Abstract
Motivated by a large number of applications, we consider the process of non-stationary growth of spherical crystals in a supercooled binary melt. The moving-boundary problem describing the unsteady-state distributions of temperature and impurity concentration around the growing crystal as well as the dynamics of its radius and growth rate is solved by means of the methods of small-parameter expansion and Laplace–Carson integral transform. We show that the growth rate of crystals contains the main contribution (which is proportional to the supercooling degree Δ) and the first correction (which is proportional to Δ2t, where t is time). The second correction is also found. The non-stationary temperature and concentration fields are determined as power functions of Δ and t. We demonstrate that the first corrections to the dynamics of crystal radius R(t) and its growth rate V (t) play an important role. It is shown that R(t) and V (t) can change more than twice in comparison with the previously known steady-state solution with the course of time. Such a behaviour will significantly modify the dynamics of a polydisperse ensemble of crystals evolving in a metastable liquid.
This article is part of the theme issue ‘Heterogeneous materials: metastable and non-ergodic internal structures’.
Keywords: crystal growth, metastable liquid, moving-boundary problem, phase transformation
1. Introduction
It is well known that the processes of nucleation and growth of individual particles determine the dynamics of the evolution of supercooled melts and supersaturated solutions in the intermediate stage of phase transformations (see, among others, [1–5]). The rate of particle growth enters the Fokker–Planck equation to determine the particle-size distribution function, and also to the boundary condition determining the flux of particles overcoming the critical barrier [6–8]. Therefore, accurate determination of the law for the growth rates of individual crystals is an important task, the solution of which completely describes the evolution of the polydisperse ensemble of particles, i.e. determines the particle size distribution function and the dynamics of desupercooling or desupersaturation of the metastable system.
In theoretical studies, the following expression is frequently used for the growth rates V of spherical crystals in supercooled single-component systems:
| 1.1 |
where R is the radius of growing crystal, β* is the kinetic coefficient, Δ = Tp − Tl is the supercooling, Tp is the phase transition temperature, Tl is the mean temperature of a metastable liquid, t is the growth time, q = ρsL/λl, ρs is the density of solid phase, L is the latent heat of phase transition, and λl is the thermal conductivity. Formula (1.1) follows from the solution of the quasi-stationary problem of the growth of a spherical crystal in a supercooled liquid [9]
| 1.2 |
where r is the spherical coordinate and T is the temperature field around the growing crystal. Note that an analogous formula for the growth rates of particles in binary systems was obtained in [10] when considering quasi-stationary fields of temperature and impurity concentration.
Generally speaking, the assumption of a stationary temperature field (∇2T = 0, r > R(t)) around the growing crystal is not correct due to the following reason. On the surface of the growing crystal, the latent heat of crystallization is released, which changes the temperature field. In the case of the growth of particles in a supersaturated solution, the interface between the solid and liquid phases displaces the dissolved impurity and increases the impurity concentration C in the near-surface layer adjacent to the growing crystal. Therefore, the concentration field is also incorrectly described by the quasi-stationary diffusion equation (∇2C = 0, r > R(t)).
Below we develop a method for determining the growth rates of spherical crystals in supercooled binary liquids with allowance for the non-stationary temperature and impurity concentration fields. It is also shown that accounting for non-stationary fields T and C, the rate of crystal growth dR/dt = V (t) significantly changes in comparison with the previously known solution obtained under quasi-stationary conditions.
2. Unsteady-state growth of spherical crystals in a metastable binary melt
Let us consider the process of evolution of spherical crystals of radius R(t) in a metastable binary melt. We will assume that the crystals are at large distances from each other so that interactions between them can be neglected. We will also assume that the growing particles are randomly arranged in the volume of the binary system and form a face-centred cubic lattice. Under such assumptions, the evolution of each individual particle can be considered within a single cell (in this case, the entire volume of the system is divided into a set of such cells). We denote by Re the radius of the equivalent sphere inscribed in such a cell (figure 1). For the sake of simplicity of the solution of the problem, we neglect the critical radius of the growing crystallites.
Figure 1.
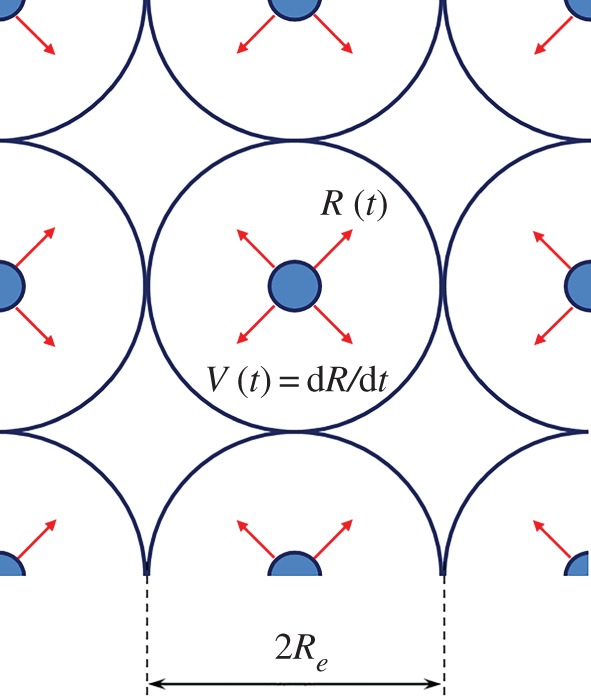
A scheme of evolution of spherical crystals in a metastable melt. The total volume of a metastable system is divided into cells (equivalent spheres) in which individual particles grow.
Let us write down the temperature conductivity and diffusion equations governing the temperature and concentration fields around the evolving crystals within a particular cell. In the spherical coordinate system, these equations take the form
| 2.1 |
where r is the radial coordinate, a is the thermal diffusivity and D is the diffusion coefficient.
Equations (2.1) must be considered under the following boundary conditions at the interphase boundary and away from it on an equivalent sphere
| 2.2 |
where k and m stand for the equilibrium distribution coefficient and liquidus slope, respectively. Note that the boundary conditions at r = Re show the absence of heat and mass fluxes on the surface of the equivalent sphere.
For the convenience of mathematical manipulations, we introduce dimensionless variables as follows:
| 2.3 |
The heat and mass transfer equations (2.1) and boundary conditions (2.2) in dimensionless variables (2.3) take the form
| 2.4 |
| 2.5 |
where γ = a/D is typically much greater than 1.
The solution of dimensionless equations (2.4) satisfying the boundary conditions (2.5) at ρ = 1 can be presented in the form [11]
| 2.6 |
where B(τ) = T(1, τ) and A(τ) = C(1, τ).
Substituting now the solutions (2.6) into the boundary conditions (2.5) at ρ = ρ0(τ), we obtain three equations for finding the functions ρ0(τ), B(τ) and A(τ)
| 2.7 |
Here we assume that γ≫1 and
An important point is that equations (2.7) obtained for binary melts transform to the corresponding equations derived in [12] for one-component melts if we formally put A = 0.
Taking into account that β(0) = β0≪1 [9,13], we seek solutions of nonlinear equations (2.7) in the form of series in the small parameter β0 as
| 2.8 |
where A(0) = C(1, 0) = C0 = const.
Now substituting the asymptotic expansions (2.8) into expressions (2.7) and equating the contributions at the same powers of small parameter β0, one can obtain
| 2.9 |
To determine the functions φ3, ψ3 and χ3, we have the following system of equations:
| 2.10 |
where
Equations (2.10) can be solved using the Laplace–Carson integral transform. To apply this method, we note that the following initial conditions follow from equations (2.10):
| 2.11 |
Now taking into account (2.11), we have from (2.10) [14]
| 2.12 |
where * shows the Laplace–Carson transform, s represents the Laplace–Carson variable, and
Keeping in mind that [11]
| 2.13 |
we get from expressions (2.12)
| 2.14 |
To find the inverse Laplace–Carson transform, we present the right-hand sides of expressions (2.14) by means of the following formula:
| 2.15 |
where
| 2.16 |
Now keeping in mind the inverse transforms [11,14]
where sk satisfies the following equation:
we have ψ3(τ) and χ3(τ) from (2.14)–(2.16)
| 2.17 |
where , μk satisfies the equation μkcosμk = sinμk, and ‘i’ is the imaginary unit. Note that ψ3(τ) coincides with the solution of thermally controlled crystal growth found in [12] in the case of single-component systems (C0 = 0).
Now the first equation (2.10) can be easily integrated so that
where ψ3 is determined by the first expression (2.17).
Now the asymptotic expansions (2.8) can be rewritten in the form
| 2.18 |
If we restrict ourselves to the main contribution to the crystal radius and its first correction, one can obtain the following dimensional expressions for R(t) and V (t) from (2.18):
| 2.19 |
where Δ = Tp − T(1, 0) − mC(1, 0) represents the system supercooling. If we formally put C0 = 0, expressions (2.19) transform to the corresponding solutions for one-component systems [12]. Note that the growth rate V (t) of crystals in binary melts is less than the rate of their growth in single-component systems (m > 0, k < 1). An important feature of expressions (2.19) is the fact that they do not depend on the radius Re of the equivalent sphere. Considering the intermediate stage of crystal growth in a metastable liquid when all particles do not interact with their neighbouring crystals, we note that T(1, 0) and C(1, 0) correspond to the mean temperature Tl and concentration Cl of a metastable binary system.
3. Discussion and conclusion
Figures 2 and 3 illustrate our asymptotic solution (2.19) for the Ti–Al alloy (its material parameters are given in table 1). Note that the first terms in the right-hand sides of expressions (2.19) represent the analytical solutions in the so-called kinetically controlled growth regime [9,10]. In this case, the crystal radius R(t) and its growth rate V (t) evolve as R(t) = R0(t) = β*Δt and V (t) = V0(t) = β*Δ.
Figure 2.
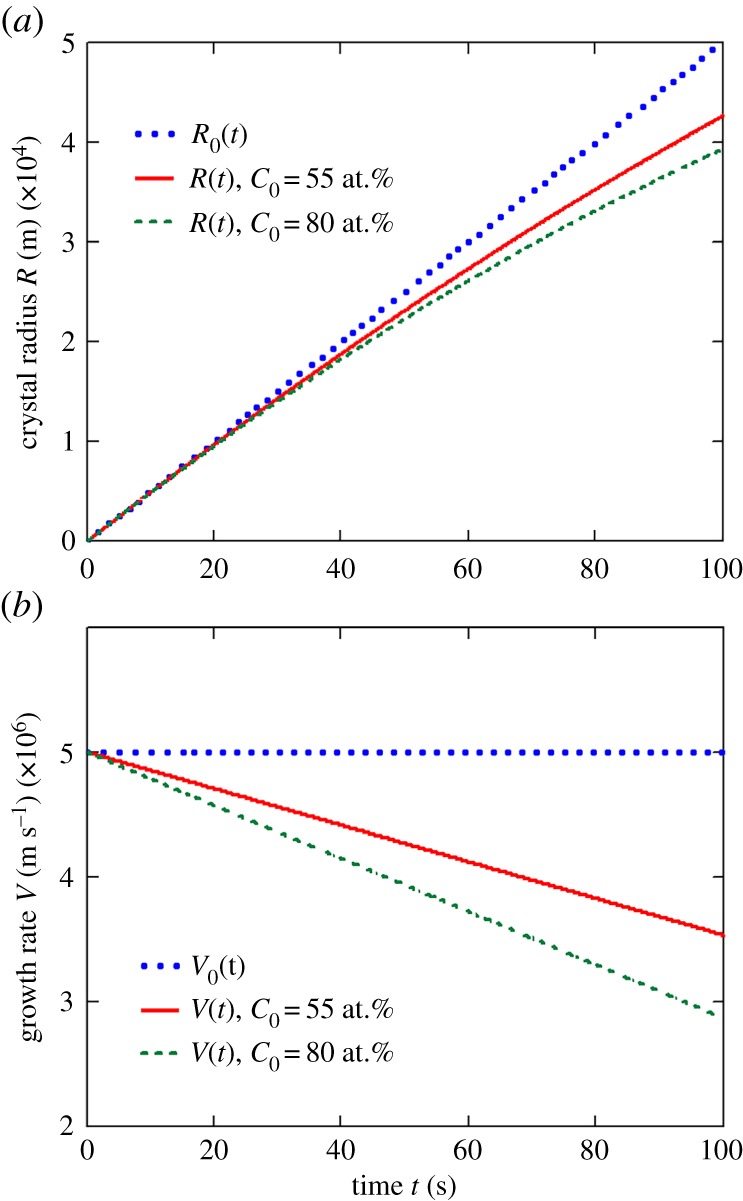
The influence of impurity concentration C0 on the evolution of crystal radius R(t) (a) and its growth rate V (t) (b), β* = 0.5 · 10−7 m K−1 s−1.
Figure 3.
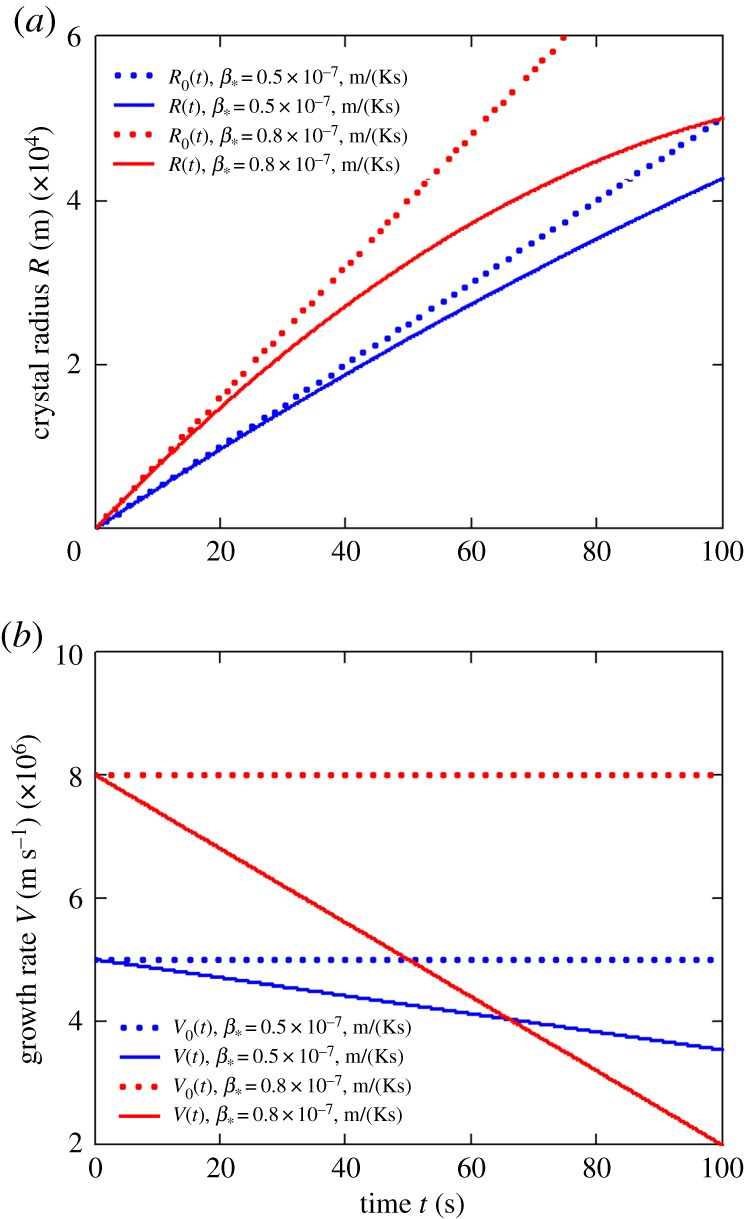
The influence of kinetic coefficient β* on the evolution of crystal radius R(t) (a) and its growth rate V (t) (b), C0 = 55 at.%.
Table 1.
Material parameters of Ti–Al alloy [17].
| parameter | value | ||
|---|---|---|---|
| thermal diffusivity | a | m2 s−1 | 7.5 × 10−6 |
| liquidus slope | m | K/at.% | 8.8 |
| partition coefficient | k | — | 0.8 |
| diffusion coefficient | D | m2 s−1 | 8.27 × 10−9 |
| supercooling | Δ | K | 100 |
| parameter | Λ | K−1 | 3.67 × 10−3 |
First, we note a strong shift of the unsteady-state solutions (2.19) from the previously known steady-state solutions R0(t) and V0(t) (compare R(t) and R0(t) as well as V (t) and V0(t) in figures 2 and 3). Secondly, as the impurity concentration C0 increases, this shift increases too (figure 2). Third, an increase in the kinetic coefficient β* also leads to a shift in the radius and growth rate of the crystals (figure 3). An important point is that the growth rate V0 does not depend on t at constant supercooling Δ, whereas V (t) from (2.19) is a decreasing function of t (figures 2b and 3b). We note that the growth rate of crystals can change several times in comparison with previously known solution V0 (figures 2b and 3b). This leads to the conclusion that in the initial stages of crystal growth (when supercooling is almost unchanged), the non-stationary rate of their growth substantially changes the solution of the Fokker–Planck equation for the crystal size distribution function, and also the solution of the heat (mass) balance equation. This question is studied in more detail in papers [15,16] from the present theme issue.
Let us now estimate the typical times when the non-stationary contributions in expressions (2.19) become significant. To do this, let us rewrite the growth rate V (t) as
| 3.1 |
The non-stationary contribution ζ is of the order of 2.9 · 10−3 s−1 for the parameter values listed in table 1 and β* = 0.5 · 10−7 m s−1 K−1, and C0 = 55 at%. Now we see that the non-stationary term (proportional to ζ) must be taken into account at times t ≳102 s (this time is counted from the nucleation of a single crystal). However, this estimate must be recalculated, for long times, when supercooling becomes dependent on time.
Let us emphasize in conclusion the key result of the present analysis. Non-stationary contributions entering in the growth rates of spherical particles, their radii, and the distribution of temperature and concentration fields must be taken into account in the theoretical description of the intermediate stage of phase transformation in melts and solutions (the analytical description of crystal growth in dilute solutions is presented in appendix A). For this reason, many theories of growth of crystals or droplike aggregates in supersaturated solutions, supercooled melts, mushy layers, colloids, magnetic fluids and other physical systems (see, among others, [18–27]) should be reconsidered with allowance for the non-stationary contributions to the particle growth rates.
Appendix A. Appendix A. Unsteady-state growth of spherical crystals in a supersaturated solution
If the growth of spherical particles occurs in a supersaturated solution, the moving-boundary problem takes the form
where Cp is the concentration at saturation. Its solution can be found by analogy with the theory presented in §2 (see, for details, [28]).
Here Δ designates the supersaturation of the liquid solution.
The last expressions for R(t) and V (t) should be used for a more precise description of nucleation and crystal growth processes in supersaturated solutions. Note that the correction of a higher order of smallness, as well as the impurity concentration around the growing spherical particle, are determined by Alexandrov et al. [28].
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed equally to the present research article.
Competing interests
We declare that we have no competing interests.
Funding
This work was supported by the Russian Science Foundation (grant no. 18-19-00008).
References
- 1.Skripov VP. 1974. Metastable liquids. New York, NY: John Wiley & Sons. [Google Scholar]
- 2.Hinz W. 1977. Nucleation and crystal growth. J. Non-Cryst. Solids 25, 215–260. ( 10.1016/0022-3093(77)90094-1) [DOI] [Google Scholar]
- 3.Buyevich YuA, Alexandrov DV, Mansurov VV. 2001. Macrokinetics of crystallization. New York, NY: Begell House. [Google Scholar]
- 4.Kelton KF, Greer AL. 2010. Nucleation in condensed matter: applications in materials and biology. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 5.Dubrovskii VG. 2014. Nucleation theory and growth of nanostructures. Heidelberg, Germany: Springer. [Google Scholar]
- 6.Buyevich YuA, Mansurov VV. 1990. Kinetics of the intermediate stage of phase transition in batch crystallization. J. Cryst. Growth 104, 861–867. ( 10.1016/0022-0248(90)90112-X) [DOI] [Google Scholar]
- 7.Alexandrov DV, Nizovtseva IG. 2014. Nucleation and particle growth with fluctuating rates at the intermediate stage of phase transitions in metastable systems. Proc. R. Soc. A 470, 20130647 ( 10.1098/rspa.2013.0647) [DOI] [Google Scholar]
- 8.Makoveeva EV, Alexandrov DV. 2018. A complete analytical solution of the Fokker-Planck and balance equations for nucleation and growth of crystals. Phil. Trans. R. Soc. A 376, 20170327 ( 10.1098/rsta.2017.0327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandrov DV, Malygin AP. 2013. Transient nucleation kinetics of crystal growth at the intermediate stage of bulk phase transitions. J. Phys. A: Math. Theor. 46, 455101 ( 10.1088/1751-8113/46/45/455101) [DOI] [Google Scholar]
- 10.Alexandrov DV. 2014. Nucleation and crystal growth in binary systems. J. Phys. A: Math. Theor. 47, 125102 ( 10.1088/1751-8113/47/12/125102) [DOI] [Google Scholar]
- 11.Lubov BYa. 1975. The theory of crystallization in large volumes. Moscow, Russia: Nauka. [Google Scholar]
- 12.Alexandrov DV. 2018. Nucleation and evolution of spherical crystals with allowance for their unsteady-state growth rates. J. Phys. A: Math. Theor. 51, 075102 ( 10.1088/1751-8121/aaa5b7) [DOI] [Google Scholar]
- 13.Alexandrov DV. 2014. Nucleation and growth of crystals at the intermediate stage of phase transformations in binary melts. Phil. Mag. Lett. 94, 786–793. ( 10.1080/09500839.2014.977975) [DOI] [Google Scholar]
- 14.Ditkin VA, Prudnikov AP. 1965. Integral transforms and operational calculus. New York, NY: Pergamon Press. [Google Scholar]
- 15.Alexandrov DV, Nizovtseva IG. 2019. On the theory of crystal growth in metastable systems with biomedical applications: protein and insulin crystallization. Phil. Trans. R. Soc. A 377, 20180214 ( 10.1098/rsta.2018.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makoveeva EV, Alexandrov DV. 2019. Effects of nonlinear growth rates of spherical crystals and their withdrawal rate from a crystallizer on the particle-size distribution function. Phil. Trans. R. Soc. A 377, 20180210 ( 10.1098/rsta.2018.0210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexandrov DV, Galenko PK. 2014. Dendrite growth under forced convection: analysis methods and experimental tests. Phys.–Usp. 57, 771–786. ( 10.3367/UFNr.0184.201408b.0833) [DOI] [Google Scholar]
- 18.Mansurov VV. 1990. The nonlinear dynamics of solidification of a binary melt with a nonequilibrium mushy region. Math. Comput. Model. 14, 819–821. ( 10.1016/0895-7177(90)90296-Y) [DOI] [Google Scholar]
- 19.Buyevich YuA, Ivanov AO. 1993. Kinetics of phase separation in colloids: II. Non-linear evolution of a metastable colloid. Physica A 193, 221–240. ( 10.1016/0378-4371(93)90027-2) [DOI] [Google Scholar]
- 20.Ivanov AO, Zubarev AYu. 1998. Non-linear evolution of a system of elongated drop-like aggregates in a metastable magnetic fluid. Physica A 251, 348–367. ( 10.1016/S0378-4371(97)00561-X) [DOI] [Google Scholar]
- 21.Buyevich YuA, Natalukha IA. 1994. Unsteady processes of combined polymerization and crystallization in continuous apparatuses. Chem. Eng. Sci. 49, 3241–3247. ( 10.1016/0009-2509(94)E0052-R) [DOI] [Google Scholar]
- 22.Aseev DL, Alexandrov DV. 2006. Unidirectional solidification with a mushy layer. The influence of weak convection. Acta Mater. 54, 2401–2406. ( 10.1016/j.actamat.2006.01.039) [DOI] [Google Scholar]
- 23.Herlach D, Galenko P, Holland-Moritz D. 2007. Metastable solids from undercooled melts. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 24.Herlach DM. 2008. Phase transformations in multicomponent melts. Weinheim, Germany: Wiley-VCH. [Google Scholar]
- 25.Alexandrov DV, Ivanov AA. 2009. Solidification of a ternary melt from a cooled boundary, or nonlinear dynamics of mushy layers. Int. J. Heat Mass Trans. 52, 4807–4811. ( 10.1016/j.ijheatmasstransfer.2009.05.029) [DOI] [Google Scholar]
- 26.Barlow DA. 2009. Theory of the intermediate stage of crystal growth with applications to protein crystallization. J. Cryst. Growth 311, 2480–2483. ( 10.1016/j.jcrysgro.2009.02.035) [DOI] [Google Scholar]
- 27.Alexandrov DV, Malygin AP. 2011. Convective instability of directional crystallization in a forced flow: the role of brine channels in a mushy layer on nonlinear dynamics of binary systems. Int. J. Heat Mass Trans. 54, 1144–1149. ( 10.1016/j.ijheatmasstransfer.2010.11.008) [DOI] [Google Scholar]
- 28.Alexandrov DV, Nizovtseva IG, Alexandrova IV. 2019. On the theory of nucleation and nonstationary evolution of a polydisperse ensemble of crystals. Int. J. Heat Mass Trans. 128, 46–53. ( 10.1016/j.ijheatmasstransfer.2018.08.119) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


