Abstract
β‐Cardiotoxin is a novel member of the snake venom three‐finger toxin (3FTX) family. This is the first exogenous protein to antagonize β‐adrenergic receptors and thereby causing reduction in heart rates (bradycardia) when administered into animals, unlike the conventional cardiotoxins as reported earlier. 3FTXs are stable all β‐sheet peptides with 60–80 amino acid residues. Here, we describe the three‐dimensional crystal structure of β‐cardiotoxin together with the identification of a molten globule intermediate in the unfolding pathway of this protein. In spite of the overall structural similarity of this protein with conventional cardiotoxins, there are notable differences observed at the loop region and in the charge distribution on the surface, which are known to be critical for cytolytic activity of cardiotoxins. The molten globule intermediate state present in the thermal unfolding pathway of β‐cardiotoxin was however not observed during the chemical denaturation of the protein. Interestingly, circular dichroism (CD) and NMR studies revealed the presence of α‐helical secondary structure in the molten globule intermediate. These results point to substantial conformational plasticity of β‐cardiotoxin, which might aid the protein in responding to the sometimes conflicting demands of structure, stability, and function during its biological lifetime.
Keywords: three‐finger toxin, beta‐blocker, molten globule, non‐hierarchical protein folding, thermal denaturation and structural transition
Short abstract
PDB Code(s): 3PLC;
Abbreviations
- β‐ARs
β‐adrenergic receptors
- 3FTXs
three‐finger toxins
- CD
circular dichroism
- CTX
cardiotoxins/cytotoxins
- CTX III
cardiotoxin analogue III
- CVDs
cardiovascular diseases
Introduction
Snake venoms contain an arsenal of bioactive proteins and peptides that target various molecules in the prey to bring about death or debilitation.1 Venom proteins have been classified under various superfamilies based on their functional and structural characteristics. Functionally, these molecules fall under two categories, namely enzymatic and nonenzymatic proteins. Few examples of the structural superfamilies are three‐finger toxins (3FTXs), C‐type lectin‐like proteins, phospholipase A2s, serine proteases, and metalloproteases.1, 2, 3, 4, 5, 6 3FTXs are a well‐characterized family of nonenzymatic polypeptides containing 60–80 amino acid residues.3, 5, 6, 7, 8 These proteins are abundant in elapid (cobras, kraits, and mambas), hydrophiid (sea snakes), and colubrid venoms,9 and their presence has also been reported in viper venoms.10, 11 They are well characterized in terms of their folding pattern. They contain four to five disulfide bridges, four of which are strictly conserved. They show a prototype of folding, in which three β‐stranded loops extend from a central core containing the four conserved disulfide bridges resembling the three outstretched fingers of a hand.3, 5 These four disulfide bridges are conserved in all the 3FTXs and are known to stabilize their unique three‐finger fold. Due to this appearance, this family of proteins is named as 3FTXs. Despite their structural similarity, 3FTXs display a wide range of functional diversity.6, 7, 8 They can be broadly classified into neurotoxins,12, 13 cardiotoxins/cytotoxins (CTX),14, 15 and anticoagulants16, 17, 18 based on their mechanism of action.
β‐Cardiotoxin is a novel 3FTX isolated from the venom of Ophiophagus hannah (king cobra). Our earlier functional studies with β‐cardiotoxin revealed that this is the first protein to show β‐adrenergic receptor (β‐AR) antagonism.19 It lacks the cytolytic activity unlike the conventional cardiotoxins. It was observed that β‐cardiotoxin, a predominant β‐sheeted protein with a molecular weight of ~7 kD (63 amino acids), has a unique circular dichroism (CD) spectrum compared to known groups of three‐finger cardiotoxins.19 A molten globule‐like state has been experimentally shown in the (un)folding pathway of cardiotoxin analogue III (CTX III), an all β‐sheet protein with 60 amino acid residues, isolated from the venom of Naja naja atra.20 Hence, we were interested in assessing the three‐dimensional structure as well as conformational plasticity of β‐cardiotoxin. In the present report, we describe the crystal structure and characterization of a molten globule intermediate in the thermal unfolding pathway of β‐cardiotoxin.
Results
Overall structure
There are three molecules of β‐cardiotoxin in each asymmetric unit. Each monomer comprises of all 63 residues from Arg1 to Lys63 [Fig. 1(a), Table 1]. The β‐cardiotoxin molecule has a conventional three‐finger fold, with a core held by disulfide bridges and three loops formed by two β‐sheets. The smaller β‐sheet consists of two small β‐strands (β1 and β2) and the large β‐sheet consists of three large β‐strands (β4, β3, and β5). In both β‐sheets the strands are antiparallel. There are four disulfide bonds in each monomer; a feature which is strictly conserved in all 3FTXs. Figure 1(b) shows the representative electron density map of the model. Although three molecules of β‐cardiotoxin were observed in each of the asymmetric unit, the gel filtration experiments suggested that β‐cardiotoxin exists as a monomer in solution (data not shown).
Figure 1.
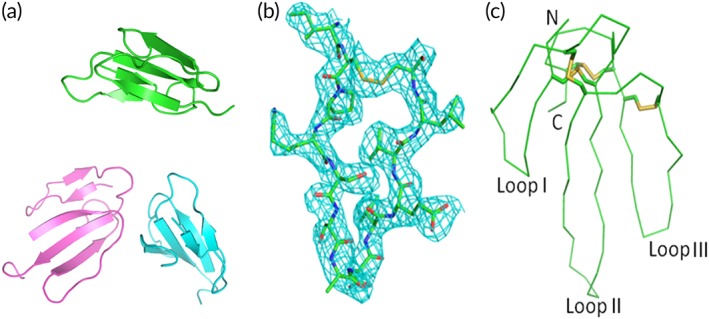
Overall crystal structure and electron density omit map of β‐cardiotoxin. (a) Cartoon representation of the β‐cardiotoxin asymmetric unit (residues Ile43‐Cys55). Monomer A: cyan; monomer B: magenta; and monomer C: green. (b) Electron density map (2Fo‐Fc) showing the loop III region. The map is calculated by CCP4 program suit and contoured at 1σ. (c) Ribbon diagram showing the β‐cardiotoxin monomer (chain B). Disulfide bonds are presented as yellow sticks. N, C terminus and the three loops are labeled.
Table 1.
Data collection and refinement statistics
| Cell axial lengths (Å) | a = b = 58.95, c = 53.03 |
| Spacegroup | P31 |
| Data collection | |
| Resolution range (Å) | 50.00–2.41 (2.50–2.41) |
| Number of observed reflections | 88567 (6978) |
| Number of unique reflections | 7999 (784) |
| Completeness (%) | 99.6 (96.0) |
| Multiplicity | 11.1 (8.9) |
| R sym a (%) | 0.137 (0.484) |
| Mean I/sigma(I) | 13.7 (2.5) |
| Solvent content (%) | 51.3 |
| Refinement | |
| Resolution range (Å) | 50.01–2.41 (2.54–2.41) |
| Number of working reflections | 7196 (1030) |
| Number of test reflections | 787 (135) |
| R work b (%) | 23.1 (25.8) |
| R free c (%) | 26.9 (32.2) |
| R.m.s. deviation bond lengths (Å) | 0.016 |
| rms deviation bond angles (°) | 1.169 |
| Average B‐factors (Å2) (# of atoms) | |
| Protein atoms | 45.60 (1446) |
| Ramachandran plot | |
| Most favored regions (%) | 83.6 |
| Allowed regions (%) | 16.4 |
| General allowed regions (%) | 0 |
| Disallowed regions (%) | 0 |
R sym a = Σ|Ii – <I>|/Σ|Ii|, where Ii is the intensity of the ith measurement, and <I> is the mean intensity for that reflection.
R work b = Σ|Fo – Fc|/|Fo|, where Fc and Fo are the calculated and observed structure factor amplitudes, respectively.
R free c calculated as for R work but for around 10% of the total reflections chosen at random and omitted from refinement for all data sets. High resolution bin values are given in parenthesis.
Structure of the individual monomer/chain is same except for small differences [Fig. 1(c)]. Chains A, B, and C are similar (rmsd 0.3 Å for all atoms). Although the main‐chain electron density of loop II residues is well defined, the electron densities for the following residues Ile27 to Tyr34 in chain A and Lys33 to Asp35 in chains B and C are disordered and hence not modeled. The electron density for the loop II region is generally weaker than the rest of the molecule, suggesting that this loop is relatively flexible.
Sequence and structural comparison
The sequence analysis of β‐cardiotoxin using BLAST against all nonredundant protein sequences in NCBI showed that cardiotoxin 9, 14, 15, 21, and 23 are highly similar to β‐cardiotoxin [>90% sequence identity; Fig. 2(a)]. Only few residues are different or replaced with similar residues, among them are 13 (Thr or Lys), 41 (Thr or Ile), 52 (Val or Glu), and 61 (Asn or Asp) as well as 21 (Lys or Arg), 23 (Val or Ile), and 54 (Val or Leu). This high conservation of sequence indicates that these proteins might have similar function(s) in vivo, but unfortunately these proteins have not been characterized functionally. Hence, β‐cardiotoxin was compared with the other conventional cardiotoxin proteins for which the function has been reported, including cardiotoxin 1–11 and several other cardiotoxins [Fig. 2(b)]. Surprisingly, the sequence identity between β‐cardiotoxin and these conventional cardiotoxins dropped to about 50%. A search for topologically similar proteins within the Protein Data Bank21 using the program DALI22 revealed significant structural homology between β‐cardiotoxin and other 3FTXs (Table 2). The closest homologs were hemachatoxin, cardiotoxins/cytotoxins, muscarinic toxin, bucain, and ringhalexin. Figure 2(c) shows the superposition of the top 12 structures with β‐cardiotoxin. Interestingly, none of the closest DALI homologs have been reported to interact with β‐ARs.
Figure 2.
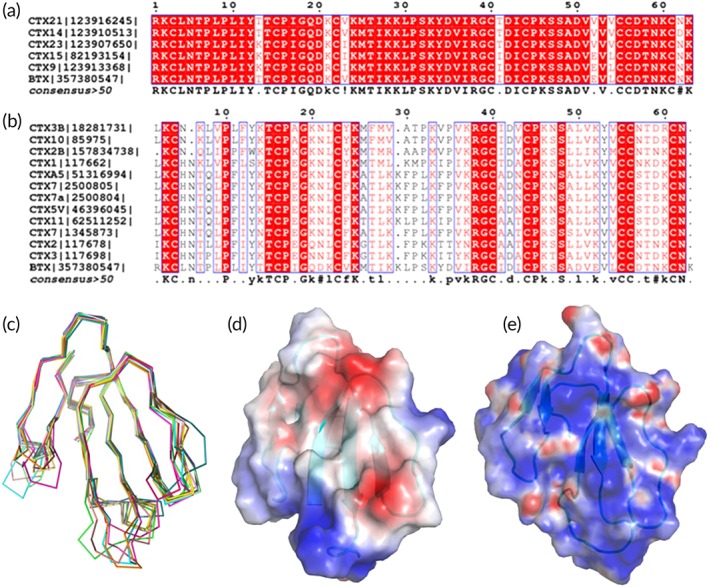
Comparison of β‐Cardiotoxin with other three‐finger toxins (3FTXs). (a) Sequence alignment of β‐cardiotoxin with all closely related 3FTX homologs (>90% identity). (b) Sequence alignment of β‐cardiotoxin with the conventional CTXs (about 50% identities). The alignment is done by ClustalW and figures are prepared by ESPRIPT. (c) Comparison of β‐cardiotoxin with its structural homologs using DALI search. β‐Cardiotoxin [3PLC] (chocolate), Hemachatoxin [3VTS] (60) (orange), cardiotoxin V [1KXI] (61) (red), cytotoxin 4 [4OM5] (62) (dark green), cytotoxin 3 [2BHI] (63) (light green), muscarinic m1‐toxin1 [2VLW] (64) (dark brown), cytotoxin 3 [1XT3] (65) (blue), cardiotoxin‐A3 [1H0J] (66) (light blue), bucain [2H8U] (67) (light brown), cytotoxin 2 [4OM4] (62) (yellow), muscarinic m1‐toxin1 [3NEQ] (68) (magenta), muscarinic toxin MT1 [4DO8] (68) (cyan), and ringhalexin (18) [4ZGY] (green). (d) Electrostatic surface potential of β‐cardiotoxin and Cardiotoxin V (61) (e). The electrostatic surface potentials in this paper are calculated using Pymol plug‐in APBS and scaled at ±10 kT. β‐Cardiotoxin does not have the cationic/hydrophobic central β‐sheet feature, which is critical for CTX's cytolytic activity.
Table 2.
Structural similarity of β‐cardiotoxin with other 3FTXs
| PDB code‐Chain id | Z | rmsd | lali | nres | %id | Description |
|---|---|---|---|---|---|---|
| 3VTS‐A | 10.7 | 0.7 | 53 | 61 | 53 | Hemachatoxin |
| 1KXI‐A | 10.1 | 0.9 | 53 | 62 | 58 | Cardiotoxin V |
| 4OM5‐A | 9.7 | 0.8 | 52 | 60 | 58 | Cytotoxin 4 |
| 2BHI‐A | 9.7 | 0.8 | 52 | 60 | 56 | Cytotoxin 3 |
| 2VLW‐A | 9.6 | 1.8 | 54 | 65 | 37 | Muscarinic m1‐toxin1 |
| 1XT3‐A | 9.6 | 0.8 | 52 | 60 | 56 | Cytotoxin 3 |
| 1H0J‐A | 9.5 | 1.0 | 52 | 60 | 56 | Cardiotoxin‐A3 |
| 2H8U‐A | 9.5 | 1.2 | 53 | 65 | 43 | Bucain |
| 4OM4‐A | 9.5 | 1.0 | 52 | 60 | 56 | Cytotoxin 2 |
| 3NEQ‐A | 9.4 | 1.9 | 54 | 66 | 37 | Muscarinic m1‐toxin1 |
| 4DO8‐A | 9.4 | 1.3 | 54 | 66 | 35 | Muscarinic toxin MT1 |
| 4ZQY‐A | 9.4 | 1.4 | 54 | 65 | 44 | Ringhalexin |
All the comparisons shown in this table are made with chain A of β‐cardiotoxin (PDB code: 3PLC). Comparisons with chains B or C of β‐cardiotoxin gave the same result within 0.3 Å.
Chain id: Denotes the chain id of the compared protein from the attached PDB code. Z: Dali Z score (calculated by DALI program for pairwise similarities between two different structures to give the significance of the results; a score above 2 usually correspond to similar folds). rmsd: root mean square deviation between the C‐α atoms in Å. lali: number of residues aligned. nres: total number of residues in the protein. %id: Sequence identity between β‐cardiotoxin and the other protein.
Overall the structures of hemachatoxin, cardiotoxins/cytotoxins, muscarinic toxin, bucain, and ringhalexin are similar, but exhibit certain variations at the three loops, especially loops I and II. Comparing to β‐cardiotoxin, muscarinic M1‐Toxin1 (cyan, pdb:2VLW) loop I is slightly longer, loops II and III are similar. Similarly, in bucain (yellow, pdb:2H8U), this loop (I) is slightly longer and loop II consists of a shorter β‐strand and larger proportion of coils, while loop III is similar in comparison to β‐cardiotoxin [Fig. 2(c)].
Similarly, the superposition of β‐cardiotoxin with other structurally related CTXs shows that β‐cardiotoxin is highly similar to CTX structures [Fig. 2(c)]. However, notable differences observed at the loop II region. Compared to all three CTX structures, loop II β‐strands of β‐cardiotoxin are slightly longer, closer spaced, and adopt a relatively straight conformation. The electrostatic surface representation shows that β‐cardiotoxin does not exhibit the cationic/hydrophobic surface in its large β‐sheet [Fig. 2(d,e)], which is critical for CTX's cytolytic activity,15, 23, 24, 25, 26 suggesting that β‐cardiotoxin and its close homologs do not belong to the conventional CTX family of proteins, but rather belong to another subfamily of snake venom toxins, as shown earlier.19 Conventional CTXs have a few well‐conserved residues apart from the eight Cys residues, which are thought to play an important role in maintaining structural integrity of the three‐finger fold. Among them are Tyr 22 and Tyr 51, which have been shown by chemical modification to play vital structural and functional (especially Tyr 22) roles in CTXs.27, 28 Roumestand and coworkers have shown that in conventional CTXs the chemical modification of Tyr 22 leads to changes in interaction of β‐sheet regions between loops I and II, and chemical modification of Tyr 51 leads to structural perturbation in the globular core region of the molecule, the overall effect being a destabilized structural core and highly perturbed dynamics in the three‐stranded β‐sheet region.29 Unlike conventional CTXs, in β‐cardiotoxin there are Val residues in both positions 23 and 53 (homologous to positions 22 and 51 of CTXs). These changes could possibly explain the observed unique secondary structural features of β‐cardiotoxin. Together, these observed conformational differences reiterate how small structural differences dictate folding stability as well as functions in CTXs.
(Un)folding studies
Molten globule intermediates in protein folding pathway have been shown to possess compact secondary structure, but lack rigid tertiary structure with dynamic disordered side‐chain interactions.30, 31, 32, 33 We observed the effect of temperature, chemical as well as combined effect of both on the conformation of β‐cardiotoxin over a wide range of pH (1.5–9.5).
Thermal denaturation studies
CD spectroscopy was employed to study the thermal unfolding of β‐cardiotoxin. The protein underwent a structural transition at higher temperatures. Double minima at 222–225 and 203 nm started to appear at 85°C indicating the presence of α‐helical secondary structural elements [Fig. 3(a)]. The structural transition from β‐sheet to α‐helix appeared at about 75°C [Fig. 3(a)]. These results indicate that a very small percentage of the protein undergoes transition to α‐helix structure. Upon cooling to 5°C β‐cardiotoxin attained the native secondary structures [Fig. 3(b)] showing that the thermal unfolding process was completely reversible. We further investigated the effect of temperature on the tertiary structure of β‐cardiotoxin using near‐UV CD spectroscopy. A 10‐times higher concentration of protein (1 mg/mL) compared to far‐UV CD experiments was used as the signals in this region are usually weak.34 Thermal denaturation experiments as described above were repeated with a wavelength scan from 350 to 250 nm. The weak positive signals obtained are probably due to the complete absence of Trp and Phe residues and the fact that the protein contains only two Tyr residues. Interestingly, at higher temperatures (>75°C) there was a sharp drop in the signal implying a total loss of tertiary structure [Fig. 4(a)]. When the sample was cooled down to 5°C, there was a recovery in the CD signal showing that the process is reversible [Fig. 4(b)]. The loss of tertiary structure observed in the near‐UV spectrum [Fig. 4(a)] coincided with the β‐sheet to α‐helix transition that was observed in the far‐UV spectrum [Fig. 3(a)] revealing the presence of a “molten globule”‐like state that retained secondary structure with concomitant loss of tertiary structure.35, 36, 37
Figure 3.
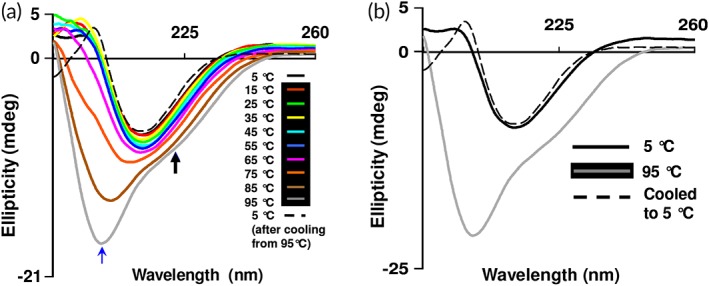
Thermal (un)folding studies of β‐cardiotoxin. (a) Effect of temperature on the secondary structure of β‐cardiotoxin. The protein was dissolved in MilliQ water (0.5 mg/mL) and far‐UV CD spectra were recorded using a 0.1 cm path‐length cuvette 5°C (after cooling from 95°C, black dotted line). The blue and black arrows indicate the new bands arising at 203 and 222 nm. (b) Refolding of β‐cardiotoxin after thermal denaturation.
Figure 4.
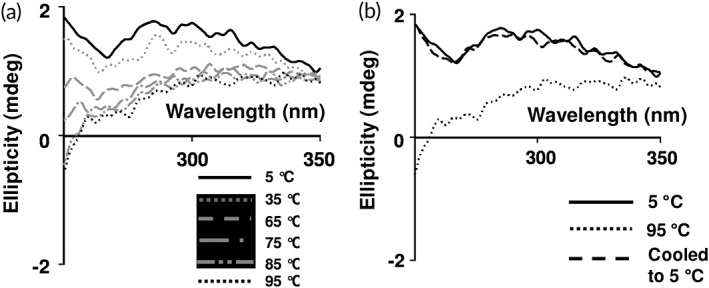
Effect of temperature on tertiary structure of β‐cardiotoxin. (a) β‐Cardiotoxin was dissolved in MilliQ water (1 mg/mL) and near‐UV CD spectra were recorded using a 0.1 cm path‐length cuvette. (b) Refolding of β‐cardiotoxin after thermal denaturation.
Chemical denaturation studies
Chemical denaturation studies of β‐cardiotoxin were performed using far‐UV CD spectroscopy. We treated the protein with increasing concentrations of the chemical denaturant guanidine HCl. The protein started to show conformational changes at 1.5 M guanidine HCl [Fig. 5(a)]. The CD spectra showed an increasing amount of random coil structures at higher concentrations of guanidine HCl (2–2.75 M) indicated by the disappearance of the minima at 215 nm [Fig. 5(a)]. Interestingly, the second minimum at ~222 nm that appeared in the thermal denaturation experiment [Fig. 3(a)] showing the presence of α‐helical elements does not appear in the chemical denaturation studies [Fig. 5(a)]. However, upon removal of the guanidine HCl, the protein attained its native secondary structure revealing the complete reversibility of the chemical unfolding process [Fig. 5(b)].
Figure 5.
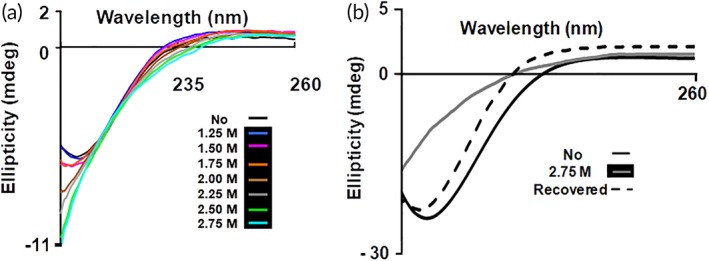
Chemical (un)folding studies of β‐cardiotoxin using guanidine hydrochloride. (a) Effect of guanidine HCl on secondary structure of β‐cardiotoxin. Chemical denaturation experiments were performed by dissolving β‐cardiotoxin in 25 mM Tris–HCL buffer pH 7.5 containing increasing concentrations of guanidine HCl. (b) Refolding of β‐cardiotoxin after the removal of guanidine HCl.
Effect of pH on secondary structure
The effect of varying pH on the secondary structure of β‐cardiotoxin was assessed using far‐UV CD spectroscopy. The protein was dissolved in buffers of different pH ranging from 1.5, 2.5, to 9.5 and the data were collected at 25°C. The CD spectra at different pH show minima at 215 nm showing the presence of β‐sheet conformation [Figs. 6 and 7(a)]. This shows that variation in the pH does not affect the secondary structure conformation of β‐cardiotoxin.
Figure 6.
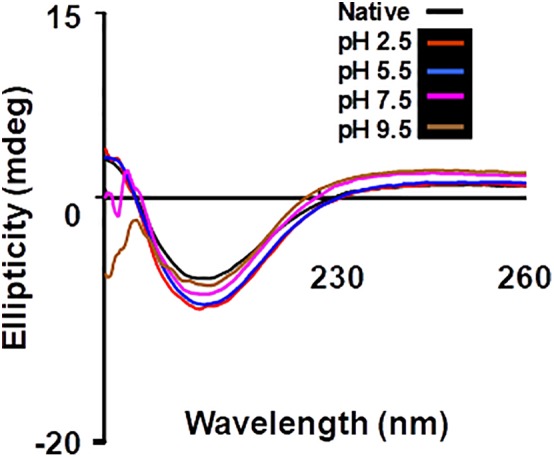
Chemical (un)folding studies of β‐cardiotoxin at different pH. (a) Effect of pH on secondary structure of β‐cardiotoxin. Chemical denaturation experiments were performed at 25°C by dissolving β‐cardiotoxin in buffers of different pH ranging from 2.5 to 9.5; 25 mM citrate pH 2.5, 25 mM MES pH 5.5, 25 mM MOPS pH 7.5, 25 mM Bis‐Tris propane pH 9.5.
Figure 7.
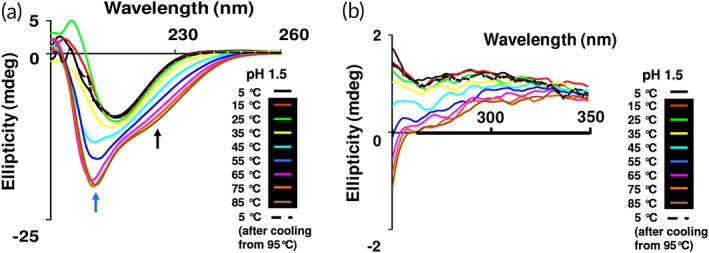
Combined effect of temperature and pH on the secondary structure of β‐cardiotoxin. Thermal denaturation of β‐cardiotoxin at pH 1.5. The protein was dissolved in MilliQ water (0.5 mg/mL) and pH adjusted to 1.5 with HCl and far‐UV CD spectra (a) and near‐UV CD spectra (b) were recorded using a 0.1 cm path‐length cuvette. Refolding was observed at 5°C after cooling from 85°C, black dotted line. The blue and black arrows indicate the new bands arising at 203 and 222 nm.
Combined effects of pH and temperature on β‐cardiotoxin structure
The combined effects of low pH and high temperature on the secondary and tertiary structure of β‐cardiotoxin were determined by far‐UV [Fig. 7(a)] and near‐UV CD spectroscopy [Fig. 7(b)], respectively. The protein undergoes similar structural transformation like the observation in the thermal denaturation experiment. However, at pH 1.5 the transition from β‐sheet to α‐helix starts to occur at much lower temperatures (from 45°C onward) [Fig. 7(a)] compared to the thermal denaturation studies where such an event started only from 75°C at neutral pH [Fig. 3(a)].
Thermodynamic calculations
The thermodynamic parameters of (un)folding for β‐cardiotoxin are calculated from Figure 8. Interestingly, the temperature induced denaturation is primarily driven by enthalpic contributions (Gibbs free‐energy of −1.4 kcal/mol at pH 1.5), which supports the view of extensive hydrogen bond formation rather than hydrophobic interactions. This is expected, since a transition from β‐sheet to α‐helical secondary structure is related to such hydrogen bond formation. The equilibrium guanidine HCl‐induced (un)folding of β‐cardiotoxin under nonreducing conditions revealed a Gibbs free‐energy of −5.0 kcal/mol, comparable to that observed for small monomeric proteins of similar size, such as myoglobin (6.6 kcal/mol).38, 39
Figure 8.
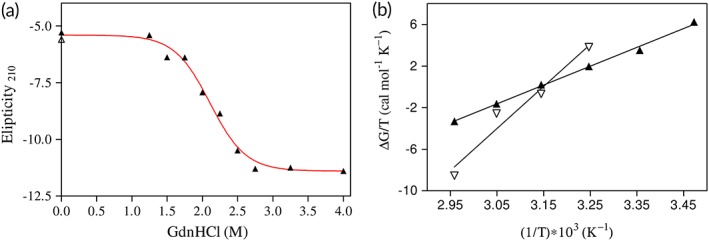
Thermodynamic plots of (un)folding of β‐cardiotoxin. (a) Guanidine HCl‐induced (un)folding of β‐cardiotoxin. The line represents a fit to a two‐state model of (un)folding. (b) Van't Hoff analysis of the temperature‐induced (un)folding of β‐cardiotoxin. Open symbols refer to (un)folding in water, while closed symbols indicate denaturation of the protein at pH 1.5. The lines are the result of linear regression analyses.
Proton NMR studies
The temperature‐dependent conformational change of β‐cardiotoxin was further examined by one‐dimensional proton NMR spectroscopy. Figure 9 shows the upfield shifted aliphatic proton resonances (left panel) and downfield shifted amide proton resonances (right panel) of β‐cardiotoxin obtained at four different temperatures. A well‐dispersed amide proton chemical shifts (7.6–9.4 ppm) and the aromatic ring‐current affected side chain resonances (0.55–0.75 ppm) clearly indicate that the protein is well folded at 25°C at pH 1.5. However, NMR spectra appear to change with increase in temperature. The spectral change is characterized by the considerable broadening of NMR signals for almost all the resonances at elevated temperatures (>25°C) (Fig. 9). The temperature‐dependent NMR line broadening is conspicuous for the most upfield shifted resonance at 0.55 ppm and a low‐field resonance at 7.7 ppm (Fig. 9). At 40°C, many of the well‐separated amide proton resonances had disappeared as a result of extreme line broadening. There was no precipitation or aggregation of the protein with increase in temperature (data not shown). Therefore, the temperature‐induced line broadening of NMR resonances clearly indicates that β‐cardiotoxin undergoes conformational exchange(s) between its native state and intermediate state(s). Such extreme line broadening also indicates that the intermediate state has very different chemical shifts as compared to the native one. It is noteworthy that molten‐globule‐like structured intermediate states of proteins including the well‐studied α‐lactalbumin are characterized by broad NMR resonances.40, 41, 42
Figure 9.
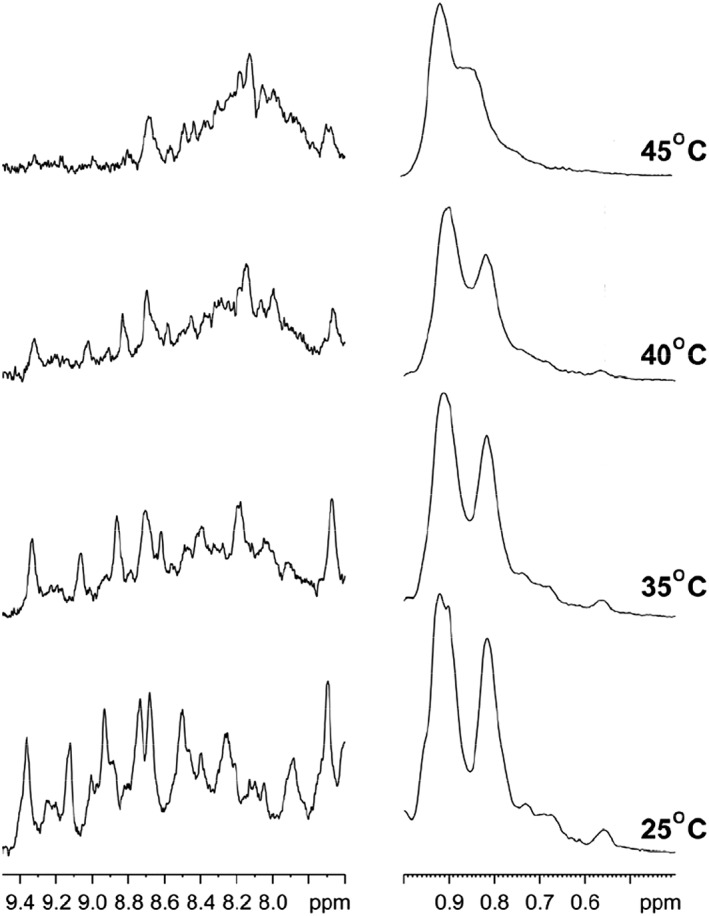
Temperature dependent structural changes of β‐cardiotoxin by NMR. One‐dimensional proton NMR spectra of β‐ cardiotoxin showing upfield shifted aliphatic proton resonances (left panel) and downfield shifted amide proton resonances (right panel) at four different temperatures. The NMR spectra were acquired in an aqueous solution, pH 1.5.
Discussion
Snake venom has provided a number of novel ligands with immense therapeutic potential. β‐cardiotoxin is the first reported natural exogenous beta‐blocker. Beta‐blockers, the antagonists of β‐ARs, are drugs of choice for cardiovascular diseases (CVDs). CVDs are the major cause of morbidity and mortality, resulting in most number of deaths in the western world. Current beta‐blockers are structural variants of aryl‐oxy propanolamines and are reported to possess many unwanted side‐effects due to lack of selectivity.43, 44 Hence, development of new beta‐blockers with higher specificity and lower side effects is crucial. β‐cardiotoxin being a natural beta‐blocker may provide a new avenue for the development of a new class of beta‐blockers with higher specificity and lower side effects.
The crystal structure of β‐cardiotoxin revealed a predominantly β‐sheeted structure similar to other three‐finger cardiotoxins with subtle alterations in the second loop. β‐cardiotoxin showed highest structural similarity to hemachatoxin, various cardiotoxins, muscarinic toxin, and bucain (Table 2). The closest structural homolog hemachatoxin from Hemachatus haemachatus is functionally uncharacterized. The DALI search did not return any 3FTX with β‐AR antagonistic activity from the PDB database and so far, this is the first structure of a 3FTX known to interact with β‐ARs. Loop II of β‐cardiotoxin is highly flexible and the overall surface charge distribution of β‐cardiotoxin differs significantly from conventional cytotoxins/cardiotoxins, which suggests the possible role of loop II residues as well as electrostatic surface potential in the unique function of this protein.
The possible conformational plasticity of β‐cardiotoxin was investigated by chemical and physical denaturation methods. In thermal denaturation studies of β‐cardiotoxin changes in the pattern and content of secondary structure was observed starting from 75°C [Fig. 3(a)]. It underwent a complete structural transition at 85°C, with the appearance of a new minima indicating the presence of α‐helical elements [Fig. 3(a)]. A complete loss of tertiary structure was observed in near UV CD spectra [Fig. 4(a)] coinciding closely with the structural transition occurring at 85°C. This indicates the formation of a stable folding intermediate with intact secondary structural elements but loss of tertiary structure, which resembles the molten globule state35, 36, 37 with α‐helical elements [Fig. 3(a)]. Interestingly, it has no α‐helix forming propensity as calculated according to Pollastri and McLysaght.45 Nonetheless, it undergoes a structural transition (from β‐sheet to α‐helix) at higher temperatures without the addition of any external agent or a reduction in pH. If the temperature is lowered from 95°C, it switches from α‐helix to β‐sheet indicating the reversibility of the (un)folding process [Fig. 3(b)]. Change in pH of the buffer did not induce any conformational changes [Fig. 6(b)]. However, a high concentration of guanidine HCl denatures the protein [Fig. 5(a)]. Interestingly, during chemical denaturation there is a highly cooperative transition from β‐sheet to random coil indicated by the loss of signal at 215 nm [Fig. 5(a)]. Also, the α‐helical intermediate identified during the thermal denaturation process is not populated during the chemical denaturation as there are no peaks detected at 203 or 222 nm [Fig. 5(a)]. The thermal denaturation performed at a pH of 1.5 showed the occurrence of the α‐helical intermediate. Interestingly, this intermediate appears at much lower temperatures (from 45°C onward) [Fig. 7(a)] enabling us to use other techniques like NMR to study this intermediate in more detail. NMR data support the existence of a populated intermediate state of the protein at 40°C whereby many of the diagnostic NMR resonances broaden as a result of conformational exchange between structurally different states (Fig. 9).
Interestingly, a variety of proteins adopting molten globule conformations have been linked to biological activity. Steroidogenic acute regulatory protein, for example, mediates cholesterol transport during steroid biosynthesis and appears to populate a partially folded molten globule state at low pH that is relevant for activity.46, 47 In addition, human α‐lactalbumin made lethal to tumor cells represent a molten globule conformation of α‐lactalbumin stabilized at low pH in the presence of oleic acid. It induces apoptosis in tumor cells and immature cells, while healthy cells are not affected and appears to be a promising lead compound in cancer treatment.48, 49 Ren and coworkers have noted that the transmembrane domain of diphtheria toxin represents a molecular chaperone but only enables translocation of the catalytic domain (A chain) of the toxin across membranes when the protein adopts a molten globule conformation, which is stabilized at acidic pH.50 A recent study by Tseng et al. revealed that crammer, a cathepsin inhibitor from Drosophila melanogaster, only shows inhibiting activity at low pH where the protein forms a molten‐globule monomeric structure as evidenced by heteronuclear NMR and CD investigations. However, the native dimeric protein at physiological pH remains inactive.51 In essence, it is tempting to propose that the molten globule intermediate together with the pronounced conformational plasticity of β‐cardiotoxin might be related biological or pharmacological activity.
In summary, we have determined the structure and studied the conformational plasticity of β‐cardiotoxin, a 3FTX with β‐blocker activity from Ophiophagus hannah. Compared to other conventional cardiotoxins, the structure reported in the present study reveals pronounced differences in the loop regions and in the charge distribution on the surface, which might be related to different pharmacological activities. In addition, thermodynamic folding studies of all this β‐sheet protein revealed the presence of an α‐helical molten‐globule intermediate which points to substantial conformational plasticity of the rather small protein. These results indicate that we are only at the beginning of understanding the whole range of functions of this fascinating snake venom protein family. This warrant further investigation and in future we would like to elucidate the detailed mechanism of action for β‐cardiotoxin and its structural determinants.
Materials and Methods
Proteins and reagents
Lyophilized O. hannah venom was obtained from PT Venom Indo Persada (Jakarta, Indonesia) and Kentucky Reptile Zoo (Slade, KY). β‐Cardiotoxin was purified from the venom of Ophiophagus hannah as described earlier.19 Acetonitrile and trifluoroacetic acid was purchased from Merck KGaA (Darmstadt, Germany). Superdex 30 Hiload (16/60) column and Jupiter C18 (5 μ, 300 Å, 4.6 mm × 150 mm) were purchased from GE Healthcare Life Sciences (Piscataway, NJ) and Phenomenex (Torrance, CA), respectively. Crystal screening solution and accessories were obtained from Hampton Research (Aliso Viejo, CA) and Qiagen GmbH (Hilden, Germany). All other chemicals were purchased from Sigma‐Aldrich (St. Louis, MO). All the reagents were of the highest purity grade. Water was purified using a MilliQ system (Millipore, Billerica, MA).
Crystallization and data collection
Lyophilized β‐Cardiotoxin protein was dissolved in 10 mM Tris–HCl buffer pH 7.4 with 100 mM NaCl. Crystallization experiments were performed at room temperature 297 K (24°C), with drops containing equal volumes (1 μL) of reservoir and protein solution, using hanging‐drop vapor diffusion method. β‐Cardiotoxin crystals were obtained in 0.1 M SPG buffer (2:7:7 – succinic acid:sodium dihydrogen phosphate:glycine) pH 7 with 25% w/v PEG 1500 (Pact suite, condition no. 4). Diffraction quality crystals with the smallest dimension measuring about 50 μm were obtained after 2–3 weeks. A 20% (v/v) glycerol was supplemented with crystallization condition as the cryo‐protectant. These crystals were flash‐frozen in a nitrogen cold stream at 100 K (−173°C). The crystals diffracted up to 2.41 Å resolution and a complete data set was collected using a CCD detector mounted on a Bruker V8 rotating anode generator (Bruker AXS, Madison, WI). The structure of β‐cardiotoxin was solved by molecular replacement method with the program MolRep52 using the coordinates of Chinese Cobra Cardiotoxin V53 (pdb code: 1KXI) as a search model. There were three molecules in the asymmetric unit. Model was initially refined using Refmac 5 program,54 and the calculated electron density map was examined in the program COOT.55 The diffraction data were twinned and amplitude‐based twin refinement was employed in Refmac. Noncrystallographic symmetry was used for all the three chains throughout the refinement. During the final stage of refinement, 55 well‐defined water molecules were included. Several cycles of map fitting alternated with refinement led to the final R work = 0.231(R free = 0.269) (Table 1).
Thermal denaturation studies using CD spectroscopy
A Jasco J‐810 spectropolarimeter connected to a Grant LTD6G water bath controlled by Jasco PTC‐423S was used for this study. All measurements were carried out using a 0.1 cm path length capped cuvette. The instrument optics and cuvette chamber were continuously flushed with 10–30 L of nitrogen/min before and during the recording of CD spectra. The spectra were recorded using a scanning speed of 50 nm/min, a resolution of 0.1 nm and a bandwidth of 1 nm. A total of three scans were recorded and averaged for each spectrum, and the baseline was subtracted. All samples were dissolved in MilliQ water, unless otherwise stated. The temperature range was from 5°C to 95°C with a pitch of 10°C. Far‐UV CD spectrum (260 to 190 nm) and near‐UV CD spectrum (350–250 nm) were recorded after each 10°C increase. The CD was also continuously monitored at 203, 222, and 280 nm with a data pitch of 0.1°C and a bandwidth of 1 nm. The temperature slope used for the study was 5°C/min.
Chemical denaturation studies using CD spectroscopy
Chemical denaturation experiments were performed using far‐UV CD spectroscopy. All CD measurement parameters were same as that described in the thermal denaturation studies. β‐Cardiotoxin (0.5 mg/mL) was dissolved in 25 mM Tris–HCl pH 7.5 buffer containing increasing concentrations of guanidine‐HCl (0–2.75 M) and the CD spectra were recorded for each concentration.
Effect of pH on secondary structure
The effect of pH on the secondary structure of β‐cardiotoxin was assessed by dissolving the protein (0.5 mg/mL) in buffers of varying pH. The buffers used were 25 mM Citrate pH 2.5, 25 mM MES [2‐(N‐morpholino) ethanesulfonic acid] pH 5.5 and 25 mM MOPS [3‐(N‐morpholino) propanesulfonic acid] pH 7.5 and 25 mM bis‐tris propane pH 9.5. All CD measurements were performed as described in the thermal denaturation studies.
Combined effects of pH and temperature on β‐cardiotoxin structure
The protein (0.5 mg/mL for far‐UV CD spectroscopy and 1 mg/mL for near‐UV CD spectroscopy) was dissolved in MilliQ water and the pH was adjusted to 1.5 using concentrated HCl and a thermal denaturation experiment was carried out as described in the previous sections.
One‐dimensional 1H NMR studies
The protein (2.5 mg) was dissolved in 550 μL of water and pH was adjusted to 1.5 using 0.1M HCl. A series of one‐dimensional proton NMR spectra were acquired in an aqueous solution containing 10% D2O on a Bruker Avance DRX 600 spectrometer equipped with cryoprobe at various temperatures. All spectra were obtained with 512 scans. NMR spectra were processed and analyzed using TOPSIN (Bruker) program. Proton chemical shifts were referenced to DSS (2,2‐dimethyl‐2‐silapentane‐5‐sulfonate sodium salt).
Thermodynamic equations
GdnHCl‐induced (un)folding of β‐cardiotoxin
The GdnHCl‐induced equilibrium (un)folding data of β‐cardiotoxin were fitted for parameter determination using a two‐state model of (un)folding according to the method of Santoro and Bolen56 using the program Prism 3.0 [Fig. 8(a)] by means of a built‐in Levenberg–Marquard nonlinear least‐squares minimization algorithm.57
Temperature‐induced (un)folding of β‐cardiotoxin
At constant pressure the temperature dependence of the equilibrium constant is described by the Van't Hoff equation:
where K T is the equilibrium constant for denaturation at temperature T and ΔG is the corresponding Gibbs free‐energy change. From a plot of ΔG/T versus the inverse temperature, the changes in enthalpy (ΔH) and entropy (ΔS) of unfolding can be extracted [Fig. 8(b)].
Data deposition
The coordinates and structure factors for β‐cardiotoxin have been deposited with the Research Collaboratory for Structural Bioinformatics (RCSB)21 PDB with the code 3PLC.
ACKNOWLEDGMENTS
This work was supported by research grant from the Biomedical Research Council (BMRC), Agency for Science and Technology, Singapore, to R.M.K. (BMRC grant no. R154‐000‐172‐316). J.S. was partially support by AcRF Tier‐1 grant (R154‐000‐A72‐114). A.R. thanks the Department of Biological Sciences, National University of Singapore, for providing graduate research scholarship.
REFERENCES
- 1. Ogawa T, Chijiwa T, Oda‐Ueda N, Ohno M (2005) Molecular diversity and accelerated evolution of C‐type lectin‐like proteins from snake venom. Toxicon 45:1–14. [DOI] [PubMed] [Google Scholar]
- 2. Kini RM (2006) Anticoagulant proteins from snake venoms: structure, function and mechanism. Biochem J 397:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kini RM, Doley R (2010) Structure, function and evolution of three‐finger toxins: mini proteins with multiple targets. Toxicon 56:855–867. [DOI] [PubMed] [Google Scholar]
- 4. Kang TS, Georgieva D, Genov N, Murakami MT, Sinha M, Kumar RP, Kaur P, Kumar S, Dey S, Sharma S, Vrielink A, Betzel C, Takeda S, Arni RK, Singh TP, Kini RM (2011) Enzymatic toxins from snake venom: structural characterization and mechanism of catalysis. FEBS J 278:4544–4576. [DOI] [PubMed] [Google Scholar]
- 5. Utkin YN (2013) Three‐finger toxins, a deadly weapon of elapid venom – milestones of discovery. Toxicon 62:50–55. [DOI] [PubMed] [Google Scholar]
- 6. McCleary RJR, Kini RM (2013) Non‐enzymatic proteins from snake venoms: a gold mine of pharmacological tools and drug leads. Toxicon 62:56–74. [DOI] [PubMed] [Google Scholar]
- 7. Kini RM (2002) Molecular moulds with multiple missions: functional sites in three‐finger toxins. Clinic Exper Pharmacol Physiol 29:815–822. [DOI] [PubMed] [Google Scholar]
- 8. Tsetlin V (1999) Snake venom alpha‐neurotoxins and other 'three‐finger' proteins. Eur J Biochem 264:281–286. [DOI] [PubMed] [Google Scholar]
- 9. Pawlak J, Mackessy SP, Fry BG, Bhatia M, Mourier G, Fruchart‐Gaillard C, Servent D, Ménez R, Stura E, Ménez A, Kini RM (2006) Denmotoxin, a three‐finger toxin from the colubrid snake Boiga dendrophila (mangrove cat snake) with bird‐specific activity. J Biol Chem 281:29030–29041. [DOI] [PubMed] [Google Scholar]
- 10. Junqueira‐de‐Azevedo ILM (2006) Lachesis muta (Viperidae) cDNAs reveal diverging pit viper molecules and scaffolds typical of cobra (Elapidae) venoms: implications for snake toxin repertoire evolution. Genetics 173:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pahari S, Mackessy SP, Kini RM (2007) The venom gland transcriptome of the Desert Massasauga rattlesnake (Sistrurus catenatus edwardsii): towards an understanding of venom composition among advanced snakes (Superfamily Colubroidea). BMC Mol Biol 8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Endo T, Tamiya N (1987) Current view on the structure‐function relationship of postsynaptic neurotoxins from snake venoms. Pharmacol Therap 34:403–406. [DOI] [PubMed] [Google Scholar]
- 13. Young HS, Herbette LG, Skita V (2003) α‐Bungarotoxin binding to acetylcholine receptor membranes studied by low angle X‐ray diffraction. Biophys J 85:943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dufton MJ, Hider RC (1988) Structure and pharmacology of elapid cytotoxins. Pharmacol Therap 36:1–40. [DOI] [PubMed] [Google Scholar]
- 15. Konshina AG, Dubovskii PV, Efremov RG (2012) Structure and dynamics of cardiotoxins. Curr Prot Pept Sci 13:570–584. [DOI] [PubMed] [Google Scholar]
- 16. Banerjee Y, Mizuguchi J, Iwanaga S, Kini RM (2005) Hemextin AB complex, a unique anticoagulant protein complex from Hemachatus haemachatus (African Ringhals cobra) venom that inhibits clot initiation and factor VIIa activity. J Biol Chem 280:42601–42611. [DOI] [PubMed] [Google Scholar]
- 17. Barnwal B, Jobichen C, Girish VM, Foo CS, Sivaraman J, Kini RM (2016) Ringhalexin from Hemachatus haemachatus: a novel inhibitor of extrinsic tenase complex. Sci Rep 6:25935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Girish VM, Kini RM (2016) Exactin: a specific inhibitor of factor X activation by extrinsic tenase complex from the venom of Hemachatus haemachatus . Sci Rep 6:32036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rajagopalan N, Pung YF, Zhu YZ, Wong PTH, Kumar PP, Kini RM (2007) β‐Cardiotoxin: a new three‐finger toxin from Ophiophagus hannah (king cobra) venom with beta‐blocker activity. FASEB J 21:3685–3695. [DOI] [PubMed] [Google Scholar]
- 20. Kumar TKS, Jayaraman G, Lee CS, Sivaraman T, Lin WY, Yu C (1995) Identification of "molten globule"‐like state in an all β‐sheet protein. Biochem Biophys Res Commun 207:536–543. [DOI] [PubMed] [Google Scholar]
- 21. Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Research 28:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holm L, Rosenstram PI (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res 38:W545–W549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen D, Kini RM, Yuen R, Khoo HE (1997) Haemolytic activity of stonustoxin from stonefish (Synanceja horrida) venom: pore formation and the role of cationic amino acid residues. Biochem J 325:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumar TKS, Jayaraman G, Lee CS, Arunkumar AI, Sivaraman T, Samuel D, Yu C (1997) Snake venom cardiotoxins‐structure, dynamics, function and folding. J Biomol Struct Dyn 15:431–463. [DOI] [PubMed] [Google Scholar]
- 25. Lee S‐C, Lin C‐C, Wang C‐H, Wu P‐L, Huang H‐W, Chang C‐I, Wu W‐G (2014) Endocytotic routes of cobra cardiotoxins depend on spatial distribution of positively charged and hydrophobic domains to target distinct types of sulfated glycoconjugates on cell surface. J Biol Chem 289:20170–20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dubovskii P, Konshina A, Efremov R (2013) Cobra cardiotoxins: membrane interactions and pharmacological potential. Curr Med Chem 21:270–287. [DOI] [PubMed] [Google Scholar]
- 27. Gatineau E, Toma F, Montenay‐Garestier T, Takechi M, Fromageot P, Menez A (1987) Role of tyrosine and tryptophan residues in the structure‐activity relationships of a cardiotoxin from Naja nigricollis venom. Biochemistry 26:8046–8055. [DOI] [PubMed] [Google Scholar]
- 28. Ménez A, Gatineau E, Roumestand C, Harvey AL, Mouawad L, Gilquin B, Toma F (1990) Do cardiotoxins possess a functional site? Structural and chemical modification studies reveal the functional site of the cardiotoxin from Naja nigricollis . Biochimie 72:575–588. [DOI] [PubMed] [Google Scholar]
- 29. Roumestand C, Gilquin B, Trémeau O, Gatineau E, Mouawad L, Ménez A, Toma F (1994) Proton NMR studies of the structural and dynamical effect of chemical modification of a single aromatic side‐chain in a snake cardiotoxin relation of the structure of the putative binding site and the cytolitic activity of the toxin. J Mol Biol 243:719–735. [DOI] [PubMed] [Google Scholar]
- 30. Semisotnov GV, Rodionova NA, Kutyshenko VP, Ebert B, Blanck J, Ptitsyn OB (1987) Sequential mechanism of refolding of carbonic anhydrase B. FEBS Lett 224:9–13. [DOI] [PubMed] [Google Scholar]
- 31. Kuwajima K (1989) The molten globule state as a clue for understanding the folding and cooperativity of globular‐protein structure. Proteins 6:87–103. [DOI] [PubMed] [Google Scholar]
- 32. Zhou Y, Karplus M (1999) Interpreting the folding kinetics of helical proteins. Nature 401:400–403. [DOI] [PubMed] [Google Scholar]
- 33. Chapeaurouge A, Johansson JS, Ferreira ST (2001) Folding intermediates of a model three‐helix bundle protein. J Biol Chem 276:14861–14866. [DOI] [PubMed] [Google Scholar]
- 34. Chmid F, Spectroscopic techniques to study protein folding and stability In: Buchner J, Kiefhaber T, Eds. (2008) Protein folding handbook. 10.1002/9783527619498.ch2 [DOI] [Google Scholar]
- 35. Kuwajima K, Nitta K, Yoneyama M, Sugai S (1976) Three‐state denaturation of α‐lactalbumin by guanidine hydrochloride. J Mol Biol 106:359–373. [DOI] [PubMed] [Google Scholar]
- 36. Dolgikh DA, Gilmanshin RI, Brazhnikov EV, Bychkova VE, Semisotnov GV, Venyaminov SY, Ptitsyn OB (1981) α‐Lactalbumin: compact state with fluctuating tertiary structure? FEBS Lett 136:311–315. [DOI] [PubMed] [Google Scholar]
- 37. Ohgushi M, Wada A (1983) ‘Molten‐globule state’: a compact form of globular proteins with mobile side‐chains. FEBS Lett 164:21–24. [DOI] [PubMed] [Google Scholar]
- 38. Barrick D, Baldwin RL (1993) Three‐state analysis of sperm whale apomyoglobin folding. Biochemistry 32:3790–3796. [DOI] [PubMed] [Google Scholar]
- 39. Jamin M (2005) The folding process of apomyoglobin. Prot Pept Lett 12:229–234. [DOI] [PubMed] [Google Scholar]
- 40. Baum J, Dobson CM, Evans PA, Hanley C (1989) Characterization of a partly folded protein by NMR methods: studies on the molten globule state of guinea pig alpha‐lactalbumin. Biochemistry 28:7–13. [DOI] [PubMed] [Google Scholar]
- 41. Bhattacharjya S, Balaram P (1997) Hexafluoroacetone hydrate as a structure modifier in proteins: characterization of a molten globule state of hen egg‐white lysozyme. Protein Sci 6:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim S, Bracken C, Baum J (1999) Characterization of millisecond time‐scale dynamics in the molten globule state of α‐lactalbumin by NMR. J Mol Biol 294:551–560. [DOI] [PubMed] [Google Scholar]
- 43. Al‐Majed AA, Bakheit AHH, Abdel Aziz HA, Alajmi FM, AlRabiah H (2017) Propranolol, Profiles of drug substances, excipients and related methodology. Academic Press, 42:287–338. 10.1016/bs.podrm.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 44. Perros F, de Man FS, Bogaard HJ, Antigny F, Simonneau G, Bonnet S, Provencher S, Galiè N, Humbert M (2017) Use of β‐blockers in pulmonary hypertension. Circulat Heart Fail 10:e003703. [DOI] [PubMed] [Google Scholar]
- 45. Pollastri G, McLysaght A (2004) Porter: a new, accurate server for protein secondary structure prediction. Bioinformatics 21:1719–1720. [DOI] [PubMed] [Google Scholar]
- 46. Christensen K, Bose HS, Harris FM, Miller WL, Bell JD (2001) Binding of steroidogenic acute regulatory protein to synthetic membranes suggests an active molten globule. J Biol Chem 276:17044–17051. [DOI] [PubMed] [Google Scholar]
- 47. Tuckey RC, Bose HS, Czerwionka I, Miller WL (2004) Molten globule structure and steroidogenic activity of N‐218 MLN64 in human placental mitochondria. Endocrinology 145:1700–1707. [DOI] [PubMed] [Google Scholar]
- 48. Svensson M, Hakansson A, Mossberg AK, Linse S, Svanborg C (2000) Conversion of alpha‐lactalbumin to a protein inducing apoptosis. Proc Natl Acad Sci USA 97:4221–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hallgren O, Aits S, Brest P, Gustafsson L, Mossberg A‐K, Wullt B, Svanborg C. Apoptosis and tumor cell death in response to HAMLET (human α‐lactalbumin made lethal to tumor cells), 2008. Advances in experimental medicine and biology. New York: Springer; p. 217–240. [DOI] [PubMed] [Google Scholar]
- 50. Ren J (1999) Interaction of diphtheria toxin T domain with molten globule‐like proteins and its implications for translocation. Science 284:955–957. [DOI] [PubMed] [Google Scholar]
- 51. Tseng T‐S, Cheng C‐S, Chen D‐J, Shih M‐F, Liu Y‐N, Hsu S‐TD, Lyu P‐C (2012) A molten globule‐to‐ordered structure transition of Drosophila melanogaster crammer is required for its ability to inhibit cathepsin. Biochem J 442:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vagin A, Teplyakov A (1997) MOLREP: an automated program for molecular replacement. J Appl Cryst 30:1022–1025. [Google Scholar]
- 53. Sun Y‐J, W‐G W, Chiang C‐M, Hsin AY, Hsiao C‐D (1997) Crystal structure of cardiotoxin V from Taiwan cobra venom: pH‐dependent conformational change and a novel membrane‐binding motif identified in the three‐finger loops of P‐type cardiotoxin. Biochemistry 36:2403–2413. [DOI] [PubMed] [Google Scholar]
- 54. Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Cryst D 67:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Emsley P, Cowtan K (2004) Coot: model‐building tools for molecular graphics. Acta Crystallogr 60:2126–2132. [DOI] [PubMed] [Google Scholar]
- 56. Santoro MM, Bolen DW (1988) Unfolding free energy changes determined by the linear extrapolation method. 1. Unfolding of phenylmethanesulfonyl alpha‐chymotrypsin using different denaturants. Biochemistry 27:8063–8068. [DOI] [PubMed] [Google Scholar]
- 57. Marquardt DW (1963) An algorithm for least‐squares estimation of nonlinear parameters. J Soc Indust Appl Math 11:431–441. [Google Scholar]


