Abstract
In many vertebrate species, early social experience generates long-term effects on later life social behaviour. These effects are accompanied by persistent modifications in the expression of genes implicated in the stress axis. It is unknown, however, whether stress axis programming can affect the development of social competence, and if so, by which mechanism(s). Here, we used pharmacological manipulations to persistently reprogramme the hypothalamic–pituitary–interrenal axis of juvenile cooperatively breeding cichlids, Neolamprologus pulcher. During the first two months of life, juveniles were repeatedly treated with cortisol, mifepristone or control treatments. Three months after the last manipulation, we tested for treatment effects on (i) social competence, (ii) the expression of genes coding for corticotropin-releasing factor (crf), glucocorticoid receptor (gr1) and mineralocorticoid receptor (mr) in the telencephalon and hypothalamus and (iii) cortisol levels. Social competence in a social challenge was reduced in cortisol-treated juveniles, which is in accordance with previous work applying early-life manipulations using different social experiences. During early life, both cortisol and mifepristone treatments induced a persistent downregulation of crf and upregulation of mr in the telencephalon. We suggest that these persistent changes in stress gene expression may represent an effective physiological mechanism for coping with stress.
This article is part of the theme issue ‘Developing differences: early-life effects and evolutionary medicine’.
Keywords: early-life effects, corticosteroid receptor, developmental plasticity, HPA/HPI axis, mifepristone, cichlids
1. Introduction
Through developmental plasticity, the early social environment can strongly influence animal social behaviour and social competence in later life stages [1,2]. Social competence, the ability of animals to optimize their social behaviour as function of the available social information [3,4], can be shaped by the quantity, quality and diversity of social interactions young animals are exposed to during early life [2]. For example, laboratory mice reared in communal nests that received more intensive maternal care and tactile stimulation [5] or encountered more peer-to-peer interactions [6] before weaning established social hierarchies faster and behaved more adequately with respect to their social rank later in life compared to mice reared by single mothers. Similar patterns have been reported in young zebra finch (Taeniopygia guttata) males [7] and in teleost fish. In the cooperatively breeding cichlid fish Neolamprologus pulcher, individuals reared in larger [8] or more complex [9,10] social groups had an improved social competence both as juveniles and adults compared to individuals that were reared in small groups or in groups consisting of a single age class. In these fish, improved social competence payed off in a number of benefits in social interactions, such as the ability to defend a resource more efficiently [9], to be accepted close to a safe shelter [10] and to integrate more easily into a new social group [10]. In highly social species with many social encounters every day, these small benefits probably accumulate over lifetime into a substantial fitness benefit.
In vertebrates, differential programming of the stress axis has repeatedly been shown to accompany long-term effects of the early social environment on social behaviour [11–13]. The hypothalamic–pituitary–adrenal (HPA) axis of mammals and birds or its homologue, the hypothalamic–pituitary–interrenal (HPI) axis of fish, is the main physiological mechanism responsible (i) for eliciting and terminating stress responses [14] and, in turn, (ii) for responding and adapting to environmental changes [15]. The presence or absence of N. pulcher parents during early life has been shown to affect the expression of genes implicated in the HPI stress axis [16,17]. Early-life experience can shape neurobiological pathways involved in stress responsiveness through organizational effects on tissue sensitivity for glucocorticoids [18,19]. Glucocorticoids, such as cortisol (in fish and most mammals) or corticosterone (in rodents and birds), are the major vertebrate stress hormones and are responsible for the regulation of the stress axis [20]. Evidence suggests that the early social environment may generate a cascade of neurobiological changes involving the vertebrate stress axis, which has implications for social behaviour [12].
Following the perception of a stressor, a stress response is elicited by an endocrine cascade, where the primary steps include the release of catecholamines in the sympathetic nervous system, followed by the hypothalamic release of corticotropin-releasing factor (CRF) [20]; this eventually leads to the secretion of glucocorticoids from the adrenal or interrenal glands into the bloodstream [21]. The control of stress responses is mediated by cortisol signalling through two types of intracellular receptors that act as ligand-dependent transcription factors [22,23]. These receptors mediate the activation or repression of different genes in the target cells [24]. Elevated concentrations of cortisol activate the glucocorticoid receptors (GR) in the nucleus preopticus [25], the hypothalamus and the pituitary gland [18], which attenuate and eventually terminate the stress response via negative feedback loops that lead to suppression of further cortisol release. This suppression happens by blocking further CRF production [25]. The second intracellular receptor type, the mineralocorticoid receptor (MR) in teleost fish [26] and in the non-epithelial limbic neurons of other vertebrates [23], has a high-affinity to cortisol and is activated at basal levels [22,23]. Nuclear MR maintains the integrity and stability of the limbic circuit, determining the threshold or sensibility of the limbic stress response system [27]. By contrast, low-affinity MR in membranes boosts the initial stress response [27].
Although early social experience alters both stress axis programming and social competence, it is as yet unknown whether stress axis programming in early life causally determines the development of social competence, and if so, by which mechanism. Few studies have pharmacologically manipulated the stress axis prenatally or in early life to test for causal links between this axis and behaviour (neophobia [28,29]; aggression [30]; defence against an intruder [31]; migration [32]; predator avoidance [33]; exploration [34]). A well-developed social competence should be particularly important for highly social species, such as cooperative breeders, in which almost all aspects of life include social interactions [4]. Here, we aimed to manipulate early-life programming of the principal pathway that regulates the stress axis by pharmacological application of either cortisol or GR blocker in the highly social cichlid N. pulcher. We predicted that our treatments would generate long-term effects on later life social competence, social performance, gene expression in the brain and fluctuating cortisol.
Mifepristone has been successfully used to block GR1 (see [35] and references therein), which is the teleost homologue of the mammalian GR. Besides its function as GR blocker, it also binds to progesterone receptors, and therefore may have an additional function in reproduction [35]. However, this second function is unlikely to play a role in this study, as the fish were in their earliest juvenile stages when they were treated with mifepristone (i.e. more than 6 months before sexual maturity). Previous work revealed contrasting behavioural effects of pharmacological administrations of cortisol and mifepristone. Blocking GR1 by mifepristone in adult N. pulcher induced a higher expression of submissive behaviours in intruders aiming to take over the territory from a resident, but nevertheless led to a higher success in the monopolization of territory [36]. The authors concluded that this increased success in territory acquisition resulted from an enhanced expression of adequate behaviours (social competence) after mifepristone treatment. Adult rainbow trout (Oncorhynchus mykiss) decreased aggression levels towards territory intruders [37] after being treated with mifepristone. By contrast, rainbow trout that were exposed to cortisol exhibited increased aggression, both when treated as adults [37] or during early ontogeny [30]. Based on these findings, we hypothesized that mifepristone treatment would increase submission and decrease aggression, but nevertheless lead to a higher success in obtaining or retaining resources (see [36]). Conversely, cortisol treatment was hypothesized to enhance aggression in N. pulcher facing a territory intrusion, but not necessarily lead to a higher success in aggressive contests over a resource.
In vertebrates, elevations of cortisol levels generate a negative feedback via GR that eventually blocks further CRF release (e.g. [24,38,39]). Therefore, we predicted that early-life cortisol treatment would upregulate gr1 gene expression, thereby enhancing the negative feedback loop on further cortisol release by decreasing crf mRNA levels. Additionally, we predicted upregulation of mr gene expression, as early-life corticosteroid treatment has been shown to upregulate mr expression in Japanese quail (Coturnix coturnix japonica) [40]. Conversely, the GR blocker mifepristone has been shown to induce downregulation of crf and gr genes in different tissues of the fish brain; for example, in the hypothalamic preoptic area of rainbow trout, O. mykiss [41], telencephalon-preoptic brain region of goldfish, Carassius auratus [42], and in whole body samples of juvenile zebrafish, Danio rerio [43]. Consequently, we predicted a decrease in the gr1 and crf expression after early-life GR blocker treatment. However, these predictions are based on studies measuring the immediate effects of mifepristone administration as studies of early-life exposure to mifepristone are not available, and should, therefore, be considered with caution.
Finally, we predicted that our treatments would translate into different mechanisms of stress response regulation, that is, the response of an individual to a stressor by cortisol release, which in turn gives rise to physiological pathways that aid in returning to a homeostatic state [44,45]. Mifepristone treatment increased endogenous cortisol levels in the fish Opsanus beta [46], but, importantly, it suppressed the amplitude of cortisol responses in both fish and rats [41,47]. After a postnatal corticosterone treatment, juvenile Japanese quail (C. c. japonica) showed a shorter stress response after a stressful stimulus than control birds [48]. Therefore, we predicted that both mifepristone and cortisol treatment would decrease stress responsiveness. Specifically, mifepristone would reduce the amplitude of the stress response and cortisol reduces the duration of the response.
2. Material and methods
(a). Study species
Neolamprologus pulcher is a cooperatively breeding cichlid fish endemic to Lake Tanganyika, East Africa. Social groups consist of a dominant breeding pair, related and unrelated helpers ranging from 1 up to 25 individuals and the offspring of the current breeding pair. A group will inhabit a joint territory of up to 1 m2 [49,50]. Helpers stay in the natal territory even after reaching sexual maturity (at a size of at least 3.5 cm SL (standard length)) [51] at about 10 and 12 months of age [36]. In natural populations, reproductive success of the dominant breeding pair increases with the number of helpers owing to improved offspring survival [52]. In turn, helpers pay-to-stay with breeders by contributing to territory defence and maintenance [53,54].
Offspring start performing social behaviour towards other siblings at about five weeks post-hatching and frequencies of these behaviours increase over time [9]. At a size of 1.5–2 cm, offspring start to join in helping tasks. These duties include the cleaning and fanning of eggs produced by dominants, removing sand from the breeding cavity and defending the breeder's territory against predators and intruders [55]. Helper task specialization is size-dependent [56]. Small helpers usually perform alloparental care in form of egg cleaning and fanning [57], while large helpers engage in territory defence and territory maintenance [56]. The composition and size of social groups during the first months of life strongly affect social behaviours (helping behaviours, social competence, life-history decisions and the expression of stress axis genes) [4,8,9,16,17,58].
(b). Animal housing
The experiments were conducted at the Hasli Ethological Station of the Institute of Ecology and Evolution, University of Bern, Switzerland, under licence BE 74/15 of the Veterinary Office of Kanton Bern. The parents of our experimental fish were laboratory-reared second- and third-generation offspring of wild-caught fish from Kasakalawe Point, Mpulungu, Zambia. We created 31 breeding pairs from haphazardly chosen adult males and females from our laboratory stock. Each breeding pair was randomly assigned to produce offspring to be exposed to one of three treatments: (i) the stress hormone cortisol (n = 11), (ii) the GR blocker mifepristone (n = 10), and (iii) a blank control treatment (n = 10). The breeding pairs were housed in individual 60 l tanks equipped with 2 cm of fine sand, a biological filter, two flowerpot halves serving as potential breeding cavities and a half-transparent PET bottle mounted near the water surface to serve as refuge. The water temperature was kept at 27 ± 1°C with a light–dark regime of 13 : 11 h and a dimmed-light phase of 10 min. The breeding pairs were fed commercial adult flake food (JBL Novo Tanganyika®) 5 days a week and frozen zooplankton 1 day a week. Additionally, frozen krill and Artemia spp. nauplia (Artemix, Dohse Aquaristik, Germany) were provided twice a week to stimulate egg production. We waited until the pairs produced a clutch and the larvae had developed into free-swimming fry. The first day of free-swimming was defined as the experimental ‘day 0’ (e.g. 10 ± 2 days post-fertilization). After day 0, each rearing group was fed TetraMin Baby® food, the amount of which was adjusted to the number of fry and their age by following the feeding regime described in [58]. Additional adult flake food was provided for the breeders. This feeding regime was adopted to ensure homogeneous growth rates among siblings within and across rearing groups.
(c). Experience phase
(i). Cortisol treatment
For the cortisol treatment, we used a concentration that was half of the cortisol plasma levels reported from stressed N. pulcher adults [59]. When this concentration was applied to developing rainbow trout eggs, it generated transitional (less than 1 day) elevations of cortisol levels and induced a long-term effect on stress sensitivity [60]. To prepare the treatment solution, hydrocortisone (Product number H4001, Sigma-Aldrich, Switzerland) was dissolved in dimethylsulfoxide (DMSO) to get a stock solution of 1 mg ml−1 cortisol concentration. The final concentration of 200 ng ml−1 was obtained by adding 100 µl of cortisol stock solution to 500 ml of tap water. Local tap water had ideal water parameters to hold Tanganyika cichlids and needed no further processing.
(ii). Mifepristone treatment
For the mifepristone treatment, we used a concentration that had been previously shown to generate short-term effects on N. pulcher social performance [36]. We dissolved mifepristone (RU486; Product number M8046 Sigma-Aldrich, Switzerland) following the modified protocol of [61]. Briefly, a mifepristone stock solution (50 ng µl−1) was used to obtain a final concentration of 400 ng l−1 by adding 4 µl of mifepristone stock solution to 500 ml of water.
(iii). Control treatment
In the control treatment, we applied the same solvents in the same concentration used to dilute mifepristone (DMSO, PBS, BSA), but without adding mifepristone.
(iv). Application of water baths
At experimental days 10, 20, 30, 40, 50 and 60 (figure 1), the assigned treatment was applied to all offspring of a rearing group. Treatments were assigned to rearing groups randomly, on the condition that the same treatment was never assigned to two neighbouring tanks and all treatments were represented equally in the different rows of the tank-rack. The treatments were applied as water baths; this non-invasive method allows repeated applications of hormones at very low manipulation stress levels [61–63]. For the water bath, a maximum of 20 juveniles were placed inside a 2 l glass beaker filled with 500 ml of tap water. All beakers were supplied with oxygen using glass Pasteur pipettes connected to an air stream. The beakers were kept in complete darkness and were isolated against noise during the entire exposure procedure, which consisted of (i) a 30 min acclimatization period; (ii) 1 h of exposure to the respective treatment; (iii) a first recovery period of 30 min in a beaker with home tank water; and (iv) a second recovery period of 30 min inside a mesh cage (14.5 × 8.5 × 7 cm) hanging in the home tank. Finally, the juveniles were gently released from the mesh cage into the home tank. The breeder pair always immediately reaccepted their offspring.
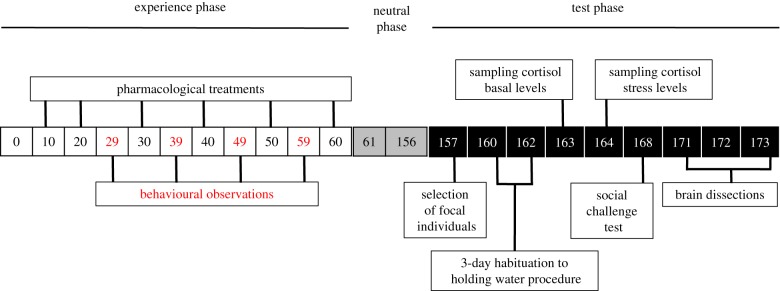
Figure 1.
Timeline of experimental manipulations between developmental day 0 (first day of free swimming) and day 173. White boxes: experience phase; individuals received pharmacological treatments and their behaviour was recorded. Grey boxes: neutral phase; broods were kept under identical conditions without their parents. Black boxes: test phase; experimental individuals were selected for hormonal measurements and social challenge tests; the brains of ‘naive’ individuals, i.e. individuals that had not taken part in either the hormone measurements or social challenge test, were dissected. (Online version in colour.)
We kept the repeated exposure to the drugs short to generate a pulsed exposure (1 h) followed by a long unexposed period (10 days), because we wanted the exposure to cortisol to have similar effects to short stressful situations in a natural context. The application of short exposures to cortisol and mifepristone is expected to lead to a temporary increase in the cortisol of juveniles once they developed full functionality of their HPI axis [61]. Veillette et al. [61] reported that in summer flounders (Paralichthys dentatus), the HPI axis was fully developed at an age of three weeks, which is even slightly earlier than the age when we exposed our fish to mifepristone (30 days of age, that is, 20 days after free swimming). Thus, we can assume that the HPI axis in N. pulcher was fully functional at first exposure. In 21-day-old summer flounders, the administration of mifepristone resulted in a rapid (within a few hours) increase in cortisol, and we assume that the same happened in N. pulcher.
(v). Behavioural recordings
During the experience phase, which lasted until day 60, we performed repeated recordings of all spontaneous social behaviours and the general activity of the experimental juveniles in their home tanks. During this time, the juveniles were still co-housed with the breeder pair (see §2b). The recordings were conducted 9 days after an application of a pharmacological or control treatment (figure 1) following the observation methods and ethogram used by [9] (see ethogram in electronic supplementary material, table S1). Thus, recording took place on days 29, 39, 49, 59 and 69, but not on day 19, as at this age, fish were still very small and did not show noticeable social behaviour. Before each recording, a transparent acetate grid of 14 × 10 cells (4 × 4 cm each cell) was attached to the front wall of the tank, which was used to randomly select the first juvenile for a recording using a random number table, and for estimating its activity. All behavioural recordings were done by an observer who was blind to the treatment. Before starting a recording, juveniles were allowed to acclimatize for 5 min to the presence of the observer, who sat motionless in front of the tank. A total of three juveniles per rearing were recorded for 5 min each, amounting to a total recording time of 15 min per tank [9].
(vi). Recorded behaviours
The recorded behaviours (cf. [9]; see ethogram in electronic supplementary material) were grouped according to their function for statistical analyses: (i) restrained aggression, which comprises all threat behaviours not involving physical contact. (ii) Overt aggression, which involves physical contact or attempted physical contact (i.e. chasing). While overt aggression by larger specimens can have a strong impact and inflict injuries in the receiver, we never observed injuries in the interactions between our experimental juveniles. Overt aggression was rare and thus could not be statistically analysed. (iii) Submissive displays, which can be shown spontaneously towards dominant individuals or in response to received aggression and most often consists of a strong vibration of tail and body. (iv) Affiliative behaviour including swimming in close proximity without showing signs of aggression, and soft body touches (‘bumping’; see ethogram in electronic supplementary material), which is mostly performed by subordinates towards dominants. (v) General activity was measured as the amount of locomotion by an individual estimated by the number of lines of the acetate grid a focal individual crossed during a 5 min observation period. All behaviours were recorded as frequencies. The sum of the behaviours shown by the three juveniles per rearing group was used for statistical analyses [9].
(d). Neutral phase
At the end of the 2 months experience phase, breeder pairs were removed and returned to the laboratory stock. The experimental juvenile groups were left in their home tanks under the same housing conditions described above and without any treatment for the next 95 days. At the beginning of the neutral phase, juvenile groups comprised a mean size of 44.1 (± 3.14 s.e.) fish.
Two-month-old N. pulcher juveniles are independent of parental care [4]. When living in a family group, at this age, they start to act as brood care helpers [57]. They can specialize in direct brood care, defence or territory maintenance, or develop a submissive non-helper type [58,64,65]. For our study, introducing a neutral phase was therefore critically necessary to prevent individual task specialization, which would have confounded the effect of the pharmacological manipulation.
(e). Test phase
(i). Selection of focal individuals
At day 157, individuals were selected for hormone measurement and the social challenge test. We determined the median SL for each rearing group and selected four focal individuals that were closest to the mean SL of their rearing group. These focal fish were later used in a social challenge test (figure 1) where they acted in one of two social roles, owners (Ow.) and intruders (Int.; see below). When selecting the focal fish (i.e. two replicate fish per social role), we preassigned them to their role and marked each individual with a fin clip according to social role for later identification. The SL of focal individuals was 3.035 ± 0.34 cm (mean ± s.e.; Int.) and 2.995 cm ± 0.25 (Ow.). Until and between the manipulations for hormone sampling and behavioural testing (figure 1), the focal juveniles were kept in individual, transparent isolation boxes (10.5 × 10 × 17 cm) floating at the surface of the home tank, which allowed for exchange of visual and olfactory cues with the other siblings housed in the tank. It facilitated repeated, quick catching of the focal individuals to reduce catching stress.
(ii). Hormone sampling
During three consecutive days (days 160–162) at 10 : 00 h, focal individuals were placed for 30 min each in a separate 2 l glass beaker containing 500 ml of clean tap water. This procedure has been shown to lead to habituation to the handling procedure in other cichlids [66], including N. pulcher, thereby minimizing the effect of handling stress on our stress measurements.
At day 163, we measured basal cortisol of all focal fish using the same manipulations and procedures used during the habituation phase. In a total of 93 individuals (cortisol, Ow.: n = 20, Int.: n = 16; mifepristone, Ow.: n = 19 Int.: n = 10; control, Ow.: n = 19, Int.: n = 9), we sampled baseline cortisol using the ‘fish-holding water method’, a non-invasive technique to sample waterborne steroid hormones in small fishes [66–68]. Finally, on day 164, we sampled cortisol responses after fish had experienced an acute stressor in the same 93 individuals and following the procedure described above. The acute stressor consisted of placing the focal individuals gently in a mesh and exposing them to air for 1 min [69,70] before placing them into the glass beaker to sample their cortisol.
All cortisol samples were obtained between 10:00 and 11:00 h to minimize variation owing to diurnal fluctuations of cortisol excretion [45]. The preparation of hormone samples followed a protocol developed by Neuchâtel Platform of Analytical Chemistry, University of Neuchâtel (see details in the electronic supplementary material). Cortisol content was analysed by ultrahigh-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS).
(iii). Social challenge test
We staged an asymmetric contest over a shelter, which is a vital resource for N. pulcher in nature, to test the ability of fish to express adequate behaviour according to their preassigned social role. Thus, this test evaluated the social competence of fish in a biologically meaningful context. Preassigned owners should defend the shelter against an intruder and are in a favourable position to maintain the shelter. Therefore, a restrained, non-escalated form of aggression (threat display) should suffice for a successful defence [9]. Preassigned intruders are usually not able to take over the shelter, but should aim to achieve tolerance from the resident owner when near the shelter [9,16]. In nature, each subordinate group member defends a private shelter for hiding from predator attacks within the group territory [52] and these shelters are crucial for survival of subordinates [10,71].
A total of 58 individuals were tested in the social challenge test (cortisol n = 20, mifepristone n = 19, control n = 19) in the role of owners, and a total of 46 individuals (cortisol n = 21, mifepristone n = 12, control n = 13) as intruders. Different sample sizes between treatments within a social role were caused by the death of some individuals between hormone sampling and behavioural testing owing to a temporary deterioration of water conditions in some rearing tanks. Furthermore, more fish were tested in the owner role than in the intruder role; for logistical reasons, we could not perform tests in the intruder role for all rearing groups. This is because in N. pulcher, larger individuals are dominant over smaller ones, so we size-matched the focal fish and their respective opponents to the nearest millimetre (difference (mean ± s.e.) between focal individual and opponent 0.16 ± 0.075 mm SL). Opponents were unrelated, unfamiliar fish from a laboratory stock tank that had never received any hormonal treatment. Each opponent was used only once, and after the contest it was immediately returned to its home tank.
One day before the social challenge, at day 167, focal individuals and opponents were acclimatized to the experimental set-up, which was a 20 l tank (20 × 20 × 30 cm) equipped with a 2 cm layer of sand divided into two compartments by an opaque partition. The owner's compartment was equipped with a flowerpot half (5.5 cm outer diameter) and a biological filter. The intruder's compartment only contained an air stone.
At day 168, we removed the opaque partition to start the asymmetric competition test. The behavioural recording started when one of the individuals crossed the virtual border between the two compartments (i.e. where the partition had previously been) and lasted for 20 min. All social behaviours performed by both individuals were recorded using the program Solomon Coder (Copyright © 2017, András Péter). The observer, who was blind to the treatments, was hidden behind a black curtain. After the recording, we determined the time point when the contest had been decided (end of contest), and who won or lost the contest following the criteria used by Nyman et al. [36]. Briefly, an individual lost when it was evicted from the vicinity of the shelter and did not attempt to gain access to it anymore; an individual was the winner when it had unrestricted access to the shelter and was not attacked by the other fish; contests were rated as ‘undecided’ when no clear winner or loser existed at the end of the recording. Contests were classified as ‘alternative outcome’ if the owner chose the aquarium filter as shelter so that the intruder could use the flowerpot without being threatened or attacked by the opponent.
(f). Gene expression
To assess whether the constitutive expression of stress axis genes was altered by our early-life pharmacological manipulations, we measured the expression of three key genes of the stress axis: crf, gr1 and mr. As outlined above, the products of these three genes play a central role in regulating stress responses.
Between days 171 and 173, we sacrificed two randomly selected replicate individuals from each rearing group by an overdose of tricaine methanesulfonate (MS-222; Sandoz, Switzerland). None of these fish had taken part in hormone sampling and/or the social challenge test beforehand. Following the procedures described in [16], we dissected the brains with a scalpel under a steromicroscope, and stored the telencephalon and the hypothalamus of each brain separately in RNAlater® (Qiagen, The Netherlands) and kept the vials at −20°C for up to 7 months until RNA extraction. Only one individual from each rearing group was used for genetic analysis (sample size per treatment and brain area: cortisol n = 11; mifepristone n = 10; control n = 10; size: 2.99 ± 0.26 cm SL) and the other was kept as backup. The two brain regions were chosen because they contain the brain tissues involved in stress regulation, namely the limbic forebrain neurons (telencephalon) and the parvocellular corticotropin-releasing hormone (CRH)-producing neurons in the paraventricular nucleus (PVN) of the hypothalamus [27]. Gene expression was measured by a standard reverse transcriptase–quantitative polymerase chain reaction (RT–qPCR) protocol (details in electronic supplementary material).
(g). Statistical analyses
We analysed treatment effect by GLMMs and LMMs (see details below). If models had non-significant interaction terms, they were excluded stepwise [72,73]. The Poisson-distributed generalized linear-mixed effect models (GLMMs) were corrected in the case of over-dispersion by including an observation-level random factor. For each factor of the model, estimates and their standard errors, and t- or z-values according to the type of model, are given in electronic supplementary material, tables S2–S5. Significance testing was based on deviance when removing respective terms from the model. The change in likelihood was compared to a χ2 distribution (likelihood ratio test, see [74]); therefore, χ2-values are given in the main text and electronic supplementary material, tables. Normality assumptions for all linear-mixed effect models (LMMs) were tested by visual inspection of quantile–quantile plots and by the Shapiro–Wilks and Lilliefors (Kolmogorov–Smirnov) normality tests. If necessary, the dependent variable was boxcox-transformed to achieve the normality of the error terms. We checked for potential outliers of model residuals by visual inspection of the Cook's distance score [75], excluding values with a Cook's distance greater than 0.5. In addition, we assessed the influence of those values with the Grubb's test [76]. Only in one dataset (crf in the telencephalon), one high-leverage data point had to be excluded based on these criteria. Post hoc analyses were done in cases of significant interaction terms in models. Data were analysed using R 3.1.2. and the packages ‘lme4’ [77], ‘car’ [78], ‘nortest’ [79], ‘MASS’ [80], ‘multcomp’ [81] and ‘outliers’ [76].
(i). Experience phase
The sums of behavioural frequencies of the three individuals recorded from each rearing group at a given developmental time point, and the sums of line crosses (our estimate of locomotory activity; see §2c(v)), were included as dependent variables of Poisson-distributed GLMMs, which were fitted with a log-link function. ‘Treatment’, ‘experimental day’ and their interaction term were included as fixed effects, and ‘rearing group of origin’ was included as a random effect.
(ii). Contest outcome
Contest outcome was analysed by fitting a binomial GLMM with logit link function. The binary outcome refers to either winning or losing the contest over the shelter. The categories ‘undecided’ and ‘alternative outcome’ were excluded from this analysis. Because social performance [82], in our case contest outcome, and social status [83] can depend on individual cortisol levels, we included waterborne cortisol levels in the models of contest outcome. We included ‘stress responsiveness' as covariate in the models; it was calculated as the difference between cortisol content in the holding water after the acute stressor (stress-level cortisol) and cortisol content in the water sample of the same individual when not stressed (basal cortisol level) [44,45]. Note that waterborne cortisol, both basal levels and stress responsiveness, per se did not differ between treatments and we do not present these results. They did, however, influence the outcome of fights (see §3). Individuals in both social roles can take over the resource; therefore, ‘social role’ (i.e. owner or intruder) was included as a covariate. Furthermore, ‘treatment’ was included as fixed factor and ‘rearing group of origin’ was included as random factor.
(iii). Social behaviour during contest
We only included behaviours performed until the end of the contest in our analysis. While after the end of a contest, social behaviour may still occur, these behaviours would not occur after natural contests, where a loser would either leave the territory or get out of reach of the winner. To account for contest duration, we analysed the behavioural data until the end of contests as rates per minute [9,16] and fitted LMMs for analysis. ‘Treatment’ was included as a fixed factor and ‘rearing group of origin’ as a random factor in all LMMs. ‘Total aggression of opponent’ was included as a covariate because the conflict can escalate and extend if both opponents behave aggressively, and neither shows submissive behaviour.
We analysed the two social roles of owners and intruders separately, because they afford different appropriate behavioural responses. For owners, we analysed restrained aggression, an appropriate type of behaviour expected in individuals that already own a resource in a competitive situation [9]; we included ‘submissive behaviour of opponent’ as covariate as the submissive tendencies of conspecifics in N. pulcher influence aggression, and vice versa [9,16]. For intruders, submissive behaviour is the most adequate social behaviour in an asymmetric situation to achieve being tolerated near the shelter, because an aggressive takeover of the shelter is nearly impossible [9,16]; we included ‘total aggression of opponent’ as covariate because of the above-mentioned mutual dependence between submission and aggression. None of the behaviours expressed in the two social roles were influenced by cortisol levels (i.e. basal or acute stress levels or stress responsiveness; data not shown).
(iv). Gene expression
In order to test the effect of treatment on the expression of the candidate genes (crf, gr1 and mr), we fitted LMs with the factors ‘treatment’ and ‘size’ (SL in cm). Size was included as a covariate only if it significantly predicted gene expression, which was only true for mr. ‘Plate number’, that is, the identification number of each plate used for quantification of each gene's transcript copy number, was included in the models because the enzyme mixture used for each plate was prepared separately and could potentially affect the measurement of gene expression.
3. Results
(a). Experience phase
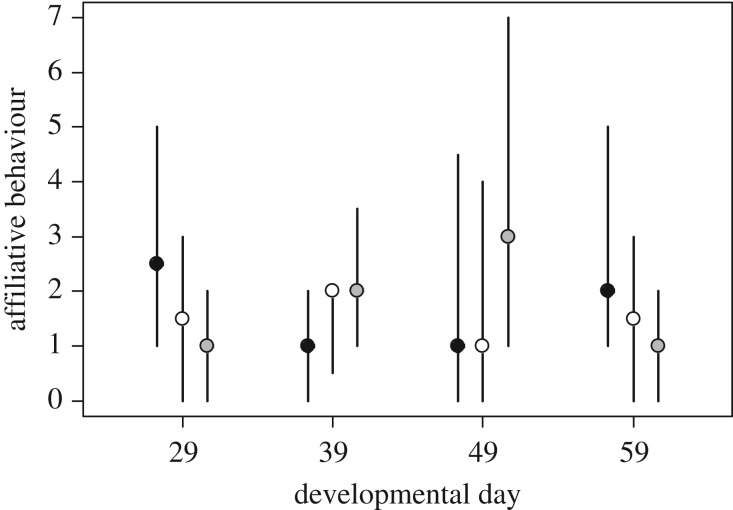
Treatment and age (i.e. experimental day) interactively influenced affiliative behaviour performed in the home tank (GLMM, n = 121, χ2 = 16.22, p = 0.0003). This effect is mainly owing to an interaction between cortisol treatment and age (cortisol × day: estimate = 0.0429 ± 0.0134 s.e., z = 3.19, p = 0.0014; mifepristone × day: estimate = 0.00611 ± 0.0141 s.e., z = −0.44, p = 0.66; electronic supplementary material, table S2; figure 2). This interaction is unlikely to be explained by locomotory activity of the fish, because activity was not affected by treatment (electronic supplementary material, table S2) or its interaction with age (non-significant interaction term was dropped from the model). No other social behaviour was affected by treatment or its interaction with age during the experience phase (results not shown).
Figure 2.
Frequency of spontaneous affiliative behaviour performed by juveniles towards their siblings (medians ± interquartile ranges are shown). Behaviours were recorded in the home tanks of rearing groups (3 times 5 min per group, see §2) during the experience phase 9 days after the application of each treatment starting on developmental day 29 until day 59. Treatments: control (black), cortisol (white) and mifepristone (grey).
(b). Social challenge
(i). Contest outcome
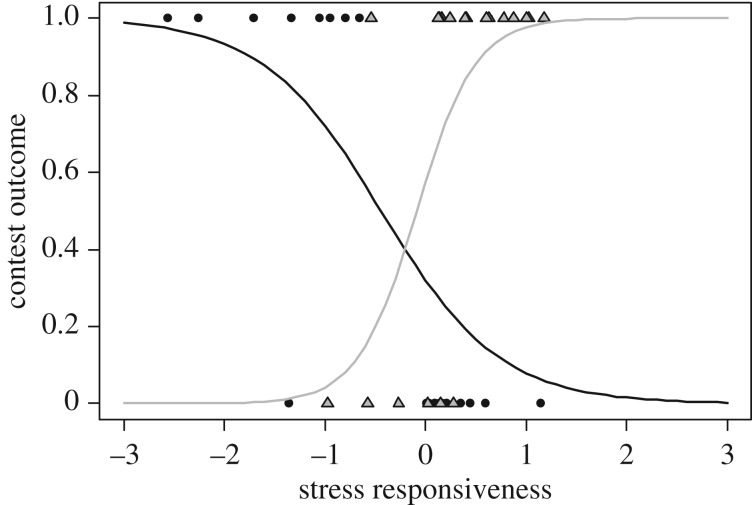
Contest outcome (winning or losing) was interactively influenced by treatment and cortisol stress responsiveness (binomial GLMM, n = 44, treatment × stress responsiveness: χ2 = 13.71, p = 0.0012). This interaction was mostly caused by an interaction between mifepristone and stress responsiveness (cortisol × stress responsiveness: estimate = 1.729 ± 1.321, z = 1.31, p = 0.19; mifepristone × stress responsiveness: estimate = 5.679 ± 2.262, z = 2.51, p = 0.012; electronic supplementary material, table S3). The likelihood of winning a contest increased with stress responsiveness in the mifepristone treatment, whereas in the control treatment, fish with a lower stress responsiveness were more likely to win (figure 3).
Figure 3.
Interactive effect of mifepristone treatment and stress responsiveness on the likelihood of winning (outcome = 1) or losing (outcome = 0) a contest. Mifepristone treatment (n = 20, grey line and triangles) and control treatment (n = 22, black line and circles).
(ii). Social behaviour during contest
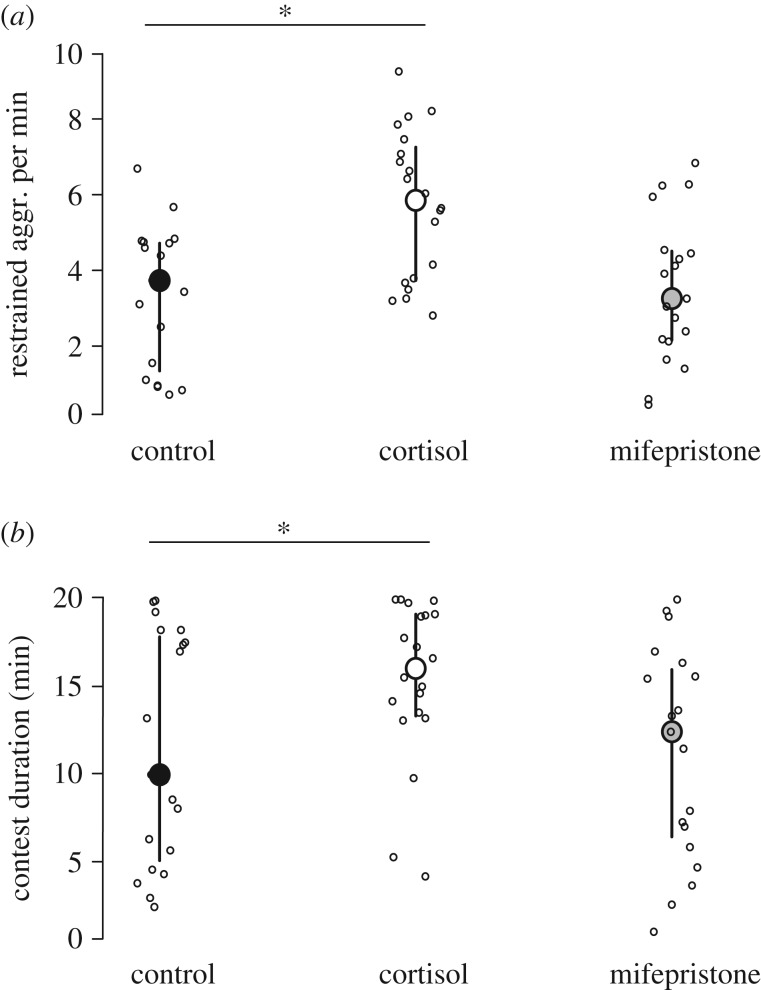
Treatment significantly affected the amount of restrained aggression shown during a contest by focal owner fish (LMM, n = 58, χ2 = 13.49, p = 0.0012) as well as contest duration (LMM, n = 58, χ2 = 8.49, p = 0.014). Focal owner fish, which received the cortisol treatment in early life, showed more restrained aggression during contests (estimate = 1.441 ± 0.418, z-value = 8.12, p = 0.0019; figure 4a; electronic supplementary material, table S4) and contests lasted longer in these fish (estimate = 3.867 ± 1.828, t-value = 2.12, p = 0.039; figure 4b; electronic supplementary material, table S4). By contrast, behaviour and contest duration were unaffected in owner fish that had received the mifepristone treatment (electronic supplementary material, table S4). Both treatments had no effect on intruder behaviour (results not shown).
Figure 4.
Social behaviours in asymmetric contest over a shelter. (a) The rate of restrained aggression per minute performed by owners. (b) Total duration (min) of contests when owners were the focal individuals. In both panels, control n = 19 (black), cortisol n = 20 (white) and mifepristone n = 19 (grey). Medians and ±interquartile range are shown. *, p < 0.05.
(c). Gene expression
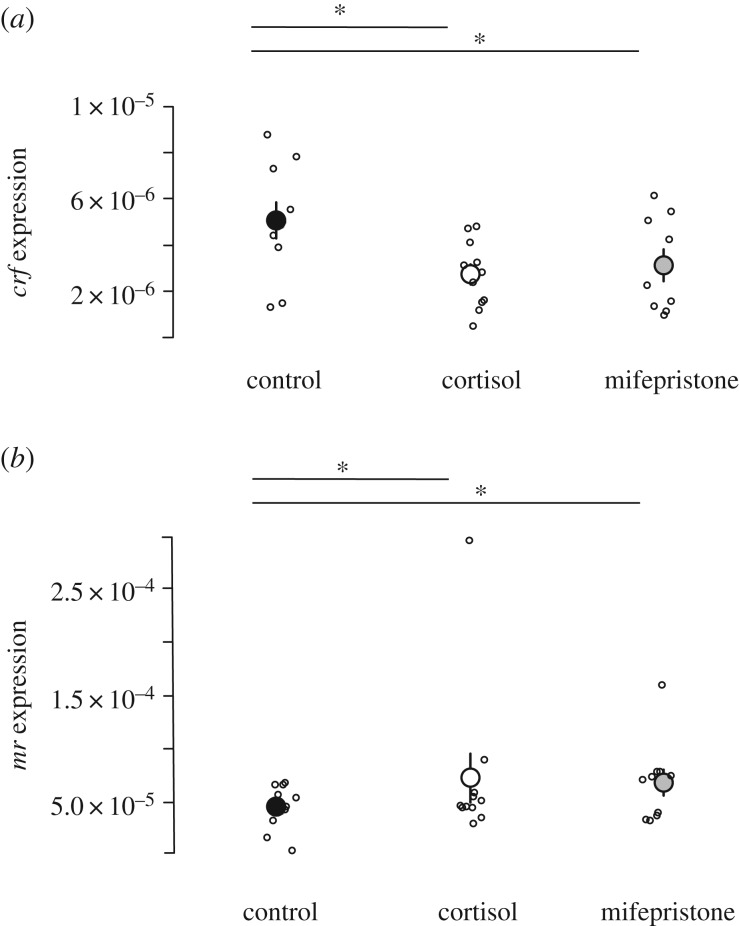
In the telencephalon, the expression of both crf and mr was affected by treatment (crf: LMM, n = 31, χ2 = −780.11, p = 0.012; mr: LMM, n = 21, χ2 = −240.28, p = 0.027). Cortisol- (estimate = −2.175 × 10−6 ± 8.480 × 10−7, t-value = −2.57, p = 0.017; electronic supplementary material, table S5) and mifepristone- (estimate = −2.237 × 10−6 ± 9.005 × 10−7, t-value = −2.48, p = 0.02, figure 5a) treated fish had a downregulated crf expression. By contrast, mr was upregulated by both treatments (cortisol treatment: estimate = 0.018 ± 0.009, t-value = 2.06, p = 0.04973; electronic supplementary material, table S5; mifepristone treatment: estimate = 0.021 ± 0.009, t-value = 2.33, p = 0.028; electronic supplementary material, table S5; figure 5b). Body size was negatively correlated with mr expression (Spearman's rank correlation, ρ = −0.36, p = 0.046) but not with the other two genes in the telencephalon. In the hypothalamus, early-life treatment did not significantly affect the expression of crf and mr (electronic supplementary material, table S5). The expression of gr1 was not affected by either treatment (results not shown).
Figure 5.
Expression of candidate genes in the telencephalon relative to the reference gene 18s. (a) Expression of crf gene. (b) Expression of mr gene. In both panels, control n = 10 (black), cortisol n = 11 (white) and tone n = 10 (grey). Means ± s.e. are shown. *, p < 0.05.
4. Discussion
Our experiment showed that social behaviour and social performance, here winning or losing a contest over an important resource, is causally evoked by early-life stress axis programming in the cooperatively breeding cichlid N. pulcher. Our pharmacological manipulations persistently altered two key players in the regulation of the vertebrate stress axis, (i) the MR and (ii) the CRF. The behavioural effects of the manipulations became apparent only over the long-term and only in fish assigned to the role of territory owners. Interestingly, social behaviour was little affected by treatment during the phase of drug application. Early cortisol application had several long-term effects. Fish showed more aggressive behaviour and contests took longer to be resolved, crf gene expression was downregulated and mr gene expression was upregulated in the telencephalon compared to control individuals. Interestingly, mifepristone application affected gene expression in the same way as the cortisol treatment. Moreover, it influenced the likelihood of winning a contest in interaction with stress responsiveness.
(a). Effects of early-life treatments on behaviour
Early-life treatment and developmental day interactively influenced affiliative behaviour during the experience phase. The interactive effect during the first weeks of life is difficult to interpret, as figure 2 suggests that the changes of affiliative behaviour occur non-linearly with age in the different treatments. Affiliative behaviours and glucocorticoid levels have been shown to be related in other vertebrates [84]. For example, in bonobos (Pan paniscus), aggressive conflicts increase HPA axis activity and promote affiliative behaviours between victims of the conflict and conspecifics [84]. Furthermore, in rhesus macaques (Macaca mulatta), playful behaviours in young males correlate with lower cortisol levels [85]. The interactive effect on affiliative behaviour was the only effect of treatment on behaviour during the experience phase, whereas all other behavioural effects became apparent only during the late juvenile period.
For the social test performed during later life, we predicted aggression to increase in cortisol-treated fish. For instance, juvenile rainbow trout given a cortisol treatment during the egg stage increased their frequency of bites and lateral displays performed towards their mirror image [30]. Accordingly, we found that cortisol-treated fish showed higher rates of restrained aggression. If this type of low-level aggressive behaviour, which does not include physical contact with opponents, tends to resolve contests faster, it can be viewed as being the most appropriate behaviour in a contest situation [9]. However, if high rates of aggression go along with longer contest durations, as was the case in our cortisol-treated fish, it suggests that cortisol evoked a decrease in social competence and consequently, decreased social performance. Longer contests should increase energy expenditure, because agonistic interactions and submissive behaviours are energetically demanding [86], and being involved in distracting, aggressive contests reduces vigilance and thereby the chance to detect dangerous predators [87]. Evidence from zebrafish and laboratory rodents suggests that our cortisol treatment had a very similar effect to that of natural stressors. After dyadic interactions of dominant and subordinate zebrafish, endogenous cortisol levels increased to levels sufficiently high to elicit changes in mr mRNA levels [88]. Furthermore, in young rodents, early exposure (between postnatal day 28 and 42) to non-social stressors or to social deprivation increased aggressiveness in adulthood [89].
Contrary to our predictions, mifepristone had no detectable influence on social behaviour during the experimental contest. Our predictions were based on findings of immediate effects of mifepristone treatment in adult N. pulcher, which have been shown to gain access to a resource by increasing their submissive behaviour when being attacked [36]. The lack of direct effects of mifepristone on behaviour in our study clearly indicates that long-term effects of early-life administration and immediate effects are not directly comparable. As discussed above, this is probably owing to the possibility of compensating for early-life impacts on the stress axis by re-programming of this axis. Interestingly, we found a striking interactive effect of mifepristone and stress responsiveness on contest outcome: control fish had an enhanced likelihood of winning a contest if they had a reduced stress response. Nyman et al. [36] argued that the likelihood of winning a contest is enhanced by low stress responsiveness, which may explain these results. However, the opposite effect occurred in fish treated with mifepristone in early life. Although this reversal of the relationship between stress responsiveness and winning is difficult to explain, we propose that it was caused by the differential programming of the stress axis of mifepristone-treated fish, which likely qualitatively altered the link between stress axis regulation and behavioural regulation.
Contrary to our findings for owners, we found that early-life treatment did not influence the contest duration or social behaviours for intruders. This finding contrasts previous work in N. pulcher, which showed a significant influence of early-life social experience on stress gene expression [17,36] and on the behaviour of both owners and intruders in the same asymmetric competition paradigm [9,16]. In these studies, N. pulcher reared with parents and other older group members (helpers) (i) had a higher expression of the gr1 gene in the telencephalon, (ii) when in the role of intruders, showed more submissive behaviour per received opponent aggression, and (iii) were less neophobic [90] compared to juveniles reared in a socially deprived setting with the same-aged siblings only. Thus, these previous findings also involved a re-programming of the stress axis, but with significant differences from the current study. Here, different genes, behaviours and social roles were affected, which suggests that different components of the stress axis were affected by social factors [16,17] and direct application of cortisol and mifepristone. This is perhaps not so surprising, given that during a social challenge, social cues and experiences accompany the stress response. In the case of cortisol administration, salient cues that can be learned and memorized are absent, and these learning processes are one important function of adaptive stress responses [89].
(b). Effects of early-life treatments on stress axis programming
Early exposure to cortisol and to mifepristone induced persistent changes in the expression of two main stress genes in the telencephalon, suggesting that glucocorticoid signalling in this brain area during later life will differ compared to control treatment fish [89]. The telencephalon is central for behavioural expression as it is involved in social decision-making [91,92] and cognitive processing of information [93]. In the following paragraphs, we discuss the long-term effects of cortisol and mifepristone, which suggest that both drugs may have temporarily increased fluctuating cortisol levels after the applications during early life. Therefore, both drugs may have induced similar changes in the telencephalon.
In line with our hypothesis, repeated exposure to cortisol during early development caused a persistently lower constitutive gene expression of crf in the telencephalon. In contrast with mammals, in which cortisol regulates CRF release in the hypothalamus [38,39,94], in fish, the crf gene is also expressed in other brain areas, including the telencephalon. In adult goldfish (C. auratus), cortisol implants downregulate crf mRNA levels in the telencephalon [25]. In the cichlid Oreochromis mossambicus, the principal source of plasma CRF is the ventral telencephalon [95], and these authors suggested that the self-inhibiting negative feedback of cortisol by blocking the secretion of further CRF acts predominantly in this brain region. CRF is related to stress-coping style, which is defined as the physiological and behavioural response to stress [96]. A proactive coping style is characterized by low stress axis activity, whereas the opposite is true for reactive individuals [96,97]. Accordingly, we hypothesize that repeated exposure to cortisol early in life generated fish with an attenuated cortisol response that produce lower levels of CRF under stressful conditions. Possibly, these effects on the cortisol response were too subtle to be detected by our rather coarse, non-invasive measurement of waterborne cortisol [68].
The MR gene (mr) was upregulated in cortisol-treated fish. Nuclear MR determines the sensitivity of the limbic stress response system [27]. MR signalling is important for coordinating the initial stress response [21,98] and preparing animals for coping with a stressor (e.g. lower sensory detection thresholds, higher alertness) [99], but MRs are also thought to be involved in the negative feedback on cortisol [100]. In Japanese quail, corticosteroid exposure before or after hatching results in mr upregulation in the hippocampus [40], which is part of the telencephalon. In line with our predictions, cortisol treatment upregulated mr gene expression in experimental fish. We speculate that a higher sensitivity to cortisol in the presence of more MR may help these fish to mount a stress response at a normal speed (i.e. a rapid response to a stressor, despite their lowered CRF response; discussed above), which is then followed by an attenuated response with lower peak values of cortisol owing to lower crf expression. The role of MR for stress responses has been rather neglected, as opposed to GR/GR1. A first step into understanding the relative importance of the two receptors would be to study the spatial distribution of the expression of genes coding for MR and GR across nodes of the social decision-making network, a network thought to be responsible for the control of social behaviour [92].
The fact that a similar change of crf expression was caused by mifepristone treatment in the telencephalon is in accordance with our hypothesis that the mifepristone applications generated increases in endogenous cortisol [46]. Furthermore, early mifepristone application induced a higher constitutive expression of mr in the telencephalon. Although this finding is opposite to our initial predictions, we speculate that the early mifepristone treatment generated a compensatory effect in the stress axis similar to the one by cortisol. Mifepristone blocks GRs, which are involved in the clearance of cortisol and blocking of further cortisol production. Correspondingly, juvenile N. pulcher exposed to mifepristone can be assumed to have experienced temporarily enhanced cortisol concentrations. In fish, temporary cortisol increases after application of mifepristone can last for 2 days in larval summer flounders, Paralichthys dentatus [61] and adult gulf toadfish, Opsanus beta [46], or 3 days in adult goldfish, C. auratus [25]. In our mifepristone-treated N. pulcher, persistent programming of higher mr expression may have developed as a compensatory mechanism to mediate the effects of high plasma cortisol concentrations, as previously shown in mice [101].
Socially challenging interactions are known to induce elevations of fluctuating glucocorticoid levels in several vertebrates, including fish [88,96] and mammals [102]. In cooperatively breeding species or other species with hierarchical social organization, individuals are exposed to frequent social interactions including repeated challenges of their social rank. For instance, subordinate members of cooperative breeders queuing for a breeder position, both in highly social vertebrate and invertebrate species, are recurrently challenged in their queue position by lower-ranking individuals [103]. Moreover, subordinate group members have to mediate conflicts with dominants over group membership [56,104] by appeasement behaviours in the form of subordination and helping [54,58,105]. Social challenges will induce frequent stress responses and thus elevations of glucocorticoid levels in the involved social partners [88]. If individuals experience frequent social stressors early in life, a re-programming of the stress axis involving altered mr and crf expression might be a mechanism allowing animals to mount normal cortisol responses even in socially stressful situations later in life. However, the potential benefits of programming depend strongly on the later life social environment. Moreover, programming of the stress axis by early cortisol surges does not come without cost, as it can negatively affect emotionality and behavioural performance in the long run, as shown in laboratory rats [19,106]. Such negative behavioural impact of glucocorticoids might explain why N. pulcher had impaired social competence and social performance following early-life cortisol exposure. Finally, further research would have to confirm whether multiple social stressors during early life have comparable effects to multiple pharmacologically induced cortisol surges.
Over recent years, evidence has been rapidly accumulating that the stress axis of vertebrates can be re-programmed early in life by social [11,16,91] and ecological [92,93] stressors in mammals, birds and fish. Understanding the physiological and behavioural mechanisms of stress coping should greatly increase our understanding of individual life-history trajectories and behavioural strategies, both in natural and perturbed environments. Vertebrates, including humans, are increasingly exposed to environmental stressors, be it because of competition for increasingly limited resources or human-made problems such as pollution. Here, we uncover a potentially general mechanism that may be involved in stress axis re-programming and stress coping, which regulates stress by increasing the sensitivity of the limbic stress response and decreasing stress axis reactivity. Further research is necessary to reveal which types of environmental social and ecological stressors will elicit this programming mechanism in vertebrates, and whether there are certain sensitive periods when it can happen [107].
5. Conclusion
The altered programming of the stress axis induced by our pharmacological treatments during early development suggests the generation of a mechanism to cope with stressors. The downregulation of crf and the upregulation of mr in the telencephalon both by early-life mifepristone and cortisol have two potential implications. First, the increase in MR expression could increase the sensitivity of the limbic stress response and result in a faster initial response to stress that is mediated by MR [65]. Second, lower levels of crf expression could lower HPI axis activity following the encounter of a stressor [96], which means that it may need stronger stress stimuli to mount a stress response compared to control fish. This suggests that individuals have developed a physiological mechanism that allows them to better cope with stressors. This may help them to avoid physiological damage caused by increases in allostatic load [108], which is the amount of energy required to maintain homeostasis [108]. However, the stress axis programming by cortisol and mifepristone comes with some costs, as these fish exhibit reduced social competence. Whether or not this stress axis programming results in net fitness benefits depends on the frequency and strength of stressors present in the environment and whether the costs are smaller than the benefits. Our results suggest that early-life exposure to stimuli known to trigger elevations in cortisol levels such as social defeat [109] or social deprivation [89] may lead to programming of stress axis genes such that this protective mechanism is implemented.
Supplementary Material
Acknowledgements
The authors thank Albert F.H. Ros and Corinna von Kuerthy for helpful comments on experimental design, Danielle Bonfils, Eva Zwygart, Laurence Lachat, Beat Suter, Paula Vazquez Pianzola and Gerald Heckel for technical laboratory support, Leif Engqvist, Mattia Maldonado and the late Hirokazu Tanaka for providing statistical advice.
Data accessibility
This article has no additional data.
Authors' contributions
M.R.-C. and B.T. designed the study, M.R.-C. collected behavioural data and biological samples, M.R.-C. and G.G. did the hormone analyses, M.R.-C. and D.J.R. did the gene expression analyses, M.R.-C., B.T. and D.J.R. did the statistical analyses, M.R.-C. and B.T. wrote the manuscript, all authors edited and approved the manuscript.
Competing interests
The authors do not declare any competing interests.
Funding
B.T. acknowledges financial support by the Swiss National Science Foundation (SNSF; grant 31003A_156881).
References
- 1.Groothuis TGG, Taborsky B. 2015. Introducing biological realism into the study of developmental plasticity in behaviour. Front. Zool. 12, S6 ( 10.1186/1742-9994-12-S1-S6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taborsky B. 2016. Opening the black box of developmental experiments: behavioural mechanisms underlying long-term effects of early social experience. Ethology 122, 267–283. ( 10.1111/eth.12473) [DOI] [Google Scholar]
- 3.Oliveira RF. 2009. Social behavior in context: hormonal modulation of behavioral plasticity and social competence. Integr. Comp. Biol. 49, 423–440. ( 10.1093/icb/icp055) [DOI] [PubMed] [Google Scholar]
- 4.Taborsky B, Oliveira RF. 2012. Social competence: an evolutionary approach. Trends Ecol. Evol. 27, 679–688. ( 10.1016/j.tree.2012.09.003) [DOI] [PubMed] [Google Scholar]
- 5.Branchi I, D'Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E. 2006. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol. Psychiatry 60, 690–696. ( 10.1016/j.biopsych.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 6.Branchi I, Curley JP, D'Andrea I, Cirulli F, Champagne FA, Alleva E. 2013. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology 38, 522–532. ( 10.1016/j.psyneuen.2012.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adkins-Regan E, Krakauer A. 2000. Removal of adult males from the rearing environment increases preference for same-sex partners in the zebra finch. Anim. Behav. 60, 47–53. ( 10.1006/anbe.2000.1448) [DOI] [PubMed] [Google Scholar]
- 8.Fischer S, Bessert-Nettelbeck M, Kotrschal A, Taborsky B. 2015. Rearing-group size determines social competence and brain structure in a cooperatively breeding cichlid. Am. Nat. 186, 123–140. ( 10.1086/681636) [DOI] [PubMed] [Google Scholar]
- 9.Arnold C, Taborsky B. 2010. Social experience in early ontogeny has lasting effects on social skills in cooperatively breeding cichlids. Anim. Behav. 79, 621–630. ( 10.1016/j.anbehav.2009.12.008) [DOI] [Google Scholar]
- 10.Taborsky B, Arnold C, Junker J, Tschopp A. 2012. The early social environment affects social competence in a cooperative breeder. Anim. Behav. 83, 1067–1074. ( 10.1016/j.anbehav.2012.01.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee SB, Arterbery AS, Fergus DJ, Adkins-Regan E. 2012. Deprivation of maternal care has long-lasting consequences for the hypothalamic–pituitary–adrenal axis of zebra finches. Proc. R. Soc. B 279, 759–766. ( 10.1098/rspb.2011.1265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curley JP, Jensen CL, Mashoodh R, Champagne FA. 2011. Social influences on neurobiology and behavior: epigenetic effects during development. Psychoneuroendocrinology 36, 352–371. ( 10.1016/j.psyneuen.2010.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maccari S, Krugers HJ, Szyf M, Brunton PJ. 2014. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J. Neuroendocrinol. 26, 707–723. ( 10.1111/jne.12175) [DOI] [PubMed] [Google Scholar]
- 14.Zimmer C, Spencer KA. 2014. Modifications of glucocorticoid receptors mRNA expression in the hypothalamic-pituitary-adrenal axis in response to early-life stress in female Japanese quail. J. Neuroendocrinol. 26, 853–860. ( 10.1111/JNE.12228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsalafouta A, Papandroulakis N, Gorissen M, Katharios P, Flik G, Pavlidis M. 2014. Ontogenesis of the HPI axis and molecular regulation of the cortisol stress response during early development in Dicentrarchus labrax. Sci. Rep. 4, 5525 ( 10.1038/srep05525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyman C, Fischer S, Aubin-Horth N, Taborsky B. 2017. Effect of the early social environment on behavioural and genomic responses to a social challenge in a cooperatively breeding vertebrate. Mol. Ecol. 26, 3186–3203. ( 10.1111/mec.14113) [DOI] [PubMed] [Google Scholar]
- 17.Taborsky B, Tschirren L, Meunier C, Aubin-Horth N. 2013. Stable reprogramming of brain transcription profiles by the early social environment in a cooperatively breeding fish. Proc. R. Soc. B. 280, 20122605 ( 10.1098/rspb.2012.2605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Regan D, Welberg LLAM, Holmes MC, Seckl JR. 2001. Glucocorticoid programming of pituitary–adrenal function: mechanisms and physiological consequences. Semin. Neonatol. 6, 319–329. ( 10.1053/siny.2001.0067) [DOI] [PubMed] [Google Scholar]
- 19.Welberg LAM, Seckl JR, Holmes MC. 2001. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience 104, 71–79. ( 10.1016/S0306-4522(01)00065-3) [DOI] [PubMed] [Google Scholar]
- 20.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Preparative actions. Endocr. Rev. 21, 55–89. ( 10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 21.Kalafatakis K, Russell GM, Zarros A, Lightman SL. 2016. Temporal control of glucocorticoid neurodynamics and its relevance for brain homeostasis, neuropathology and glucocorticoid-based therapeutics. Neurosci. Biobehav. Rev. 61, 12–25. ( 10.1016/j.neubiorev.2015.11.009) [DOI] [PubMed] [Google Scholar]
- 22.Greenwood AK, Butler PC, White RB, Demarco U, Pearce D, Fernald RD. 2003. Multiple corticosteroid receptors in a teleost fish: distinct sequences, expression patterns, and transcriptional activities. Endocrinology 144, 4226–4236. ( 10.1210/en.2003-0566) [DOI] [PubMed] [Google Scholar]
- 23.Joëls M. 2006. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol. Sci. 27, 244–250. ( 10.1016/j.tips.2006.03.007) [DOI] [PubMed] [Google Scholar]
- 24.Stolte EH, Nabuurs SB, Bury NR, Sturm A, Flik G, Savelkoul HFJ, Lidy Verburg-van Kemenade BM. 2008. Stress and innate immunity in carp: corticosteroid receptors and pro-inflammatory cytokines. Mol. Immunol. 46, 70–79. ( 10.1016/j.molimm.2008.07.022) [DOI] [PubMed] [Google Scholar]
- 25.Bernier NJ, Lin X, Peter RE. 1999. Differential expression of corticotropin-releasing factor (CRF) and urotensin I precursor genes, and evidence of CRF gene expression regulated by cortisol in goldfish brain. Gen. Comp. Endocrinol. 116, 461–477. ( 10.1006/gcen.1999.7386) [DOI] [PubMed] [Google Scholar]
- 26.Prunet P, Sturm A, Milla S. 2006. Multiple corticosteroid receptors in fish: from old ideas to new concepts. Gen. Comp. Endocrinol. 147, 17–23. ( 10.1016/j.ygcen.2006.01.015) [DOI] [PubMed] [Google Scholar]
- 27.Joëls M, Karst H, DeRijk R, de Kloet ER. 2008. The coming out of the brain mineralocorticoid receptor. Trends Neurosci. 31, 1–7. ( 10.1016/j.tins.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 28.Emmerson MG, Spencer KA. 2017. Long-term effects of adolescent stress on neophobic behaviors in zebra finches are modulated by social context when in adulthood. Horm. Behav. 90, 48–55. ( 10.1016/j.yhbeh.2017.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer KA, Verhulst S. 2007. Delayed behavioral effects of postnatal exposure to corticosterone in the zebra finch (Taeniopygia guttata). Horm. Behav. 51, 273–280. ( 10.1016/j.yhbeh.2006.11.001) [DOI] [PubMed] [Google Scholar]
- 30.Sloman KA. 2010. Exposure of ova to cortisol pre-fertilisation affects subsequent behaviour and physiology of brown trout. Horm. Behav. 58, 433–439. ( 10.1016/j.yhbeh.2010.05.010) [DOI] [PubMed] [Google Scholar]
- 31.Sopinka NM, Hinch SG, Healy SJ, Harrison PM, Patterson DA. 2015. Egg cortisol treatment affects the behavioural response of coho salmon to a conspecific intruder and threat of predation. Anim. Behav. 104, 115–122. ( 10.1016/j.anbehav.2015.03.011) [DOI] [Google Scholar]
- 32.Pakkala JJ, Norris DR, Sedinger JS, Newman AEM. 2016. Experimental effects of early-life corticosterone on the hypothalamic–pituitary–adrenal axis and pre-migratory behaviour in a wild songbird. Funct. Ecol. 30, 1149–1160. ( 10.1111/1365-2435.12603) [DOI] [Google Scholar]
- 33.Grace JK, Martin-Gousset L, Angelier F. 2017. Delayed effect of early-life corticosterone treatment on adult anti-predator behavior in a common passerine. Physiol. Behav. 177, 82–90. ( 10.1016/j.physbeh.2017.04.018) [DOI] [PubMed] [Google Scholar]
- 34.Zimmer C, Larriva M, Boogert NJ, Spencer KA, Spencer KA, Garcia L, MacDougall-Shackleton SA. 2017. Transgenerational transmission of a stress-coping phenotype programmed by early-life stress in the Japanese quail. Sci. Rep. 7, 46125 ( 10.1038/srep46125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ros AFH, Vullioud P, Bshary R. 2012. Treatment with the glucocorticoid antagonist RU486 reduces cooperative cleaning visits of a common reef fish, the lined bristletooth. Horm. Behav. 61, 37–43. ( 10.1016/J.YHBEH.2011.09.013) [DOI] [PubMed] [Google Scholar]
- 36.Nyman C, Fischer S, Aubin-Horth N, Taborsky B. 2018. Evolutionary conserved neural signature of early life stress affects animal social competence. Proc. R. Soc. B 285, 20172344 ( 10.1098/rspb.2017.2344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schjolden J, Basic D, Winberg S. 2009. Aggression in rainbow trout is inhibited by both MR and GR antagonists. Physiol. Behav. 98, 625–630. ( 10.1016/j.physbeh.2009.09.018) [DOI] [PubMed] [Google Scholar]
- 38.Meaney MJ, Szyf M. 2005. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 28, 456–463. ( 10.1016/J.TINS.2005.07.006) [DOI] [PubMed] [Google Scholar]
- 39.Ziv L, Muto A, Schoonheim P, Meijsing S, Strasser D, Ingraham H, Schaaf M, Yamamoto K, Baier H. 2012. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol. Psychiatry 18, 681–691. ( 10.1038/mp.2012.64) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marasco V, Herzyk P, Robinson J, Spencer KA. 2016. Pre- and post-natal stress programming: developmental exposure to glucocorticoids causes long-term brain-region specific changes to transcriptome in the precocial Japanese quail. J. Neuroendocrinol. 28, article no. 5525. ( 10.1111/jne.12387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alderman SL, McGuire A, Bernier NJ, Vijayan MM. 2012. Central and peripheral glucocorticoid receptors are involved in the plasma cortisol response to an acute stressor in rainbow trout. Gen. Comp. Endocrinol. 176, 79–85. ( 10.1016/j.ygcen.2011.12.031) [DOI] [PubMed] [Google Scholar]
- 42.Bernier NJ, Flik G, Klaren PHM. 2009. Regulation and contribution of the corticotropic, melanotropic and thyrotropic axes to the stress response in fishes. In Fish neuroendocrinology (eds NJ Bernier, G Van Der Kraak, AP Farrell, CJ Brauner), pp. 235–311. Academic Press. 1st edn New York, NY: Academic. [Google Scholar]
- 43.Blüthgen N, Sumpter JP, Odermatt A, Fent K. 2013. Effects of low concentrations of the antiprogestin mifepristone (RU486) in adults and embryos of zebrafish (Danio rerio): 2. Gene expression analysis and in vitro activity. Aquat. Toxicol. 144–145, 96–104. ( 10.1016/j.aquatox.2013.09.030) [DOI] [PubMed] [Google Scholar]
- 44.Barton BA. 2002. Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 42, 517–525. ( 10.1093/icb/42.3.517) [DOI] [PubMed] [Google Scholar]
- 45.Archard GA, Earley RL, Hanninen AF, Braithwaite VA. 2012. Correlated behaviour and stress physiology in fish exposed to different levels of predation pressure. Funct. Ecol. 26, 637–645. ( 10.1111/j.1365-2435.2012.01968.x) [DOI] [Google Scholar]
- 46.Medeiros LR, McDonald MD. 2013. Cortisol-mediated downregulation of the serotonin 1A receptor subtype in the Gulf toadfish, Opsanus beta. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 164, 612–621. ( 10.1016/j.cbpa.2013.01.014) [DOI] [PubMed] [Google Scholar]
- 47.Zalachoras I, et al. 2013. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proc. Natl Acad. Sci. USA 110, 7910–7915. ( 10.1073/pnas.1219411110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marasco V, Robinson J, Herzyk P, Spencer KA. 2012. Pre- and post-natal stress in context: effects on the stress physiology in a precocial bird. J. Exp. Biol. 215, 3955–3964. ( 10.1242/jeb.071423) [DOI] [PubMed] [Google Scholar]
- 49.Stiver KA, Dierkes P, Taborsky M, Balshine S. 2004. Dispersal patterns and status change in a co-operatively breeding cichlid Neolamprologus pulcher: evidence from microsatellite analyses and behavioural observations. J. Fish Biol. 65, 91–105. ( 10.1111/j.0022-1112.2004.00427.x) [DOI] [Google Scholar]
- 50.Taborsky M. 1984. Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim. Behav. 32, 1236–1252. ( 10.1016/S0003-3472(84)80241-9) [DOI] [Google Scholar]
- 51.Taborsky M. 1985. Breeder-helper conflict in a cichlid fish with broodcare helpers: an experimental analysis. Behaviour 95, 45–75. ( 10.1163/156853985X00046) [DOI] [Google Scholar]
- 52.Heg D, Bachar Z, Brouwer L, Taborsky M. 2004. Predation risk is an ecological constraint for helper dispersal in a cooperatively breeding cichlid. Proc. R. Soc. Lond. B 271, 2367–2374. ( 10.1098/rspb.2004.2855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergmüller R, Heg D, Taborsky M. 2005. Helpers in a cooperatively breeding cichlid stay and pay or disperse and breed, depending on ecological constraints. Proc. R. Soc. B 272, 325–331. ( 10.1098/rspb.2004.2960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zöttl M, Heg D, Chervet N, Taborsky M. 2013. Kinship reduces alloparental care in cooperative cichlids where helpers pay-to-stay. Nat. Commun. 4, 1341 ( 10.1038/ncomms2344) [DOI] [PubMed] [Google Scholar]
- 55.Shackelford TK, Weekes-Shackelford V. 2017. Encyclopedia of evolutionary psychological science. Berlin, Germany: Springer Verlag. [Google Scholar]
- 56.Fischer S, Zöttl M, Groenewoud F, Taborsky B. 2014. Group-size-dependent punishment of idle subordinates in a cooperative breeder where helpers pay to stay. Proc. R. Soc. B 281, 20140184 ( 10.1098/rspb.2014.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruintjes R, Taborsky M. 2011. Size-dependent task specialization in a cooperative cichlid in response to experimental variation of demand. Anim. Behav. 81, 387–394. ( 10.1016/J.ANBEHAV.2010.10.004) [DOI] [Google Scholar]
- 58.Fischer S, Bohn L, Oberhummer E, Nyman C, Taborsky B. 2017. Divergence of developmental trajectories is triggered interactively by early social and ecological experience in a cooperative breeder. Proc. Natl Acad. Sci. USA 114, E9300–E9307. ( 10.1073/pnas.1705934114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mileva VR, Fitzpatrick JL, Marsh-Rollo S, Gilmour KM, Wood CM, Balshine S. 2009. The stress response of the highly social African cichlid Neolamprologus pulcher. Physiol. Biochem. Zool. 82, 720–729. ( 10.1086/605937) [DOI] [PubMed] [Google Scholar]
- 60.Auperin B, Geslin M. 2008. Plasma cortisol response to stress in juvenile rainbow trout is influenced by their life history during early development and by egg cortisol content. Gen. Comp. Endocrinol. 158, 234–239. ( 10.1016/j.ygcen.2008.07.002) [DOI] [PubMed] [Google Scholar]
- 61.Veillette PA, Serrano X, Garcia MM, Specker JL. 2007. Evidence for the onset of feedback regulation of cortisol in larval summer flounder. Gen. Comp. Endocrinol. 154, 105–110. ( 10.1016/j.ygcen.2007.05.033) [DOI] [PubMed] [Google Scholar]
- 62.Hillegass JM, Villano CM, Cooper KR, White LA. 2007. Matrix metalloproteinase-13 is required for zebra fish (Danio rerio) development and is a target for glucocorticoids. Toxicol. Sci. 100, 168–179. ( 10.1093/toxsci/kfm192) [DOI] [PubMed] [Google Scholar]
- 63.Veillette PA, Merino M, Marcaccio ND, Garcia MM, Specker JL. 2007. Cortisol is necessary for seawater tolerance in larvae of a marine teleost the summer flounder. 151, 116–121. ( 10.1016/j.ygcen.2006.12.019) [DOI] [PubMed] [Google Scholar]
- 64.Kasper C, et al. 2017. Genetics and developmental biology of cooperation. Mol. Ecol. 26, 4364–4377. ( 10.1111/mec.14208) [DOI] [PubMed] [Google Scholar]
- 65.Kasper C, Colombo M, Aubin-Horth N, Taborsky B. 2018. Brain activation patterns following a cooperation opportunity in a highly social cichlid fish. Physiol. Behav. 195, 37–47. ( 10.1016/J.PHYSBEH.2018.07.025) [DOI] [PubMed] [Google Scholar]
- 66.Wong S, Dykstra M, Campbell J, Earley R. 2008. Measuring water-borne cortisol in convict cichlids (Amatitlania nigrofasciata): is the procedure a stressor? Behaviour 145, 1283–1305. ( 10.1163/156853908785765863) [DOI] [Google Scholar]
- 67.Bender N, Heg-Bachar Z, Oliveira RF, Canario AVM, Taborsky M. 2008. Hormonal control of brood care and social status in a cichlid fish with brood care helpers. Physiol. Behav. 94, 349–358. ( 10.1016/j.physbeh.2008.02.002) [DOI] [PubMed] [Google Scholar]
- 68.Scott AP, Ellis T. 2007. Measurement of fish steroids in water—a review. Gen. Comp. Endocrinol. 153, 392–400. ( 10.1016/j.ygcen.2006.11.006) [DOI] [PubMed] [Google Scholar]
- 69.Silva PIM, et al. 2010. Individual differences in cortisol levels and behaviour of Senegalese sole (Solea senegalensis) juveniles: evidence for coping styles. Appl. Anim. Behav. Sci. 124, 75–81. ( 10.1016/j.applanim.2010.01.008) [DOI] [Google Scholar]
- 70.Castanheira MF, Herrera M, Costas B, Conceição LEC, Martins CIM. 2013. Linking cortisol responsiveness and aggressive behaviour in gilthead seabream Sparus aurata: Indication of divergent coping styles. Appl. Anim. Behav. Sci. 143, 75–81. ( 10.1016/j.applanim.2012.11.008) [DOI] [Google Scholar]
- 71.Wong MYL, et al. 2012. Mating systems in cooperative breeders: the roles of resource dispersion and conflict mitigation. Behav. Ecol. 23, 521–530. ( 10.1093/beheco/arr218) [DOI] [Google Scholar]
- 72.Engqvist L. 2005. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim. Behav. 70, 967–971. ( 10.1016/J.ANBEHAV.2005.01.016) [DOI] [Google Scholar]
- 73.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 74.Crawley MJ. 2013. The R book, 2nd edn West Sussex, UK: Wiley, A John Wiley & Sons, Ltd. [Google Scholar]
- 75.Bommae K.2015. Understanding diagnostic plots for linear regression analysis. See https://data.library.virginia.edu/diagnostic-plots/
- 76.Komsta L. 2011. outliers: Tests for outliers. R package version 0.14. https://CRAN.Rproject.org/package=outliers.
- 77.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 78.Fox J, Weisberg S. 2011. An {R} companion to applied regression, 2nd edn Thousand Oaks, CA: Sage; See http://socserv.socsci.mcmaster.ca/jfox/books/companion. [Google Scholar]
- 79.Gross J, Ligges U. 2015. nortest: Tests for Normality. R package version 1.0-4. https://CRAN.R-project.org/package=nortest.
- 80.Venables WN, William N, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 81.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 82.Øverli Ø, Korzan WJ, Larson ET, Winberg S, Lepage O, Pottinger TG, Renner KJ, Summers CH. 2004. Behavioral and neuroendocrine correlates of displaced aggression in trout. Horm. Behav. 45, 324–329. ( 10.1016/j.yhbeh.2004.01.001) [DOI] [PubMed] [Google Scholar]
- 83.Bender N, Heg D, Hamilton IM, Bachar Z, Taborsky M, Oliveira RF. 2006. The relationship between social status, behaviour, growth and steroids in male helpers and breeders of a cooperatively breeding cichlid. Horm. Behav. 50, 173–182. ( 10.1016/j.yhbeh.2006.02.009) [DOI] [PubMed] [Google Scholar]
- 84.Raulo A, Dantzer B. 2018. Associations between glucocorticoids and sociality across a continuum of vertebrate social behavior. Ecol. Evol. 8, 7697–7716. ( 10.1002/ece3.4059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wooddell LJ, Hamel AF, Murphy AM, Byers KL, Kaburu SSK, Meyer JS, Suomi SJ, Dettmer AM. 2017. Relationships between affiliative social behavior and hair cortisol concentrations in semi-free ranging rhesus monkeys. Psychoneuroendocrinology 84, 109–115. ( 10.1016/J.PSYNEUEN.2017.06.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grantner A, Taborsky M. 1998. The metabolic rates associated with resting, and with the performance of agonistic, submissive and digging behaviours in the cichlid fish Neolamprologus pulcher (Pisces: Cichlidae). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 168, 427–433. ( 10.1007/s003600050162) [DOI] [Google Scholar]
- 87.Hess S, Fischer S, Taborsky B. 2016. Territorial aggression reduces vigilance but increases aggression towards predators in a cooperatively breeding fish. Anim. Behav. 113, 229–235. ( 10.1016/J.ANBEHAV.2016.01.008) [DOI] [Google Scholar]
- 88.Pavlidis M, Sundvik M, Chen Y-C, Panula P. 2011. Adaptive changes in zebrafish brain in dominant–subordinate behavioral context. Behav. Brain Res. 225, 529–537. ( 10.1016/J.BBR.2011.08.022) [DOI] [PubMed] [Google Scholar]
- 89.Sandi C, Haller J. 2015. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 16, 290–304. ( 10.1038/nrn3918) [DOI] [PubMed] [Google Scholar]
- 90.Bannier F, Tebbich S, Taborsky B. 2017. Early experience affects learning performance and neophobia in a cooperatively breeding cichlid. Ethology 123, 712–723. ( 10.1111/eth.12646) [DOI] [Google Scholar]
- 91.O'Connell LA, Hofmann HA. 2011. Genes, hormones, and circuits: an integrative approach to study the evolution of social behavior. Front. Neuroendocrinol. 32, 320–335. ( 10.1016/j.yfrne.2010.12.004) [DOI] [PubMed] [Google Scholar]
- 92.O'Connell LA, Hofmann HA. 2012. Evolution of a vertebrate social decision-making network. Science 336, 1154–1157. ( 10.1126/science.1218889) [DOI] [PubMed] [Google Scholar]
- 93.Vindas MA, Fokos S, Pavlidis M, Höglund E, Ebbesson LOE, Papandroulakis N, Dermon CR. 2018. Early life stress induces long-term changes in limbic areas of a teleost fish: the role of catecholamine systems in stress coping. Sci. Rep. 8, Article number 5638 ( 10.1038/s41598-018-23950-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. 1991. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J. Neurosci. 11, 585.–. ( 10.1523/JNEUROSCI.11-03-00585.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pepels PPLM, Van Helvoort H, Wendelaar Bonga SE, Balm PHM. 2004. Corticotropin-releasing hormone in the teleost stress response: rapid appearance of the peptide in plasma of tilapia (Oreochromis mossambicus). J. Endocrinol. 180, 425–438. ( 10.1677/JOE.0.1800425) [DOI] [PubMed] [Google Scholar]
- 96.Backström T, Winberg S. 2013. Central corticotropin releasing factor and social stress. Front. Neurosci. 7, 117 ( 10.3389/fnins.2013.00117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boulton K, Couto E, Grimmer AJ, Earley RL, Canario AVM, Wilson AJ, Walling CA. 2015. How integrated are behavioral and endocrine stress response traits? A repeated measures approach to testing the stress-coping style model. Ecol. Evol. 5, 618–633. ( 10.1002/ece3.1395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wommack JC, Delville Y. 2007. Cortisol controls the pubertal development of agonistic behavior in male golden hamsters via type II corticosteroid receptors. Horm. Behav. 51, 306–312. ( 10.1016/j.yhbeh.2006.11.007) [DOI] [PubMed] [Google Scholar]
- 99.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. 2005. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 29, 3–38. ( 10.1016/j.neubiorev.2004.08.009) [DOI] [PubMed] [Google Scholar]
- 100.Spencer KA. 2017. Developmental stress and social phenotypes: integrating neuroendocrine, behavioural and evolutionary perspectives. Phil. Trans. R. Soc. B 372, 20160242 ( 10.1098/rstb.2016.0242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Kloet ER. 2013. Lifetime achievement from a brain-adrenal perspective: on the CRF–urocortin–glucocorticoid balance. J. Chem. Neuroanat. 54, 42–49. ( 10.1016/j.jchemneu.2013.10.003) [DOI] [PubMed] [Google Scholar]
- 102.Woodruff JA, Lacey EA, Bentley GE, Kriegsfeld LJ. 2013. Effects of social environment on baseline glucocorticoid levels in a communally breeding rodent, the colonial tuco-tuco (Ctenomys sociabilis). Horm. Behav. 64, 566–572. ( 10.1016/J.YHBEH.2013.07.008) [DOI] [PubMed] [Google Scholar]
- 103.Field J, Cant MA. 2009. Social stability and helping in small animal societies. Phil. Trans. R. Soc. B 364, 3181–3189. ( 10.1098/rstb.2009.0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cant MA, Hodge SJ, Bell MBV, Gilchrist JS, Nichols HJ. 2010. Reproductive control via eviction (but not the threat of eviction) in banded mongooses. Proc. R. Soc. B 277, 2219–2226. ( 10.1098/rspb.2009.2097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bergmüller R, Taborsky M. 2005. Experimental manipulation of helping in a cooperative breeder: helpers ‘pay to stay’ by pre-emptive appeasement. Anim. Behav. 69, 19–28. ( 10.1016/j.anbehav.2004.05.009) [DOI] [Google Scholar]
- 106.Veenit V, Cordero MI, Tzanoulinou S, Sandi C. 2013. Increased corticosterone in peripubertal rats leads to long-lasting alterations in social exploration and aggression. Front. Behav. Neurosci. 7, 26 ( 10.3389/fnbeh.2013.00026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Panchanathan K, Frankenhuis WE. 2016. The evolution of sensitive periods in a model of incremental development. Proc. R. Soc. B 283, 20152439 ( 10.1098/rspb.2015.2439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goymann W, Wingfield JC. 2004. Allostatic load, social status and stress hormones: the costs of social status matter. Anim. Behav. 67, 591–602. ( 10.1016/j.anbehav.2003.08.007) [DOI] [Google Scholar]
- 109.Jeffrey JD, Gollock MJ, Gilmour KM. 2014. Social stress modulates the cortisol response to an acute stressor in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 196, 8–16. ( 10.1016/j.ygcen.2013.11.010) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.