Abstract
Maternal effects can adaptively modulate offspring developmental trajectories in variable but predictable environments. Hormone synthesis is sensitive to environmental factors, and maternal hormones are thus a powerful mechanism to transfer environmental cues to the next generation. Birds have become a key model for the study of hormone-mediated maternal effects because the embryo develops outside the mother's body, facilitating the measurement and manipulation of prenatal hormone exposure. At the same time, birds are excellent models for the integration of both proximate and ultimate approaches, which is key to a better understanding of the evolution of hormone-mediated maternal effects. Over the past two decades, a surge of studies on hormone-mediated maternal effects has revealed an increasing number of discrepancies. In this review, we discuss the role of the environment, genetic factors and social interactions in causing these discrepancies and provide a framework to resolve them. We also explore the largely neglected role of the embryo in modulating the maternal signal, as well as costs and benefits of hormone transfer and expression for the different family members. We conclude by highlighting fruitful avenues for future research that have opened up thanks to new theoretical insights and technical advances in the field.
This article is part of the theme issue ‘Developing differences: early-life effects and evolutionary medicine’.
Keywords: parental effects, phenotypic plasticity, context dependency, parent–offspring conflict, steroids, yolk hormones
1. Hormone-mediated maternal effects: bridging traditional discipline boundaries
The optimal phenotype depends on the environment an individual is going to encounter. Because phenotypic plasticity is not infinite and often associated with significant costs [1], and developing young have limited information about the environment, parental guidance of offspring developmental trajectories is assumed to be favoured by natural selection in variable but predictable environments [2,3]. Over the past 20 years parental effects have witnessed a steep increase in attention, in the context of understanding individual differences in human health and disease, the capacity of organisms to respond to global change, the limited explanatory power of genetic information alone and the surge of interest in epigenetics. In this review we focus on prenatal parental effects mediated by hormones of maternal origin. Hormones not only provide a well-described and measureable pathway for parental effects, especially in the sensitive early developmental period, but are also an ideal tool to translate environmental cues from the mother to the offspring as environmental conditions strongly affect maternal hormone production and transfer.
Although studied in various taxa, from insects to mammals, oviparous species have been a key model for research into hormone-mediated maternal effects because, unlike in viviparous species, the embryo develops outside of the mother's body. This facilitates the measurement and manipulation of embryonic hormone exposure without interfering with the mother, whereas such confounding interference is inevitable in viviparous taxa. Moreover, after egg laying, there is much less hormonal communication between mother and offspring possible than in placental animals, facilitating interpretation. However, as we will see below, recent findings suggest that both mothers and offspring may still influence the fate of maternal hormones transferred to the yolk, in which the extra-embryonic membranes may function as a ‘quasi’-placenta.
For eco-evolutionary studies, birds are especially well suited because their survival and reproductive success can be relatively easily studied in the field, in contrast to many other taxa. Moreover, systematic differences in avian yolk hormone concentrations within clutches (i.e. eggs of the same nest), between clutches, between mothers, and between species have been demonstrated, and the effect of a variety of ecological variables on hormone transfer (i.e. in the quantity of maternally produced hormone that ends up in eggs, which is influenced by maternal hormone production and active or passive transfer processes) documented [4]. As a consequence the study of hormones in bird eggs has strongly contributed to ultimate explanations for the transfer of maternal hormones to the next generation, with relevance for other fields, including human health and disease [5,6].
After the initial discovery of gonadal hormones in bird eggs [7], the field has seen a surge of publications. However, as more studies are published, the patterns also become more complex with an increase in discrepancies among studies and species. Rather than seeing these discrepancies as something negative, they are intriguing and should stimulate new ideas and research approaches, as scientific progress often comes from challenging established hypotheses rather than supporting them. Therefore, goals of this paper are to highlight potential explanations for observed discrepancies among studies, discuss recent discoveries and technical advances, and outline promising avenues for future research.
2. Understanding discrepancies: the role of pleiotropy and context dependency
Hormones are well known for having pleiotropic effects, influencing a range of different aspects of an individual's phenotype, and synchronizing changes in brain and body periphery [8]. The first studies on maternally derived hormones in bird eggs showed that maternal yolk androgens boost early offspring growth, either directly or indirectly via stimulating begging behaviour [7,9]. Although this pattern has been confirmed in several studies and species, the opposite pattern or no effect on growth have been reported in the course of time (reviewed in [4]). In addition, effects of maternal yolk androgens on a wide range of other physiological, behavioural, morphological and life-history traits have been reported, including, for example, immune suppression, an increase in metabolic rate, modulation of antioxidant status, hormone receptor densities, the expression of secondary sexual characters, exploratory behaviour or natal dispersal (reviewed in [4,10,11]). Several of these effects, such as immune suppression, modulation of oxidative status or an increase in metabolic rate, are likely to be costly for the offspring, potentially resulting in a trade-off between beneficial and detrimental effects of hormone transfer.
This has two consequences: first, facing this trade-off, mothers may transfer hormones to their offspring differently depending on contextual cues that influence these costs and benefits. For example, in an environment where parasite abundance is high or food availability is low, the immunological or metabolic costs of exposure to elevated androgen concentrations during development may outweigh the benefits in terms of enhanced growth, and mothers may therefore reduce the amount of hormones they transfer [12]. Similarly, females may transfer higher concentrations of androgens to their last laid egg when food conditions are favourable to increase this chick's success in the competition with earlier hatched and stronger siblings, whereas they may do the opposite when food is scarce to facilitate brood reduction. In line with this hypothesis, an effect of food availability on the transfer of hormones to eggs has been observed in some bird species (e.g. [13,14]). Other studies, however, found no effect of food availability on egg hormone concentrations (e.g. [15,16]). These discrepancies might be due to differences in the stability and/or predictability of food abundance in different study systems [17]. Indeed, if there is low temporal autocorrelation between food availability during egg laying and chick rearing, mothers cannot predict the conditions their offspring are going to encounter, and anticipatory maternal effects would not be expected to evolve [2].
Second, pleiotropic effects of yolk androgens may influence the outcomes of egg hormone injection experiments depending on the prevailing context under which they are performed. Support for this hypothesis was found in a recent experiment in rock pigeons (Columba livia) which lay a modal clutch size of two eggs, with the second hatching later than the first and containing substantially higher androgen concentrations [18]. Manipulation of both yolk androgen content and food availability in a full-factorial design showed that elevated yolk androgen levels had clear beneficial effects under favourable food conditions, facilitating survival of both the first and second chick, but strong negative effects under poor food conditions, facilitating brood reduction in times of food shortage. The effects of yolk hormone manipulations may also depend on the egg's position in the laying order (and thus the chick's position in the sibling rivalry), where the positive effects are present only when the chick is in a competitive disadvantage [19,20].
Overall, a better understanding of the mechanisms underlying the pleiotropic effects hormones have on various body traits and functions will be key to advance our understanding of hormone-mediated trait integration, as well as trade-offs and constraints associated with maternal hormone transfer. For example, it is still unclear if pleiotropy is the consequence of independent effects of hormones on multiple targets, or if hormones affect a single regulatory system with downstream effects on various traits. Under the former scenario, constraints on evolution and limitations for the mother to influence offspring phenotype are likely less pronounced than under the latter. However, at the same time, the latter may reduce the risk of improper integration of individual traits into a single phenotype (see [21] for further discussion). Gene expression analysis (i.e. whole transcriptome sequencing) in combination with gene set enrichment and pathway analysis is a promising approach to gain insights into the mechanisms underlying pleiotropic effects of maternal hormones, and we encourage studies on the mechanisms and functions of hormone-mediated maternal effects to incorporate such technologies.
In addition to environmental factors discussed above, genetic factors (such as offspring sex) can influence the expression of hormone mediated-maternal effects and mediate their fitness consequences. Interestingly, there is no general pattern to which sex is more strongly affected by maternal yolk hormones across bird species (reviewed in [22,23]). This variation is at least partly explained by differences in the strength of selection acting on male and female body size, respectively, with growth of female offspring being more strongly boosted by maternal yolk hormones in species where selection favours a large female body size [22]. It suggests that (natural and/or sexual) selection may shape the sex-specific sensitivities to maternal yolk hormones within species, and highlights that an ultimate perspective is key to understanding the seemingly inconsistent patterns across species. It also highlights that wrong conclusions may be drawn when not accounting for offspring sex. Yet, to date, only a minority of studies on hormone-mediated maternal effects have explicitly modelled such sex-specific consequences [23]. Molecular sexing methods are now available for most bird species [24] and we urge researchers to consider the sex of their focal individuals when studying phenotypic consequences of maternal hormone transfer.
As exposure to elevated maternal hormone concentrations is associated with costs for the offspring (see above), only offspring of highest genetic quality may be able to bear these costs. This might explain why females deposit higher androgen concentrations in eggs sired by an attractive male [25], as well as discrepancies in the effects of egg hormone manipulations across broods. Egg hormone manipulations in combination with artificial insemination with sperm from high and low quality males would allow light to be shed on this hypothesis.
3. Complex eggs: the challenge of performing biologically meaningful manipulations
(a). Interactions among hormonal and non-hormonal egg constituents
Most experimental studies on hormone-mediated maternal effects have manipulated one or two related hormones in isolation [23], which allows pinpointing of the effect of a particular hormone on offspring phenotype, but may lead to unnatural imbalances within eggs, and thus create artefacts. This is the case if different yolk constituents are mutually co-adjusted within eggs, as is observed in a number of bird species [26–30]. Selection may favour co-deposition of different egg components if they have opposite, compensatory effects on offspring phenotype (reviewed in [31]). For example, hormone-induced changes in growth and metabolism may increase levels of free radicals that can cause physiological damage if not buffered adequately by antioxidants [32–35]. Similarly, exposure to high androgen concentrations during development may cause immunosuppression [36], which might render offspring particularly susceptible to infection unless compensated for by alternative protection mechanisms such as the transfer of maternal immunoglobulins [27]. Thus, by only increasing hormone levels, without simultaneously increasing other co-adjusted egg constituents, an unnatural imbalance may be created that may negatively affect offspring development. To our knowledge, only one study has experimentally tested this hypothesis by simultaneously manipulating two co-adjusted yolk constituents (i.e. yolk testosterone and carotenoid concentrations) in a full-factorial design [37]. This study found evidence that imbalances in the egg have negative consequences for the chicks [37], highlighting that single-egg-constituent manipulations, as is usually done, might disturb a fragile equilibrium within eggs. A multivariate approach to quantifying and manipulating egg constituents is therefore recommended, which will be facilitated by on-going technical advances (e.g. in mass spectrometry or proteomics), but will nevertheless remain challenging. As an example of such a multivariate approach, a recent study used high-performance liquid chromatography with tandem mass spectroscopy (LC-MS-MS) to simultaneously quantify 27 yolk steroids from the eggs of seven shrubland bird species [38]. Such approaches open up new avenues for the quantification of hormonal profiles and their association with ecological variables both within and across taxa.
Artefacts may not only arise when egg constituents are manipulated in isolation but also when hormone doses for injection experiments are not carefully chosen (i.e. are not biologically and ecologically meaningful). Dose-response effects have been documented for several hormones [23,39], yet specific dose-response curves are missing for most study systems. An additional complication is that it is not trivial to quantify concentrations of hormones in eggs (see section ‘What is in an egg? Challenges and advances in the measurement of yolk hormones’ below), which makes it difficult to determine biologically and ecologically meaningful doses for injection experiments. Together, these technical difficulties and gaps in our knowledge significantly hamper further progress in our understanding of functional, ecological and evolutionary consequences of prenatal hormone exposure.
In addition, a significant hurdle in manipulative studies is that we can only increase embryonic exposure to hormones, but not decrease it. Currently the dynamics of blockers of relevant receptors or hormone conversions in the egg are unknown, and they may not only affect maternal hormone conversion or action, but also the embryo's endogenous hormone production, which can start early during incubation (see below).
Finally, testing whether hormone-mediated maternal effects are anticipatory (i.e. prepare the offspring for the ecological conditions they are going to encounter) requires a full-factorial match–mismatch design, in which the manipulated environmental variable is ecologically relevant and shows a pattern of temporal autocorrelation that allows parents to ‘predict the future’ [40]. Unless these conditions are met, no benefits of matched conditions are expected to be observed.
(b). Which hormone matters?
Traditionally, there has been a strong focus on maternally transferred androgens [7]. Yet, mothers transfer a range of other hormones that may mediate (anticipatory) maternal effects. Gonadal hormones, such as testosterone, androstenedione or progesterone are produced in the follicle wall very close to the ovum [41]. They are at least partly independently regulated from hormone concentrations in the mother's circulation (reviewed [41]), suggesting that females can flexibly bestow their eggs with different concentrations of gonadal hormones depending on the ecological context. Indeed, both within [10] and across species [38,42] variation in yolk androgen deposition can be partly explained by ecological variables. Although concentrations of progesterone in yolks is often much higher than that of testosterone or androstenedione [43,44], this hormone has received comparably little attention to date. Progesterone has been shown to vary across the laying sequence and in relation to a range of ecological variables [45–47] and it has been suggested to affect the chicks' fear behaviour [48] and facilitate brood reduction [46]. However, no study has yet experimentally and selectively manipulated this hormone in the egg and more studies are required to fully understand interactions with other egg components and the role of this hormone in mediating maternal effects [28].
Another important class of maternal hormones to which developing young may be exposed to prenatally are glucocorticoids, such as corticosterone and cortisol, which are produced in the mother's adrenals, and have thus to be transported via the mother's circulation to the follicles, and then enter the yolk. Whether an increase in circulating glucocorticoids in the mother automatically leads to increased concentrations in the yolk is as yet unclear, and rather than having direct effects on offspring development, circulating glucocorticoids may affect the embryo via indirect pathways, such as suppressed maternal nutrient provisioning (reviewed in Henriksen et al. [49]). In mammals there is evidence that the placenta might act to prevent maternal glucocorticoids from reaching the embryo [50]. To what extent this is the case in oviparous species is as yet unclear (but see below).
A third class of hormones that may mediate (anticipatory) maternal effects are thyroid hormones. Although produced by the mother's thyroid gland distant from the ovaries, there is some evidence that mothers may be able to regulate concentrations of these hormones in their eggs independently of their own circulation [51]. These hormones are particularly interesting because their production requires iodine that cannot be synthesized de novo and of which availability in the environment may be limited. If so, this would induce an additional trade-off for the mother between allocating limited thyroid hormone to herself or her offspring. Thyroid hormones are important for normal development of the brain, peripheral tissues, thermoregulation and metabolism [52]. After the initial studies of McNabb and her co-workers more than twenty years ago [53,54], these maternally transmitted hormones have, until very recently, received little attention. The few experimental studies performed, however, show clear effects of prenatal thyroid exposure on hatching success, early growth, and thyroid hormone production [55,56].
In addition to the hormones discussed above there is a wide range of other hormones in the yolk (e.g. growth hormones and other peptide hormones) that have received little to no attention to date. A better understanding of the role of these understudied hormones in mediating maternal effects may thus be a promising avenue for future research and proteomics and metabolomics approaches might be particularly fruitful for explorative studies into such understudied egg components.
(c). What is in an egg? Challenges and advances in the measurement of yolk hormones
In the past, most studies have relied on analytical methods such as radioimmunoassay (RIA) or enzyme immunoassay (EIA) to determine hormone concentrations in eggs. It now becomes clear that these methods often yield hormone concentrations that are too high [57,58]. This is probably not surprising, as most assays have been designed for particular substrates (mostly plasma) and species, and not for lipophilic egg yolk. Furthermore, it has recently been shown that a wide array of steroids found in egg yolk can cross-react with antibodies used in these assays, leading to artefacts when the extraction, clean-up and separation processes preceding RIA/EIA are inadequate [59–61]. These preparatory processes are often not properly conducted, and rarely validated for the target hormones under study. But even when preparatory processes such as classical chromatography over Celite or HPLC are conducted, recent work has shown that they may not be sufficient (e.g. [58,61]). This raises serious questions about which hormones, and in what concentrations, are actually present in eggs. It also raises concerns about the dosages used in egg injection experiments, as they are often calibrated on the basis of RIA/EIA results. This is particularly concerning because the effects of most hormones are strongly dose-dependent [23,39]. Thereby, such technical issues can contribute to discrepancies in findings across studies. The re-evaluation of previously reported hormone-deposition patterns in relation to environmental variables and the quantification of hormone concentrations using modern, sensitive liquid chromatography–mass spectrometry approaches (i.e. LC-MS-MS or GC/LC-M(M)S) is therefore recommended (e.g. as used in [38,62–64]).
Finally, a recent study revealed that maternal hormones are already partly metabolized in the oviduct after ovulation [59]. This provides another hurdle for the interpretation and experimentation with maternal hormones, as levels measured at oviposition may not adequately reflect what the mother initially deposited in the egg.
4. Hormone-mediated maternal effects—a family affair
Parental effects are assumed to have evolved as a mechanism that allows parents to adaptively shape offspring phenotype in variable but predictable environments [3]. As a consequence most behavioural ecology research on hormone-mediated maternal effects is based on the assumption that changes in maternal hormone transfer in response to environmental factors lead to higher offspring fitness [10]. However, this assumption ignores the possibility of conflicts of interest within families [65,66]. A whole-family perspective is therefore essential to further our understanding of the selective forces that shape variation in maternal hormone transfer as well as the response to these maternal cues [67].
(a). Conflicts between mothers and their offspring
If maternal and offspring interests are aligned, offspring should be sensitive and reactive to maternal cues. However, maternal and offspring interests are not always aligned, resulting in mother–offspring conflict [65–67]. For example when maternal investments favour future reproduction over current reproduction, current offspring will pay a fitness cost. Similarly, when mothers favour certain offspring within a reproductive event (e.g. offspring early in the laying sequence [68]), the disfavoured offspring will pay a fitness cost. If such conflicts arise, parental effects will not increase the fitness of (all) offspring, as is indeed found in many studies [17,49,69], and natural selection will thus favour resistance mechanisms that help offspring to ignore or modify maternal manipulation attempts [67,70].
Hormone receptors for androgens and glucocorticoids are present before the embryo starts to produce its own hormones, both in mammals [71] and birds [72,73], suggesting adaptation to receive the maternal hormonal signals. Interestingly, part of these receptors (as well as hormone-metabolizing enzymes) are present in the placenta in mammals and in the extra-embryonic membranes of egg-laying species [74,75], indicating homologous functions of both tissues. Hormone receptors are also found in the brain of developing embryos. However, to what extent maternal hormones reach the brain is, surprisingly, as yet not clear [76,77].
Although the machinery is thus in place to perceive maternal hormonal cues, there is also growing evidence that embryos rapidly and heavily metabolize maternal hormones in the egg [62,78,79]. Intriguingly, most of these metabolites are (supposedly) inactive forms of the free steroids, such as conjugated forms or etiocholanolone in the case of androgens [80]. It has been suggested that this conversion may facilitate uptake by the embryo, after which the inactive hormones might be converted back to the original, biologically active form [78,81]. Alternatively, these metabolites may have an as yet unknown biological effect on the embryo, such as erythropoiesis [80]. Clearly, these are intriguing avenues for future studies.
The active role of the embryo opens up the possibility that they are not just passive responders to the maternal signal but may influence how they react to it and thus how maternal effects are expressed. For example, other egg components may inform the embryo about ecologically relevant cues such as food availability, parasite abundance, or the position in the laying sequence and therefore the level of sibling competition. Based on such information the embryo may differentially metabolize maternal hormones. A first, recent study testing this hypothesis found that rock pigeon embryos in the second egg (being the last laid egg with relatively high testosterone concentrations, see earlier) metabolize yolk androgens significantly more than embryos in the first egg [62]. Understanding if this is just a consequence of the second egg having much higher natural maternal testosterone concentrations, or if this increased metabolization is instead related to the egg's position in the laying order will be an important next step and will help to clarify the adaptive significance of embryo-induced hormone metabolization.
Although these empirical studies thus show that there is scope for offspring to influence the expression of hormone-mediated maternal effects, theoretical models predict that if conflict occurs, mothers will likely have the upper hand or that the transgenerational information transfer will partly or fully break down [66]. More likely, both parties thus benefit on average from hormone-mediated signalling systems, ensuring their stability [66]. Together, these recent findings demonstrate how ultimate thinking and theory can prompt and inform studies on mechanisms, and vice versa.
(b). Costs for the mother
In addition to potential conflicts of interest between mothers and their offspring, quantifying the costs of hormone transfer for the mother is key to a better understanding of the evolution of hormone-mediated maternal effects. So far, very few studies have quantified the fitness consequences of maternal hormone transfer for the mother, and, to our knowledge, none has quantified the costs of maternal plasticity in hormone transfer in response to environmental variation.
Artificial selection is an elegant but underused experimental tool to reveal such maternal costs, and costs associated with different reproductive strategies in general [82,83]. Okuliarová et al. [84] used this approach to create genetic lines of Japanese quail (Coturnix japonica) that differ in the amount of testosterone females transfer to their eggs. This selection experiment revealed that an increase in yolk testosterone concentrations can be achieved without a correlated increase in testosterone concentrations in the female's circulation, highlighting that the mother's hormone levels do not constrain the evolution of yolk testosterone transfer. Rather, correlative work in the same species suggests that yolk testosterone transfer is associated with metabolic costs for the female [85], which might ultimately underlie the negative association between yolk testosterone transfer and female longevity observed in wild birds [86]. Thus, yolk testosterone transfer is associated with significant metabolic and longevity costs for the female, which ensure that egg hormones are an honest signal. These costs increase the stability of hormone-mediated transgenerational signalling systems [66,67,69] and create a trade-off between current and future reproductive investment that is central to models of life-history evolution [87].
Mechanistic studies are now required to gain insights into the proximate basis of variation in maternal hormone deposition and its associated costs, and artificial selection experiments [84,88] in combination with gene expression profiling [89] would be a particularly fruitful approach to shed light on these questions.
(c). The role of fathers
Conflicts cannot only arise between mothers and their offspring but also between mothers and fathers [90]. In species with biparental care, mothers and fathers disagree over the amount of care each parent provides to the offspring: the fitness benefits of parental investment depend on the summed contribution of both partners. However, the costs of parental investment are paid by each parent individually [65]. Thus, conflicts over parental care arise because each individual benefits if the partner works disproportionally hard [65].
It has been hypothesized that females may manipulate males into increasing their contribution to parental care by transferring high concentrations of yolk androgens into their eggs, and thereby enhance offspring begging behaviour (‘Manipulating Androgen Hypothesis (MAH)’; [67,91]). Several studies have tested this hypothesis, but found no evidence for this [92–96]. One study even found that mothers are more sensitive to yolk hormone-mediated begging signals than fathers [97], which is the opposite of what is predicted under MAH [91]. There was some support for MAH in a study on yellow-legged gulls (Larus michahellis), which found that yolk testosterone affects a specific component of the chicks' begging calls to which fathers are particularly sensitive [98]. Although this study did not analyse provisioning rates of the two parents, it suggests that at least in some species females can shift the outcome of the sexual conflict over care in their favour by means of maternal hormones. Methodological improvements of measuring parental provisioning may however be required to detect such manipulative effects [99].
Whereas hormone-mediated maternal effects may affect the father's behaviour (see above), inversely males also have the potential to influence their partner's transfer of hormones to the eggs. Several studies have reported that females deposit higher concentrations of androgens into their eggs when mated to an attractive, supposedly high quality male, and this effect appears to be particularly strong in species where only females provide parental care (reviewed in [25]). This finding is in line with the idea that females should invest more in broods with a higher reproductive value [87,100], assuming that ‘more is better’, which is not necessarily the case for yolk androgens ([10]; see also above). To our knowledge, the adaptive value of this differential allocation of yolk androgens in response to partner attractiveness has not been experimentally tested to date.
Besides such indirect effects of male attractiveness on egg hormone transfer, males may directly influence their offspring's response to maternally-derived hormones. In mammals, it is well documented that paternally imprinted genes expressed in the offspring can influence the mother's prenatal resource provisioning [101]. In birds, the potential for such male manipulation of female care has been questioned because the embryo develops outside of the mother's body, and females transfer all the resources they provide prenatally to the developing young before (paternally imprinted) genes in offspring are expressed [102,103]. Besides genomic imprinting, males may be able to modify the offspring's response to maternal cues through, for example, the transfer of variable epigenetic marks [104] or the transfer of non-coding RNA or proteins via sperm [105]. Exploring such paternal effects, their interaction with maternal effects, and their consequences for the resolution of within-family conflicts will be one of the most exciting avenues for future research in our opinion.
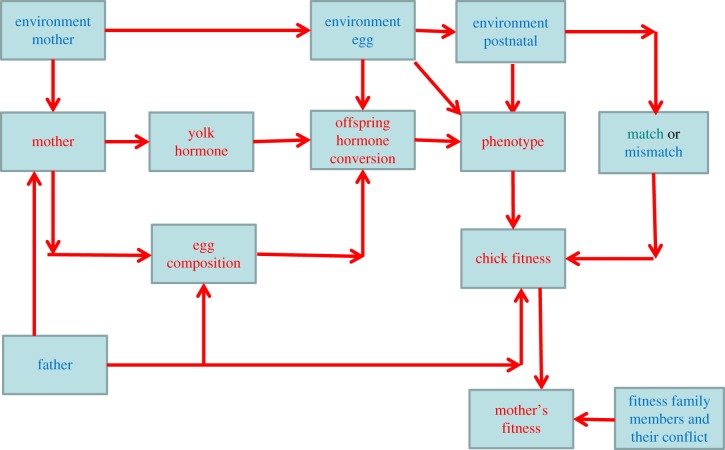
In conclusion, although the study of hormone-mediated maternal effects has exposed an increasing number of discrepancies over the last two decades, recent technical and theoretical advances have revealed that these are not simply uninteresting inconsistencies among studies, but rather intriguing phenomena that deserve further research. There is a diversity of environmental, social and genetic contexts that modify the transfer of maternal hormones, their expression as well as their fitness consequences, as summarized in figure 1. To complicate things further, these interactions can come to expression in a range of different ways, from nullifying to strengthening and even reversing phenotypic consequences, and from acting as permissive or compensatory effects [40]. Identifying such contexts that explain discrepancies among studies will thus be an important next step. In addition, recent technical advances have revealed that the embryo itself may play an active role in modifying the maternal signal. This may have consequences for the resolution of parent–offspring conflicts, and is an intriguing avenue for further research, as is the potential role of the father and interaction among different egg components in shaping the expression of hormone-mediated maternal effects.
Figure 1.
Interactions between mother, father, embryo and the environment in shaping the expression of hormone-mediated maternal effects. (Online version in colour.)
Acknowledgement
The authors thank two anonymous referees and the editor for constructive comments on the manuscript.
Data accessibility
This article has no additional data.
Authors' contribution
T.G.G.G. and B.T. wrote the paper. Parts of the context dependency, hormone conversion and receptor activity sections are based on the PhD thesis of B.-Y.H. and N.K., respectively, under supervision of T.G.G.G.
Competing interests
The authors have no conflicting interests.
Funding
We received no funding for this study.
References
- 1.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 2.Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963. ( 10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 3.Mousseau TA, Fox CW. 1998. Maternal effects as adaptations. New York, NY: Oxford University Press. [Google Scholar]
- 4.von Engelhardt N, Groothuis TGG. 2011. Maternal hormones in avian eggs. In Hormones and reproduction of vertebrates (eds Norris DO, Lopez KH), pp. 91–127. Cambridge, MA: Academic Press. [Google Scholar]
- 5.Gluckman PD, Hanson MA, Beedle AS. 2007. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol. 19, 1–19. ( 10.1002/ajhb.20590) [DOI] [PubMed] [Google Scholar]
- 6.Bateson P, et al. 2004. Developmental plasticity and human health. Nature 430, 419–421. ( 10.1038/nature02725) [DOI] [PubMed] [Google Scholar]
- 7.Schwabl H. 1993. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA 90, 11 446–11 450. ( 10.1073/pnas.90.24.11446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adkins-Regan E. 2005. Hormones and animal social behaviour. Princeton, NJ: Princeton University Press. [Google Scholar]
- 9.Schwabl H. 1996. Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol. A Physiol. 114, 271–276. ( 10.1016/0300-9629(96)00009-6) [DOI] [PubMed] [Google Scholar]
- 10.Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352. ( 10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 11.Gil D. 2008. Hormones in avian eggs: physiology, ecology and behavior. Adv. Study Behav. 38, 337–398. ( 10.1016/s0065-3454(08)00007-7) [DOI] [Google Scholar]
- 12.Tschirren B, Richner H, Schwabl H. 2004. Ectoparasite-modulated deposition of maternal androgens in great tit eggs. Proc. R. Soc. Lond. B 271, 1371–1375. ( 10.1098/rspb.2004.2730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verboven N, Monaghan P, Evans DM, Schwabl H, Evans N, Whitelaw C, Nager RG. 2003. Maternal condition, yolk androgens and offspring performance: a supplemental feeding experiment in the lesser black-backed gull (Larus fuscus). Proc. R. Soc. Lond. B 270, 2223–2232. ( 10.1098/rspb.2003.2496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergauwen J, Goerlich VC, Groothuis TGG, Eens M, Müller W. 2012. Food conditions affect yolk testosterone deposition but not incubation attendance. Gen. Comp. Endocrinol. 176, 112–119. ( 10.1016/j.ygcen.2012.01.003) [DOI] [PubMed] [Google Scholar]
- 15.Giordano M, Groothuis TGG, Tschirren B. 2014. Interactions between prenatal maternal effects and posthatching conditions in a wild bird population. Behav. Ecol. 25, 1459–1466. ( 10.1093/beheco/aru149) [DOI] [Google Scholar]
- 16.Hsu BY, Dijkstra C, Darras VM, de Vries B, Groothuis TGG. 2016. Maternal adjustment or constraint: differential effects of food availability on maternal deposition of macro-nutrients, steroids and thyroid hormones in rock pigeon eggs. Ecol. Evol. 6, 397–411. ( 10.1002/ece3.1845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uller T, Nakagawa S, English S. 2013. Weak evidence for anticipatory parental effects in plants and animals. J. Evol. Biol. 26, 2161–2170. ( 10.1111/jeb.12212) [DOI] [PubMed] [Google Scholar]
- 18.Hsu BY, Müller MS, Gahr CL, Dijkstra C, Groothuis TGG. Submitted. Co-evolved maternal effects selectively eliminate offspring depending on resource availability.
- 19.Müller MS, Roelofs Y, Erikstad KE, Groothuis TGG. 2012. Maternal androgens increase sibling aggression, dominance, and competitive ability in the siblicidal black-legged kittiwake (Rissa tridactyla). PLoS ONE 7, e47763 ( 10.1371/journal.pone.0047763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschirren B, Saladin V, Fitze PS, Schwabl H, Richner H. 2005. Maternal yolk testosterone does not modulate parasite susceptibility or immune function in great tit nestlings. J. Anim. Ecol. 74, 675–682. ( 10.1111/j.1365-2656.2005.00963.x) [DOI] [Google Scholar]
- 21.Groothuis TGG, Trillmich F. 2011. Unfolding personalities: the importance of studying ontogeny. Dev. Psychobiol. 53, 641–655. ( 10.1002/dev.20574) [DOI] [PubMed] [Google Scholar]
- 22.Tschirren B. 2015. Differential effects of maternal yolk androgens on male and female offspring: a role for sex-specific selection? PLoS ONE 10, e0133673 ( 10.1371/journal.pone.0133673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podmokla E, Drobniak SM, Rutkowska J. 2018. Chicken or egg? Outcomes of experimental manipulations of maternally transmitted hormones depend on administration method—a meta-analysis. Biol. Rev. 93, 1499–1517. ( 10.1111/brv.12406) [DOI] [PubMed] [Google Scholar]
- 24.Morinha F, Cabral JA, Bastos E. 2012. Molecular sexing of birds: a comparative review of polymerase chain reaction (PCR)-based methods. Theriogenology 78, 703–714. ( 10.1016/j.theriogenology.2012.04.015) [DOI] [PubMed] [Google Scholar]
- 25.Horváthová T, Nakagawa S, Uller T. 2012. Strategic female reproductive investment in response to male attractiveness in birds. Proc. R. Soc. B 279, 163–170. ( 10.1098/rspb.2011.0663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royle NJ, Surai PF, Hartley IR. 2001. Maternally derived androgens and antioxidants in bird eggs: complementary but opposing effects? Behav. Ecol. 12, 381–385. ( 10.1093/beheco/12.4.381) [DOI] [Google Scholar]
- 27.Postma E, Siitari H, Schwabl H, Richner H, Tschirren B. 2014. The multivariate egg: quantifying within- and among-clutch correlations between maternally derived yolk immunoglobulins and yolk androgens using multivariate mixed models. Oecologia 174, 631–638. ( 10.1007/s00442-013-2803-8) [DOI] [PubMed] [Google Scholar]
- 28.Okuliarová M, Kankova Z, Bertin A, Leterrier C, Möstl E, Zeman M. 2014. Maternally derived egg hormones, antibodies and antimicrobial proteins: common and different pathways of maternal effects in Japanese quail. PLoS ONE 9, e112817 ( 10.1371/journal.pone.0112817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giraudeau M, Ducatez S. 2016. Co-adjustment of yolk antioxidants and androgens in birds. Biol. Lett. 12, 20160676 ( 10.1098/rsbl.2016.0676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navara KJ, Badyaev AV, Mendonca MT, Hill GE. 2006. Yolk antioxidants vary with male attractiveness and female condition in the house finch (Carpodacus mexicanus). Physiol. Biochem. Zool. 79, 1098–1105. ( 10.1086/507661) [DOI] [PubMed] [Google Scholar]
- 31.Williams TD, Groothuis TGG. 2015. Egg quality, embryonic development, and post-hatching phenotype: an integrated perspective. In Nests, eggs, and incubation (eds Deeming DC, Reynolds SJ). Oxford, UK: Oxford University Press. [Google Scholar]
- 32.Tobler M, Nilsson JA, Nilsson JF. 2007. Costly steroids: egg testosterone modulates nestling metabolic rate in the zebra finch. Biol. Lett. 3, 408–410. ( 10.1098/rsbl.2007.0127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobler M, Sandell MI. 2009. Sex-specific effects of prenatal testosterone on nestling plasma antioxidant capacity in the zebra finch. J. Exp. Biol. 212, 89–94. ( 10.1242/jeb.020826) [DOI] [PubMed] [Google Scholar]
- 34.Treidel LA, Whitley BN, Benowitz-Fredericks ZM, Haussmann MF. 2013. Prenatal exposure to testosterone impairs oxidative damage repair efficiency in the domestic chicken (Gallus gallus). Biol. Lett. 9, 20130684 ( 10.1098/rsbl.2013.0684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruuskanen S, Lehikoinen E, Nikinmaa M, Siitari H, Waser W, Laaksonen T. 2013. Long-lasting effects of yolk androgens on phenotype in the pied flycatcher (Ficedula hypoleuca). Behav. Ecol. Sociobiol. 67, 361–372. ( 10.1007/s00265-012-1456-7) [DOI] [Google Scholar]
- 36.Groothuis TGG, Eising CM, Dijkstra C, Müller W. 2005. Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol. Lett. 1, 78–81. ( 10.1098/rsbl.2004.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giraudeau M, Ziegler AK, Pick JL, Ducatez S, Canale CI, Tschirren B. 2017. Interactive effects of yolk testosterone and carotenoid on prenatal growth and offspring physiology in a precocial bird. Behav. Ecol. 28, 31–38. ( 10.1093/beheco/arw127) [DOI] [Google Scholar]
- 38.Merrill L, Chiavacci SJ, Paitz RT, Benson TJ. 2018. Quantification of 27 yolk steroid hormones in seven shrubland bird species: interspecific patterns of hormone deposition, and links to life history, development, and predation risk. Can. J. Zool. 97, 1–2. ( 10.1139/cjz-2017-0351) [DOI] [Google Scholar]
- 39.Muriel J, Perez-Rodriguez L, Puerta M, Gil D. 2015. Diverse dose-response effects of yolk androgens on embryo development and nestling growth in a wild passerine. J. Exp. Biol. 218, 2241–2249. ( 10.1242/jeb.118257) [DOI] [PubMed] [Google Scholar]
- 40.Groothuis TGG, Taborsky B. 2015. Introducing biological realism into the study of developmental plasticity in behaviour. Front. Zool. 12, S6 ( 10.1186/1742-9994-12-s1-s6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groothuis TGG, Schwabl H. 2008. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Phil. Trans. R. Soc. B 363, 1647–1661. ( 10.1098/rstb.2007.0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin TE, Schwabl H. 2008. Variation in maternal effects and embryonic development rates among passerine species. Phil. Trans. R. Soc. B 363, 1663–1674. ( 10.1098/rstb.2007.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lipar JL, Ketterson ED, Nolan V, Casto JM. 1999. Egg yolk layers vary in the concentration of steroid hormones in two avian species. Gen. Comp. Endocrinol. 115, 220–227. ( 10.1006/gcen.1999.7296) [DOI] [PubMed] [Google Scholar]
- 44.Lipar JL. 2001. Yolk steroids and the development of the hatching muscle in nestling European starlings. J. Avian Biol. 32, 231–238. ( 10.1111/j.0908-8857.2001.320305.x) [DOI] [Google Scholar]
- 45.Bertin A, Chanson M, Delaveau J, Mercerand F, Möstl E, Calandreau L, Arnould C, Leterrier C, Collin A. 2013. Moderate heat challenge increased yolk steroid hormones and shaped offspring growth and behavior in chickens. PLoS ONE 8, e57670 ( 10.1371/journal.pone.0057670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poisbleau M, Demongin L, Parenteau C, Eens M. 2011. Intra-clutch ratio of yolk progesterone level changes with laying date in rockhopper penguins: a strategy to influence brood reduction? PLoS ONE 6, e27765 ( 10.1371/journal.pone.0027765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bertin A, Richard-Yris MA, Houdelier C, Lumineau S, Möstl E, Kuchar A, Hirschenhauser K, Kotrschal K. 2008. Habituation to humans affects yolk steroid levels and offspring phenotype in quail. Horm. Behav. 54, 396–402. ( 10.1016/j.yhbeh.2008.04.012) [DOI] [PubMed] [Google Scholar]
- 48.Bertin A, Hirschenhauser K, Kotrschal K. 2009. Trans-generational influence of human disturbances in Japanese quail: egg quality influences male social and sexual behaviour. Ethology 115, 879–887. ( 10.1111/j.1439-0310.2009.01672.x) [DOI] [Google Scholar]
- 49.Henriksen R, Rettenbacher S, Groothuis TGG. 2011. Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci. Biobehav. Rev. 35, 1484–1501. ( 10.1016/j.neubiorev.2011.04.010) [DOI] [PubMed] [Google Scholar]
- 50.Del Giudice M. 2012. Fetal programming by maternal stress: insights from a conflict perspective. Psychoneuroendocrinology 37, 1614–1629. ( 10.1016/j.psyneuen.2012.05.014) [DOI] [PubMed] [Google Scholar]
- 51.Ruuskanen S, Hsu BY. 2018. Maternal thyroid hormones: an unexplored mechanism underlying maternal effects in an ecological framework. Physiol. Biochem. Zool. 91, 904–916. ( 10.1086/697380) [DOI] [PubMed] [Google Scholar]
- 52.Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. 2017. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 342, 68–100. ( 10.1016/j.neuroscience.2015.09.070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNabb FMA, Dicken SG, Cherry JA. 1985. The effects of different maternal dietary iodine concentrations on Japanese quail. Thyroid function in embryos and hatchlings. Domest. Anim. Endocrinol. 2, 35–42. ( 10.1016/0739-7240(85)90024-4) [DOI] [Google Scholar]
- 54.Wilson CM, McNabb FMA. 1997. Maternal thyroid hormones in Japanese quail eggs and their influence on embryonic development. Gen. Comp. Endocrinol. 107, 153–165. ( 10.1006/gcen.1997.6906) [DOI] [PubMed] [Google Scholar]
- 55.Ruuskanen S, Darras VM, Visser ME, Groothuis TGG. 2016. Effects of experimentally manipulated yolk thyroid hormone levels on offspring development in a wild bird species. Horm. Behav. 81, 38–44. ( 10.1016/j.yhbeh.2016.03.006) [DOI] [PubMed] [Google Scholar]
- 56.Hsu BY, Dijkstra C, Darras VM, de Vries B, Groothuis TGG. 2017. Maternal thyroid hormones enhance hatching success but decrease nestling body mass in the rock pigeon (Columba livia). Gen. Comp. Endocrinol. 240, 174–181. ( 10.1016/j.ygcen.2016.10.011) [DOI] [PubMed] [Google Scholar]
- 57.Kumar N, van Faassen M, de Vries B, Almansa J, Hsu BY, Lelono KA, Gahr M, Groothuis TGG. Submitted. Testosterone concentrations differ substantially between radioimmunoassay and mass spectrometry measurements in blood plasma and especially egg yolk.
- 58.Rettenbacher S, Möstl E, Groothuis TGG. 2009. Gestagens and glucocorticoids in chicken eggs. Gen. Comp. Endocrinol. 164, 125–129. ( 10.1016/j.ygcen.2009.05.019) [DOI] [PubMed] [Google Scholar]
- 59.Kumar N, van Faassen M, de Vries B, Kema I, Gahr M, Groothuis TGG. 2018. Gonadal steroid levels in rock pigeon eggs do not represent adequately maternal allocation. Sci. Rep. 8, 11213 ( 10.1038/s41598-018-29478-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quillfeldt P, Poisbleau M, Parenteau C, Trouve C, Demongin L, van Noordwijk HJ, Möstl E. 2011. Measuring corticosterone in seabird egg yolk and the presence of high yolk gestagen concentrations. Gen. Comp. Endocrinol. 173, 11–14. ( 10.1016/j.ygcen.2011.05.005) [DOI] [PubMed] [Google Scholar]
- 61.Rettenbacher S, Groothuis TG, Henriksen R, Möstl E. 2013. Corticosterone in bird eggs: the importance of analytical validation. Wien. Tierarztl. Monatsschr. 100, 283–290. [Google Scholar]
- 62.Kumar N, van Faassen M, Kema I, Gahr M, Groothuis TGG. 2018. Early embryonic modification of maternal hormones differs systematically among embryos of different laying order: a study in birds. Gen. Comp. Endocrinol. 269, 53–59. ( 10.1016/j.ygcen.2018.08.014) [DOI] [PubMed] [Google Scholar]
- 63.Larsen TR, Fairhurst GD, De Baere S, Croubels S, Muller W, De Neve L, Lens L. 2015. Novel insights into relationships between egg corticosterone and timing of breeding revealed by LC-MS/MS. J. Avian Biol. 46, 643–647. ( 10.1111/jav.00735) [DOI] [Google Scholar]
- 64.De Baere S, Larsen TR, Devreese M, De Backer P, De Neve L, Fairhurst G, Lens L, Croubels S. 2015. Use of LC-MS-MS as an alternative to currently available immunoassay methods to quantitate corticosterone in egg yolk and albumen. Anal. Bioanal. Chem. 407, 4351–4362. ( 10.1007/s00216-014-8269-7) [DOI] [PubMed] [Google Scholar]
- 65.Trivers RL. 1974. Parent-offspring conflict. Am. Zool. 14, 249–264. ( 10.1093/icb/14.1.249) [DOI] [Google Scholar]
- 66.Kuijper B, Johnstone RA. 2018. Maternal effects and parent-offspring conflict. Evolution 72, 220–233. ( 10.1111/evo.13403) [DOI] [PubMed] [Google Scholar]
- 67.Müller W, Lessells CM, Korsten P, von Engelhardt N. 2007. Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. Am. Nat. 169, E84–E96. ( 10.1086/511962) [DOI] [PubMed] [Google Scholar]
- 68.Schwabl H, Mock DW, Gieg JA. 1997. A hormonal mechanism for parental favouritism. Nature 386, 231 ( 10.1038/386231a0)9069278 [DOI] [Google Scholar]
- 69.Smiseth PT, Scott MP, Andrews C. 2011. Hormonal regulation of offspring begging and mediation of parent-offspring conflict. Anim. Behav. 81, 507–517. ( 10.1016/j.anbehav.2010.11.029) [DOI] [Google Scholar]
- 70.Reed WL, Clark ME. 2011. Beyond maternal effects in birds: responses of the embryo to the environment. Integr. Comp. Biol. 51, 73–80. ( 10.1093/icb/icr032) [DOI] [PubMed] [Google Scholar]
- 71.Pedernera E, Gómora MJ, Meneses I, De Ita M, Méndez C. 2017. Androgen receptor is expressed in mouse cardiomyocytes at prenatal and early postnatal developmental stages. BMC Physiol. 17, 7 ( 10.1186/s12899-017-0033-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Godsave SF, Lohmann R, Vloet RPM, Gahr M. 2002. Androgen receptors in the embryonic zebra finch hindbrain suggest a function for maternal androgens in perihatching survival. J. Comp. Neurol. 453, 57–70. ( 10.1002/cne.10391) [DOI] [PubMed] [Google Scholar]
- 73.Kumar N, Lohrentz A, Gahr M, Groothuis TGG. Submitted. Steroid receptors in avian extra-embryonic membranes: a novel mechanism for hormone-mediated maternal effects. [DOI] [PMC free article] [PubMed]
- 74.Albergotti LC, Hamlin HJ, McCoy MW, Guillette LJ. 2009. Endocrine activity of extraembryonic membranes extends beyond placental amniotes. PLoS ONE 4, e5452 ( 10.1371/journal.pone.0005452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griffith OW, Brandley MC, Whittington CM, Belov K, Thompson MB. 2017. Comparative genomics of hormonal signaling in the chorioallantoic membrane of oviparous and viviparous amniotes. Gen. Comp. Endocrinol. 244, 19–29. ( 10.1016/j.ygcen.2016.04.017) [DOI] [PubMed] [Google Scholar]
- 76.von Engelhardt N, Henriksen R, Groothuis TGG. 2009. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. Gen. Comp. Endocrinol. 163, 175–183. ( 10.1016/j.ygcen.2009.04.004) [DOI] [PubMed] [Google Scholar]
- 77.Benowitz-Fredericks ZM, Hodge M. 2013. Yolk androstenedione in domestic chicks (Gallus gallus domesticus): uptake and sex-dependent alteration of growth and behavior. Gen. Comp. Endocrinol. 193, 48–55. ( 10.1016/j.ygcen.2013.07.005) [DOI] [PubMed] [Google Scholar]
- 78.Paitz RT, Bowden RM. 2011. Biological activity of oestradiol sulphate in an oviparous amniote: implications for maternal steroid effects. Proc. R. Soc. B 278, 2005–2010. ( 10.1098/rspb.2010.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vassallo BG, Paitz RT, Fasanello VJ, Haussmann MF. 2014. Glucocorticoid metabolism in the in ovo environment modulates exposure to maternal corticosterone in Japanese quail embryos (Coturnix japonica). Biol. Lett. 10, 20140502 ( 10.1098/rsbl.2014.0502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paitz RT, Bowden RM, Casto JM. 2011. Embryonic modulation of maternal steroids in European starlings (Sturnus vulgaris). Proc. R. Soc. B 278, 99–106. ( 10.1098/rspb.2010.0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paitz RT, Bowden RM. 2013. Sulfonation of maternal steroids is a conserved metabolic pathway in vertebrates. Integr. Comp. Biol. 53, 895–901. ( 10.1093/icb/ict027) [DOI] [PubMed] [Google Scholar]
- 82.Pick JL, Hutter P, Ebneter C, Ziegler AK, Giordano M, Tschirren B. 2016. Artificial selection reveals the energetic expense of producing larger eggs. Front. Zool. 13, 38 ( 10.1186/s12983-016-0172-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pick JL, Hutter P, Tschirren B. 2016. In search of genetic constraints limiting the evolution of egg size: direct and correlated responses to artificial selection on a prenatal maternal effector. Heredity 116, 542–549. ( 10.1038/hdy.2016.16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okuliarová M, Groothuis TGG, Skrobanek P, Zeman M. 2011. Experimental evidence for genetic heritability of maternal hormone transfer to offspring. Am. Nat. 177, 824–834. ( 10.1086/659996) [DOI] [PubMed] [Google Scholar]
- 85.Tschirren B, Ziegler AK, Canale CI, Okuliarová M, Zeman M, Giraudeau M. 2016. High yolk testosterone transfer is associated with an increased female metabolic rate. Physiol. Biochem. Zool. 89, 448–452. ( 10.1086/687571) [DOI] [PubMed] [Google Scholar]
- 86.Tschirren B, Postma E, Gustafsson L, Groothuis TGG, Doligez B. 2014. Natural selection acts in opposite ways on correlated hormonal mediators of prenatal maternal effects in a wild bird population. Ecol. Lett. 17, 1310–1315. ( 10.1111/ele.12339) [DOI] [PubMed] [Google Scholar]
- 87.Roff DA. 1992. The evolution of life histories. New York, NY: Chapman & Hall. [Google Scholar]
- 88.Okuliarová M, Meddle SL, Zeman M. 2018. Egg deposition of maternal testosterone is primarily controlled by the preovulatory peak of luteinizing hormone in Japanese quail. Gen. Comp. Endocrinol. 256, 23–29. ( 10.1016/j.ygcen.2017.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Egbert JR, Jackson MF, Rodgers BD, Schwabl H. 2013. Between-female variation in house sparrow yolk testosterone concentration is negatively associated with CYP19A1 (aromatase) mRNA expression in ovarian follicles. Gen. Comp. Endocrinol. 183, 53–62. ( 10.1016/j.ygcen.2012.12.001) [DOI] [PubMed] [Google Scholar]
- 90.Lessells CM. 1999. Sexual conflict in animals. In Levels of selection in evolution (ed. Keller L.), pp. 75–99. Princeton, NJ: Princeton University Press. [Google Scholar]
- 91.Moreno-Rueda G. 2007. Yolk androgen deposition as a female tactic to manipulate paternal contribution. Behav. Ecol. 18, 496–498. ( 10.1093/beheco/arl106) [DOI] [Google Scholar]
- 92.Müller W, Boonen S, Groothuis TGG, Eens M. 2010. Maternal yolk testosterone in canary eggs: toward a better understanding of mechanisms and function. Behav. Ecol. 21, 493–500. ( 10.1093/beheco/arq010) [DOI] [Google Scholar]
- 93.Ruuskanen S, Doligez B, Tschirren B, Pitala N, Gustafsson L, Groothuis TGG, Laaksonen T. 2009. Yolk androgens do not appear to mediate sexual conflict over parental investment in the collared flycatcher Ficedula albicollis. Horm. Behav. 55, 514–519. ( 10.1016/j.yhbeh.2009.01.010) [DOI] [PubMed] [Google Scholar]
- 94.Laaksonen T, Adamczyk F, Ahola M, Möstl E, Lessells CM. 2011. Yolk hormones and sexual conflict over parental investment in the pied flycatcher. Behav. Ecol. Sociobiol. 65, 257–264. ( 10.1007/s00265-010-1034-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barnett CA, Clairardin SG, Thompson CF, Sakaluk SK. 2011. Turning a deaf ear: a test of the manipulating androgens hypothesis in house wrens. Anim. Behav. 81, 113–120. ( 10.1016/j.anbehav.2010.09.019) [DOI] [Google Scholar]
- 96.Estramil N, Groothuis TGG, Eens M, de Vries B, Müller W. 2017. Coadaptation of offspring begging and parental provisioning: a role for prenatal maternal effects? Horm. Behav. 87, 129–136. ( 10.1016/j.yhbeh.2016.11.005) [DOI] [PubMed] [Google Scholar]
- 97.Tschirren B, Richner H. 2008. Differential effects of yolk hormones on maternal and paternal contribution to parental care. Anim. Behav. 75, 1989–1994. ( 10.1016/j.anbehav.2008.01.007) [DOI] [Google Scholar]
- 98.Noguera JC, Kim SY, Velando A. 2013. Maternal testosterone influences a begging component that makes fathers work harder in chick provisioning. Horm. Behav. 64, 19–25. ( 10.1016/j.yhbeh.2013.04.008) [DOI] [PubMed] [Google Scholar]
- 99.Paquet M, Smiseth PT. 2016. Maternal effects as a mechanism for manipulating male care and resolving sexual conflict over care. Behav. Ecol. 27, 685–694. ( 10.1093/beheco/arv230) [DOI] [Google Scholar]
- 100.Burley N. 1986. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 127, 415–445. ( 10.1086/284493) [DOI] [Google Scholar]
- 101.Moore T, Haig D. 1991. Genomic imprinting in mammalian development—a parental tug-of-war. Trends Genet. 7, 45–49. ( 10.1016/0168-9525(91)90040-w) [DOI] [PubMed] [Google Scholar]
- 102.Tuiskula-Haavisto M, Vilkki J. 2007. Parent-of-origin specific QTL—a possibility towards understanding reciprocal effects in chicken and the origin of imprinting. Cytogenet. Genome Res. 117, 305–312. ( 10.1159/000103192) [DOI] [PubMed] [Google Scholar]
- 103.Frésard L, et al. 2014. Transcriptome-wide investigation of genomic imprinting in chicken. Nucleic Acids Res. 42, 3768–3782. ( 10.1093/nar/gkt1390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jenkins TG, Aston KI, Trost C, Farley J, Hotaling JM, Carrell DT. 2015. Intra-sample heterogeneity of sperm DNA methylation. Mol. Hum. Reprod. 21, 313–319. ( 10.1093/molehr/gau115) [DOI] [PubMed] [Google Scholar]
- 105.Immler S. 2018. The sperm factor: paternal impact beyond genes. Heredity 121, 239–247. ( 10.1038/s41437-018-0111-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.