Abstract
Purpose
Hypofractionated radiation therapy (HFRT) remains underused, despite multiple randomized trials showing the equivalence of HFRT to conventional fractionated radiation therapy (CFRT). We sought to retrospectively review the relationship between breast volume and toxicity for HFRT versus CFRT.
Methods and materials
Data from 114 patients who received a diagnosis of early stage breast cancer and were treated with lumpectomy and whole breast radiation alone were reviewed. Breast cancer laterality, stage, grade, estrogen/progesterone receptor and human epidermal growth factor receptor 2 status, and systemic therapy use were recorded. Length of follow-up was calculated using the last day of radiation treatment and the date of the most recent follow-up.
Results
The median follow-up was 42 months. A total of 83 patients were treated with HFRT and 31 with CFRT. Rates of grade ≥2 skin toxicity were significantly higher in patients undergoing CFRT compared with HFRT (76% vs 28%; P < .0001). In a subset of patients with breast volume ≥1000 cm3, grade ≥2 skin toxicity trended higher for CFRT patients (73% vs 38%; P = .057). For posterior separation >25 cm, the percentage of patients with grade 2 skin toxicity was 43% for HFRT versus 57% for CFRT (P = .67). The volume of breast tissue receiving >105% of the total prescription dose, including boost, was not significantly different for HFRT versus CFRT (P = .70).
Conclusions
Use of HFRT resulted in lower acute skin toxicity rates compared with CFRT. Large breast size (volume ≥1000 cm3) was associated with lower acute grade 2 toxicity with the use of HFRT despite achieving similar dosimetry compared with CFRT.
Introduction
Breast cancer is the second most common cancer in the world and is the most common cancer in women.1 Historically, women who developed breast cancer were treated with mastectomy. However, lumpectomy followed by whole breast radiation therapy has since been shown to carry equivalent risk of recurrence compared with mastectomy, thereby allowing women to conserve their breasts.2 The survival benefits of adjuvant radiation therapy for early stage breast cancer were shown by a systematic review of 17 randomized studies that compared mastectomy with lumpectomy and radiation.3
Most of the trials reviewed used the conventional fractionation radiation therapy (CFRT) regimen of 50 Gy in 25 fractions of 2 Gy. There is increasing interest in reducing the duration of radiation treatment with the use of fraction sizes >2 Gy, which is known as hypofractionation. Several recent large randomized prospective studies have shown equal efficacy of hypofractionation radiation therapy (HFRT) regimens and conventional regimens.4, 5, 6 Whelan et al found that HFRT of 42.5 Gy in 16 fractions is not inferior to CFRT with 50 Gy in 25 fractions in women with early stage breast cancer treated with breast-conserving surgery with negative margins and negative lymph nodes.5 Likewise, Haviland et al found that HFRT of 40.05 Gy in 15 fractions was as effective as 50 Gy in 25 fractions and was associated with lower rates of breast shrinkage, telangiectasia, and breast edema.6
Despite studies that show equivalency in efficacy for HFRT, there has been no widespread uptake in its use.7 Concerns exist with regard to the use of HFRT in women with larger posterior breast separation (larger volume breasts) because of fears of greater skin toxicity and poor cosmesis.8, 9 Women with breast separation >25 cm were excluded from the Canadian hypofractionation trial.5 However, there were no constraints with regard to breast size in the UK START study.6 A study by Shaitelman et al included a large proportion of obese women and found lower rates of skin reactions in patients treated with HFRT, suggesting HFRT should not be withheld in this population.4 With these results, the newest guidelines on hypofractionation do not restrict eligibility based on breast size.10
At our institution, we have been routinely offering HFRT over the past 6 years to patients with early stage breast cancer, regardless of their breast size. Early during this period, patients were presented at consultation with the data for hypofractionation and allowed to play a role in the decision-making process for dose and fractionation. After anecdotally noticing less toxicity in patients who were treated with hypofractionation, we transitioned more recently to only using hypofractionation in patients with early stage disease.
We sought to retrospectively review the relationship between breast volume and toxicity in patients treated with HFRT versus CFRT to further assess the appropriateness of HFRT in patients with larger breast volume.
Methods and Materials
Patient selection
This study was approved by the institutional review board. After approval, we retrospectively identified patients at our institution who were diagnosed with American Joint Committee on Cancer stage T0-3N0-1 breast cancer and treated with breast-conserving surgery, followed by postoperative radiation therapy, between January 2012 and September 2015. Male patients were excluded, as were patients who were treated with a third field to the supraclavicular fossa. Patients treated with inverse planned intensity modulated radiation therapy (IMRT) were excluded, but forward planned field in-field optimization was allowed.
Patient and tumor characteristics
Patient, tumor, and treatment characteristics were collected from the electronic medical records, and the following variables were recorded: T stage, N stage, breast cancer laterality, tumor grade, estrogen/progesterone receptor status, human epidermal growth factor receptor 2/Neu status, use of hormone therapy, and use of neoadjuvant or adjuvant chemotherapy. Length of follow-up was calculated using the last day of radiation treatment and the date of the most recent follow-up visit.
All patients were seen weekly during treatment and for follow-up within 3 months of completion of treatment, with skin toxicity reported at each visit. The median time between completion of treatment and first follow-up was 27 days, with a range of 3 to 75 days. The highest Radiation Therapy Oncology Group (RTOG) acute skin toxicity level documented in the electronic medical records during this period was recorded.11
Dosimetric and planning variables
All patients were simulated and treated in the supine position on a breast board. Breast tissue was contoured in accordance with the RTOG breast atlas.12 The lumpectomy bed was contoured using clinical and radiographic information, including surgical clips, excision cavity volume, lumpectomy scar, and seroma. The Varian Eclipse treatment planning system was used to collect contouring and radiation treatment dosimetric variables, including radiation dose, fractionation, use of lumpectomy bed boost, posterior separation of breasts (defined as the distance along the posterior border of the treatment tangents from the medial to lateral edge of the treated tissue), breast volume, maximum hot spot, volume of breast receiving 105% of the prescription dose with or without boost, and dosimetric breast coverage determined using the dose-volume histogram. HFRT was defined as radiation fractions >2 Gy (most commonly 4005 cGy in 15 fractions), and CFRT was defined as radiation fractions ≤2 Gy (most commonly 5000 cGy in 25 fractions).
Radiation therapy was delivered using 3-dimensional conformal radiation therapy. The breast planning target volume was defined as the breast volume less 5 mm from the surface of the skin. Breast posterior separation was defined as the greatest distance along the posterior edge of the tangent fields through the chest. For patients with large posterior separation, higher-energy photon beams were often employed to reduce hot spots. Plans with mixed energies were allowed. Forward planned field in-field optimization was used, with a minimum of 4 monitor units per subfield. Per institutional policy, all hypofractionation plans had a maximum hot spot of 107%.
The primary endpoint of this study was the highest level of acute skin toxicity during treatment. Secondary endpoints included breast tissue coverage and dosimetric hotspots, as well as pain and fatigue.
Statistical analysis
Univariate and multivariable logistic regression models were fit to test for associations among patient, tumor, and treatment characteristics. To fit the multivariable logistic regression model, variables with a global P value < .20 in the univariate analysis were included.13 P values < .05 were considered significant. All statistical analyses were conducted using SAS, version 9.3 software (SAS Institute, Cary, NC).
Results
Patients
We identified a total of 114 patients who met the inclusion criteria, of whom 83 were treated with HFRT and 31 were treated with CFRT. A boost of 8 to 12 Gy to the lumpectomy cavity was administered in 55% of patients. The median follow-up time was 42 months. The patient characteristics were well balanced between the HFRT and CFRT treatment regimens, including age, stage, and receptor status (Table 1), with the exception of follow-up time because HFRT was more recently adopted. Local control was excellent. There was only 1 local recurrence in a patient who was treated with CFRT, which occurred 23 months after completion of the radiation therapy. There were no distant recurrences.
Table 1.
Patient characteristics by fractionation regimen
| Variables | HFRT (n = 83) | CFRT (n = 31) | P-value |
|---|---|---|---|
| Age | |||
| Median (y) | 63 | 59 | |
| Mean (y) | 62 | 59 | .088 |
| Follow-up time | |||
| Median (mo) | 34 | 55 | |
| Mean (y) | 35 | 54 | <.001 |
| T Stage | |||
| Tis | 21 | 4 | .13 |
| T1 (unspecified) | 5 | 0 | |
| T1a | 9 | 1 | |
| T1b | 14 | 8 | |
| T1c | 22 | 11 | |
| T2 | 9 | 7 | |
| T3 | 3 | 0 | |
| N stage | |||
| N0 | 74 | 28 | .93 |
| N1mic | 4 | 1 | |
| N1a | 5 | 2 | |
| Side | |||
| Left | 42 | 15 | .79 |
| Right | 40 | 16 | |
| ER | |||
| ER negative | 13 | 4 | .78 |
| ER positive | 69 | 27 | |
| PR | |||
| PR negative | 23 | 10 | .72 |
| PR positive | 57 | 21 | |
| HER2 | |||
| Her2 negative | 59 | 27 | .75 |
| Her2 positive | 10 | 3 | |
| Volume | |||
| Median | 827 | 729 | |
| Mean | 932 | 997 | .60 |
| Separation | |||
| Median | 21 | 22 | |
| Mean | 22 | 23 | .15 |
| Lumpectomy bed | |||
| Yes | 37 | 6 | |
| Boost | |||
| No | 46 | 25 | .0002 |
Abbreviations: CFRT = conventional fractionated radiation therapy; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; HFRT = hypofractionated radiation therapy; PR = progesterone receptor.
Plan dosimetry
For patients treated with either HFRT or CFRT, lumpectomy bed coverage was acceptable. There was no statistically significant difference in preferred lumpectomy bed coverage (95% prescription dose to >95% lumpectomy volume) between HFRT and CFRT patients: 95% of HFRT plans had optimal coverage compared with 100% of CFRT plans (P = .57). With regard to whole breast coverage, there was significantly better coverage of the contoured breast volume with CFRT, with 93% of CFRT patients treated with a 95% dose to >95% of the volume, compared with 71% of HFRT patients (P = .016).
The analysis of patients with breast volume >1000 cm3 and breast separation >25 cm showed no differences in whole breast or lumpectomy bed coverage. The maximum hot spot was 106.0% for HFRT plans and 105.9% for CFRT plans (P = .77). There was also no significant difference for hypofractionation versus conventional fractionation in maximum hot spot for patients with breast volume >1000 cm3 (106.3% vs 106.6%; P = .62) or for patients with separation >25 cm (106.3% vs 107%; P = .2).
The volume of breast tissue receiving >105% of the total prescription dose (including boost) was not significantly different for patients receiving HFRT versus CFRT (25.1 vs 33.7 cm3). The volume of breast tissue receiving >105% of the whole breast dose (not including boost) was also not significantly different between HFRT and CFRT. In addition, there was no significant difference when including only patients with breast volume >1000 cm3 (46.9 cm3 in HFRT vs 85.5 cm3 in CFRT; P = .55). Patients with breast separation >25cm also did not show a significant difference (48.3 cm3 for HFRT vs 111.9 cm3 for CFRT; P = .45).
Skin toxicity
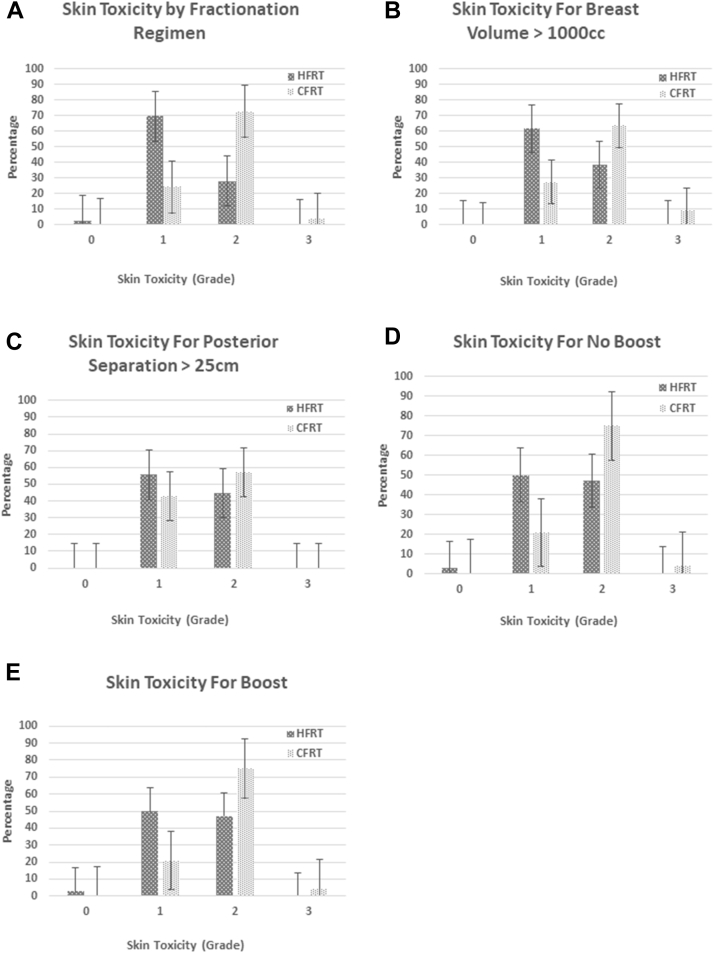
There was significantly less skin reaction in patients treated with HFRT compared with patients treated with CFRT (Figs. 1A-E). Only 28% of patients treated with HFRT had grade ≥2 skin toxicity, versus 76% of patients treated with CFRT (P < .0001). Grade 3 skin toxicity occurred in 1 patient (CFRT group). In patients with breast volume ≥1000 cm3, there was a trend toward higher rates of grade ≥2 toxicity for CFRT (73% vs 38% with HFRT; P = .057). For patients with posterior breast separation >25 cm, there was no significant difference in grade ≥2 skin toxicity (57% for CFRT; 43% for HFRT; P = .67). A significant increased rate of acute grade 2 skin toxicity with CFRT was seen regardless of the use of boost.
Fig. 1.
Skin toxicity. Highest grade of Radiation Therapy Oncology Group acute toxicity for patients treated with hypofractionated radiation therapy versus conventional radiation therapy. (A) Skin toxicity for all patients included in the study. (B) Skin toxicity for patients with large breasts, defined as volume of breast tissue >1000 cm3. (C) Skin toxicity for patients with large breasts, defined as posterior separation >25 cm, with posterior separation defined as the greatest distance traveled by the posterior edge of the radiation beam. (D) Skin toxicity for patients who were treated to the whole breast alone, without a boost. (E) Skin toxicity for patients treated to the whole breast followed by a sequential boost.
Univariate analysis showed that the use of boost, CFRT, and a higher-than-average volume of the breast receiving 105% of the whole breast prescription dose was associated with increased grade ≥2 acute skin toxicity (Table 2). On multivariate analysis, the use of boost and CFRT remained significant predictors for acute skin toxicity (P = .017 and .0053, respectively; Table 3).
Table 2.
Univariate analysis for grade ≥2 acute skin toxicity
| Variables | Odds ratio | P-value |
|---|---|---|
| T stage | ||
| Tis | 1 | |
| T1 | 0.76 | .49 |
| T2 | 0.28 | .20 |
| T3 | 0.47 | .87 |
| ER status | ||
| ER negative | 1 | |
| ER positive | 0.46 | .16 |
| PR status | ||
| PR negative | 1 | |
| PR positive | 0.49 | .09 |
| HER2 status | ||
| HER2 negative | 1 | |
| HER2 positive | 1.6 | .43 |
| Boost | ||
| No boost | 1 | |
| Boost | 6.8 | <.0001 (95% CI, 2.8-17) |
| Fractionation regimen | ||
| HFRT | 1 | |
| CFRT | 8.1 | <.0001 (95% CI, 3.0-21) |
| Systemic therapy | ||
| None | 1 | |
| Hormone, chemotherapy, or both | 2.7 | .079 |
| Volume of 105% of whole breast dose (without boost) | ||
| <average (252.4 cm3) | 1 | |
| >average (252.4 cm3) | 4.1 | .0009 (95% CI, 1.8-9.3) |
| Volume of 105% of total prescription (breast plus boost) | ||
| <average (27.3 cm3) | 1 | |
| >average (27.3 cm3) | 0.47 | .15 |
| Breast volume | ||
| <1000 cm3 | 1 | |
| >1000 cm3 | 1.6 | .22 |
| Posterior separation | ||
| <25 cm | ||
| >25 cm | 1.48 | .39 |
Abbreviations: CFRT = conventional fractionated radiation therapy; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; HFRT = hypofractionated radiation therapy; PR = progesterone receptor.
Individual chemotherapy, hormone, was omitted because there was not enough of each for the analysis.
Table 3.
Multivariate analysis for acute skin toxicity, controlling for fractionation regimen
| Variables | Odds ratio | P-value |
|---|---|---|
| Boost | ||
| No boost | 1 | |
| Boost | 4.6 | .017 |
| Fractionation regimen | ||
| HFRT | 1 | |
| CFRT | 4.5 | .0053 |
Abbreviations: CFRT = conventional fractionated radiation therapy; HFRT = hypofractionated radiation therapy.
Discussion
Our study found that patients who were treated with HFRT have significantly less grade ≥2 acute skin toxicity than patients treated with conventional radiation therapy (72% vs 28%; P < .0001). These results are in line with the skin toxicity findings reported in the literature. A trial at MD Anderson Cancer Center randomized 287 women with breast cancer after breast-conserving surgery to either CFRT (50 Gy/25 fractions + 10-14 Gy boost) or HFRT (42.56 Gy/16 fractions + 10-12.5Gy boost).4 The results showed that acute grade ≥2 dermatitis was present in 36% of women treated with HFRT versus 69% of women treated with CFRT (P < .001).
Likewise, a study of 2309 patients in the Michigan Radiation Oncology Quality Consortium found that grade ≥2 skin toxicity developed in 27.4% of patients treated with HFRT versus 62.6% of patients treated with CFRT (P < .001).14 Another retrospective study of 266 patients allowed IMRT for planning to reduce hot spots, and CFRT remained associated with greater grade 2 radiation dermatitis on multivariate analysis compared with HFRT (19.3% vs 1.7%; P < .001).15
Despite continued findings of lower skin toxicity with HFRT in multiple studies, concerns persist with regard to HFRT in women with larger breast volumes because of fears of greater skin toxicity and poor cosmesis.8, 9 The aforementioned randomized MD Anderson Cancer Center trial similarly found that even for the highest quartile of central axis separation, HFRT was equivalent to (and even perhaps slightly better) than CFRT in terms of acute skin reactions. The rate of grade ≥2 acute skin reaction was 63% for patients treated with HFRT and 78% for patients treated with CFRT (P = .17). The same was true for grade ≥3 acute skin toxicity (0% HFRT vs 8% CFRT; P = .24).4
A previously reported, single-institution, retrospective review of 143 patients with stage 0 to III breast cancer who were treated with HFRT showed that volume of tissue radiated was associated with higher grade ≥3 acute skin toxicity, but high-volume disease was categorized as addition of a third field for supraclavicular radiation.16 Our study is distinct because of its focus on patients with breast-only radiation; we excluded patients with a third field to the supraclavicular region. In our study, women with breast volume ≥1000 cm3 had fewer grade 2 acute skin reactions when treated with HFRT. This is the first study to our knowledge to categorize patients on the basis of breast volume in addition to central axis separation. Our findings support the new guidelines that state that hypofractionation should be used regardless of breast size.10
Our study is not the first to assess the relationship among breast volume, dose homogeneity, and toxicity. However, we feel confident that our treatment regimen closely matches current practice because patients were most often treated with a regimen of 4005 cGy in 15 fractions, with a maximum hot spot of 107%. This differs from a study of dosimetric parameters predictive for toxicity by Lazzari et al, which allowed doses of >110% hot spots using a regimen with a higher total dose (4256 cGy in 16 fractions).17 Another study of predictors of toxicity during hypofractionation suggested that breast volume does predict for higher toxicity.18 However, our study shows that, compared with patients treated with conventional radiation therapy, there is less toxicity with hypofractionation for patients with large breasts.
A retrospective study of 339 patients treated with CFRT (50 Gy/25 fractions) versus HFRT (44 Gy/16 fractions) found that breast volume receiving >107% of the prescription dose (V107) was associated with increased skin toxicity (P < .001).19 In our study, we chose to analyze the volume of breast receiving 105% of the whole breast dose (V105) because the maximum accepted hot spot at our institution is 107%. We found that V105 was associated with greater skin toxicity, which is in line with current HFRT guidelines that recommend minimization of the volume of the breast receiving 105% of the prescription dose, with a suggestion to keep this volume <200 cm3.10
IMRT was used in a study of 129 patients who received HFRT of 42.4 Gy in 16 fractions to the breast, followed by a 9.6 Gy boost in 4 fractions via IMRT.20 In review of dosimetry, the study found that V105 was 3.66% for patients with large breasts (chest wall separation >25 cm, or planning target volume >1500 cm3) and 1.91% for patients with small breasts (P = .04). The researchers reported grade 2 acute skin toxicity of 28% in patients with large breasts and 12% in patients with small breasts (P = .008). In our study, patients treated with HFRT had lower V105 than those with CFRT, which persisted in the groups with larger breast size, but this was not statistically significant. On multivariate analysis, there was a trend toward decreased grade ≥2 skin toxicity with HFRT compared with CFRT in patients with large breast size, which may be related to the decreased V105 in this group. We therefore recommend keeping the V105 of the whole breast dose <50 cm3, if possible, which was the mean in our patients with large breasts undergoing HFRT.
At our institution, for patients treated with HFRT, keeping the maximum hotspot to <107% of the prescription dose is a priority. To achieve this goal, higher-energy beams are often used, leading to decreased dose near the surface of the whole breast. Occasionally, a compromise in coverage of the whole breast is accepted to meet this goal as long as the lumpectomy bed has adequate coverage. As a result, we found in our review that several patients who were treated with HFRT did not meet the ideal American Society for Radiation Oncology guideline criteria of 95% prescription dose to 95% of the breast volume. Despite this difference in coverage, there was no difference in local control between the 2 groups. A single local recurrence event occurred within the CFRT group. Longer follow-up is needed to determine whether there is a significant difference in local control between the groups.
This is a retrospective study with a small sample size and unbalanced arms because higher number of patients receiving hypofractionation. The point of highest toxicity was not uniformly recorded at the same time point, and arguably skin reaction peak may have been in the interval between the last on-treatment visit and the first follow-up. Data from randomized studies in the era of 3-dimensional treatment, such as RTOG 1005, will be more definitive in determining appropriate dosimetric criteria for treatment planning in this population. Despite these limitations, we believe that this study lends support to the current recommendations for hypofractionation regardless of breast size, as long as physicians pay close attention to dosimetry and minimization of hot spots.
Limitations of this study also include lack of long-term follow-up. Concerns remain that patients with larger breasts will have increased fibrosis with hypofractionation compared with conventional treatment. However, some studies suggest that acute toxicity correlates with long-term toxicity, and UK data to date suggest that at 10 years there is less cosmetic change with hypofractionation.6, 21
Conclusions
Our data illustrated that HFRT is as effective as CFRT in terms of local control and has decreased grade ≥2 acute skin toxicity. This trend persists for patients with larger breasts measured as a function of breast volume. Our findings lend further support to the use of HFRT for patients of all breast sizes. Care should be taken to minimize the volume of the breast receiving >105% of the prescription dose to further reduce the risk of acute toxicity. Our findings lend further support to the newly published American Society for Radiation Oncology consensus guideline that now recommends HFRT for patients of all breast sizes given that dose-homogeneity requirements are met.10
Acknowledgments
The authors thank Amanda Schroeder for her help with the preparation and editing of this manuscript.
Footnotes
Sources of support: This work had no specific funding.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Ghoncheh M., Pournamdar Z., Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17:43–46. doi: 10.7314/apjcp.2016.17.s3.43. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B., Anderson S., Bryant J. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Darby S., McGale P., Correa C. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomized trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaitelman S.F., Schlembach P.J., Arzu I. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation. JAMA Oncol. 2015;1:931–941. doi: 10.1001/jamaoncol.2015.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelan T.J., Pignol J., Levine M.N. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 6.Haviland J.S., Owen J.R., Dewar J.A. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 7.Bekelman J.E., Sylwestrzak G., Barron J. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States. JAMA. 2014;12:2542–2550. doi: 10.1001/jama.2014.16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goffman T.E., Glatstein E. Hypofractionation redux? J Clin Oncol. 2004;22:589–591. doi: 10.1200/JCO.2004.07.174. [DOI] [PubMed] [Google Scholar]
- 9.Mowery Y.M., Blitzblau R.C. Whole-breast radiation therapy: The long and short of it. Int J Radiat Oncol Biol Phys. 2014;90:990–992. doi: 10.1016/j.ijrobp.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Smith B.D., Bellon J.R., Blitzblau R. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8:145–152. doi: 10.1016/j.prro.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 12.White J., Tai A., Arthur D. Breast cancer atlas for radiation therapy planning: Consensus definitions. https://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx Available at:
- 13.Hosmer D.W., Lemeshow S. Wiley; New York, NY: 2000. Applied Logistic Regression. [Google Scholar]
- 14.Jagsi R., Griffith K.A., Boike T.P. Differences in the acute toxic effects of breast radiotherapy by fractionation schedule. JAMA Oncol. 2015;1:918–930. doi: 10.1001/jamaoncol.2015.2590. [DOI] [PubMed] [Google Scholar]
- 15.Rudat V., Nour A., Ghaida S.A., Alaradi A. Impact of hypofractionation and tangential beam IMRT on the acute skin reaction in adjuvant breast cancer radiotherapy. Radiat Oncol. 2016;11:100. doi: 10.1186/s13014-016-0674-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linares I., Tovar M.I., Zurita M., Guerrero R., Expósito M., Moral R.D. Hypofractionated breast radiation: Shorter scheme, lower toxicity. Clin Breast Cancer. 2016;16:262–268. doi: 10.1016/j.clbc.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Lazzari G., Terlizzi A., Vittoria Scarpati G. Predictive parameters in hypofractionated whole-breast 3D conformal radiotherapy according to the Ontario Canadian trial. Onco Targets Ther. 2017;10:1835–1842. doi: 10.2147/OTT.S127833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciammella P., Podgornii A., Galeandro M. Toxicity and cosmetic outcome of hypofractionated whole-breast radiotherapy: Predictive clinical and dosimetric factors. Radiat Oncol. 2014;9:97. doi: 10.1186/1748-717X-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tortorelli G., Murro L.D., Barbarino R. Standard or hypofractionated radiotherapy in the postoperative treatment of breast cancer: A retrospective analysis of acute skin toxicity and dose in homogeneities. BMC Cancer. 2013;13:230. doi: 10.1186/1471-2407-13-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannan R., Thompson R.F., Chen Y. Hypofractionated whole-breast radiation therapy: Does breast size matter? Int J Radiat Oncol Biol Phys. 2012;84:894–901. doi: 10.1016/j.ijrobp.2012.01.093. [DOI] [PubMed] [Google Scholar]
- 21.Lilla C., Ambrosone C.B., Kropp S. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;106:143–150. doi: 10.1007/s10549-006-9480-9. [DOI] [PubMed] [Google Scholar]