcoxon p < 5.1 × 10−22, Fig. 3c), with this increase being detected across all ranges of gene expression (Wilcoxon p < 5.1 × 10−5, Fig. 3d). To verify that this observation was not explained by the improved detection of cryptic splice sites after stimulation due to higher gene expression, we measured the change in the number of cryptic splice sites and noisy splicing per gene, according to fold changes in gene expression upon stimulation. We found that while the detection of cryptic splice sites does correlate with gene expression, the rate of noisy splicing increases for both up and downregulated genes (Supplementary Fig. 4). We further observed that the increase in noisy splicing detected upon stimulation was associated to a decreased expression of NMD genes (Spearman’s ρ = −0.81, Student’s p < 4.5 × 10−226, Fig. 3e). These results indicate that while splicing errors increase in response to immune stimulation due to reduced NMD activity, such errors are less common amongst highly transcribed genes, thereby mitigating their impact on immune gene expression.
Characterising the genetic bases of splicing regulation
To investigate the genetic regulation of splicing variability, we mapped sQTLs, i.e., genetic variants associated with changes in AS. We tested the 16,173 AS events for association, in cis, with 5,634,819 single nucleotide polymorphisms (SNPs) at a minor allele frequency (MAF) > 0.05, located within a 1 Mb-window of AS events. At a 5% FDR, we identified 1,464 AS events significantly associated with a SNP, corresponding to 21% of the 4,739 AS genes (Supplementary Data 3). We found an excess of sQTLs among immune response genes (non-central hypergeometric test (GOseq); OR = 1.7, p < 1.5 × 10−8), receptors (OR = 2.3, GOseq p < 3.2 × 10−6) and genes located at the cell periphery (OR = 1.5, GOseq p < 2.5 × 10−9) (Supplementary Data 3). These enrichments remained significant when excluding the HLA region (GOseq p < 2.4 × 10−5), and accounting for gene expression levels (resampling p < 1.0 × 10−4) or a higher rate of AS events at immune response genes (resampling p < 1.0 × 10−4).
After excluding the HLA region, 66% of sQTLs were located within 10 kb of their respective AS event (Fig. 4a), with 38% falling directly within the boundaries of the AS event. We found that sQTLs were strongly enriched in exonic and near-exonic regions (<300 nucleotides from splice site, OR > 5.7, Fisher’s exact p < 1.5 × 10−84), and in loci predicted to alter splicing (SPIDEX database36, OR = 4.7, Fisher’s exact p < 1.7 × 10−19, Fig. 4b). Consistently, we found a strong enrichment of sQTLs in splicing regulatory regions (e.g., branchpoint, OR = 17.9, Fisher’s exact p < 3.5 × 10−75), RNA-binding motifs (e.g., RBM4; OR = 4.5, Fisher’s exact p < 3.1 × 10−96), highly conserved sites (GerpRS > 4; OR = 3.1, Fisher’s exact p < 1.7 × 10−7), as well as promoter regions (OR = 8.6, Fisher’s exact p < 4.61 × 10−94; Supplementary Fig. 5a).
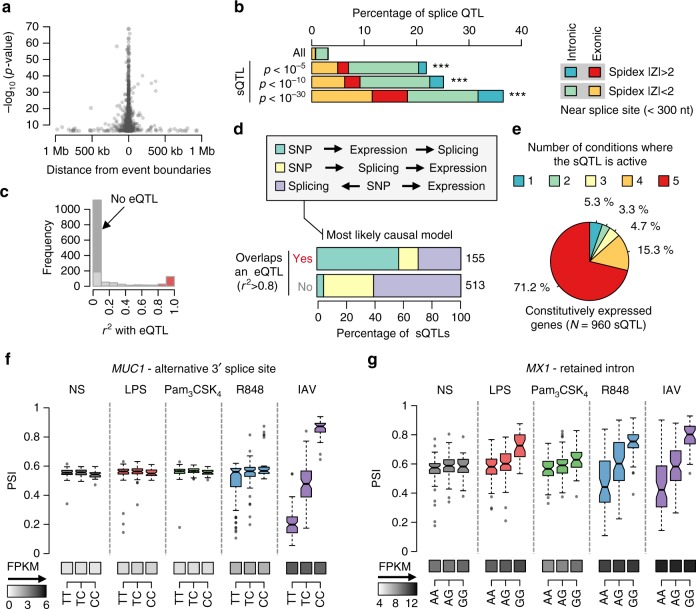
Fig. 4.
Genetic regulation of splicing variation in response to immune stimulation. a Distance of splice QTLs (sQTLs) to their associated AS event. Most sQTLs are located in close proximity to their respective AS event. b Enrichment of sQTLs in near-exonic regions and sites predicted to alter splicing by SPIDEX. Twenty percent of sQTLs are located in exonic or intronic regions located <300 nucleotides (nt) apart of a splice site. The enrichment increases with the levels of significance of sQTLs (***Fisher’s p < 1.1 × 10−16). c Overlap between all sQTLs and eQTLs identified for the same gene and condition. sQTLs of genes without eQTLs are shown in dark grey, and sQTLs that colocalize with an eQTL (r2 > 0.8) are shown in red. d Frequency of inferred causal model among sQTLs associated with gene expression. Causality is shown separately for sQTLs overlapping a cis-eQTL (r2 > 0.8), and sQTLs that show marginal associations with gene expression (pexpr < 0.05 in the condition where the sQTL is the strongest) but do not overlap an eQTL. For each group, the total number of sQTLs is reported. e Sharing of sQTLs across conditions. For the 960 sQTLs where the corresponding gene is expressed across all conditions, the pie chart represents the number of conditions where the sQTL is active. f Example of a context-specific sQTL that manifests only upon IAV infection because the expression of the corresponding MUC1 gene is restricted to the IAV condition. For each genotype and condition, the boxplots show the distribution of PSI (centre line, median; box limits, upper and lower quartiles; whiskers, 1.5× interquartile range; points, outliers). For each genotype and conditions, grey squares indicate FPKM levels (darker shade = higher gene expression). g Response sQTL (rsQTL) leading to differential intron retention in the 5′-UTR region of the MX1 gene in response to LPS, R848 and IAV (boxplots drawn as previously). f, g Boxplots are coloured according to the condition of stimulation. Source data are provided as a Source Data file
Untangling the genetic control of splicing and transcription
We next investigated whether the same regulatory variants were associated with changes in splicing (sQTLs) and gene expression (expression quantitative trait loci (eQTLs)) (Methods; Supplementary Data 3). Remarkably, 11% of sQTLs overlap an eQTL (N = 155, r2 > 0.8, Fig. 4c), and ~35% of sQTLs (N = 513) showed marginal associations with the expression levels of the gene they regulate (peQTL < 0.05). Three scenarios can explain the link between splicing and gene expression: (i) changes in isoform levels may alter gene expression through NMD, (ii) transcription could lead to AS by activating, for example, an alternative transcription start site (TSS) or by increasing transcriptional elongation rate and preventing exon recognition, and (iii) the co-occurrence of a sQTL with an eQTL results from linkage disequilibrium (LD) between variants that regulate splicing and transcription1,20,43.
To discern between these scenarios, we used a two-step approach; we first tested for a link between splicing and gene expression when accounting for the effects of genetics, to subsequently assess the direction of this link by comparing the likelihood of each causality model44 (Methods; Supplementary Fig. 6; Supplementary Note 3). Among the 155 sQTLs overlapping an eQTL, splicing remained significantly correlated to gene expression, after adjusting for genotype, in ~70% of the cases (Fig. 4d). Among these, we identified 21 sQTLs where splicing modifications lead to gene expression changes (causal sQTLs, ~14% of tested sQTLs). For example, the rs2927608 variant at ERAP2 is associated with an increased proportion of nonsense isoforms (Student’s test; βsQTL = −0.3, psQTL = 1.7 × 10−66) that lead to decreased gene expression via NMD (Student’s test; βeQTL = −0.6, peQTL = 3.2 × 10−53)19.
However, the most frequent scenario (~57% of tested sQTLs) was that of a reactive sQTL (Supplementary Fig. 6), where splicing changes are mediated by gene expression. This is illustrated by the rs1317397 variant, located in the promoter flanking region of the chemokine receptor CMKLR1, which is associated to a strong decrease in expression (Student’s test; βeQTL = −0.6, peQTL = 1.6 × 10−20) that leads to an increased inclusion of exon 3 (Student’s test; modified protein, βsQTL = 0.03, psQTL = 7.3 × 10−10). Extending our analysis to the 513 sQTLs that present marginal associations with gene expression but do not overlap an eQTL, we found that the occurrence of causal sQTLs increased to 34%, whereas that of reactive sQTLs decreased to ~5% (Fig. 4d). This suggests that the majority of causal sQTLs have a moderate effect on gene expression.
Finally, to assess whether reactive sQTLs could act through the modulation of transcriptional elongation rates, we analysed the effect of sQTLs on transcription rate, using intronic reads as a proxy for nascent mRNA levels45. We detected a significant effect for 89% of reactive sQTLs, compared with 25% of sQTLs that do not correlate with expression (OR = 24.2, Fisher’s exact p < 9.1 × 10−28). Together, these analyses indicate that the genetic determinants of isoform usage and gene expression are largely independent; yet, when they are not, the regulation of AS through modulation of the transcription rate seems to be the predominant model.
Mapping the genetic bases of context-specific splicing
We subsequently explored the context specificity of sQTLs, using a Bayesian model selection approach (Supplementary Note 4) to identify cases where the genetic regulation of isoform usage is altered depending on the presence or absence of immune stimuli. Focusing on genes expressed in all conditions, we found that 71% of sQTLs (N = 684) were shared across all conditions, whereas 5.3% of them (N = 51) were detected in a single condition (Fig. 4e; Supplementary Fig. 5b).
We then searched for sQTLs that manifest only in the presence of immune stimuli (Supplementary Data 3), because either the gene is expressed only upon immune challenge (sQTLs of stimulation-specific genes) or the genetic variant alters splicing in a stimulation-specific manner (response sQTLs, rsQTLs) reflecting G × E interactions. Focusing of the 274 stimulation-specific genes (FPKM < 10 at basal state and log2FC > 1 in at least one condition), we detected 74 genes with at least one sQTL, for a total of 108 sQTLs. For example, the rs4072037 variant induces a change in 3′ splice sites of MUC1, encoding a protein at the cell surface of lung epithelial cells, which is expressed only upon IAV infection (modified protein, Student’s test; psQTL < 1.1 × 10−54, Fig. 4f). To search for rsQTLs, we focused on genes expressed both at the basal state and after stimulation (FPKM > 10), and found 127 rsQTLs showing a significantly stronger effect in response to stimulation (prsQTL < 0.01, corresponding to ~5% FDR). For instance, the influenza resistance gene MX1, while being expressed at basal state, displays genotype-dependent intron retention specifically upon stimulation by LPS, R848 or IAV (rs462687, modified protein, Student’s test; prsQTL < 6.7 × 10−16, Fig. 4g). These examples highlight how cellular perturbation with external stimuli can reveal, or alter, the genetic regulation of AS in the context of immunity to infection.
AS contributes to immune disorders
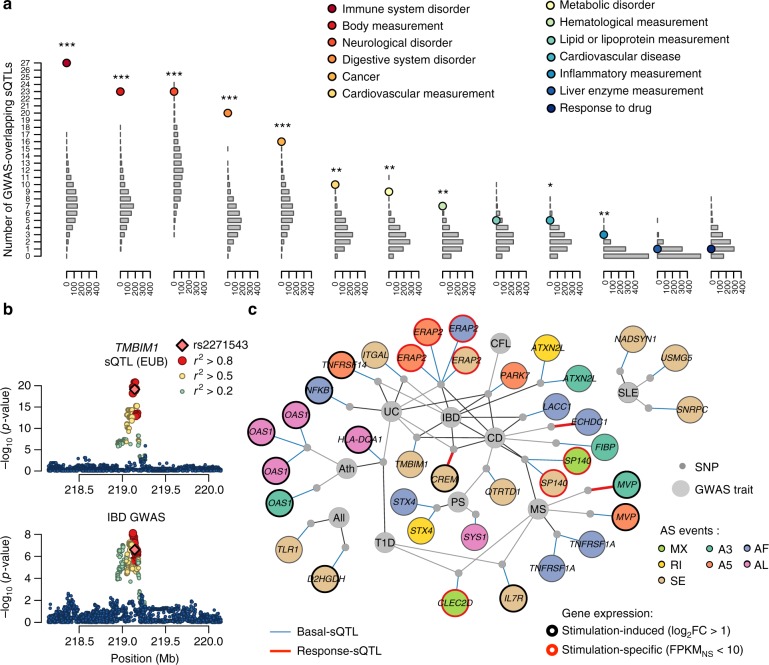
To understand how splice regulatory variants may ultimately impact organismal traits, we searched for overlaps between sQTLs and loci associated with complex traits or diseases by genome-wide association studies (GWAS)46. We identified 195 sQTLs that overlap GWAS hits (Methods; Supplementary Data 3). When grouping traits according to their Experimental Factor Ontology (EFO) category47, we found that loci associated with immune system disorders such as ulcerative colitis or type-1 diabetes (N = 27, OR = 4.6, resampling p < 0.001) and body measurements such as height or body mass index (N = 25, OR = 3.1, resampling p < 0.001) were the most significantly enriched (Fig. 5a).
Fig. 5.
Innate immunity sQTLs contribute to complex traits and immune disorders. a Enrichment of sQTLs in GWAS hits. For each trait/disease category, coloured dots show the number of sQTLs overlapping a GWAS locus. Grey bars represent the distribution of the expected number of GWAS hits when resampling 1000 random SNPs matched for minor allele frequency (resampling p-value, *p < 0.05, **p < 0.01, ***p < 0.001). b Log10(p-value) profiles of the TMBIM1 sQTL (upper panel) and GWAS for inflammatory bowel disease (IBD, lower panel). SNPs are coloured according to their LD (r2) with the sQTL peak SNP and putative causal variant rs2271543. Only SNPs where both sQTLs and GWAS summary statistics were available are shown. c sQTLs colocalizing with GWAS loci for 10 major immune-related disorders (grey nodes, Ath asthma, All allergy, CEL celiac disease, CD Crohn’s disease, IBD inflammatory bowel disease, MS multiple sclerosis, PS psoriasis, SLE systemic lupus erythematous, T1D type-1 diabetes, UC ulcerative colitis). For each sQTL, genetic variants are represented by grey dots and are linked to the associated AS event (coloured nodes, named after their associated gene). AS events are coloured by class of event and circled according to gene expression patterns upon stimulation (black: upregulated—log2FC > 1 in at least one condition, red: specific to stimulation—FPKM < 10 at basal state and log2FC > 1). Links between AS events and sQTLs indicate stimulation-specificity of the genetic association (blue edge for sQTLs, red for response sQTLs—p-value > 0.001 at basal state and prsQTL < 0.01). Links between sQTLs and GWAS traits indicate colocalization (r2 > 0.8 between sQTLs and GWAS SNPs in Europeans) and are coloured according to the strength of the GWAS association (grey: GWAS p < 10−5, black: GWAS p < 10−8). Source data are provided as a Source Data file
Among disease-associated sQTLs at the basal state, we identified a variant (rs2271543) that promotes the inclusion of a 5′UTR exon in TMBIM1 (modified protein, Student’s test; psQTL < 3.1 × 10−33, R2 > 0.54, |SPIDEX’s Z| = 2.1) and colocalizes with a susceptibility locus for inflammatory bowel disease48 (Fig. 5b). Similarly, a variant (rs1333973) affecting the exclusion of exon 2 of the antiviral gene IFI44L (modified protein, Student’s test; psQTL < 1.1 × 10−56, R2 = 0.72, SPIDEX’s Z = 3.9) is associated with measles-specific humoral immunity and increased risk of febrile seizures49,50. Finally, a non-synonymous variant (rs1127354) leading to the removal of the second exon of ITPA (modified protein, Student’s test; psQTL < 2.8 × 10−12, R2 > 0.22, |SPIDEX’s Z| = 2.6) associates with increased susceptibility to hepatitis C virus infection and ribavirin-induced anaemia51.
Focusing on the 235 sQTLs that manifest only upon immune stimulation, we found 20 sQTLs of stimulation-specific genes and 14 rsQTLs that overlap GWAS hits. Of these 34 sQTLs, 12 were associated with immune disorders (Fig. 5c; Supplementary Data 3). In addition to known disease-causing sQTLs at SP140 or ERAP219,52,53, we detected novel cases at immune genes such as MVP and CLEC2D, which encode key regulators of the JAK/STAT and interferon-γ pathways, respectively. The MVP variant (rs13332078) promotes the usage of an alternative 3′ splice site upon stimulation with R848 (modified protein, Student’s test prsQTL < 0.007), and is associated with increased risk of multiple sclerosis54. The CLEC2D variant (rs7968401), involving a switch between mutually exclusive exons after TLR activation (modified protein, Student’s test psQTL < 9.4 × 10−10, R2 = 0.17), overlaps hits associated with multiple sclerosis and type-1 diabetes55,56. These results not only support the importance of AS as a mechanism explaining variation of complex traits2,19,20,27,35,36, but also highlight immune pathways where differential isoform usage following cellular perturbation may influence immune disease outcomes.
Differential splicing as a substrate of local adaptation
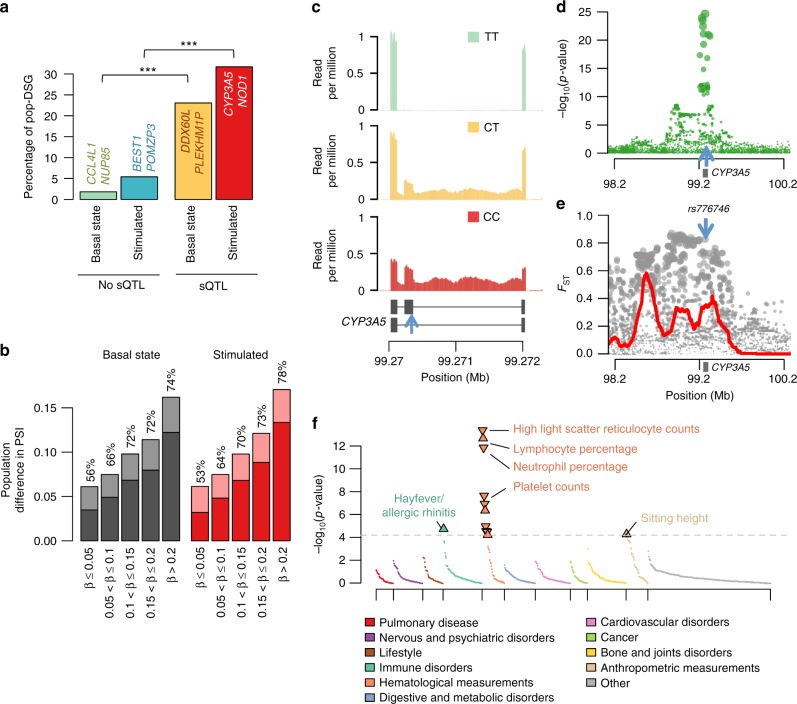
To investigate the role of splicing as a mechanism of local adaptation, we first searched for AS events that presented different PSI values between African- and European-descent individuals (FDR < 5%, |ΔPSI| > 0.05). We found 515 genes that were differentially spliced between populations in at least one condition (pop-DSG, Supplementary Data 4), of which 60% (N = 309) were detected only after stimulation (Supplementary Fig. 7a). Among pop-DSG presenting the largest population differences (|ΔPSI| > 0.1), we found key regulators of the immune response, such as the microbial sensor NOD1, upon R848 and IAV stimulation (modified protein, Wilcoxon p < 6.5 × 10−20, |ΔPSI| > 0.27, max |ΔPSI| = 0.37), and the transcription factor NFKB1, after all TLR stimulations (modified protein, Wilcoxon p < 6.1 × 10−12, |ΔPSI| > 0.16, max |ΔPSI| = 0.22). Furthermore, pop-DSG were strongly enriched in sQTLs, both at the basal state and after stimulation (OR > 8.1, Fisher’s p < 3.5 × 10−88, Fig. 6a). Using causal mediation analysis57, we found that among pop-DSG with sQTLs, differences in sQTL allele frequency between populations account for 67% of population differences in splicing levels, a figure that reached 78% for the strongest sQTLs (i.e., βsQTL > 0.2, Fig. 6b and Supplementary Fig. 7b).
Fig. 6.
Population differences in alternative splicing and impact of positive selection. a Percentage of genes showing differential splicing between populations (pop-DSG) at the basal state and after stimulation, classified according to the presence of a sQTL (Fisher’s exact test for equal proportion, ***p < 10−20). For each group, examples of genes that present the strongest population differences are reported. For the stimulated state, the reported genes are chosen among those that are differentially spliced specifically after stimulation. b Contribution of genetic factors to the detected population differences in splicing patterns. The mean difference in PSI value between populations is represented, at the basal state (grey) and after stimulation (red), for genes presenting an sQTL with various levels of effect size (β). For each group, the average difference in PSI value that is mediated by the sQTL is shown in dark shade, and the corresponding percentage is indicated above the bar. c The derived allele (C) of the sQTL rs776746 (blue arrow) is associated with the inclusion of an additional exon at the CYP3A5 gene. d Association between SNPs in a 2 Mb window around the sQTL rs776746 and splicing levels of CYP3A5 in the Pam3CSK4 condition. e Signals of positive selection around the CYP3A5 locus, measured by the FST metric. The red curve shows the proportion of SNPs with extreme FST values (>95% percentile), in a 100 kb window around each SNP. f Phenome-wide association study of the SNP rs776746, based on 778 phenotypic traits measured by the UK biobank study63. The dashed line indicates the Bonferroni-corrected significance threshold. For significantly associated traits, arrows indicate the direction of the effect of the derived allele (c). Source data are provided as a Source Data file
Once established that a large fraction of population differences in splicing can be attributed to genetic factors, we searched for sQTLs that presented signatures of positive selection, based on levels of population differentiation (FST) and extended haplotype homozygosity (integrated haplotype score (iHS))58,59. To reduce false positives, we considered as selection candidates only loci presenting both the strongest selection signatures (>95th percentile genome-wide for FST or |iHS|) and an enrichment of SNPs with such signals around the sQTL59,60. In doing so, we detected 29 sQTLs presenting extreme population differentiation (FST > 0.4, Supplementary Data 4). Among these hits, the strongest FST was found at the RAB GTPase activator RABGAP1 (rs59393793, FST = 0.84, pemp = 1.2 × 10−4, beta-binomial test penrich < 0.01), with Europeans showing an increased frequency of the derived allele associated with a diminished amount of the protein-coding isoform. Focusing on immune genes, the sQTL with the strongest FST was found for the CD226 locus, encoding a glycoprotein involved in T-cell activation (rs1823778, FST = 0.75, pemp < 9.2 × 10−4, beta-binomial test penrich < 0.007), also showing a higher frequency of the derived allele in Europeans but, in this case, leading to a qualitative change in the resulting protein-coding isoform.
With respect to |iHS| signals, which reflect more recent events of positive selection59, we detected 33 sQTLs presenting outlier values (13 in European and 20 in Africans, Supplementary Data 4). Among these, the strongest signals of adaptation were found for the lysozyme regulator HEATR7A in Africans (rs6558326, |iHS| = 3.1, pemp = 0.003, beta-binomial test penrich < 0.05), and for the meiosis regulator CPEB2 in Europeans (rs12502866, |iHS| = 3.6, pemp = 0.0007, beta-binomial test penrich < 0.003). The largest |iHS| among immune genes was found at the P2RX7 locus (rs208293, |iHS| = 2.95 in Europeans, pemp = 0.003, beta-binomial test penrich < 0.05), which is involved in inflammasome activation and intracellular pathogen clearance61.
Among sQTLs of stimulation-specific genes, we found the strongest signal of selection for the rs776746 variant, which C allele promotes the inclusion of an additional exon in CYP3A5 upon all TLR stimulations. This allele, which decreases the percentage of protein-coding isoform, presents a very high frequency in Europeans (FST = 0.73, pemp = 0.001, beta-binomial test penrich < 0.005, Fig. 6c–e; Supplementary Fig. 7c). Interestingly, the C allele has been reported to protect against paediatric tuberculosis62 and, using the UK Biobank atlas of genetic associations63, we found it associated with varying proportions of neutrophils, reticulocytes and lymphocytes, and increased risk of allergic rhinitis (Fig. 6f). Together, these results highlight the important role of splicing not only as a source of population variation of immune responses but also as a potential driver of local adaptation.
Archaic introgression contributed to splicing variability
Because admixture between modern humans and Neanderthals has been shown to contribute to present-day population differences in immune responses29,38,64, we explored how Neanderthal introgression has affected splicing. We overlapped our list of sQTLs with a set of 100,755 SNPs putatively brought into European genomes by Neanderthal introgression (Supplementary Note 5). We detected eight loci where the presence of Neanderthal haplotypes promotes differential isoform usage (Supplementary Data 4), representing a 2.8-fold enrichment with respect to genome-wide expectations (resampling p < 7.0 × 10−3).
Among archaic sQTLs, we observed the previously described rs10774671 associated with AS at OAS1 (ref. 53). We also found novel cases, including sQTLs at the microbial sensor TLR1 (rs5743593, archaic allele associated with a change in protein-coding isoform, Student’s test p < 2.1 × 10−11 in the non-stimulated state, MAFEU = 0.165), the cytosolic sensor DDX60L (rs56999040, decreasing protein-coding transcripts, p < 3.2 × 10−8 in the non-stimulated state, MAFEU = 0.165), shown to inhibit viral replication in vitro65, and the Fc-γ receptor FCGR2A (rs373579, increasing protein-coding transcripts, p < 1.6 × 10−9 after stimulation by LPS, MAF = 0.14), triggering phagocytosis of bacterial antigens after IgG stimulation66. Overall, these results show that introgression of splice regulatory variants from Neanderthals has also contributed to diversify the patterns of immune response variation currently observed among individuals of European ancestry.
Discussion
Several important insights can be drawn from our study. First, we show that activation of major innate immunity pathways, such as TLR1/2, TLR4 and TLR7/8, and infection with a live strain of IAV increase isoform diversity and elicit a marked remodelling of the isoform repertoire. Our results are consistent with previous findings of global shifts in response to bacterial and viral stimuli detected in macrophages or dendritic cells17–20, but show that such an elevated isoform diversity can be attributed, to a large extent, to a global increase in splicing errors leading to non-functional transcripts. Erroneous splicing has been previously detected in lymphoblastoid cell lines, in the absence of any stimulation23,24. We find that noisy splicing increases not only upon stimulation by live pathogens but also with synthetic stimuli, such as TLR ligands, suggesting that this phenomenon is driven by the host itself. A plausible explanation could be a reduction in the activity of NMD upon stimulation, leading to reduced degradation of mis-spliced transcripts. This is consistent with the increase in the number of truncated/non-translatable transcripts reported after infection of macrophages by Mycobacterium tuberculosis17. It has been shown that highly expressed genes are less prone to erroneous splicing, following the removal of genetic variants leading to non-functional isoforms by purifying selection24. Our findings indicate that, although immune stimulation increases the degree of splicing errors, highly expressed genes tend to maintain a reduced error rate after stimulation, allowing for an efficient immune response.
We also provide evidence for condition-specific genetic regulation of isoform usage. We found a total of 993 genes with evidence of genetic control of AS in resting or stimulated monocytes. Although such genetic regulation was largely shared across conditions, we detected a large number of regulatory variants acting in a context-dependent manner (up to 28% of the total number of detected sQTLs). Notably, ~9% of sQTLs altered splicing only in the presence of immune stimulation, regardless of gene expression levels, suggesting the occurrence of G × E interactions. Furthermore, our analyses indicate that the genetic control of AS is largely independent to that of transcription, as hitherto observed19,20,25,27,31, but extend previous findings by inferring causality between regulatory mechanisms when these are not independent. Through the analysis of the conditional independence structure between genotypes, gene expression and isoform ratios, we show that changes in splicing are primarily mediated by changes in gene expression, rather than the opposite. This finding, supported by our analysis of nascent transcript abundance, illustrates the complex interactions between the transcription and splicing machineries1 and highlights the contribution of variants that modulate transcription to AS.
From an evolutionary perspective, this study shows decreased inter-species conservation of immune gene splicing, which is due to an increased rate of splice site emergence at immune genes, as attested by the human-specific splice sites detected at CSF3 and NUB1. Further support to the lower constraint acting on immune gene splicing comes from their enrichment in sQTLs, with respect to the remainder of the coding genome. Given that the immune system is our primary interface with the environment, including pathogens8–10, these findings may also support a selective model that favours diversity. Under such a model, while most variants leading to non-functional transcripts (i.e., noisy splicing) are purged by purifying selection24, genetic variants that alter splicing of immune genes may also be favoured by different forms of adaptive evolution, allowing for a broader repertoire of functional isoforms.
The signals of positive selection we detect at splicing regulatory variants and their enrichment in Neanderthal ancestry further support that differential isoform usage can be a vehicle for local adaptation, or at least contribute to diversify immune responses. For example, the strong frequency increase of the rs776746-C allele at the CYP3A5 locus in Europeans, which triggers the inclusion of an additional exon in response to TLR activation, may reflect the advantageous nature of this variant. Plausible adaptive phenotypes associated with the rs776746-C allele include xenobiotic-metabolism, sodium-sensitive hypertension or decreased susceptibility to paediatric tuberculosis62,67. Similarly, our analyses reveal that the high Neanderthal ancestry we and others have detected at the TLR1 locus68,69 is related to the presence of various alleles that alter TLR1 isoform usage. Together, these results suggest a role of AS as a substrate of human adaptation, following various evolutionary mechanisms that operate at different time-scales. Additional simulation-based work, accompanied by studies of ancient DNA time transects for the direct detection of natural selection (i.e., analyses of temporal changes of allelic frequencies), is now required to validate the action of positive selection at the sQTLs identified.
The case of CYP3A5 also illustrates how past adaptation can have deleterious consequences in the present day, as the putatively selected rs776746-C allele is associated with increased allergic sensitivity in Europeans63. The strong enrichments of sQTLs in variants associated with complex diseases19,20,27,35, in particular chronic inflammatory disorders in our setting, suggest the contribution of splice regulatory variants to the genetic architecture of deleterious phenotypes. Future work using colocalization analyses and Mendelian randomisation approaches should help establishing a causal role of AS in disease risk at the loci identified. As such, the regulatory variants we report here, whether adaptive or not, constitute a valuable resource to explore further how AS contributes to individual and population variability in host responses to infection and immune disease susceptibility.
Methods
Ethics statement
All experiments involving human primary monocytes from healthy volunteers, who gave informed consent, were approved by the Ethics Board of Institut Pasteur (EVOIMMUNOPOP-281297) and the relevant French authorities (CPP, CCITRS and CNIL).
Samples and dataset
The high-density genotyping and RNA-sequencing data used in this study were generated as part of the EvoImmunoPop project38. Briefly, the EvoImmunoPop cohort is composed of 200 healthy, male donors of self-reported African and European ancestry, all living in Belgium (100 individuals of each population).
Genotyping data was obtained, for all individuals, using both Illumina HumanOmni5-Quad BeadChips and whole-exome sequencing with the Nextera Rapid Capture Expanded Exome kit, leading to a total of 3,782,260 SNPs after stringent quality control. This dataset was then used for imputation, based on the 1,000 Genomes Project imputation reference panel (Phase 1 v3.2010/11/23), leading to a final set of 19,619,457 high-quality SNPs, including 7,650,709 SNPs with a MAF >5% in our cohort. Details on filtering criteria and quality control have been provided elsewhere38.
RNA was collected from non-stimulated and stimulated monocytes, which were purified from peripheral blood mononuclear cells (PBMCs) with magnetic CD14 microbeads, and sequenced at a depth of ~34 million single-end reads per sample, yielding a final dataset of 970 transcriptional profiles. The purity of the CD14+ monocyte fraction and its lack of contamination by neutrophils were assessed by flow cytometry (Supplementary Note 6; Supplementary Fig. 8). Monocytes from each individual were exposed, for 6 h, to four stimuli including LPS (bacterial lipopolysaccharide, activating TLR4, n = 184), Pam3CSK4 (synthetic triacylated lipopeptide, activating TLR1/2, n = 196), R848, (imidazoquinoline compound, activating TLR7/8, n = 191) and to a human seasonal IAV (strain A/USSR/90/1977(H1N1), n = 199), and compared with resting monocytes from the same individuals (n = 200). Normalised gene expression values (FPKM) were computed with CuffDiff and corrected for GC content, ratio of average gene body coverage at 5′/3′ ends of the gene, using linear models and for date of experiment and date of library preparation using ComBat70. In addition, htseq-count was used to measure, for each sample, the total number of intronic read counts per gene (defining intronic regions as genic regions that are not part of any known exon). Intronic read counts were then divided by total intron length and total read count of the sample, to yield intronic RPKM that were used as a proxy of the transcription rate in downstream analyses.
Quantification and definition of splice sites
Reads from all samples were aligned on hg19 genome using the HISAT2 software version 2.0.171 and the publicly available genome_snp index, which includes all common SNPs from dbSNP 144. Known splice sites were extracted from Ensembl Genes v70 using the hisat2_extract_splice_sites script, and used as input to the –known-splicesite-infile option to guide the mapping of spliced reads. All other parameters were left to their default value. Spliced reads with an overhang of at least six nucleotides into each exon were then extracted from HISAT2 aligned reads using the filter_cs script from leafcutter package72, and reads spanning over 100 kb, or falling outside genic regions, were discarded to reduce the risk of mapping errors. The resulting set of >7.2 billion spliced reads was used to identify the boundaries of spliced-out introns, and we defined donor and acceptor sites as the two-base pairs located at each end of the detected introns. We then compared the detected splice sites with those of the intropolis database73, which regroups 42,882,032 introns previously observed in the European Nucleotide Archive, and estimated the frequency of non-GT/AG splice sites as a measure of false positive rate (Supplementary Fig. 1a–c). For all subsequent analyses, we filtered spliced reads overlapping multiple genes, reads mapping low expressed genes (FPKM < 1), and reads for which the corresponding intron was absent from intropolis73.
To distinguish splice sites that are present in a majority of transcripts from those that are present only in a subset of transcripts, we considered, for each splice site, the splice site to which it is most frequently joined, and extracted all spliced reads that overlap the corresponding intron (i.e., the most frequently spliced intron). We then estimated the percentage of transcripts where the splice site is active as the ratio of the number of reads that support the splice site, to those that overlap the most frequently spliced intron. We defined high-activity splice sites as those supported by an average of one read per sample in at least one condition, and that are used in at least 5% of transcripts. Remaining sites were considered as weakly active (weak splice sites). Weak splice sites that are absent from Ensembl annotations and show no evidence of conservation (GerpRS < 2, see below) were considered as cryptic. Among high-activity splice sites, those that are used in over 95% of transcripts were considered as constitutive, whereas others were considered as alternative. In addition, splice sites were annotated as coding if they were located next to coding exons in Ensembl v70, and as non-coding otherwise.
Long-term evolutionary analysis of splice sites
To measure the conservation of splice sites across mammals, we retrieved pre-computed base-wise GerpRS scores42 for hg19 from the website of the Sidow Lab (http://mendel.stanford.edu/SidowLab/downloads/gerp/index.html). To reduce the risk of misalignments, we excluded from our analysis positions that (i) had a null GerpRS score, (ii) were absent from the UCSC MULTIZ-46way alignments74 or (iii) had a negative score in the MULTIZ-46way alignments. We then considered, for each splice site, the mean GerpRS score over the 2 bp that constitute the essential splice site (AG/GT sequence, for canonical splice sites) as a measure of splice site conservation. Gene Ontology (GO) enrichment of genes harbouring non-conserved splice sites was performed with GOSeq75, adjusting for the total number of active splice sites in the gene and using all genes with at least one active splice site as background set. Enrichments of non-conserved splice sites among genes that are upregulated upon stimulation was assessed using logistic regression. Specifically, we modelled the probability that a given splice site is conserved as a function of the gene’s fold-change in expression and maximal level of expression across all stimulations. We then tested for a significant effect of being upregulated (log2FC > 1) on the probability of being conserved, adjusting on gene expression, with a likelihood-ratio test. The following bins were used for gene expression: [1 < FPKM ≤ 5], [5 < FPKM ≤ 10], [10 < FPKM ≤ 50], [50 < FPKM ≤ 100], [100 < FPKM ≤ 500], [500 < FPKM ≤ 1000], [1000 < FPKM]. Phylogenetic ages of splice sites were estimated from MULTIZ-46way alignments by reconstructing the ancestral sequence and dating the first occurrence of the splice site based on the ancestors of modern humans (Supplementary Note 1).
To test the enrichment of recent splice sites among immune genes—defined based on either GO (immune response term, GO:0006955) or their response to immune stimulation (log2FC > 1 in at least one condition, with respect to the basal state)—we first estimated the phylogenetic age of each gene as the age of its most ancient splice. We then compared the mean phylogenetic age of splice sites of immune genes with the age of 1000 random sets of splice sites of non-immune genes matched for phylogenetic age and levels of gene expression (using the same bins as previously). When computing p-values, a normal distribution was fit to the null distribution obtained from the resampling to increase the precision beyond 10−3.
Definition and quantification of AS events
Human transcripts were retrieved from Biomart (Ensembl Genes 70—GRCh37), and SUPPA eventGenerator script was used to annotate local AS events (AS events; script available at https://github.com/comprna/SUPPA). This resulted in a total of 163,525 annotated AS events (Alternative first exon—AF: 68,751, Alternative last exon—AL: 17,536, Alternative 3′ splice site—A3: 15,054, Alternative 5′ splice site—A5: 14,274, Mutually exclusive exons—MX: 4,768, Retained intron—RI: 5,789, Skipped exon—SE: 37,353).
To ensure sufficient power in the detection of both isoforms at AS events, we first focused, for each condition of immune stimulation, on genes with an average FPKM > 10, and AS events where each possible alternative intron was supported by at least 30 spliced reads. We then quantified the resulting 39,030 AS events using MISO to obtain PSI values12 and filtered out, in each condition, rare AS events with a mean PSI value under 5% or over 95%, as well as AS events with >5% of missing values (due to the lack of informative reads in the sample). Remaining missing values were imputed by K-nearest neighbours with the impute.knn function from the Impute R package. As a result of these filters, we obtained a set of 30,796 frequent AS events, for which the less frequent isoform has FPKM > 0.5 in at least one condition and 95% of samples have informative reads. In our setting (~35 M reads per sample) and at this level of expression (FPKM = 0.5), we expect to find ~15 reads per sample for each kb of the minor transcript, providing high power for the detection of both isoforms.
For each AS event, we defined its boundaries as the most upstream and downstream splice site of the alternatively spliced introns. In addition, we extended these boundaries to the most upstream TSS for alternative first exon and the most downstream transcription end site (TES) for alternative last exons. We next computed correlations between PSI value profiles of overlapping AS events, and considered AS events as related events when their PSI values showed an absolute correlation > 0.5. We thus defined a graph of AS events relationships, and used the cluster_walktrap function from the igraph R package to define clusters of highly connected AS events. We then selected a single representative event for each cluster, based on total counts of spliced reads supporting the events. After filtering overlapping AS events, we obtained a final set of 16,173 AS events for analysis (A3: 2,625, A5: 2,062, AF: 2,018, AL: 1,167, MX: 566, RI: 1,415, SE: 6,320), which we adjusted for batch effects and technical covariates using ComBat70 (Supplementary Note 2; Supplementary Fig. 2f).
To assess the putative consequences of AS events at the protein level, we retrieved transcript annotations from Ensembl v94 and considered for each AS event, the set of transcripts that are compatible with each possible splicing (reference and alternative isoforms) and the corresponding protein(s). We annotated AS events as modified protein when both isoforms are protein-coding, loss-of-function if only the reference isoform is protein-coding, gain-of-function if only the alternative isoform is protein-coding, and non-coding if both isoforms are non-coding.
Differential splicing and isoform diversity
For each annotated AS event, differential splicing between stimulation conditions or populations was assessed using Wilcoxon rank test, and p-values were corrected for multiple testing across all conditions using the’fdr’ method from the p.adjust R function (Benjamini–Hochberg FDR correction). When testing differential splicing between conditions, only genes with FPKM > 10, both at basal state and after stimulation, were considered. We considered a change in isoform ratios as significant if it satisfied the following criteria: (i) adjusted p-value < 0.05 and (ii) difference in PSI > 0.05. We considered genes as being differentially spliced (between conditions or populations) when they significantly changed their isoform ratios (PSI), for at least one of their AS events. For each gene and condition, isoform diversity was measured by Shannon entropy, assuming that all AS events of a gene occur independently. Shannon entropy (H) was computed as follows
| 1 |
where Ψj is the mean PSI ratio of the jth AS event of gene i.
Quantification of noisy splicing
To quantify the degree of noisy splicing per gene, we considered, for each constitutive splice site next to a coding exon, the set of all spliced reads that link the splice site to a cryptic splice site (i.e., weak, non-conserved splice site absent from Ensembl annotations) as splicing errors. We then computed, for each condition, the proportion of such splicing errors among all reads that start/end at the splice site, and averaged these values across all constitutive splice sites of a gene to obtain its rate of noisy splicing. In addition, we computed these proportions separately for each sample, and averaged the levels of noisy splicing across all genes, to yield an estimate of the degree of noisy splicing per sample. We assessed the contribution of NMD to noisy splicing by correlating the degree of noisy splicing per sample, with the expression of the key NMD genes UPF1–3 and SMG1–9 (summarised by their first PC). For each gene, mean intron length was determined based on the transcript with the largest number of exons among transcripts with FPKM > 1. To assess the impact of transcription rate on noisy splicing, we used the mean intronic RPKM observed at the basal state as a proxy of the transcription rate. We assessed the impact of these features on noisy splicing by Spearman’s rank correlation tests, and used Wilcoxon rank test to assess the difference in levels of noisy splicing across conditions. The partial correlation between gene expression and noisy splicing, adjusted for transcription rate, was obtained by regressing ranks of gene expression and noisy splicing at basal state on those of transcription rates, and correlating the residuals.
Mapping of splice QTLs
To map genetic variants associated with differences in splicing, i.e., sQTL, we used variants with a MAF ≥ 0.05 in both populations combined, resulting in a set of 7,650,709 SNPs, of which 5,634,819 were located < 1 Mb from an AS event. We mapped local sQTLs, likely cis-acting, with MatrixEQTL76, using a 1 Mb window on each side of the splicing event boundaries. sQTL mapping was performed separately for each condition and merging both populations. PC1 and PC2 of the genotype matrix were included in the model to account for population stratification, and PSI values were rank transformed to a normal distribution before mapping, to reduce the impact of outliers.
FDR was computed by mapping sQTLs on 100 permuted datasets, in which genotypes were randomly permuted within each population. We then kept, for each permutated dataset, the most significant p-value per AS event, across all conditions, and computed the FDR associated with various p-value thresholds ranging from 10−3 to 10−50. We subsequently selected the p-value threshold that provided a 5% FDR (p < 4.8 × 10−7). To compare sQTLs across conditions, we used a Bayesian framework to select the most likely model of sQTL sharing across conditions (Supplementary Note 4). sQTLs for which the gene was expressed specifically after stimulation (FPKM < 10 in the non-stimulated state and log2FC > 1), were annotated as sQTLs of stimulation-specific genes. rsQTLs were defined as those where (i) the gene was expressed at FPKM > 10 both before and after stimulation, (ii) the sQTL was significant specifically after stimulation (p > 0.001 in the non-stimulated state) and (iii) the sQTL was significantly associated to changes in PSI value upon stimulation (p < 0.01, corresponding to ~5% FDR, when testing all sQTLs across the 4 stimuli).
Regulatory elements and predicted impact on splicing
The SPIDEXTM database36 was retrieved from the deepgenomics website (https://www.deepgenomics.com/spidex-noncommercial-download), and functionality scores (Z index) were extracted from all SNPs present in the database (i.e., located < 300 nucleotides apart of a known splice site). Scored SNPs were annotated as exonic or intronic based on Ensembl v70 annotation, and considered deleterious when their absolute Z-score was above 2, and benign otherwise. We then used Fisher’s exact test to assess the enrichment of sQTLs in exonic and intronic regions, as well as in predicted splice-altering regions, considering the peak SNP at each locus.
To further determine the set of regulatory elements that contribute to regulate splicing, we annotated SNPs for various types of regulatory features including overlap with (i) donor/acceptor sites (2-bp upstream or downstream of each exon), (ii) splice site flanking regions (3–8-bp upstream or downstream of an exon), (iii) expected branchpoint regions (18–39-bp upstream of acceptor site), (iv) transcriptional regulatory elements (promoter, promoter flanking, enhancer, and CTCF binding sites) that are active in monocytes, based on Ensembl Regulatory Build v8077 and (v) conserved regions (GerpRS > 2). In addition, to explore how sQTLs overlap motifs of RNA-binding proteins, we retrieved a set of 107 motifs bound, in vitro, by 81 human splicing factors and RNA-binding proteins78. We then kept a single motif per protein, giving priority to the motif with the highest information content, and used Homer79 to scan the reference genome sequence for these motifs. Namely, we used findMotifsGenome.pl script with -mask and -rna to identify motifs of human RNA-binding proteins in a 20 nucleotides window around each SNP (considering both strands), and excluded motifs that did not directly overlap the SNP or presented a motifScore of 5 or less. We used a Fisher’s exact test to assess the enrichment of sQTLs in SNPs that overlap the regulatory motifs and features described above, with respect to genome-wide expectations. For all analyses, SNPs with a MAF ≥ 0.05 and located < 1 Mb away from an AS event were used as background set.
sQTL/eQTL overlap and causality inference
To measure the impact of sQTLs on gene expression, we also mapped eQTLs from rank-transformed gene-level FPKM values, with MatrixEQTL76. Local eQTLs were mapped in a 1 Mb window around each gene, merging the two populations and adjusting for PC1 and PC2 of the genotype matrix. FDR was computed through permutations as performed for sQTLs. To identify shared genetic control of splicing and gene expression, we considered for each sQTL, the levels of LD (r2), computed across both populations, between the sQTLs and eQTLs detected for the same gene and condition, as a measure of the colocalization between the genetic determinants of splicing and gene expression. Causal relationships between splicing and expression changes observed at sQTLs were assessed using a two-step approach and a modified version of the Likelihood-based Causal Model Selection Framework described in44 (Supplementary Note 3; Supplementary Fig. 6). To assess the impact of sQTLs on transcription rate, we tested, for each sQTL, the effect of the peak sQTL SNP on intronic RPKM in the condition where the sQTL was the most significant, adjusting for population of origin. sQTLs associated to intronic RPKM with p < 0.05 were considered as significantly associated with transcription rate.
Overlap of sQTLs with GWAS loci
We downloaded the NHGRI GWAS catalogue46 from EBI (date: 26 June 2017), and selected all SNPs that were significantly associated with a complex trait or disease at p-value of 10−5 (N = 27,884). GWAS traits were annotated based on the EFO mappings47, and grouped according to their parental EFO categories. Note that a single locus/trait can be assigned to multiple EFO categories. To account for uncertainty of GWAS mapping, we extended the set of GWAS SNPs to all SNPs in LD with a GWAS SNP in Europeans (r2 > 0.8), given that most GWAS are performed on individuals of European ancestry. Using this criterion, we estimate that 5.7% of all SNPs considered in our analyses are associated with at least one trait, with no >0.2% of the tested SNPs being associated to a single phenotype (e.g., height). After excluding nonspecific EFO categories (i.e., other disease, other trait, other measurement and biological process), we obtained a final set of 229,425 SNPs associated with at least one of the 14 EFO terms (4.1% of all tested SNPs).
For enrichment analyses, we removed sQTLs that were in LD (r2 > 0.8) to keep a single SNP per haplotype (1,271 independent SNPs) and compared, for each EFO category, the number of sQTLs overlapping GWAS loci, with respect to genome-wide expectations: all SNPs initially used for sQTL mapping, pruning them for LD (r2 > 0.8), were used as background set (568,973 SNPs). For each EFO category, 1000 resamples were used, matching for bins of MAF at intervals of 2%. Enrichment p-values were calculated by counting the frequency at which the number of GWAS loci in the 1000 resampled sets of SNPs, exceeded the number of GWAS loci observed in our data. For the example of the TMBIM1 sQTL, we downloaded inflammatory bowel disease (IBD) summary statistics from https://www.ibdgenetics.org/downloads.html, and extracted GWAS p-values from the European-based GWAS meta-analysis. The TMBIM1 sQTL was remapped in European individuals only, to allow comparison with the GWAS p-values.
Population differences in splicing and mediation analysis
For genes that are differentially spliced between populations and harbour a sQTL, we evaluated the fraction of population differences in splicing that are attributable to the sQTL, using the mediate function from the mediation R package57. Briefly, mediation analyses decompose the population differences in isoform ratios between the effect that is due to differences in allelic frequency at the sQTL (mediated or indirect effect, ) and the effect of population that occur independently of the SNP under consideration (independent or direct effect δ).
| 2 |
with
| 3 |
and
| 4 |
Under a linear model, the mediated effect can be expressed as:
| 5 |
where fAFB and fEUB are the allelic frequency of the SNP under consideration, and is the impact of a single allele of the sQTL on PSI values.
The proportion mediated τ can then be estimated as:
| 6 |
Detection of signals of positive selection at sQTLs
To detect signatures of population-specific natural selection, we used two metrics, FST and iHS, which detect signals of local adaptation. FST quantifies the variance of allele frequencies within and between populations to detect outlier values of population differentiation58, which may result from the action of positive selection in one specific population. The iHS59 compares the degree of extended haplotype homozygosity of the derived and ancestral alleles, allowing to identify differences in haplotype length between alleles that can result from the rapid increase in frequency of the putatively selected allele.
To identify candidate sQTLs under selection, we used an outlier approach by computing the top 5% values of FST and |iHS| at the genome-wide level, in each population separately. To support further the adaptive nature of candidate sQTLs, we tested for local enrichments in outliers of FST or |iHS| (top 5% of signals) within a 100 kb window around each sQTL (focusing on SNPs with MAF ≥ 0.05), as previously performed38,59,60. Indeed, it has been shown that searching for the occurrence of local enrichments in outliers increases the rate of true selected loci among genome-wide outliers59. We compared the number of outliers within each window with a beta-binomial distribution with parameters (α, β, N), where N is the number of informative SNPs (MAF ≥ 0.05) in the window, and the parameters (α, β) are obtained by fitting a beta distribution to the proportion of outliers of FST or |iHS| per 100 kb window genome-wide.
Detection of Neanderthal-introgressed sQTLs
To measure the effect of admixture with Neanderthals on splicing, we used the complete genome sequence of the Neanderthal Altai80 to identify a total of 100,755 frequent SNPs (MAF > 5%) that are exclusively present on Neanderthal haplotypes in Europe (aSNPs, Supplementary Note 5). We then counted the number of independent loci where the peak SNP is either an aSNP or tags an aSNP in Europe (r2 > 0.8), and compared this number with genome-wide expectations by resampling. All SNPs initially used for sQTL mapping, with a MAF > 5% in Europe, were LD pruned (r2 > 0.8) and used as background set (568,973 SNPs). We then randomly resampled 1000 sets of independent SNPs matched for allelic frequencies with sQTLs, and counted the number of tag-aSNPs in each set. Enrichment p-values were calculated by counting the frequency at which the number of tag-aSNPs in our 1000 resampled sets of SNPs exceeded the number of tag-aSNPs observed in our data. To assess the robustness of our enrichment analyses to changes in the definition of Neanderthal haplotypes, we repeated the resampling with more stringent definitions of archaic haplotypes (i.e., increasing the haplotype length and number of Neanderthal alleles required to call a haplotype as archaic, Supplementary Note 5).
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This project was funded by the European Research Council under the European Union’s Seventh Framework Programme (FP/2007–2013)/ERC grant agreement 281297 (to L.Q.-M.). The Quintana-Murci lab is funded by the French Government’s Investissement d’Avenir program, Laboratoires d’Excellence “Integrative Biology of Emerging Infectious Diseases” (ANR-10-LABX-62-IBEID) and the Fondation pour la Recherche Médicale (Equipe FRM DEQ20180339214). M.R. was supported by a Marie Skłodowska-Curie fellowship (DLV-655417). We thank Etienne Patin and Mary O’Neill for helpful comments and suggestions.
Author contributions
M.R. designed and performed the computational analyses, and analysed the data. M.R., H.Q. and L.Q.-M. interpreted results and wrote the paper. L.Q.-M. supervised the research and obtained the funding.
Data availability
Genome-wide SNP genotyping, whole-exome sequencing and RNA-sequencing data used in this study have been deposited in the European Genome-phenome Archive (EGA) under accession code EGA: EGAS00001001895. Source data for figure(s) 1–6 are provided with the paper. All other relevant data are available upon request.
Code availability
All scripts have been deposited on github under MIT License (https://github.com/mrotival/EvoImmunoPop_Splicing).
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Colin Logie, Rajiv McCoy and Yi Xing for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maxime Rotival, Email: maxime.rotival@pasteur.fr.
Lluis Quintana-Murci, Email: quintana@pasteur.fr.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09689-7.
References
- 1.Kornblihtt AR, et al. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nat. Rev. Mol. Cell Biol. 2013;14:153–165. doi: 10.1038/nrm3525. [DOI] [PubMed] [Google Scholar]
- 2.Barash Y, et al. Deciphering the splicing code. Nature. 2010;465:53–59. doi: 10.1038/nature09000. [DOI] [PubMed] [Google Scholar]
- 3.Barbosa-Morais NL, et al. The evolutionary landscape of alternative splicing in vertebrate species. Science. 2012;338:1587–1593. doi: 10.1126/science.1230612. [DOI] [PubMed] [Google Scholar]
- 4.Keren H, Lev-Maor G, Ast G. Alternative splicing and evolution: diversification, exon definition and function. Nat. Rev. Genet. 2010;11:345–355. doi: 10.1038/nrg2776. [DOI] [PubMed] [Google Scholar]
- 5.Merkin J, Russell C, Chen P, Burge CB. Evolutionary dynamics of gene and isoform regulation in Mammalian tissues. Science. 2012;338:1593–1599. doi: 10.1126/science.1228186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skandalis A, Frampton M, Seger J, Richards MH. The adaptive significance of unproductive alternative splicing in primates. RNA. 2010;16:2014–−2022. doi: 10.1261/rna.2127910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramensky VE, Nurtdinov RN, Neverov AD, Mironov AA, Gelfand MS. Positive selection in alternatively spliced exons of human genes. Am. J. Hum. Genet. 2008;83:94–98. doi: 10.1016/j.ajhg.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintana-Murci, L. Human immunology through the lens of evolutionary genetics. Cell 177, 184–199 (2019). [DOI] [PubMed]
- 9.Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nat. Rev. Genet. 2014;15:379–393. doi: 10.1038/nrg3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fumagalli M, et al. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS. Genet. 2011;7:e1002355. doi: 10.1371/journal.pgen.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GTEx Consortium. Human genomics. The Genotype-tissue expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science348, 648–660 (2015). [DOI] [PMC free article] [PubMed]
- 12.Wang ET, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ergun A, et al. Differential splicing across immune system lineages. Proc. Natl. Acad. Sci USA. 2013;110:14324–14329. doi: 10.1073/pnas.1311839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, et al. Genetic drivers of epigenetic and transcriptional variation in human immune cells. Cell. 2016;167:1398–1414. doi: 10.1016/j.cell.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter S, Ricci EP, Mercier BC, Moore MJ, Fitzgerald KA. Post-transcriptional regulation of gene expression in innate immunity. Nat. Rev. Immunol. 2014;14:361–376. doi: 10.1038/nri3682. [DOI] [PubMed] [Google Scholar]
- 16.Martinez NM, Lynch KW. Control of alternative splicing in immune responses: many regulators, many predictions, much still to learn. Immunol. Rev. 2013;253:216–236. doi: 10.1111/imr.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalam H, Fontana MF, Kumar D. Alternate splicing of transcripts shape macrophage response to Mycobacterium tuberculosis infection. PLoS. Pathog. 2017;13:e1006236. doi: 10.1371/journal.ppat.1006236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pai AA, et al. Widespread shortening of 3′ untranslated regions and increased exon inclusion are evolutionarily conserved features of innate immune responses to infection. PLoS. Genet. 2016;12:e1006338. doi: 10.1371/journal.pgen.1006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye CJ, et al. Genetic analysis of isoform usage in the human anti-viral response reveals influenza-specific regulation of ERAP2 transcripts under balancing selection. Genome Res. 2018;28:1812–1825. doi: 10.1101/gr.240390.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alasoo K, et al. Genetic effects on promoter usage are highly context-specific and contribute to complex traits. eLife. 2019;8:e41673. doi: 10.7554/eLife.41673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards AL, et al. Environmental perturbations lead to extensive directional shifts in RNA processing. PLoS. Genet. 2017;13:e1006995. doi: 10.1371/journal.pgen.1006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melamud E, Moult J. Stochastic noise in splicing machinery. Nucleic Acids Res. 2009;37:4873–4886. doi: 10.1093/nar/gkp471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickrell JK, Pai AA, Gilad Y, Pritchard JK. Noisy splicing drives mRNA isoform diversity in human cells. PLoS. Genet. 2010;6:e1001236. doi: 10.1371/journal.pgen.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saudemont B, et al. The fitness cost of mis-splicing is the main determinant of alternative splicing patterns. Genome. Biol. 2017;18:208. doi: 10.1186/s13059-017-1344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalonde E, et al. RNA sequencing reveals the role of splicing polymorphisms in regulating human gene expression. Genome Res. 2011;21:545–554. doi: 10.1101/gr.111211.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickrell JK, et al. Understanding mechanisms underlying human gene expression variation with RNA sequencing. Nature. 2010;464:768–772. doi: 10.1038/nature08872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YI, et al. RNA splicing is a primary link between genetic variation and disease. Science. 2016;352:600–604. doi: 10.1126/science.aad9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ongen H, Dermitzakis ET. Alternative Splicing QTLs in European and African populations. Am. J. Hum. Genet. 2015;97:567–575. doi: 10.1016/j.ajhg.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nedelec Y, et al. Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell. 2016;167:657–669. doi: 10.1016/j.cell.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery SB, et al. Transcriptome genetics using second generation sequencing in a Caucasian population. Nature. 2010;464:773–777. doi: 10.1038/nature08903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Battle A, et al. Characterizing the genetic basis of transcriptome diversity through RNA-sequencing of 922 individuals. Genome Res. 2014;24:14–24. doi: 10.1101/gr.155192.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez-Arcelus M, et al. Tissue-specific effects of genetic and epigenetic variation on gene regulation and splicing. PLoS. Genet. 2015;11:e1004958. doi: 10.1371/journal.pgen.1004958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung R, et al. A multiplexed assay for exon recognition reveals that an unappreciated fraction of rare genetic variants cause large-effect splicing disruptions. Mol. Cell. 2019;73:183–194. doi: 10.1016/j.molcel.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soemedi R, et al. Pathogenic variants that alter protein code often disrupt splicing. Nat. Genet. 2017;49:848–855. doi: 10.1038/ng.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat. Rev. Genet. 2007;8:749–761. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- 36.Xiong HY, et al. RNA splicing. The human splicing code reveals new insights into the genetic determinants of disease. Science. 2015;347:1254806. doi: 10.1126/science.1254806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lord J, et al. Pathogenicity and selective constraint on variation near splice sites. Genome Res. 2018;29:159–170. doi: 10.1101/gr.238444.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quach H, et al. Genetic adaptation and neandertal admixture shaped the immune system of human populations. Cell. 2016;167:643–656. doi: 10.1016/j.cell.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lappalainen T, et al. Transcriptome and genome sequencing uncovers functional variation in humans. Nature. 2013;501:506–511. doi: 10.1038/nature12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park E, Pan Z, Zhang Z, Lin L, Xing Y. The expanding landscape of alternative splicing variation in human populations. Am. J. Hum. Genet. 2018;102:11–26. doi: 10.1016/j.ajhg.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gamazon ER, Konkashbaev A, Derks EM, Cox NJ, Lee Y. Evidence of selection on splicing-associated loci in human populations and relevance to disease loci mapping. Sci. Rep. 2017;7:5980. doi: 10.1038/s41598-017-05744-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davydov EV, et al. Identifying a high fraction of the human genome to be under selective constraint using GERP++ PLoS. Comput. Biol. 2010;6:e1001025. doi: 10.1371/journal.pcbi.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis BP, Green RE, Brenner SE. Evidence for the widespread coupling of alternative splicing and nonsense-mediated mRNA decay in humans. Proc. Natl. Acad. Sci. USA. 2003;100:189–192. doi: 10.1073/pnas.0136770100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schadt EE, et al. An integrative genomics approach to infer causal associations between gene expression and disease. Nat. Genet. 2005;37:710–717. doi: 10.1038/ng1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaidatzis D, Burger L, Florescu M, Stadler MB. Analysis of intronic and exonic reads in RNA-seq data characterizes transcriptional and post-transcriptional regulation. Nat. Biotechnol. 2015;33:722–729. doi: 10.1038/nbt.3269. [DOI] [PubMed] [Google Scholar]
- 46.MacArthur J, et al. The new NHGRI-EBI catalog of published genome-wide association studies (GWAS Catalog) Nucleic Acids Res. 2017;45:D896–D901. doi: 10.1093/nar/gkw1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malone J, et al. Modeling sample variables with an experimental factor ontology. Bioinformatics. 2010;26:1112–1118. doi: 10.1093/bioinformatics/btq099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jostins L, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feenstra B, et al. Common variants associated with general and MMR vaccine-related febrile seizures. Nat. Genet. 2014;46:1274–1282. doi: 10.1038/ng.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haralambieva IH, et al. Genome-wide associations of CD46 and IFI44L genetic variants with neutralizing antibody response to measles vaccine. Hum. Genet. 2017;136:421–435. doi: 10.1007/s00439-017-1768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fellay J, et al. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010;464:405–408. doi: 10.1038/nature08825. [DOI] [PubMed] [Google Scholar]
- 52.Mehta S, et al. Maintenance of macrophage transcriptional programs and intestinal homeostasis by epigenetic reader SP140. Sci. Immunol. 2017;2:eaag3160. doi: 10.1126/sciimmunol.aag3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sams AJ, et al. Adaptively introgressed Neandertal haplotype at the OAS locus functionally impacts innate immune responses in humans. Genome. Biol. 2016;17:246. doi: 10.1186/s13059-016-1098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andlauer TF, et al. Novel multiple sclerosis susceptibility loci implicated in epigenetic regulation. Sci. Adv. 2016;2:e1501678. doi: 10.1126/sciadv.1501678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.International Multiple Sclerosis Genetics Consortium. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature476, 214–219 (2011). [DOI] [PMC free article] [PubMed]
- 56.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature447, 661–678 (2007). [DOI] [PMC free article] [PubMed]
- 57.Tingley, D., Yamamoto, T., Hirose, K., Keele, L. & Imai, K. mediation: R package for causal mediation analysis. J. Stat. Softw. 10.18637/jss.v059.i05 (2014).
- 58.Holsinger KE, Weir BS. Genetics in geographically structured populations: defining, estimating and interpreting F(ST) Nat. Rev. Genet. 2009;10:639–650. doi: 10.1038/nrg2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voight BF, Kudaravalli S, Wen X, Pritchard JK. A map of recent positive selection in the human genome. PLoS Biol. 2006;4:e72. doi: 10.1371/journal.pbio.0040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grossman SR, et al. Identifying recent adaptations in large-scale genomic data. Cell. 2013;152:703–713. doi: 10.1016/j.cell.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 62.Feng WX, et al. Functional polymorphisms in CYP2C19 & CYP3A5 genes associated with decreased susceptibility for paediatric tuberculosis. Indian J. Med. Res. 2012;135:642–649. [PMC free article] [PubMed] [Google Scholar]
- 63.Canela-Xandri O, Rawlik K, Tenesa A. An atlas of genetic associations in UK Biobank. Nat. Genet. 2018;50:1593–1599. doi: 10.1038/s41588-018-0248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enard D, Petrov DA. Evidence that RNA viruses drove adaptive introgression between Neanderthals and modern humans. Cell. 2018;175:360–371. doi: 10.1016/j.cell.2018.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grunvogel O, et al. DDX60L is an interferon-stimulated gene product restricting hepatitis C virus replication in cell culture. J. Virol. 2015;89:10548–10568. doi: 10.1128/JVI.01297-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogelpoel LT, Baeten DL, de Jong EC, den Dunnen J. Control of cytokine production by human fc gamma receptors: implications for pathogen defense and autoimmunity. Front. Immunol. 2015;6:79. doi: 10.3389/fimmu.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuehl P, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 68.Dannemann M, Andres AM, Kelso J. Introgression of Neandertal- and Denisovan-like haplotypes contributes to adaptive variation in human Toll-like receptors. Am. J. Hum. Genet. 2016;98:22–33. doi: 10.1016/j.ajhg.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deschamps M, et al. Genomic signatures of selective pressures and introgression from archaic hominins at human innate immunity genes. Am. J. Hum. Genet. 2016;98:5–21. doi: 10.1016/j.ajhg.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li YI, et al. Annotation-free quantification of RNA splicing using LeafCutter. Nat. Genet. 2018;50:151–158. doi: 10.1038/s41588-017-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nellore A, et al. Human splicing diversity and the extent of unannotated splice junctions across human RNA-seq samples on the sequence read archive. Genome. Biol. 2016;17:266. doi: 10.1186/s13059-016-1118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blanchette M, et al. Aligning multiple genomic sequences with the threaded blockset aligner. Genome Res. 2004;14:708–715. doi: 10.1101/gr.1933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome. Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–1358. doi: 10.1093/bioinformatics/bts163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zerbino DR, Wilder SP, Johnson N, Juettemann T, Flicek PR. The ensembl regulatory build. Genome. Biol. 2015;16:56. doi: 10.1186/s13059-015-0621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ray D, et al. A compendium of RNA-binding motifs for decoding gene regulation. Nature. 2013;499:172–177. doi: 10.1038/nature12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prufer K, et al. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature. 2014;505:43–49. doi: 10.1038/nature12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Genome-wide SNP genotyping, whole-exome sequencing and RNA-sequencing data used in this study have been deposited in the European Genome-phenome Archive (EGA) under accession code EGA: EGAS00001001895. Source data for figure(s) 1–6 are provided with the paper. All other relevant data are available upon request.
All scripts have been deposited on github under MIT License (https://github.com/mrotival/EvoImmunoPop_Splicing).