Abstract
In recent years, studies in Caenorhabditis elegans nematodes have shown that different stresses can generate multigenerational changes. Here, we show that worms that grow in liquid media, and also their plate-grown progeny, are different from worms whose ancestors were grown on plates. It has been suggested that C. elegans might encounter liquid environments in nature, although actual observations in the wild are few and far between. By contrast, in the laboratory, growing worms in liquid is commonplace, and often used as an alternative to growing worms on agar plates, to control the composition of the worms' diet, to starve (and synchronize) worms or to grow large populations for biochemical assays. We found that plate-grown descendants of M9 liquid medium-grown worms were longer than control worms, and the heritable effects were already apparent very early in development. We tested for the involvement of different known epigenetic inheritance mechanisms, but could not find a single mutant in which these inter-generational effects are cancelled. While we found that growing in liquid always leads to inter-generational changes in the worms’ size, trans-generational effects were found to be variable, and in some cases, the effects were gone after one to two generations. These results demonstrate that standard cultivation conditions in early life can dramatically change the worms' physiology in adulthood, and can also affect the next generations.
This article is part of the theme issue ‘Developing differences: early-life effects and evolutionary medicine’.
Keywords: trans-generational, inter-generational, liquid, inheritance, epigenetics, C. elegans
1. Introduction
You could not step twice into the same river (Heraclitus)
In comparison with Mendelian inheritance, which entails faithful transmission of discrete packets of information which do not blend or dilute [1], heritable epigenetic data are ‘soft’, or ‘fluid’, as they change as a function of time, and in response to physiological processes [2].
Generally, mechanisms that allow trans-generational epigenetic effects, namely transmission of information to progeny not exposed to the original trigger, are still poorly understood [3]. However, in Caenorhabditis elegans, substantial understanding has been gained regarding the molecular mechanisms that allow transmission of responses via small RNAs across multiple generations (reviewed in [4]). Further, many studies with C. elegans have demonstrated that different environmental challenges leave a trace in the progeny, for example, viral infections [5–7], starvation [8–11], high temperatures [12–14], high osmolality [15,16] and exposure to toxins [15]. Such stressors can lead to short- or long-term gene expression alterations and physiological changes. The inheritance of some of these responses is associated with changes in heritable small RNAs [8,13,14] and/or is correlated with alteration in histone modifications (for example, of H3K4me [15,17]).
In the laboratory, C. elegans are commonly grown on agar plates, but when large numbers of worms are needed, worms are often grown in liquid media [18]. Caenorhabditis elegans are grown in liquid for other purposes as well, for example, to synchronize the worms' growth at the L1 stage [18], to control for the composition of the worms’ diet [19] and sometimes for studying starvation [18]. Liquid media were also used to study how swim exercise affects the worm's physiology [20]. In addition to C. elegans, other nematodes, such as the entomopathogenic species Steinernema [21,22] and Heterorhabditis [23,24], are routinely grown in liquid culture, and recently, growth in liquid medium of different Pristionchus species was shown to generate striking phenotypic differences [25] (see also Discussion).
In nature, C. elegans worms are most often isolated from solid decomposing plant material. However, there are rare reports of isolation of C. elegans from freshwater as well [26]. In addition, it has been hypothesized, but not yet documented, that C. elegans could encounter liquid environments in the wild, in cases of excessive decomposition, especially of fruits [27].
Here, we report that growth of worms in liquid media changes the morphology of the liquid-grown worms and also the morphology of their plate-grown progeny. We found that plate-grown descendants of liquid-grown worms are on average longer than control worms. This morphological change is apparent early during the worms' life, and persists even in spite of experimental synchronization of the worms’ development. In addition, the inheritance of this phenotype does not appear to depend on pheromone production or any single known epigenetic inheritance pathway, as we could not find a single mutant in which these inter-generational effects are cancelled. While the effect is robustly inherited to the immediate F1 progeny, the inheritance to the F2 and the F3 generations exhibits variability. These results demonstrate that commonplace cultivation conditions can dramatically change the worms' physiology in adulthood, and can also affect the next generations. We suggest that the growth and handling conditions of at least a few generations should be monitored before experimentation begins.
2. Results
Even in the laboratory, C. elegans often encounters different environments, for example, worms are frequently starved or grown at slightly stressful temperatures, either accidentally or intentionally. It is very common to synchronize or control the speed of the worms’ growth by starving them or by switching the growth temperature. Similarly, in many cases, researchers are forced to grow temperature-sensitive worms at low temperatures, or to cultivate the worms at high temperatures to avoid transgene silencing [28]. Contaminations with different pathogens are very common in C. elegans cultures, and while this has not been studied in detail, such infections could also leave heritable imprints [29]. As described above, worms are often grown in liquid in the laboratory (the standard liquid used is M9 buffer [18]). In the course of conducting many experiments with liquid-grown worms, we noticed that after growth in liquid medium, the worms exhibit a different morphology in comparison with plate-grown worms. We first set out to characterize this phenomenon, and then to examine whether the effects could be inherited (figure 1a).
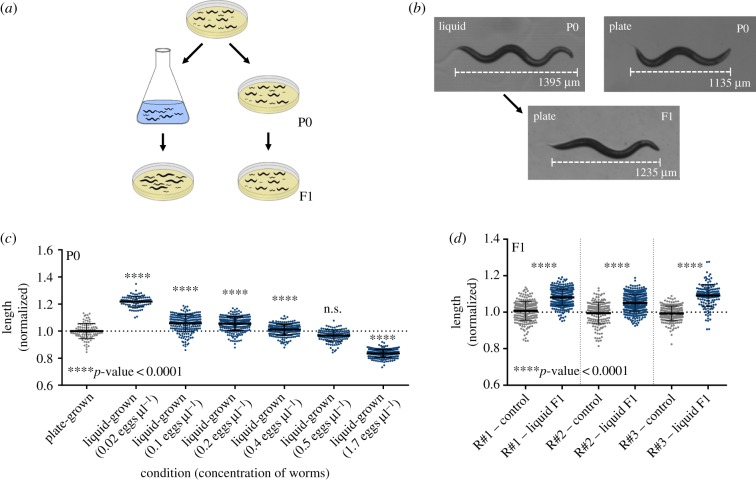
Figure 1.
Progeny of liquid-grown worms have longer morphology. (a) Experimental scheme of inter-generational inheritance of growth in liquid medium. Eggs were produced from isogenic worm lines and placed either on plates or in liquid medium. When the parental P0 worms reached adulthood, they were placed on plates to lay their eggs. The body length of their resulting F1 adult progeny was measured. (b) Representative photographs of adult plate-grown (P0), liquid-grown (P0) or plate-grown progeny (F1) of liquid-grown worms. The differences in length between the photographed worms match the average length differences found between the examined populations. A scale bar that shows the total stretched length of the worm is presented. (c) Liquid-grown worms are longer when grown in low concentration. The normalized lengths of the parental plate-grown, and liquid-grown worms are shown. Worms were grown in liquid in different concentrations (x-axis). The measured length was normalized to that of the plate-grown worms. The p-value was determined using one-way ANOVA. n.s., not significant. (d) Plate-grown progeny of worms grown in liquid medium are longer. The length of F1 plate-grown progeny of liquid-grown worms and progeny of plate-grown controls are shown. The measured length was normalized to that of the corresponding control worms. Data from three independent biological repeats are presented (N > 150 worms per group). The p-value was determined using two-way ANOVA with the two categorical variables: (i) biological condition (growth in liquid or on plates) and (ii) biological repeat (independent experiments conducted on separate groups of animals, on different days). Both factors have a significant effect on the measured worm length (p < 0.0001). In addition, an interaction effect was found (p < 0.0001). The biological condition explained far more variance than the biological repeat or the interaction (32.44 compared with 2.07 or 1.69%, respectively). Error bars represent standard deviations.
(a). Progeny of liquid-grown worms are longer
We noticed that worms that grow in liquid become narrow, and that their body length becomes more variable in comparison with plate-grown worms. These observations are in accordance with a previous observation [18], and a recent study showing that worms grown in axenic (chemically defined and bacteria-free) liquid medium (specifically the ‘Habitation and Reproduction’ medium used by NASA when growing worms in space [19]) are also narrower and longer than plate-grown worms [30].
We found that the worms' concentration in liquid affects their size (p < 0.0001, one-way ANOVA), and that while in low concentrations, the liquid-grown worms are longer (figure 1b,c); in very high concentrations, liquid-grown worms become shorter (perhaps in this condition, not enough food is available). As starvation is known to generate heritable effects in C. elegans [8–11], in all the subsequent experiments, we grew worms in liquid in low concentrations (maximum 0.05 worms per microlitre, see Methods). In this concentration, liquid-grown worms are approximately 22% longer than plate-grown worms (p < 0.0001, Sidak's multiple comparisons test, figure 1b,c).
Next, we tested whether plate-grown progeny of liquid-grown worms differ in their body length (figure 1a,b). Interestingly, we found that the F1 progeny of liquid-grown worms were on average approximately 8% longer than control worms (p < 0.0001, two-way ANOVA, figure 1b,d). The heritable effect was very robust, repeated across many biological repeats, and was independent of the concentration of the parent worms in the liquid medium (electronic supplementary material, figure S1; in addition, experiments were replicated by multiple different experimenters).
When placed in liquid media, worms change their movement, to a swim-like behaviour, which involves continuous ‘thrashing’. Previously, this swim-like behaviour was used to study the effects of exercise on the worm's physiology [20]. We wondered whether this energetically demanding behaviour could be involved in the morphological change found in the liquid-grown worms and their plate-grown progeny. To test this, we examined the morphology of liquid-grown immobile unc-119 mutants across generations. We found that liquid-grown unc-119 mutants do not become longer in liquid (p < 0.0001, two-way ANOVA, electronic supplementary material, figure S2A,B). While experimentation with such Unc mutants is challenging (the mutants exhibit higher length variability, and do not grow well in liquid), the plate-grown progeny of liquid-grown mutants were nevertheless longer than controls (p < 0.0001, two-way ANOVA, electronic supplementary material, figure S2C). These results suggest that swimming in liquid is not required for the inter-generational effect.
Recently, Çelen et al. analysed the transcriptome of worms that grew in a special axenic liquid medium [31]. They found that the genes that were upregulated in axenic medium were enriched with genes related to cuticle formation and morphology. Further, some transcriptional changes were also inherited by plate-grown F1 progeny. The authors did not examine whether the morphology of the progeny of the worms that grew in axenic medium changed; however, it is possible that these transcriptional changes correspond to the inherited morphological changes that we observe in the progeny of M9 liquid-grown worms.
(b). The change in the morphology of the progeny of liquid-grown worms is apparent early during development, and persists even when the progeny's development is synchronized
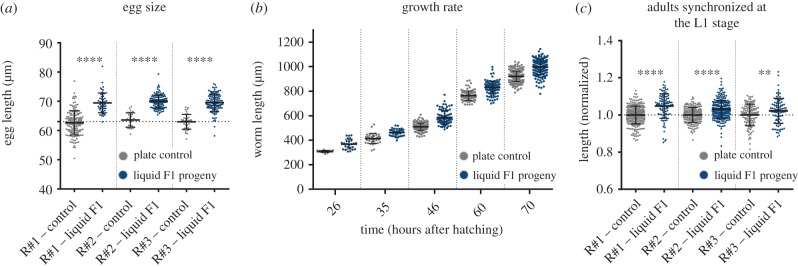
It is possible that the progeny of the liquid-grown worms is longer because these worms develop faster. To test this possibility, we monitored the worms' length throughout development. To our surprise, we found that the progeny of liquid-grown worms were already longer as eggs (p < 0.0001, two-way ANOVA, figure 2a). The progeny of the liquid-grown worms were also longer immediately after hatching, at the L1 stage (figure 2b; electronic supplementary material, figure S3; p = 0.022, see Statistical analysis (§4g)), they maintained the elongated morphology throughout development, and their rate of development was identical to the control group (figure 2b; electronic supplementary material, figure S3; p = 0.7806, see Statistical analysis (§4g)). Moreover, the effect persisted in adults, even when we synchronized the worms’ development at the L1 stage by starvation (for 12 h, see Methods; p < 0.0001, two-way ANOVA, figure 2c). In summary, the elongated morphology of the F1 progeny of liquid-grown worms does not stem from differences in the rate of development.
Figure 2.
Progeny of liquid-grown worms are already longer early in development. (a) The length of eggs laid by liquid-grown worms and plate control worms. Data from three independent biological repeats are presented (N > 30). ****p < 0.0001, two-way ANOVA (biological condition is significant: p < 0.0001, biological repeat and interaction effect factors are not significant: p = 0.8642 and p = 0.2045, respectively). Sidak's multiple comparisons test p-values are presented. (b) The length of progeny of liquid-grown worms and control worms is presented at different stages of the worms' development. (c) The length of adult worm progeny of liquid-grown and plate-grown controls, 72 h after being released from starvation-induced developmental arrest at the L1 stage. Data from three independent biological repeats are presented (N > 100). ****p < 0.0001, **p = 0.0066. Two-way ANOVA (biological condition is significant: p < 0.0001, biological repeat and interaction effect factors are also significant: p = 0.0093. These factors explain 0.81% of the variance, while the biological condition explains 7.96% of the variance). Sidak's multiple comparisons test p-values are presented. Error bars represent standard deviations.
(c). Progeny of liquid-grown worms are longer even in mutants defective in chemosensation and pheromone biosynthesis
In response to various environmental changes, C. elegans alter the composition of the molecular social cues (or pheromones) that they secrete [32,33]. In turn, the secreted pheromones greatly affect the worms' physiology [34,35]. For example, in response to crowded culture conditions, C. elegans secrete pheromones that cause starved larval worms to arrest their development and enter the dauer stage (an alternative developmental stage during which worms can endure harsh conditions) [35]. We examined whether sensation of pheromones or other secreted molecules could be the factor that transforms the progeny of the liquid-grown worms. To test this, we conducted experiments with mutants defective in pheromone production in general (daf-22) and dauer pheromones sensation specifically (srg-36/37). We also examined mutants that are generally defective in chemosensation (che-2).
DAF-22 is a fatty acid β-oxidase important for the production of short-chain ascarosides [36] and dauer pheromones [37]. SRG-36/37 are two redundant G-protein-coupled receptors for the dauer pheromone ascaroside C3 [38]. Mutations in these genes that confer resistance to dauer formation were identified in two independent long-term liquid-grown strains (in worms that were grown in liquid for many decades [38]). We found that liquid-grown daf-22 and srg-36/37 mutants generate a functional inter-generational response and, similarly to wild-type worms, plate-grown progeny of the liquid-grown mutants were longer than controls (figure 3). Thus, pheromones are probably not involved in this inter-generational effect.
Figure 3.
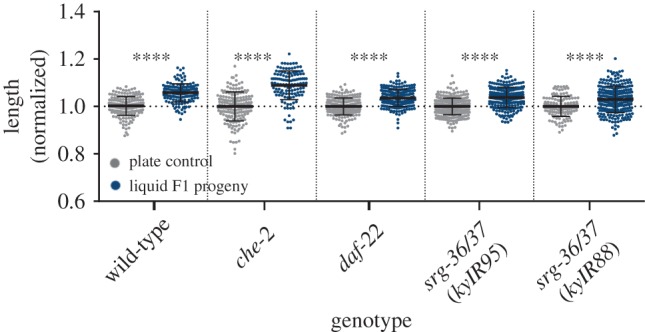
Progeny of liquid-grown worms are longer even in mutants defective in chemosensation and pheromone biosynthesis. The normalized lengths of progeny of liquid-grown mutants defective in genes involved in dauer pheromone synthesis (daf-22), pheromone sensation (srg-36/37), stress response (daf-16) and chemosensation (che-2) are presented. N > 100 per group. ****p < 0.0001, one-way ANOVA, Sidak's multiple comparisons test. Error bars represent standard deviations.
In che-2 mutants the cilia of the sensory neurons are severely defective [39]. Accordingly, these mutants show defects in osmotic pressure avoidance [40], and are defective in chemotaxis to NaCl [39,40] and to many odorants [41]. We found that the progeny of liquid-grown che-2 mutants are also elongated, similarly to wild-type worms. Therefore, sensation of secreted molecules does not appear to mediate the inter-generational response that we describe here (figure 3).
DAF-16 is a transcription factor playing a pivotal role in the insulin/IGF-1 pathway, regulating stress responses, stress survival, longevity, fat metabolism, immunity and dauer formation [42]. DAF-16 was further implicated in inter-generational responses to stress [16]. Surprisingly, plate-grown progeny of liquid-grown daf-16 mutants were elongated, similarly to wild-type worms (figure 3). This suggests that the DAF-16-dependent stress response is not involved in the liquid-induced heritable effect.
(d). Progeny of liquid-grown worms are longer even in mutants defective in inheritance of small RNAs and chromatin modifications
To examine whether the heritable changes in body length depend on inheritance of small RNAs or histone modifications, we examined mutants that are defective in production or inheritance of small RNAs and histone modifications. The nuclear argonautes HRDE-1 and NRDE-3 carry heritable small RNAs, and are required for the inheritance of dsRNA-induced silencing [43,44]. While NRDE-3 is expressed in somatic tissues and is important for inter-generational inheritance of somatic RNA interference [45], HRDE-1 is expressed in the germline and is required for the trans-generational inheritance (lasting multiple generations) of exogenous, dsRNA-derived small interfering RNAs (siRNAs) and endogenous siRNAs that target germline genes [43]. The amplified siRNAs that are carried by the NRDE-3 and HRDE-1 argonautes are produced by RNA-dependent RNA polymerases (RdRPs), such as RRF-1. RRF-1 is involved in the inheritance of Piwi-interacting RNA (piRNA)-triggered endogenous siRNAs and viral-derived siRNAs [5,46]. We found that hrde-1, nrde-3 and rrf-1 mutants, as well as additional mutants defective in siRNA biogenesis (rde-1, rde-4, rrf-3 and mut-16), are not required for the inheritance of the elongated morphology (figure 4). Previously, histone-H3-lysine-9 (H3K9) methylations were implicated in trans-generational inheritance of small RNAs [47–50]. H3K9 mono- and di-methylations are deposited by the MET-2 methyltransferase [51], while H3K9me3 is deposited by the SET-25 and SET-32 methyltransferases [51,52]. We found that met-2, set-25 and set-32 mutants also inherit the elongated morphology from their liquid-grown parents (figure 4). To conclude, the inter-generational inheritance of this phenotype does not appear to depend on the canonical epigenetic inheritance pathways that we examined.
Figure 4.
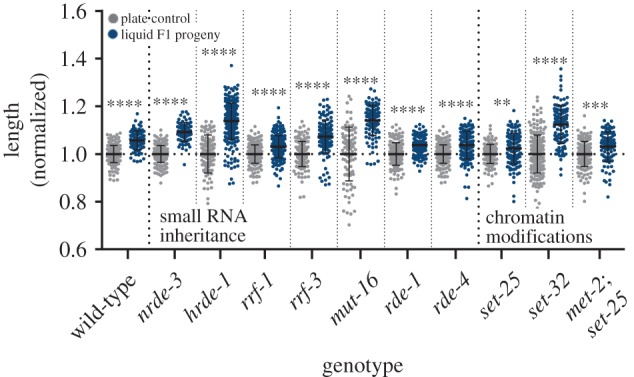
Mutants defective in inheritance of small RNAs and histone methylations are also longer, if they derive from ancestors that were grown in liquid. The normalized lengths of progeny of liquid-grown worms mutated in genes involved in small RNA biogenesis and inheritance (hrde-1, nrde-3, rrf-1, rrf-3, rde-1, rde-4 and mut-16 mutants) or chromatin methylation (set-25, set-32 and met-2; set-25 mutants) are presented. N > 100 per group. ****p < 0.0001, ***p = 0.0001, **p = 0.0084, one-way ANOVA, Sidak's multiple comparisons test. Error bars represent standard deviations.
(e). Unlike the inter-generational effects, trans-generational inheritance of the morphological changes exhibits variability
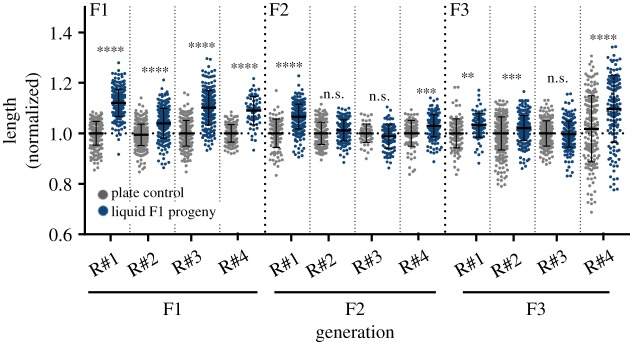
Previously, certain environmental stresses, such as L1 starvation [8], starvation during dauer arrest [11] and exposure to high temperatures [12], were shown to induce trans-generational effects that persist for more than two generations. To test whether the elongated body morphology can be inherited for more than one generation, we measured the body length of plate-grown worms that derive from liquid-grown parents in the F1, F2 and F3 generations. In contrast with the inter-generational changes to the worms' size which were robustly inherited by the F1 generation, the trans-generational effect in the F2 and F3 generations was variable. While in some experiments we did observe trans-generational heritable changes, in other cases the effect was gone after one to two generations (figure 5).
Figure 5.
The liquid-induced longer morphology is not always inherited trans-generationally (in some experiments the effects peter out after one generation). The normalized lengths of progeny of liquid-grown worms and progeny of plate-grown controls in the F1, F2 and the F3 generations are presented. The measured length is normalized to the corresponding control worms of the matching generation and biological repeat. Data from four independent biological repeats are presented (N > 150 worms per group). ****p < 0.0001, ***p = 0.0003 for F3 R#3 ***p = 0.0010 for F2 R#4, **p = 0.0015, n.s. not significant. One-way ANOVA, Sidak's multiple comparisons test. Error bars represent standard deviations.
3. Discussion
In this manuscript, we show that growth in liquid medium under laboratory conditions changes the morphology of C. elegans worms and the morphology of their progeny. As briefly described above, there are rare reports of isolation of C. elegans from freshwater habitats [26]. There are also phenotypes which are specific to worms that grow in liquid. For example, infection of C. elegans in liquid by the pathogenic bacterium Leucobacter celer was found to induce aggregation of worms (termed ‘worm stars’) [53]. Whether the morphological changes that we describe are adaptive in natural circumstances remains to be determined.
In addition to C. elegans, other nematodes are routinely cultured in liquid cultures. Entomopathogenic nematodes such as Steinernema [21,22] and Heterorhabditis [23,24] are grown in liquid cultures as a means of mass production of a biocontrol agent. However, liquid-grown entomopathogenic nematodes were found to have lower dauer stage recovery rates and pathogenicity [21,54,55], and much effort was invested in optimizing the different aspects of their mass production in liquid cultures [22]. Recently, a different nematode model organism, Pristionchus pacificus, was found to exhibit altered, narrow and elongated morphology when grown in liquid cultures [25]. Interestingly, the dimorphic mouth-form of P. pacificus was found to respond to growth in liquid medium; however, the response was not heritable [25]. Several other Pristionchus species, but not all, also showed mouth-form alteration in response to growth in liquid culture [25].
During the course of this study, we could not identify mutants in which epigenetic pathways are affected that are defective for the inter-generational inheritance of the elongated morphology. It is possible that such mutants do exist, and could be detected in more comprehensive screens. Alternatively, the inter-generational effects that we examined could transmit via many different molecular ‘agents’ that do not necessarily affect epigenetics. Pheromone production and sensation do not seem to be required for this effect, as mutants in the relevant pathways also exhibited the heritable liquid-induced effect. Changes in other molecules, such as metabolites or hormones, which are not necessarily self-propagating or ‘amplifiable’ (in contrast with heritable small RNAs), could mediate such short-term inter-generational inheritance as well.
Previously, environmental changes such as starvation and temperature shifts, both common in the laboratory, were shown to change the physiology of the C. elegans worms and of their progeny. Together with growth in liquid, these manipulations should be taken into consideration when designing experiments with C. elegans. It is reasonable to assume that many other subtle characters are affected in the progeny of liquid-grown worms (for example, behaviour). To control for such changes, the worms' growth conditions should be monitored for several generations before experimentation.
Inter-generational and trans-generational inheritance present a unique challenge: to compare between different experiments, the experimenter must keep track of the history of the culture, to make sure different experiments begin from the same starting point. There are currently no standard protocols for achieving this goal. We do not know yet how to ‘re-set’ epigenetic inheritance. Previously, we used CRISPR/cas9 to deactivate HRDE-1, a factor that is important for small RNA inheritance, consequently erasing small RNA memory. This led to the re-setting of the accumulated sterility of chromatin mutants (Mrt phenotype) [49]. Since not all heritable effects are necessarily small-RNA-mediated, other re-setting strategies should be devised in parallel.
The same principles that were discussed above with regard to inter- and trans-generational epigenetic inheritance in C. elegans could apply also to other organisms, and even to experiments conducted with cell lines. Several years ago, a controversy arose regarding the mislabelling of many cell lines [56]. While this is certainly a serious problem, it is worth noting that even when the correct cell line is used, cells in culture acquire mutations [57] (and differ in ploidy), and moreover, non-genetic inheritance could create variability between cultures in different laboratories. Indeed, the ‘generation time’ of the culture is a factor, as cells that have been passaged many times can differ from recently thawed stocks [58,59]. RdRPs, which are crucial for small RNA inheritance in C. elegans, are not known to be conserved in humans [4]; however, even if this mechanism is missing in other organisms, epigenetic memories could be transmitted to daughter cells via different feedback mechanisms [60,61]. For example, in human cells, genes known to be induced by interferon-γ (IFN-γ) were found to exhibit a more robust induction in cells that have previously experienced IFN-γ [62,63]. Importantly, this effect was maintained for four to seven cell divisions, and was associated with H3K4 dimethylation [63]. Therefore, while the causative agents are not always characterized, it is clear that some information that is not encoded in the DNA sequence can persist for long durations. This type of inheritance should be taken into account in the experimental design.
4. Methods
(a). Cultivation of worms
Standard culture techniques were used to maintain C. elegans on nematode growth medium (NGM) plates seeded with OP50 Escherichia coli bacteria. Extreme care was taken to avoid contamination or starvation, and contaminated plates were discarded from the analysis. For inheritance experiments, worms were synchronized either by a standard sodium hypochlorite bleaching technique or by letting adult worms lay eggs on OP50-seeded plates for 2 h.
(b). Growth in liquid medium
Worm eggs were seeded into flasks with 20 ml of M9 buffer (including 1 mM MgSO4 [18]) supplemented with 200 µl penicillin–streptomycin–nystatin (penicillin G sodium salt: 10 000 units ml−1, streptomycin sulfate: 10 mg ml−1, nystatin: 1250 units ml−1) and 20 µl cholesterol (5 mg ml−1) and were fed with 200 µl of concentrated OP51 E. coli bacteria (the bacteria were grown overnight in LB buffer + 100 µg ml−1 streptomycin, pelleted and resuspended in 1 ml of M9 buffer). The flasks were shaken (120 r.p.m.) at 20°C.
(c). L1 arrest experiments
Adult worms were washed using M9 buffer [18]. After removal of the buffer supernatant, an approximately 100 µl pellet of worms was treated for 5 min with 1 ml of bleach solution (2 ml of 5% sodium hypochlorite, 7.5 ml double distilled water, 0.5 ml NaOH 10 M). Next, 0.5 ml of M9 buffer was added and the solution was centrifuged (8000 r.p.m., 1 min). The resulting egg pellet was washed three times by removing the supernatant, adding fresh M9 buffer and centrifugation of 8000 r.p.m. for 1 min to re-pellet the eggs. Around 400 eggs were left to hatch and arrest in the larval L1 stage on fresh plates for approximately 12 h. Next, larval worms were transferred using M9 buffer to NGM plates seeded with OP50 bacteria. Worms were photographed 72 h later.
(d). Strains used in this study
YY720: hrde-1(tm1200) III., YY158: nrde-3(gg66) X., RB798: rrf-1(ok589) I., GW638: set-25(n5021) III.; met-2(n4256) III., VC967: set-32(ok1457) I., MT17463: set-25(n5021) III., WM27: rde-1(ne219) V., WM49: rde-4(ne301) III., YY13: rrf-3(mg373) II., GR1823: mut-16(mg461) I., CF1038: daf-16(mu86) I. CB1033: che-2(e1033) X., DR476: daf-22(m130) II., EG6699: ttTi5605 II.; unc-119(ed3) III., CX13249: srg-37; srg-37(kyIR88) X., CX13591: srg-36; srg-37(kyIR95) X.
(e). Worm length measurements
Worms were placed on empty NGM plates and photographed using a ScopeTek DCM310 binocular camera. The worms' length was measured using WormSizer [64].
(f). Egg length measurements
Worms were allowed to lay eggs on an empty NGM plate, and the laid eggs were photographed using a ScopeTek DCM310 binocular camera. Egg length was measured using the particle analysis tool of the ImageJ software.
(g). Statistical analysis
Two-way ANOVA was used to compare the effect of two categorical variables on the length of the worms' progeny (this statistical analysis is relevant to the experiments shown in figures 1d and 2a,c and in electronic supplementary material, figures S2B,C): (i) biological condition (growth in liquid or on plates) and (ii) biological repeat (independent experiments conducted on separate groups of animals, on different days). One-way ANOVA was used to compare the effect of growth in liquid medium on worm length between different genetic backgrounds (figures 3 and 4), between different food regimes (figure 1c; electronic supplementary material, figure S1) or between conditions across generations (figure 5). In cases of multiple comparisons between genotypes conditions, Sidak's multiple comparison test was applied. Linear regression analysis and a statistical method equivalent to analysis of covariance was used to compare the growth rates of progeny of liquid-grown worms and control worms throughout development (figure 2b; electronic supplementary material, figure S3). Biological repeats are defined as independent experiments conducted on separate populations of animals, on different days. Statistical tests were performed using GraphPad Prism software version 6.
Supplementary Material
Acknowledgements
We thank all the Rechavi lab members for the helpful comments and fruitful discussions. We thank Yoav Zeevi, Yoav Benjamini's group, for his help with the statistical analysis. We also thank the reviewers for their excellent comments and suggestions that greatly improved the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
I.L., R.B. and O.R. conceived and designed the experiments; I.L., R.B., Y.L. and L.I.C. performed the experiments; I.L. and O.R. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by the ERC (grant no. 335624) and the Israel Science Foundation (grant no. 1339/17) and O.R. gratefully acknowledges support by the Allen Discovery Center through the Paul G. Allen Frontiers Group (12171) and the support of the Adelis foundation (no. 01430001000).
References
- 1.Radick G. 2015. Beyond the ‘Mendel–Fisher controversy’. Science 350, 159–160. ( 10.1126/science.aab3846) [DOI] [PubMed] [Google Scholar]
- 2.Anava S, Posner R, Rechavi O. 2014. The soft genome. Worm 3, e989798 ( 10.4161/21624054.2014.989798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heard E, Martienssen RA. 2014. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157, 95–109. ( 10.1016/j.cell.2014.02.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rechavi O, Lev I. 2017. Principles of transgenerational small RNA inheritance in Caenorhabditis elegans. Curr. Biol. 27, R720–R730. ( 10.1016/j.cub.2017.05.043) [DOI] [PubMed] [Google Scholar]
- 5.Rechavi O, Minevich G, Hobert O. 2011. Transgenerational inheritance of an acquired small RNA-based antiviral response in C. elegans. Cell 147, 1248–1256. ( 10.1016/j.cell.2011.10.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gammon DB, Ishidate T, Li L, Gu W, Silverman N, Mello CC. 2017. The antiviral RNA interference response provides resistance to lethal arbovirus infection and vertical transmission in Caenorhabditis elegans. Curr. Biol. 27, 795–806. ( 10.1016/j.cub.2017.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo X, Li W-X, Lu R. 2012. Silencing of host genes directed by virus-derived short interfering RNAs in Caenorhabditis elegans. J. Virol. 86, 11 645–11 653. ( 10.1128/JVI.01501-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rechavi O, Houri-Ze'evi L, Anava S, Goh WSS, Kerk SY, Hannon GJ, Hobert O. 2014. Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell 158, 277–287. ( 10.1016/j.cell.2014.06.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jobson MA, Jordan JM, Sandrof MA, Hibshman JD, Ashley L, Baugh LR. 2015. Transgenerational effects of early life starvation on growth, reproduction and stress resistance in C. elegans. Genetics 201, 1–37. ( 10.1534/genetics.115.178699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demoinet E, Li S, Roy R. 2017. AMPK blocks starvation-inducible transgenerational defects in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 114, E2689–E2698. ( 10.1073/pnas.1616171114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webster AK, Jordan JM, Hibshman JD, Chitrakar R, Baugh LR. 2018. Transgenerational effects of extended Dauer diapause on starvation survival and gene expression plasticity in Caenorhabditis elegans. Genetics 210, 263–274. ( 10.1534/genetics.118.301250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, Lehner B. 2017. Transgenerational transmission of environmental information in C. elegans. Science 356, 320–323. ( 10.1126/science.aah6412) [DOI] [PubMed] [Google Scholar]
- 13.Schott D, Yanai I, Hunter CP. 2014. Natural RNA interference directs a heritable response to the environment. Sci. Rep. 4, 7387 ( 10.1038/srep07387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni JZ, Kalinava N, Chen E, Huang A, Trinh T, Gu SG. 2016. A transgenerational role of the germline nuclear RNAi pathway in repressing heat stress-induced transcriptional activation in C. elegans. Epigenet. Chromatin 9, 3 ( 10.1186/s13072-016-0052-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishimoto S, Uno M, Okabe E, Nono M, Nishida E. 2017. Environmental stresses induce transgenerationally inheritable survival advantages via germline-to-soma communication in Caenorhabditis elegans. Nat. Commun. 8, 14031 ( 10.1038/ncomms14031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burton NO, Furuta T, Webster AK, Kaplan REW, Baugh LR, Arur S, Horvitz HR. 2017. Insulin-like signalling to the maternal germline controls progeny response to osmotic stress. Nat. Cell Biol. 19, 252 ( 10.1038/ncb3470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tauffenberger A, Parker JA. 2014. Heritable transmission of stress resistance by high dietary glucose in Caenorhabditis elegans. PLoS Genet. 10, e1004346 ( 10.1371/journal.pgen.1004346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiernagle T. 2006. Maintenance of C. elegans. WormBook. ( 10.1895/wormbook.1.101.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szewczyk NJ, Kozak E, Conley CA. 2003. Chemically defined medium and Caenorhabditis elegans. BMC Biotechnol. 3, 19 ( 10.1186/1472-6750-3-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laranjeiro R, Harinath G, Burke D, Braeckman BP, Driscoll M. 2017. Single swim sessions in C. elegans induce key features of mammalian exercise. BMC Biol. 15, 30 ( 10.1186/s12915-017-0368-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han R, Ehlers R-U. 2000. Pathogenicity, development, and reproduction of Heterorhabditis bacteriophora and Steinernema carpocapsae under axenic in vivo conditions. J. Invertebr. Pathol. 75, 55–58. ( 10.1006/jipa.1999.4900) [DOI] [PubMed] [Google Scholar]
- 22.Peters A, Han R, Yan X, Leite LG. 2017. Production of entomopathogenic nematodes. In Microbial control of insect and mite pests (ed. L Lacey), pp. 157–170. London, UK: Academic Press. [Google Scholar]
- 23.Surrey MR, Davies RJ. 1996. Pilot-scale liquid culture and harvesting of an entomopathogenic nematode, Heterorhabditis bacteriophora. J. Invertebr. Pathol. 67, 92–99. ( 10.1006/jipa.1996.0013) [DOI] [Google Scholar]
- 24.Upadhyay D, Mandjiny S, Bullard-Dillard R, Storms M, Menefee M, Holmes LD. 2015. Lab-scale in vitro mass production of the entomopathogenic nematode Heterorhabditis bacteriophora using liquid culture fermentation technology. Am. J. Biosci. Bioeng. 3, 203 ( 10.11648/j.bio.20150306.19) [DOI] [Google Scholar]
- 25.Werner MS, Sieriebriennikov B, Loschko T, Namdeo S, Lenuzzi M, Dardiry M, Renahan T, Sharma DR, Sommer RJ. 2017. Environmental influence on Pristionchus pacificus mouth form through different culture methods. Sci. Rep. 7, 7207 ( 10.1038/s41598-017-07455-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiontke K. 2006. Ecology of Caenorhabditis species. WormBook, 6–7. ( 10.1895/wormbook.1.37.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulenburg H, Félix M-A. 2017. The natural biotic environment of Caenorhabditis elegans. Genetics 206, 55–86. ( 10.1534/genetics.116.195511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frøkjær-Jensen C, et al. 2016. An abundant class of non-coding DNA can prevent stochastic gene silencing in the C. elegans germline. Cell 166, 343–357. ( 10.1016/j.cell.2016.05.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timmons L, Tabara H, Mello CC, Fire AZ. 2003. Inducible systemic RNA silencing in Caenorhabditis elegans. Mol. Biol. Cell 14, 2972–2983. ( 10.1091/mbc.E03-01-0858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doh JH, Moore AB, Celen I, Moore MT, Sabanayagam CR. 2016. ChIP and chips: introducing the WormPharm for correlative studies employing pharmacology and genome-wide analyses in C. elegans. J. Biol. Methods 3, 44 ( 10.14440/jbm.2016.109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Çelen İ, Doh JH, Sabanayagam CR. 2018. Effects of liquid cultivation on gene expression and phenotype of C. elegans. BMC Genomics 19, 562 ( 10.1186/s12864-018-4948-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan F, et al. 2011. Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage. PLoS One 6, e17804 ( 10.1371/journal.pone.0017804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Reuss SH, Bose N, Srinivasan J, Yim JJ, Judkins JC, Sternberg PW, Schroeder FC. 2012. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans. J. Am. Chem. Soc. 134, 1817–1824. ( 10.1021/ja210202y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludewig AH, et al. 2017. Larval crowding accelerates C. elegans development and reduces lifespan. PLoS Genet. 13, e1006717 ( 10.1371/journal.pgen.1006717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu PJ. 2007. Dauer. WormBook, 1–3. ( 10.1895/wormbook.1.144.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan J, et al. 2008. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 454, 1115–1118. ( 10.1038/nature07168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golden JW, Riddle DL. 1985. A gene affecting production of the Caenorhabditis elegans dauer-inducing pheromone. MGG Mol. Gen. Genet. 198, 534–536. ( 10.1097/01.EPX.0000417960.79703.06) [DOI] [PubMed] [Google Scholar]
- 38.McGrath PT, Xu Y, Ailion M, Garrison JL, Butcher RA, Bargmann CI. 2011. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature 477, 321–325. ( 10.1038/nature10378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis JA, Hodgkin JA. 1977. Specific neuroanatomical changes in chemosensory mutants of the nematode Caenorhabditis elegans. J. Comp. Neurol. 172, 489–510. ( 10.1002/cne.901720306) [DOI] [PubMed] [Google Scholar]
- 40.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117, 456–487. ( 10.1016/0012-1606(86)90314-3) [DOI] [PubMed] [Google Scholar]
- 41.Bargmann CI, Hartwieg E, Horvitz HR. 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515–527. ( 10.1016/0092-8674(93)80053-H) [DOI] [PubMed] [Google Scholar]
- 42.Murphy CT, Hu PJ. 2013. Insulin/insulin-like growth factor signaling in C. elegans. WormBook, 1–43. ( 10.1895/wormbook.1.164.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.BA Buckley, Burkhart KB, Gu SG, Spracklin G, Kershner A, Fritz H, Kimble J, Fire A, Kennedy S. 2012. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature 489, 447–451. ( 10.1038/nature11352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guang S, Bochner AF, Pavelec DM, Burkhart KB, Harding S, Lachowiec J, Kennedy S. 2008. An Argonaute transports siRNAs from the cytoplasm to the nucleus. Science 321, 537–541. ( 10.1126/science.1157647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burton NO, Burkhart KB, Kennedy S. 2011. Nuclear RNAi maintains heritable gene silencing in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 108, 19 683–19 688. ( 10.1073/pnas.1113310108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapetschnig A, Sarkies P, Lehrbach NJ, Miska EA. 2015. Tertiary siRNAs mediate paramutation in C. elegans. PLoS Genet. 11, e1005078 ( 10.1371/journal.pgen.1005078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu SG, Pak J, Guang S, Maniar JM, Kennedy S, Fire A. 2012. Amplification of siRNA in Caenorhabditis elegans generates a transgenerational sequence-targeted histone H3 lysine 9 methylation footprint. Nat. Genet. 44, 157–164. ( 10.1038/ng.1039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lev I, Gingold H, Rechavi O. In press. H3K9me3 is required for transgenerational inheritance of small RNAs that target a unique subset of newly evolved genes. eLife. ( 10.1101/338582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lev I, Seroussi U, Gingold H, Bril R, Anava S, Rechavi O. 2017. MET-2-dependent H3K9 methylation suppresses transgenerational small RNA inheritance. Curr. Biol. 27, 1138–1147. ( 10.1016/j.cub.2017.03.008) [DOI] [PubMed] [Google Scholar]
- 50.McMurchy AN, et al. 2017. A team of heterochromatin factors collaborates with small RNA pathways to combat repetitive elements and germline stress. eLife 6, e21666 ( 10.7554/eLife.21666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Towbin BDD, González-Aguilera C, Sack R, Gaidatzis D, Kalck V, Meister P, Askjaer P, Gasser SMM. 2012. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 150, 934–947. ( 10.1016/j.cell.2012.06.051) [DOI] [PubMed] [Google Scholar]
- 52.Spracklin G, Fields B, Wan G, Vijayendran D, Wallig A, Shukla A, Kennedy S. 2017. Identification and characterization of C. elegans RNAi inheritance machinery. Genetics 206, 1–19. ( 10.20944/preprints201702.0096.v1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hodgkin J, Félix M-A, Clark LC, Stroud D, Gravato-Nobre MJ. 2013. Two Leucobacter strains exert complementary virulence on Caenorhabditis including death by worm-star formation. Curr. Biol. 23, 2157–2161. ( 10.1016/j.cub.2013.08.060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehlers R-U, Lunau S, Krasomil-Osterfeld K, Osterfeld KH. 1998. Liquid culture of the entomopathogenic nematode-bacterium-complex Heterorhabditis megidis/Photorhabdus luminescens. BioControl 43, 77–86. ( 10.1023/A:1009965922794) [DOI] [Google Scholar]
- 55.Strauch O, Ehlers R-U. 1998. Food signal production of Photorhabdus luminescens inducing the recovery of entomopathogenic nematodes Heterorhabditis spp. in liquid culture. Appl. Microbiol. Biotechnol. 50, 369–374. ( 10.1007/s002530051306) [DOI] [Google Scholar]
- 56.Capes-Davis A, et al. 2010. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int. J. Cancer 127, 1–8. ( 10.1002/ijc.25242) [DOI] [PubMed] [Google Scholar]
- 57.Ben-David U, et al. 2018. Genetic and transcriptional evolution alters cancer cell line drug response. Nature 560, 325–330. ( 10.1038/s41586-018-0409-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merrill GF. 1998. Cell synchronization. Methods Cell Biol. 57, 229–249. ( 10.1016/S0091-679X(08)61582-4) [DOI] [PubMed] [Google Scholar]
- 59.Niida H, Matsumoto T, Satoh H, Shiwa M, Tokutake Y, Furuichi Y, Shinkai Y. 1998. Severe growth defect in mouse cells lacking the telomerase RNA component. Nat. Genet. 19, 203–206. ( 10.1038/580) [DOI] [PubMed] [Google Scholar]
- 60.Campos EI, Stafford JM, Reinberg D. 2014. Epigenetic inheritance: histone bookmarks across generations. Trends Cell Biol. 24, 664–674. ( 10.1016/j.tcb.2014.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, et al. 2013. Tissue culture-induced genetic and epigenetic alterations in rice pure-lines, F1 hybrids and polyploids. BMC Plant Biol. 13, 77 ( 10.1186/1471-2229-13-77) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gialitakis M, Arampatzi P, Makatounakis T, Papamatheakis J. 2010. Gamma interferon-dependent transcriptional memory via relocalization of a gene locus to PML nuclear bodies. Mol. Cell. Biol. 30, 2046–2056. ( 10.1128/MCB.00906-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Light WH, Freaney J, Sood V, Thompson A, D'Urso A, Horvath CM, Brickner JH. 2013. A conserved role for human Nup98 in altering chromatin structure and promoting epigenetic transcriptional memory. PLoS Biol. 11, e1001524 ( 10.1371/journal.pbio.1001524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore BT, Jordan JM, Baugh LR. 2013. WormSizer: high-throughput analysis of nematode size and shape. PLoS ONE 8, e57142 ( 10.1371/journal.pone.0057142) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.