Abstract
Animal models have been indispensable in elucidating the potential causative mechanisms underlying the effects of maternal diet on offspring health. Of these, the mouse has been widely used to model maternal overnutrition and/or maternal obesity and to study its effects across one or more generations. This review discusses recent findings from mouse models, which resemble the human situation, i.e. overnutrition/obesity across pregnancy and lactation. It also highlights the importance of embryo transfer models in identifying critical developmental period(s) during which specific metabolic changes are programmed in the offspring. The mouse is also an excellent tool for maternal intervention studies aimed at elucidating the longer-term effects on the offspring and for defining possible maternal factors underling the programming of metabolic adversity in offspring. While knowledge of the mouse genome and the molecular tools available have allowed great progress to be made in the field, it is clear that we need to define if the effects on the offspring are mediated by maternal obesity per se or if specific components of the maternal metabolic environment are more important. We can then begin to identify at-risk offspring and to design more effective interventions for the mother and/or her child.
This article is part of the theme issue ‘Developing differences: early-life effects and evolutionary medicine’.
Keywords: maternal overnutrition, animal models, epigenetics, intervention studies
1. Introduction
It is well established that a mother's nutritional/metabolic environment both before and during pregnancy can have a lifelong impact on the health of her offspring. The global rise in the prevalence of overweight/obesity in women of child-bearing age has led to an emphasis, in more recent years, on understanding the long-term impact of maternal overweight/obesity and its associated metabolic co-morbidities on offspring health. While there are known genetic factors that either cause or predispose to obesity that may be transferred from mother to child, the picture is far from complete. For example, studies in humans that have controlled for shared genetics by comparing siblings discordant for a particular exposure, e.g. gestational diabetes mellitus (GDM) [1,2] or siblings born before versus after maternal bariatric surgery [3] found that exposed individuals had a greater risk for diabetes and obesity than unexposed siblings. In order to fully understand how maternal parameters contribute to offspring disease, it is necessary to dissect the relative contributions of maternal diet and components of her metabolic milieu to offspring phenotypes, which is impossible in humans given that a mother's genes and environment coexist.
Studies in animals have been indispensable in elucidating the potential causative mechanisms underlying the effects of maternal diet on offspring metabolic health. The mouse is commonly used as an experimental model relating to the ‘programming’ of obesity and type 2 diabetes (T2D) risk in the offspring by maternal overnutrition/high-fat feeding and/or maternal obesity. Knowledge of the mouse genome and the molecular tools available make it easier to perform genetic and epigenetic studies in inbred mouse strains, thus making mice an advantageous choice. It is, however, important to take into account the strain when examining metabolic outcomes because genetic background can influence the metabolic responses to dietary challenges such as high-fat feeding [4].
Mouse models of maternal obesity across pregnancy and lactation, which most closely resemble the human situation, have been key in our understanding of the non-genetic transfer of metabolic disease risk from mother to offspring. On the other hand, experimental studies involving in vitro fertilization or embryo transfer have allowed for the identification of critical periods in development during which exposure to maternal obesity/high-fat diet (HFD) programs specific metabolic changes in the offspring. This is important because in order to develop targeted interventions, it is critical to determine the separate contribution of maternal physiological status during the periconceptional, gestational and lactational periods to the longer-term metabolic outcomes of the offspring. Another requirement is the inclusion of both male and female offspring when investigating offspring outcomes. This has proven to be essential because many of the studies that have looked at males and females have found sex differences in metabolic disease susceptibility as a result of exposure to maternal obesity, which could have significant implications especially in offspring interventional studies to ameliorate the negative effect of exposure to maternal obesity/HFD.
2. Models of maternal high-fat feeding and/or obesity during gestation and lactation program metabolic dysregulation in subsequent generations
Most mouse models have involved maternal consumption of either a high-fat only or high-fat, high-sugar diet, the latter being more reflective of an obesogenic western diet in humans, from before and during pregnancy. In some studies, this period was also extended to encompass lactation. The difference in fat content between control and HFD-fed groups in these studies ranges from 25 to 50% kcal from fat and the latter type of diet is mainly composed of saturated fats. The high-sugar component generally consists of either carbohydrate in the form of sucrose in the pellet [5,6] or in the form of sweetened condensed milk [7–10]. Furthermore, there are also differences relating to when the high-fat feeding commenced, with the earliest being at 13 weeks before mating [8] and the latest being the day of conception [11]. Unsurprisingly, this has resulted in models with dissimilarities in maternal phenotype at the start of pregnancy. For example, 12 weeks of high-fat, high-sugar feeding in C57BL/6 mice (58% kcal fat, 25.5% kcal carbohydrate as sucrose versus 10.5% kcal fat and 73.1% kcal carbohydrate as corn starch in controls) led to increased adiposity as well as hyperglycaemia and hyperinsulinaemia during a glucose tolerance test before pregnancy [5]. Similarly, 10 to 12 weeks of HFD (60.3% from fat (lard and soya bean oil)) resulted in an 80% higher body weight when compared with controls, presumably owing to increased adiposity. Glucose tolerance was also impaired in these mice prior to pregnancy [12]. Feeding C57BL/6 mice either an HFD or high-fat, high-sugar diet for half the amount of time, i.e. for six weeks before pregnancy, was also sufficient to induce an obese phenotype with glucose intolerance [13,14]. Interestingly, McPherson and colleagues [15] showed that feeding mice an HFD consisting of a lower fat content (21% versus 6% kcal from fat in controls) for six weeks was sufficient to increase adiposity but without the introduction of additional glycaemic or hyperinsulinaemic influences. This model could be useful for studying the effects of maternal obesity per se.
By contrast, studies in which the nutritional challenge commenced shortly before or on the day of conception [11,16,17] may not necessarily reflect the situation in humans but are a useful model to study the more acute effects of maternal high-fat feeding on the offspring. For example, in a study by Yokomizo and colleagues [11], one week of high-fat feeding (approx. 60% kcal from fat) from the start of pregnancy increased maternal fasting blood glucose levels compared to controls. Both male and female offspring of HFD-fed dams had increased fat pad weight and fasting hyperinsulinaemia by six weeks of age. Furthermore, male offspring also displayed fasting hyperglycaemia at this age. This metabolic vulnerability worsened with age in male but not female offspring, thus raising the possibility that males may be particularly vulnerable when challenged acutely during early development and are, therefore, inherently more at risk than females. When challenged with a HFD for 14 weeks, however, both male and female offspring had increased adiposity, fasting hyperglycaemia and were glucose intolerant [11]. The mechanisms involved in driving these sex differences are yet to be determined and remain a critical area of research in this field. One interesting possibility is the role of sex hormones; for example, oestrogen has been shown to have anti-diabetic effects [18] and targeted oestrogen delivery was able to reverse features of the metabolic syndrome in diet-induced obese male mice [19]. Furthermore, males also age more rapidly than females and have a higher growth rate in utero, which may make them more vulnerable to a suboptimal in utero environment; a fetus growing faster in utero can be considered to have a greater effective exposure to a given insult than one that undergoes fewer cell cycles during the period of exposure [20].
The presence of the offspring obesity phenotype is not always a given even in studies where maternal obesity encompassed the period before and throughout pregnancy and lactation. Differences in the composition of maternal and offspring diet, whether or not the offspring were born smaller and displayed catch-up growth and the age of the offspring when adiposity measurements were made, may contribute to this variation. For example, in a study by Samuelsson et al. [10], exposure to maternal obesity resulted in obesity in both chow-fed male and female offspring at 12 weeks of age, whereas Wankhade et al. [21] did not observe any difference in offspring adiposity at 17 weeks of age. This is not to say that these offspring are not metabolically compromised. Exposure to maternal obesity resulted in differences in the expression of hepatic genes associated with inflammatory and fibrogenic pathways in the offspring [21]. Furthermore, there was also an increased risk of non-alcoholic fatty liver disease, especially following a postnatal HFD challenge [21–24]. King et al. [5] and Jungheim et al. [25] both showed that exposure to maternal obesity resulted in offspring that were smaller at birth and then underwent catch-up growth. King et al. [5] found, however, no difference in adiposity or glucose tolerance between male and female adult offspring. Interestingly, there was a more pronounced metabolic defect in F2 male offspring [5]. By contrast, Jungheim et al. [25] found a transient increase in adiposity, impaired glucose tolerance and other features of the metabolic syndrome that were more prominent in male offspring. Although metabolic phenotypes are not always entirely consistent between studies for reasons stated above, it is clear from the numerous experimental studies published across a number of different species that exposure to maternal overnutrition/obesity leads to metabolic dysfunction in the offspring, including obesity and impaired glucose tolerance (reviewed in [26,27]).
3. The importance of the identifying ‘critical’ developmental periods in the programming of metabolic dysfunction in the offspring
Mouse models of maternal obesity secondary to high-fat feeding from before and throughout gestation and lactation have been used to reflect the situation facing humans in the current global obesity epidemic. Consequently, these studies have been key in our understanding of the longer-term impact of maternal overweight/obesity on the health of her offspring. In order to gain mechanistic insight relating to the phenotypic observations, however, it is helpful to identify the critical period(s) of developmental plasticity in response to maternal environmental influences.
Although less common in mice compared with rats, cross-fostering of offspring born to a lean dam to be suckled by an obese dam and vice versa has been important in identifying the suckling period as a critical stage of development. Oben et al. [24] showed that offspring exposed to maternal obesity during lactation only had increased body weight, plasma insulin and leptin in adult life compared with offspring of lean or obese dams, suckled by lean dams. Moreover, these mice also displayed more severe evidence of liver injury and had a greater propensity to develop hepatic fibrosis. What is not known, however, is whether these effects were worse or better when compared to offspring exposed to maternal obesity during gestation and lactation. A recent study by Monks and colleagues [28] cross-fostered pups from low-fat fed dams to either low-fat fed lean dams, high-fat fed obesity-resistant dams (i.e. lowest tertile of weight gain) or high-fat fed obesity-prone dams (highest tertile of weight gain dams). Surprisingly, they found the worst metabolic outcomes in male pups nursed by dams that were resistant to diet-induced obesity. Weight gain was also most rapid in these offspring (although the reasons why remain unclear), suggesting a correlation between the rate of postnatal weight gain and development of disease.
While cross-fostering experiments in mice are useful for characterizing the importance of the early postnatal environment independently from a prenatal insult, results also need to be considered in light of the ‘predictive adaptive response’ hypothesis. Bateson & Gluckman [29,30] proposed that developing organisms receive information about the quality of their external environment and in response formulate predictions about future extrauterine conditions and prepare themselves adaptively for this environment. Based on this, it is, therefore, possible that by cross-fostering we are introducing a mismatch between the prenatal and postnatal environment. This seems particularly detrimental for offspring that were not ‘programmed’ for a high level of nutrition in postnatal life but were subsequently exposed to overnutrition. Cross-fostering per se can, depending on the protocol used, also induce stress in the offspring, leading to metabolic and cardiovascular dysfunction in adulthood [31]. Thus, if exposure to maternal high-fat feeding results in a heightened stress response in the offspring compared with controls [32], this could be a possible confounder when interpreting results.
In recent years, a number of human studies have highlighted the association between maternal pre-pregnancy body mass index (BMI) and her offspring's BMI, as well as other metabolic parameters including waist-to-height ratio, insulin and triglycerides [33,34], suggesting that the time around conception may be a sensitive developmental window with long-lasting effects on the offspring. This was further strengthened by studies in mice, which showed that when fertilized in vitro, oocytes from obese mice exhibited slower development from the four- to eight-cell stage and through to the blastocyst stage [35]. Indeed, examination of oocytes from obese mice showed aberrations in oocyte development, including delayed maturation [25].
Experimental studies involving embryo transfer have also been invaluable in investigating the impact of maternal obesity during the periconceptional period alone on offspring metabolic health. Interestingly, when exposure to maternal obesity is confined to pre-pregnancy events, i.e. oocyte maturation and follicular development and conception until the two-cell stage, this results in fetal growth restriction [12,14,15], which persists at birth [12]. This is then followed by catch-up growth so that there was no difference in body weight in adult offspring exposed to maternal obesity during the periconceptional period [12]. In their model, Huypens et al. [13] also challenged the offspring with a HFD to test whether they were more susceptible to developing metabolic dysfunction and they found that female but not male offspring had increased adiposity, which corresponded with a more rapid gain in body weight. Glucose tolerance was, however, impaired in both male and female offspring, which was not owing to a reduction in insulin levels and, therefore, likely caused by insulin resistance.
4. Epigenetic mechanisms underlying the developmental origins of metabolic disease in response to maternal obesity/HFD
During the early stages of development, the differentiation and development of different cell types are regulated by epigenetic mechanisms, which play a role in modulating chromatin architecture [36]. Furthermore, epigenetic regulation plays a key role in conferring phenotype plasticity, which allows organisms to adapt their gene expression and function in response to the environment [26]. Each cell type, therefore, has its own epigenetic signature that reflects genotype, developmental history and environmental influences. This is ultimately reflected in the phenotype of the cell and organism [37]. Early embryogenesis in mammals is a critical period for the establishment of the epigenome [38]; during the period between conception and implantation, there is de-methylation of the genome, followed by a wave of re-methylation shortly after implantation [37]. This period, therefore, represents a critical window in development during which the embryo is vulnerable to environmental and/or nutritional cues that disrupt the establishment of epigenetic marks such as DNA methylation, histone modification and microRNAs (miRs) [39], a class of small, non-coding RNAs that play an important role in post-transcriptional gene regulation by cleavage and/or translational repression of their specific target mRNAs. Importantly, although the genome of an individual is largely stable, the epigenome has the potential to be reversibly modified by exposure to a range of nutritional and environmental factors [40].
(a). The agouti viable yellow (Avy) mouse model: a tool to study offspring epigenetic changes in response to maternal obesity
While the mouse models outlined above have been useful in showing persistent and heritable changes in offspring metabolism, the differences in diet composition and timing of manipulation have made it challenging to determine whether the observed offspring effects are attributable to maternal obesity per se or to the high-fat and/or high-sugar diet. In order to overcome this, the agouti viable yellow (Avy) mouse has been used to model maternal obesity. The Avy allele is subject to stochastic epigenetic silencing in an isogenic background so that genetically identical mice display distinctly different phenotypes. The agouti signalling molecule induces yellow pigmentation in hair follicles and antagonizes satiety signalling through the melanocortin 4 receptor in the hypothalamus. Avy/a mice, therefore, have yellow coats and are obese owing to hyperphagia. Adult-onset obesity occurs in Avy/a mice provided ad libitum access to standard chow and recapitulates the gradual onset of metabolic disease in humans. By contrast, mice carrying a silent allele have agouti fur and a metabolically normal phenotype [41–43]. Seminal work by Waterland and colleagues [43] using the Avy mouse showed that effects of maternal obesity accumulate over successive generations, leading to a shift towards increased adult body weight in the population. Additionally, by using two separate but contemporaneous populations of mice, one provided with a standard diet and the other a methyl-supplemented diet that induces DNA hypermethylation during development, they found that the transgenerational effects on body weight were mediated by epigenetic changes. Further characterization of offspring of obese yellow Avy/a dams by Li and colleagues [42] found them to have a latent predisposition to metabolic disease, which was unmasked by a HFD challenge. This was more prominent in male offspring, which were significantly heavier and developed glucose intolerance and insulin resistance after only three weeks of high-fat feeding. An unbiased, genome-wide profiling of DNA methylation found that while maternal obesity induced methylation changes across the genome and expression changes at a variety of genes, these were mainly clustered in developmental ontologies. The authors concluded that genes required for developmental processes are more susceptible to environmental perturbations compared with other parts of the genome [42,44] and that these perturbations could well underlie the metabolic phenotype programmed by exposure to maternal obesity [42].
Moving forward, it is essential that we profile DNA methylation in both male and female offspring as this will likely provide important information about sex-specific programming of metabolic dysfunction by maternal obesity. A recent study by McCormick et al. [45] showed that there was sexual dimorphism in DNA methylation patterns across different tissues derived from all three embryonic germ layers. In the majority of instances, sex differences were manifest as a strong female bias towards hypermethylation. Importantly, this was not merely owing to the de-methylating action of testosterone in males. The exact mechanism, however, is still unknown.
(b). A role for mitochondrial epigenetics in the transmission of obesity and T2D risk from mother to offspring?
The transmission of obesity and T2D risk from mother to her offspring may also occur through mitochondria, which are maternally inherited. Mitochondria are the power house of the cell and consequently play a key role in regulating many cellular processes, including metabolism, apoptosis and redox homeostasis. Defects in mitochondrial function and behaviour have been linked to cancer, metabolic disorders and neurodegenerative diseases [46]. Importantly, in the context of developmental programming, transgenerational inheritance of altered mitochondrial phenotypes through the maternal germline has been shown to occur. Using a mouse model of high-fat, high-sugar feeding to induce metabolic syndrome in the dam before and during pregnancy, Saben et al. [6] showed mitochondrial dysfunction as well as impaired mitochondrial dynamics in skeletal muscle of the first, second and third generations of female offspring. Mitochondrial abnormalities were also present in the germ cells of F1 and F2 females, suggesting that transmission of the mitochondrial phenotype occurred through the maternal germline. It will be interesting in the future to determine whether changes in mtDNA methylation/hydroxymethylation have a role to play in this context. DNA methylation occurs at both CpG and non-CpG sites in mtDNA and this has been shown to correlate positively with gene expression [47]. The highest density of hydroxymethylated cytosine (5hmC) has been found in mitochondrial DNA (mtDNA) [48]. Epigenetic changes impacting the expression of mtDNA could have an impact on mitochondrial function and consequently lead to dysregulation of metabolic processes. This will have an impact on disease risk; mitochondrial dysfunction has been shown to occur in skeletal muscle [49] and pancreatic islets [50] in T2D and obesity.
(c). Epigenetic programming of offspring adipose tissue by maternal obesity
Tissue-specific epigenetic changes have also been characterized in the context of programming by maternal obesity with a particular focus on adipose tissue. Yang and colleagues [51] examined the role of Zfp-423, a zinc-finger protein that was previously shown to initiate an early pre-adipocyte commitment to mature adipose tissue, in adipogenesis during fetal development. Zfp-423 has been classified as a key developmental gene owing to the high density of CpG sites and islands in its promoters. They found that both DNA methylation and repressive histone methylation (H3K27me3) were lower in the promoter of Zfp-423 following exposure to maternal obesity. This resulted in a corresponding increase in gene expression in maternal obesity-exposed fetuses at embryonic day 14.5, which is when early adipogenic commitment is initiated. The authors concluded that these changes will elevate the adipogenic differentiation of adipose tissue progenitor cells in adult tissue, thus programming adiposity and metabolic dysfunction in later life. Indeed, reduced DNA methylation in the Zfp-423 promoter and increased expression persisted in the offspring of obese dams at weaning, resulting in elevated (premature) adipogenic differentiation of progenitor cells. This, in turn, limited adipose tissue expandability when these offspring were challenged with an HFD, leading to adipocyte hypertrophy, a cause of hypoxia and inflammation [52].
In addition to DNA methylation and histone modification, miRs have also been implicated in the regulation of adipose tissue metabolism and are also susceptible to programming in adipose tissue [8,53]. For example, Fernandez-Twinn et al. [8] showed that exposure to maternal diet-induced obesity programmes increased expression of miR-126 in adipose tissue of lean offspring. This was associated with decreased protein abundance of one of its targets, the insulin signalling protein IRS-1. Importantly, the programmed loss of IRS-1 protein and increase in miR-126 were maintained in primary adipocyte precursors isolated from epididymal adipose tissue from offspring of obese dams, which were then expanded in culture and differentiated into adipocytes, strongly suggesting that the underlying mechanisms are cell autonomous and retained following multiple rounds of cell division.
5. Intervention studies in the mother: what can we learn?
There are a number of on-going randomized intervention studies in humans involving pharmacological [54,55], dietary and exercise interventions [56,57] to target different potential maternal programming factors. It is, however, going to take several years before we can be certain about the longer-term effects of maternal interventions on the offspring. Animal models are, therefore, excellent tools for intervention studies. While the examples highlighted in this section are not exhaustive, they highlight the importance of these types of studies in defining the possible maternal factors, e.g. hyperinsulinaemia, hyperglycaemia and hyperlipidemia, underlying the programming of metabolic adversity across the life course of the offspring.
Furthermore, as outlined above, high-fat feeding in mice results in several defects in the oocyte including mitochondrial dysfunction leading to reduced ATP and increased reactive oxygen species production, thus resulting in dysregulated metabolism. This has been attributed partly to high oxidative stress in an obese, hyperglycaemic environment [14,58]. Supplementation with co-enzyme Q10 (CoQ10), an antioxidant component of the electron transport chain in mice fed a high-fat, high-sugar diet, was able to alleviate the mitochondrial dysfunction in oocytes from obese mice and improve their reproductive outcomes [59]. There were, however, no follow-up studies to determine whether maternal CoQ10 supplementation has a longer-term impact on the offspring. In vivo administration of melatonin, a free radical scavenger, is also able to prevent oxidative stress in oocytes from HFD-fed mice, promoting the developmental potential of early embryos derived from obese mice [60].
It is important that not only does any potential maternal intervention not have a negative impact on the mother and her offspring during development, and it is also key to ensure that there are no longer-term effects in the offspring in later life. To this end, Jonscher and colleagues [61] tested the impact of pyrroloquinoline quinone (PQQ) supplementation provided prenatally at conception and throughout lactation in obese mice on the offspring in adult life. PQQ is an important antioxidant in mammals and is enriched in human breast milk, making it an attractive dietary therapeutic. They found that maternal PQQ supplementation improved offspring metabolic outcomes by enhancing oxidative defence. PQQ also altered fuel utilization and increased metabolic flexibility, leading to reduced adiposity and an overall healthier metabolic state [61].
Exercise intervention either in the mother or the offspring has also gained considerable interest because exercise is one of the most effective ways of reducing the incidence of overweight/obesity, T2D and other metabolic disorders. Exercise intervention in the form of a voluntary running wheel in mice fed a HFD was able to improve glucose tolerance and also reduced HFD-induced lipid accumulation in the oocyte [62]. Furthermore, we have shown that maternal exercise intervention can also be used to establish causality and to help define underlying mechanisms; our findings suggested that maternal hyperinsulinaemia is a key programming factor that mediates the effects of maternal high-fat, high-sugar feeding on offspring insulin sensitivity. Following a regime of maternal peri-gestational exercise, which improved her insulin sensitivity (without impacting adiposity), offspring hyperinsulinaemia and adipose tissue insulin resistance were rescued [63]. Similar offspring findings were also observed in HFD-fed dams with access to a running wheel for two weeks prior to and throughout pregnancy [64,65]. The mechanisms involved have not been fully elucidated but may involve epigenetic changes, e.g. DNA methylation of key metabolic regulators [66].
In their study, Aye et al. [67] showed that maternal adiponectin supplementation (during the last 4 days of pregnancy, which is a period of rapid fetal growth) normalized fetal blood glucose levels and weight, which was higher in fetuses exposed to maternal obesity. Maternal fasting insulin was also normalized along with placental function [67], further supporting the role of maternal insulin as a key programming factor that mediates the effects of maternal obesity on offspring health.
The suckling period has also been identified as a critical stage when offspring are vulnerable to the effects of maternal obesity. Thus, maternal intervention during this period can also have beneficial outcomes for the offspring. For example, maternal dietary intervention during lactation in dams previously fed an HFD during gestation was able to improve anxiety and sociability characteristics in female offspring [68]. The level of neuroinflammation was also alleviated [68]. It should be noted that interventions during the lactational period are likely to be most beneficial if they are designed to minimize the unwanted effects of a mismatch in the pre- and postnatal environments [29,30].
6. Mice versus humans
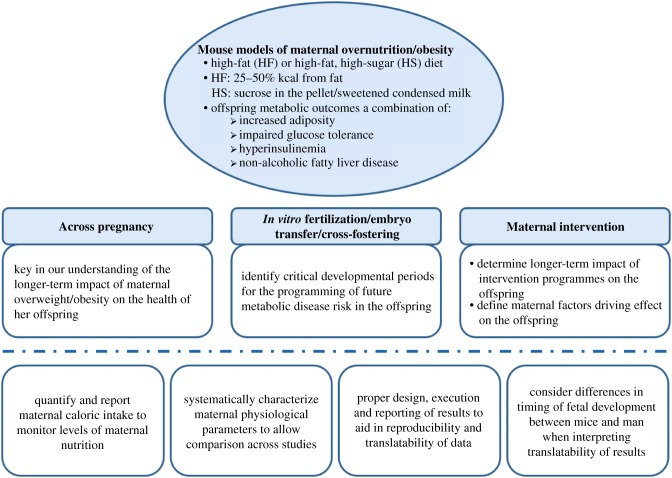
While mice have proven to be a useful animal model for research relating to early life programming by maternal obesity, it is important to consider that the timing of fetal development as well as offspring number are different between mice and humans. Care should, therefore, be taken when interpreting the translatability of studies in mice. This is especially true when considering the impact of exposure during specific developmental windows. For example, events that occur during lactation in mice may represent mid- to late gestation in the human and thus experiments designed to test the importance of the postnatal environment (including the effectiveness of interventions during this period) are not directly translatable to humans. On balance, however, mouse models of maternal obesity remain indispensable for our understanding of the mechanisms driving the onset of non-communicable diseases in the offspring and are an essential part of clinical progress (figure 1). It is only when we have a fundamental understanding of what goes wrong and why it goes wrong that can we begin to influence clinical practice in a meaningful way.
Figure 1.
The importance of mouse models of maternal overnutrition/obesity in understanding the non-genetic transfer of metabolic disease risk from mother to offspring. (Online version in colour.)
7. Conclusion
It is clear from the studies highlighted in this review that mice are an invaluable model for studying the impact of maternal obesity on offspring health across multiple generations. This has led to its popularity in studies of maternal obesity. Currently, however, there is no consensus relating to high-fat, high-sugar diet composition and not all studies report or fully characterize maternal physiological parameters, e.g. adiposity, glycaemia, insulin and lipid levels systematically before and during pregnancy and lactation, making it difficult to compare findings across studies. Furthermore, maternal high-fat/high-sugar feeding may also represent a form of malnutrition. Studies often do not consider if a change in the dietary composition also impacts on caloric intake, with some high-fat diets in particular not being that palatable. Furthermore, diets high in fat are often low in protein, so although high in some nutrients may be deficient in others. Additionally, there is still further need to define if detrimental effects on the offspring are mediated by maternal obesity per se or if specific components of the metabolic milieu are more important. Given the wide variation in diets used and experimental design, it is crucial that the reporting of such details of animal models is sufficiently clear and detailed in publications. This will aid in the reproducibility and translatability of pre-clinical data. It is also key that we define the timing for most effective interventions. It is when we have an understanding of this that we can begin to identify at-risk offspring and to design effective interventions for the mother and/or her child.
Data accessibility
This article has no additional data.
Competing interests
We have no competing interests.
Funding
L.M.N. is funded by the Isaac Newton Trust [17.37(l)] and S.E.O. is funded by the Medical Research Council (MC_UU_12012/4) and the British Heart Foundation (RG/17/12/33167).
References
- 1.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. 2000. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49, 2208–2211. ( 10.2337/diabetes.49.12.2208) [DOI] [PubMed] [Google Scholar]
- 2.Lawlor DA, Lichtenstein P, Langstrom N. 2011. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 123, 258–265. ( 10.1161/CIRCULATIONAHA.110.980169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith J, et al. 2009. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J. Clin. Endocrinol. Metab. 94, 4275–4283. ( 10.1210/jc.2009-0709) [DOI] [PubMed] [Google Scholar]
- 4.Dickinson H, et al. 2016. A review of fundamental principles for animal models of DOHaD research: an Australian perspective. J. Dev. Orig. Health Dis. 7, 449–472. ( 10.1017/S2040174416000477) [DOI] [PubMed] [Google Scholar]
- 5.King V, Dakin RS, Liu L, Hadoke PW, Walker BR, Seckl JR, Norman JE, Drake AJ. 2013. Maternal obesity has little effect on the immediate offspring but impacts on the next generation. Endocrinology 154, 2514–2524. ( 10.1210/en.2013-1013) [DOI] [PubMed] [Google Scholar]
- 6.Saben JL, et al. 2016. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep. 16, 1–8. ( 10.1016/j.celrep.2016.05.065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alfaradhi MZ, Fernandez-Twinn DS, Martin-Gronert MS, Musial B, Fowden A, Ozanne SE. 2014. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R26–R34. ( 10.1152/ajpregu.00049.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Twinn DS, Alfaradhi MZ, Martin-Gronert MS, Duque-Guimaraes DE, Piekarz A, Ferland-McCollough D, Bushell M, Ozanne SE. et al. 2014. Downregulation of IRS-1 in adipose tissue of offspring of obese mice is programmed cell-autonomously through post-transcriptional mechanisms. Mol. Metab. 3, 325–333. ( 10.1016/j.molmet.2014.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Twinn DS, Blackmore HL, Siggens L, Giussani DA, Cross CM, Foo R, Ozanne SE. 2012. The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation. Endocrinology 153, 5961–5971. ( 10.1210/en.2012-1508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuelsson AM, et al. 2008. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51, 383–392. ( 10.1161/HYPERTENSIONAHA.107.101477) [DOI] [PubMed] [Google Scholar]
- 11.Yokomizo H, et al. 2014. Maternal high-fat diet induces insulin resistance and deterioration of pancreatic beta-cell function in adult offspring with sex differences in mice. Am. J. Physiol. Endocrinol. Metab. 306, E1163–E1175. ( 10.1152/ajpendo.00688.2013) [DOI] [PubMed] [Google Scholar]
- 12.Sasson IE, Vitins AP, Mainigi MA, Moley KH, Simmons RA. 2015. Pre-gestational vs gestational exposure to maternal obesity differentially programs the offspring in mice. Diabetologia 58, 615–624. ( 10.1007/s00125-014-3466-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huypens P, et al. 2016. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat. Genet. 48, 497–499. ( 10.1038/ng.3527) [DOI] [PubMed] [Google Scholar]
- 14.Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. 2012. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS ONE 7, e49217 ( 10.1371/journal.pone.0049217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McPherson NO, Bell VG, Zander-Fox DL, Fullston T, Wu LL, Robker RL, Lane M. 2015. When two obese parents are worse than one! Impacts on embryo and fetal development. Am. J. Physiol. Endocrinol. Metab. 309, E568–E581. ( 10.1152/ajpendo.00230.2015) [DOI] [PubMed] [Google Scholar]
- 16.Dudele A, et al. 2017. Chronic maternal inflammation or high-fat-feeding programs offspring obesity in a sex-dependent manner. Int. J. Obes. 41, 1420–1426. ( 10.1038/ijo.2017.136) [DOI] [PubMed] [Google Scholar]
- 17.Hartil K, et al. 2009. Maternal substrate utilization programs the development of the metabolic syndrome in male mice exposed to high fat in utero. Pediatr. Res. 66, 368–373. ( 10.1203/PDR.0b013e3181b33375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louet JF, LeMay C, Mauvais-Jarvis F. 2004. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr. Atheroscler. Rep. 6, 180–185. ( 10.1007/s11883-004-0030-9) [DOI] [PubMed] [Google Scholar]
- 19.Finan B, et al. 2012. Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med. 18, 1847–1856. ( 10.1038/nm.3009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aiken CE, Ozanne SE. 2013. Sex differences in developmental programming models. Reproduction 145, R1–13. ( 10.1530/REP-11-0489) [DOI] [PubMed] [Google Scholar]
- 21.Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Thakali KM, Shankar K. 2017. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE 12, e0175675 ( 10.1371/journal.pone.0175675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruce KD, et al. 2009. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 50, 1796–1808. ( 10.1002/hep.23205) [DOI] [PubMed] [Google Scholar]
- 23.Gregorio BM, Souza-Mello V, Carvalho JJ, Mandarim-de-Lacerda CA, Aguila MB. 2010. Maternal high-fat intake predisposes nonalcoholic fatty liver disease in C57BL/6 offspring. Am. J. Obstet. Gynecol. 203, e1–e8. ( 10.1016/j.ajog.2010.06.042) [DOI] [PubMed] [Google Scholar]
- 24.Oben JA, et al. 2010. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J. Hepatol. 52, 913–920. ( 10.1016/j.jhep.2009.12.042) [DOI] [PubMed] [Google Scholar]
- 25.Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. 2010. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology 151, 4039–4046. ( 10.1210/en.2010-0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duque-Guimaraes DE, Ozanne SE. 2013. Nutritional programming of insulin resistance: causes and consequences. Trends Endocrinol. Metab. 24, 525–535. ( 10.1016/j.tem.2013.05.006) [DOI] [PubMed] [Google Scholar]
- 27.Nicholas LM, Morrison JL, Rattanatray L, Zhang S, Ozanne SE, McMillen IC. 2016. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int. J. Obes. 40, 229–238. ( 10.1038/ijo.2015.178) [DOI] [PubMed] [Google Scholar]
- 28.Monks J, et al. 2018. Maternal obesity during lactation may protect offspring from high fat diet-induced metabolic dysfunction. Nutr. Diab. 8, 18 ( 10.1038/s41387-018-0027-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bateson P, Bateson PPG, Martin PR. 1999. Design for a life: how behaviour develops. London, UK: Vintage. [Google Scholar]
- 30.Gluckman PD, Hanson MA, Beedle AS. 2007. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol. 19, 1–19. ( 10.1002/ajhb.20590) [DOI] [PubMed] [Google Scholar]
- 31.Matthews PA, Samuelsson AM, Seed P, Pombo J, Oben JA, Poston L, Taylor PD. 2011. Fostering in mice induces cardiovascular and metabolic dysfunction in adulthood. J. Physiol. 589, 3969–3981. ( 10.1113/jphysiol.2011.212324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki A, de Vega WC, St-Cyr S, Pan P, McGowan PO. 2013. Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood. Neuroscience 240, 1–12. ( 10.1016/j.neuroscience.2013.02.044) [DOI] [PubMed] [Google Scholar]
- 33.Hochner H, et al. 2012. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation 125, 1381–1389. ( 10.1161/CIRCULATIONAHA.111.070060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oostvogels AJ, Stronks K, Roseboom TJ, van der Post JA, van Eijsden M, Vrijkotte TG. 2014. Maternal prepregnancy BMI, offspring's early postnatal growth, and metabolic profile at age 5–6 years: the ABCD Study. J. Clin. Endocrinol. Metab. 99, 3845–3854. ( 10.1210/jc.2014-1561) [DOI] [PubMed] [Google Scholar]
- 35.Minge CE, Bennett BD, Norman RJ, Robker RL. 2008. Peroxisome proliferator-activated receptor-α agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology 149, 2646–2656. ( 10.1210/en.2007-1570) [DOI] [PubMed] [Google Scholar]
- 36.Isagawa T, Nagae G, Shiraki N, Fujita T, Sato N, Ishikawa S, Kume S, Aburatani H. 2011. DNA methylation profiling of embryonic stem cell differentiation into the three germ layers. PLoS ONE 6, e26052 ( 10.1371/journal.pone.0026052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan HD, Santos F, Green K, Dean W, Reik W. 2005. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 14(SPEC. ISS. 1), R47–R58. ( 10.1093/hmg/ddi114) [DOI] [PubMed] [Google Scholar]
- 38.Jiménez-Chillarón JC, Díaz R, Martínez D, Pentinat T, Ramón-Krauel M, Ribó S, Plösch T. 2012. The role of nutrition on epigenetic modifications and their implications on health. Biochimie 94, 2242–2263. ( 10.1016/j.biochi.2012.06.012) [DOI] [PubMed] [Google Scholar]
- 39.Margueron R, Reinberg D. 2010. Chromatin structure and the inheritance of epigenetic information. Nat. Rev. Genet. 11, 285–296. ( 10.1038/nrg2752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semple RK, Chatterjee VK, O'Rahilly S. 2006. PPAR γ and human metabolic disease. J. Clin. Invest. 116, 581–589. ( 10.1172/JCI28003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS. 1994. Neomorphic agouti mutations in obese yellow mice. Nat. Genet. 8, 59–65. ( 10.1038/ng0994-59) [DOI] [PubMed] [Google Scholar]
- 42.Li CC, et al. 2013. Maternal obesity and diabetes induces latent metabolic defects and widespread epigenetic changes in isogenic mice. Epigenetics 8, 602–611. ( 10.4161/epi.24656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. 2008. Methyl donor supplementation prevents transgenerational amplification of obesity. Int. J. Obes. 32, 1373–1379. ( 10.1038/ijo.2008.100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li CC, Cropley JE, Cowley MJ, Preiss T, Martin DI, Suter CM. 2011. A sustained dietary change increases epigenetic variation in isogenic mice. PLoS Genet. 7, e1001380 ( 10.1371/journal.pgen.1001380) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCormick H, Young PE, Hur SSJ, Booher K, Chung H, Cropley JE, Giannoulatou E, Suter CM. 2017. Isogenic mice exhibit sexually-dimorphic DNA methylation patterns across multiple tissues. BMC Genomics 18, 966 ( 10.1186/s12864-017-4350-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman JR, Nunnari J. 2014. Mitochondrial form and function. Nature 505, 335–343. ( 10.1038/nature12985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobayashi H, et al. 2012. Contribution of intragenic DNA methylation in mouse gametic DNA methylomes to establish oocyte-specific heritable marks. PLoS Genet. 8, e1002440 ( 10.1371/journal.pgen.1002440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Z, et al. 2013. High-resolution enzymatic mapping of genomic 5-hydroxymethylcytosine in mouse embryonic stem cells. Cell Rep. 3, 567–576. ( 10.1016/j.celrep.2013.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelley DE, He J, Menshikova EV, Ritov VB. 2002. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944–2950. ( 10.2337/diabetes.51.10.2944) [DOI] [PubMed] [Google Scholar]
- 50.Fex M, Nicholas LM, Vishnu N, Medina A, Sharoyko VV, Nicholls DG, Spégel P, Mulder H. 2018. The pathogenetic role of β-cell mitochondria in type 2 diabetes. J. Endocrinol. 236, R145–R159. ( 10.1530/JOE-17-0367) [DOI] [PubMed] [Google Scholar]
- 51.Yang QY, Liang JF, Rogers CJ, Zhao JX, Zhu MJ, Du M. 2013. Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes 62, 3727–3735. ( 10.2337/db13-0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang X, Yang Q, Fu X, Rogers CJ, Wang B, Pan H, Zhu M-J, Nathanielsz PW, Du M. 2016. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J. Physiol. 594, 4453–4466. ( 10.1113/JP272123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferland-McCollough D, et al. 2012. Programming of adipose tissue miR-483-3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ. 19, 1003–1012. ( 10.1038/cdd.2011.183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiswick C, et al. 2015. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diab. Endocrinol. 3, 778–786. ( 10.1016/S2213-8587(15)00219-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanem LG, Stridsklev S, Júlíusson PB, Salvesen Ø, Roelants M, Carlsen SM, Ødegård R, Vanky E. 2018. Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 years of age: follow-up of two RCTs. J. Clin. Endocrinol. Metab. 103, 1612–1621. ( 10.1210/jc.2017-02419) [DOI] [PubMed] [Google Scholar]
- 56.Dodd JM, et al. 2016. The effect of antenatal dietary and lifestyle advice for women who are overweight or obese on emotional well-being: the LIMIT randomized trial. Acta Obstet. Gynecol. Scand. 95, 309–318. ( 10.1111/aogs.12832) [DOI] [PubMed] [Google Scholar]
- 57.Poston L, et al. 2015. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diab. Endocrinol. 3, 767–777. ( 10.1016/S2213-8587(15)00227-2) [DOI] [PubMed] [Google Scholar]
- 58.Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, Moley KH. 2009. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol. Endocrinol. 23, 1603–1612. ( 10.1210/me.2009-0033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boots CE, Boudoures A, Zhang W, Drury A, Moley KH. 2016. Obesity-induced oocyte mitochondrial defects are partially prevented and rescued by supplementation with co-enzyme Q10 in a mouse model. Hum. Reprod. 31, 2090–2097. ( 10.1093/humrep/dew181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han L, Wang H, Li L, Li X, Ge J, Reiter RJ, Wang Q. 2017. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J. Pineal Res. 63, e12431 ( 10.1111/jpi.12431) [DOI] [PubMed] [Google Scholar]
- 61.Jonscher KR, et al. 2017. Early PQQ supplementation has persistent long-term protective effects on developmental programming of hepatic lipotoxicity and inflammation in obese mice. FASEB J. 31, 1434–1448. ( 10.1096/fj.201600906R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boudoures AL, Chi M, Thompson A, Zhang W, Moley KH. 2016. The effects of voluntary exercise on oocyte quality in a diet-induced obese murine model. Reproduction 151, 261–270. ( 10.1530/REP-15-0419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez-Twinn DS, et al. 2017. Exercise rescues obese mothers' insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Sci. Rep. 7, 44650 ( 10.1038/srep44650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanford KI, Lee MY, Getchell KM, So K, Hirshman MF, Goodyear LJ. 2015. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 64, 427–433. ( 10.2337/db13-1848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stanford KI, et al. 2017. Maternal exercise improves glucose tolerance in female offspring. Diabetes 66, 2124–2136. ( 10.2337/db17-0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laker RC, Lillard TS, Okutsu M, Zhang M, Hoehn KL, Connelly JJ, Yan Z. 2014. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes 63, 1605–1611. ( 10.2337/db13-1614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aye IL, Rosario FJ, Powell TL, Jansson T. 2015. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc. Natl Acad. Sci. USA 112, 12858–12863. ( 10.1073/pnas.1515484112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang SS, Kurti A, Fair DA, Fryer JD. 2014. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflammation 11, 156 ( 10.1186/s12974-014-0156-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.