Abstract
Background
The objective of this study was to establish the efficacy and safety of procalcitonin (PCT)-guided antibiotic discontinuation in critically ill patients with sepsis in a country with a high prevalence of antimicrobial resistance and a national health insurance system.
Methods
In a multi-center randomized controlled trial, patients were randomly assigned to a PCT group (stopping antibiotics based on a predefined cut-off range of PCT) or a control group. The primary end-point was antibiotic duration. We also performed a cost-minimization analysis of PCT-guided antibiotic discontinuation.
Results
The two groups (23 in the PCT group and 29 in the control group) had similar demographic and clinical characteristics except for need for renal replacement therapy on ICU admission (46% vs. 14%; P = 0.010). In the per-protocol analysis, the median duration of antibiotic treatment for sepsis was 4 days shorter in the PCT group than the control group (8 days; interquartile range [IQR], 6–10 days vs. 14 days; IQR, 12–21 days; P = 0.001). However, main secondary outcomes, such as clinical cure, 28-day mortality, hospital mortality, and ICU and hospital stays were not different between the two groups. In cost evaluation, PCT-guided therapy decreased antibiotic costs by USD 30 (USD 241 in the PCT group vs. USD 270 in the control group). The results of the intention-to-treat analysis were similar to those obtained for the per-protocol analysis.
Conclusion
PCT-guided antibiotic discontinuation in critically ill patients with sepsis could reduce the duration of antibiotic use and its costs with no apparent adverse outcomes.
Trial Registration
ClinicalTrials.gov Identifier: NCT02202941
Keywords: Sepsis, Biomarkers, Anti-Bacterial Agents, Calcitonin, Intensive Care Unit, Economics
Graphical Abstract
INTRODUCTION
The duration of antibiotic therapy in critically ill patients with sepsis is currently based on empirical rules depending on the severity of the patients' clinical condition and the site of infection.1 As a result of the lack of clear guidance in difficult clinical situations dealing with septic patients, however, there is an inherent tendency for antibiotic overuse in the intensive care unit (ICU).2 In addition, clinicians may be reluctant to shorten the duration of antibiotic therapy in patients with sepsis.3 These factors could increase the risk of developing antimicrobial resistance and treatment-related costs.4 Use of a biomarker of resolution of infection could assist clinicians in making the decision to discontinue empiric antibiotics.1 Among sepsis biomarkers, procalcitonin (PCT), a precursor of calcitonin, has been most widely studied to guide antibiotic therapy in patients with sepsis.5,6,7,8,9,10,11,12,13,14,15 Data from randomized-controlled trials confirms the safety and efficacy of PCT-guided discontinuation of antibiotics in patients with sepsis.6,7,8,9,10,11,12,13 However, most of these studies were conducted in western countries with a relatively low consumption of antibiotics16 and a low prevalence of antimicrobial resistance17 compared to Asian countries. In addition, data on the cost-effectiveness of PCT-guided antibiotic therapy are difficult to generalize to countries with public insurance systems,11,13 because insured patients are less affected by antibiotic costs.4
In Korea, which has a compulsory national health insurance system, prevalence of antimicrobial resistance to major bacterial pathogens has increased gradually.18 Therefore, the Korean National Evidence-based Healthcare Collaborating Agency organized a study group on issues regarding efficacy, safety, and cost-effectiveness of PCT-guided antibiotic therapy in critically ill patients with sepsis. The objectives of this study were to assess the efficacy and safety of PCT-guided antibiotic therapy in critically ill patients with sepsis in a country with a high prevalence of antimicrobial resistance, and to perform an economic evaluation in the context of a national health insurance system.
METHODS
Study design and participants
This prospective, single-blinded, randomized, controlled, investigator-initiated trial on the duration and cost of antibiotic treatment for sepsis in the ICU (PROcalcitonin-guided treatment on Duration of Antibiotic therapy and cost in septic patients: PRODA) was conducted in four medical ICUs in Korea. To be eligible, patients had to be older than 18 years of age, enrolled within 24 hours of admission to ICU with suspected severe sepsis or septic shock, and expected to remain in the ICU for longer than 48 hours if they had received antibiotics for less than 48 hours. Exclusion criteria were patients with proven bacterial infection requiring more than 3 weeks' antibiotic therapy; antibiotic therapy started 48 hours or more before enrollment; severely immunocompromised patients, such as patients infected with human immunodeficiency virus and with a CD4 count of less than 200 cells/mm3, neutropenic patients (< 500 neutrophils/mm3), or patients on immunosuppressive therapy; subjects not expected to survive to hospital discharge or those with do-not-resuscitate orders; or known pregnancy.
Interventions
Prospective written informed consent was obtained from all patients or legally authorized representatives. Patients were randomly assigned at a 1:1 ratio to PCT-guided or clinician-guided groups. A computer-generated randomization list in blocks of four was used for treatment allocation. Randomization procedure and allocation of treatment were performed by the research coordinator. The sequence was concealed from patients and investigators until interventions were assigned. However, investigators were aware of treatment assignment after randomization. Investigator and the coordinators were unaware of outcomes during the study, and primary endpoints were strictly defined and not patient-reported. Data were collected by professional research personnel at each site.
All patients included in the study had their serum PCT concentration measured at baseline as close to initiation of antibiotics as possible. For patients randomly assigned to the PCT-guided group, measurements of PCT concentrations were taken every other day until day 14 after inclusion or until antibiotics were stopped. The study protocol advised discontinuation of prescribed antibiotics if PCT concentration had decreased by 80% or more of its peak value (relative stopping threshold) or when it reached a value of 0.5 µg/L or lower (absolute stopping threshold) based on a previously published algorithm.9,13 Of note, the final decision concerning the duration of antibiotic use was left to the discretion of the attending physician. Cases in whom antibiotic therapy was continued despite the encouragement of the investigators to stop it were classified as ‘overruling’ and the reasons for overruling were recorded. For patients assigned to the clinician-guided group, the investigators did not interfere with the duration of antibiotic therapy.
For both groups, antibiotic selection was at the discretion of the attending physicians. Nevertheless, broad-spectrum antibiotics were recommended for initial empirical treatment (i.e., before the susceptibility patterns of the responsible pathogens became known) of sepsis. Antibiotic de-escalation with narrower-spectrum antibiotics was strongly recommended on the basis of culture results when possible.
Outcomes
The primary endpoint was the duration of antibiotic therapy, expressed as days between start and end of antibiotics in the two groups for all randomized patients who were not excluded (modified intention-to-treat population). Secondary endpoints were the percentage of patients who had a cure or recurrence of the initial infection, 28-day mortality, in-hospital mortality, length of stay in ICU and hospital, and costs of antibiotics and care for sepsis. The costs of care for individual patients were obtained via a fee-for-service based costing system that extracted all information relating to the clinical procedures performed on each patient during the study period. However, this information was available for only two of the four participating hospitals (Seoul National University Hospital and Samsung Medical Center). Economic evaluation examined whether the PCT-guided strategy was cost-effective compared to the clinician-guided group. We constructed a cost-minimization approach because mortality, which is a major clinical outcome in this population, was not significantly different between the PCT-guided group and clinician-guided group in this study. A healthcare system perspective that includes medical and non-medical costs was employed. Regarding medical costs, hospitalization costs to care for sepsis paid by the national health insurance and copayments by patients were included. We did not consider non-medical costs, such as time or transportation costs, because our study population comprised hospitalized sepsis patients. Costs were not discounted as the time horizon was the period of hospitalization. To facilitate comparison of outcomes with other countries, total costs were expressed in US dollars based on an exchange rate of 1,010.98 Korean Won/USD, which was the existing exchange rate at the time of initiation of the study (July 1, 2014).
Statistical analysis
The goal of this study was to establish whether a PCT-guided strategy was superior to a clinician-guided strategy in terms of antibiotic duration and cost-effectiveness, and to demonstrate the non-inferiority of PCT-guided antibiotic therapy with regard to recurrent infections. We assumed mean durations of 14 days of antibiotic therapy in the control group and 9 days in the PCT group, and a standard deviation of 5 days in both groups based on data from previous studies6,19 and Korean antibiotic consumption characteristics.20 To assess the superiority of the primary outcome with an α error of 0.05 and a power of 80%, we calculated that we needed to enroll at least 23 patients in each group to detect a 36% (five days) difference in duration of antibiotic therapy for the initial infection between the two groups. Assuming a 10% drop-out rate, we planned enrollment of 50 subjects.
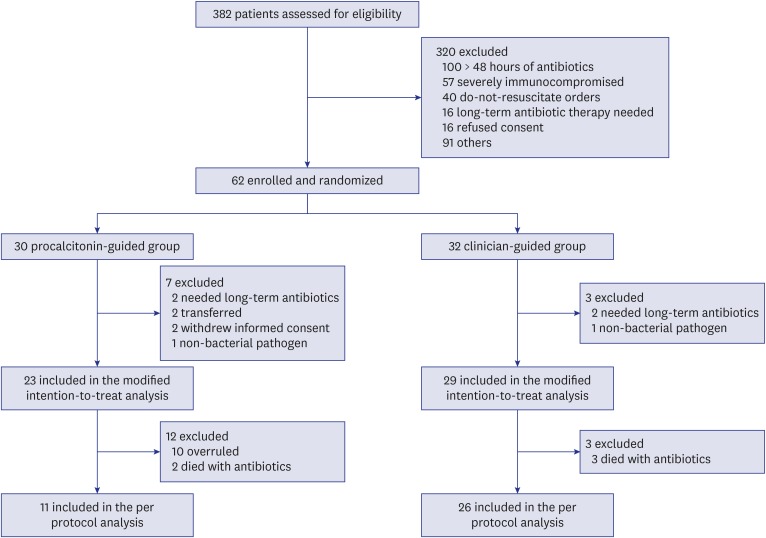
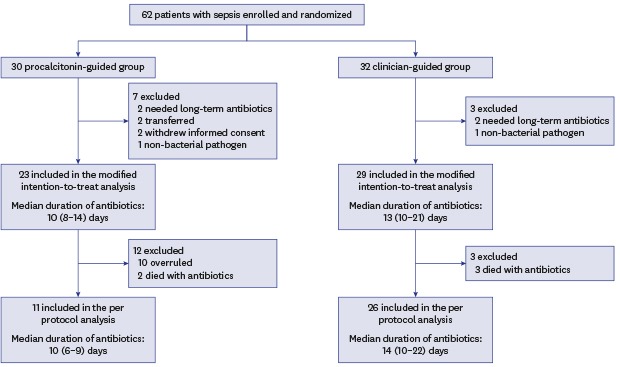
Continuous data are presented as medians with interquartile ranges (IQRs) while categorical variables are presented as numbers and percentages. Data were compared using the Mann-Whitney U test for continuous variables and Pearson's χ2 test or Fisher's exact test for categorical variables. The Kaplan-Meier method was used to estimate the cumulative rates of antibiotic cessation, which were subsequently compared using the log-rank test. Primary endpoints were first analyzed on the basis of modified intention-to-treat analysis for all randomized patients who were not excluded. Then, we performed a per-protocol analysis excluding patients who did not follow the PCT algorithm and patients who died before a decision to stop antibiotics could be taken, because this was the targeted population in this study (i.e., patients in whom a decision to stop antibiotics could be taken on the basis of the PCT levels) (Fig. 1). For economic evaluation of PCT-guided antibiotic therapy, we performed a cost-minimization analysis. To evaluate the cost-effectiveness of the PCT-guided therapy, we compared the total cost per patient with sepsis by cumulating the hospital costs including general ward and ICU stay, antibiotic treatment cost, and PCT cost. Each cost was the multiplication of the mean daily costs per patient. All tests were two-sided, and P < 0.05 was considered significant. Data were analyzed using SAS version 9.4 (SAS Institute Inc. Cary, NC, USA).
Fig. 1. Flow chart of the screening and randomization process.
Ethics statement
The study protocol was approved by The Institutional Review Board (IRB) of National Evidence-based Healthcare Collaborating Agency, Ministry of Health and Welfare, Korea (IRB No. 14-081-1), and the ethics committees of all participating sites. The study was monitored by an independent data safety and monitoring committee, with no interim analysis performed. This study was registered at ClinicalTrials.gov under the identifier NCT02202941.
RESULTS
Study population
From July 2014, through June 2015, 382 patients were assessed for eligibility. Of these, 62 (16%) patients were enrolled, including nine patients who needed long-term antibiotic therapy, transferred to other hospital, or withdrew from the study, resulting in a modified intention-to-treat population of 53 patients (23 in the PCT group and 29 in the control group, Fig. 1). Baseline characteristics of all included patients are shown in Table 1. The two groups had similar demographic and clinical characteristics except for need for renal replacement therapy on ICU admission (46% in the PCT group vs. 14% in the control group; P = 0.010). Similar proportions of patients with community-acquired, healthcare-associated, and hospital-acquired sepsis were included in both groups. Forty-eight percent of patients in the PCT group required vasopressor support as compared with 55% of those in the control group (P = 0.707).
Table 1. Baseline characteristics of the septic patients.
| Characteristics | Modified intention-to-treat analysis | Per-protocol analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Procalcitonin group (n = 23) | Control group (n = 29) | P value | Procalcitonin group (n = 11) | Control group (n = 26) | P value | |||
| Age, yr | 69 (61–75) | 70 (63–77) | 0.537 | 69 (63–74) | 71 (61–77) | 0.781 | ||
| Gender, men | 8 (33) | 14 (48) | 0.272 | 4 (36) | 12 (46) | 0.723 | ||
| Comorbidities | ||||||||
| Diabetes | 13 (54) | 11 (38) | 0.237 | 6 (55) | 9 (35) | 0.295 | ||
| Cardiovascular disease | 7 (29) | 8 (28) | 0.899 | 3 (27) | 6 (23) | > 0.999 | ||
| Chronic lung disease | 6 (25) | 7 (24) | 0.942 | 3 (27) | 5 (19) | 0.672 | ||
| Chronic renal disease | 8 (33) | 7 (24) | 0.459 | 4 (36) | 5 (19) | 0.404 | ||
| Chronic liver disease | 6 (25) | 4 (14) | 0.482 | 2 (18) | 4 (15) | > 0.999 | ||
| Malignancy | 5 (21) | 7 (24) | 0.775 | 2 (18) | 6 (23) | > 0.999 | ||
| Charlson comorbidity index | 4 (2–5) | 3 (2–4) | 0.301 | 2 (2–4) | 3 (2–4) | 0.919 | ||
| Acquisition of infection | 0.387 | 0.293 | ||||||
| Community-acquired | 9 (37) | 14 (48) | 4 (36) | 14 (54) | ||||
| Healthcare-associated | 9 (37) | 10 (35) | 4 (36) | 8 (31) | ||||
| Hospital-acquired | 6 (25) | 5 (17) | 3 (27) | 4 (15) | ||||
| Sites of infection | 0.927 | 0.908 | ||||||
| Pulmonary | 5 (21) | 10 (35) | 2 (18) | 8 (31) | ||||
| Intraabdominal | 8 (33) | 11 (38) | 5 (46) | 11 (42) | ||||
| Urinary | 9 (38) | 4 (14) | 3 (27) | 4 (15) | ||||
| Skin and soft tissue | 1 (4) | 0 | 0 | 0 | ||||
| Catheter-related | 0 | 0 | 0 | 0 | ||||
| Others | 1 (4) | 3 (10) | 1 (9) | 2 (8) | ||||
| Unknown | 0 | 1 (3) | 0 | 1 (4) | ||||
| Bacterial pathogen identified | 12 (52) | 15 (52) | 0.560 | 5 (45) | 13 (50) | 0.543 | ||
| Gram positive pathogens | ||||||||
| S. pneumoniae | 1 | 0 | 0 | 0 | ||||
| Enterococcus | 1 | 1 | 1 | 0 | ||||
| Methicillin-resistant S. aureus | 1 | 1 | 1 | 1 | ||||
| Other gram positive bacteria | 0 | 1 | 0 | 1 | ||||
| Gram negative pathogens | ||||||||
| P. aeruginosa | 1 | 0 | 0 | 0 | ||||
| K. pneumoniae | 2 | 3 | 1 | 3 | ||||
| E. coli | 5 | 6 | 1 | 5 | ||||
| Enterobacter species | 0 | 3 | 1 | 3 | ||||
| Other gram negative bacteria | 1 | 0 | 0 | 0 | ||||
| MDR pathogen | 4 (17) | 1 (3) | 0.164 | 2 (18) | 1 (4) | 0.205 | ||
| Appropriateness of empirical antibiotics | 0.748 | 0.646 | ||||||
| Appropriate | 20 (83) | 22 (76) | 10 (91) | 21 (81) | ||||
| Inappropriate | 1 (4) | 1 (3) | 0 | 0 | ||||
| Not available | 3 (13) | 6 (21) | 1 (9) | 5 (19) | ||||
| Clinical status on ICU admission | ||||||||
| Need for MV | 9 (38) | 12 (41) | 0.774 | 5 (46) | 10 (39) | 0.728 | ||
| Need for vasopressor support | 12 (50) | 16 (55) | 0.707 | 4 (36) | 14 (54) | 0.331 | ||
| Need for RRT | 11 (46) | 4 (14) | 0.010 | 5 (46) | 2 (8) | 0.016 | ||
| Corticosteroids | 6 (25) | 8 (28) | 0.832 | 1 (9) | 6 (23) | 0.649 | ||
| Laboratory findings | ||||||||
| White blood cells, ×103/µL | 15.9 (12.3–30.8) | 15.8 (9.8–24.5) | 0.353 | 12.7 (9.7–17.5) | 14.9 (9.2–24.9) | 0.658 | ||
| Procalcitonin, µg/L | 24.7 (8.5–55.4) | 26.8 (4.8–48.2) | 0.665 | 19.5 (4.7–42.1) | 28.1 (6.3–50.0) | 0.727 | ||
| C-reactive protein, mg/dL | 17.2 (6.6–27.9) | 15.5 (7.9–26.9) | 0.962 | 18.1 (7.7–23.8) | 16.8 (9.4–30.1) | 0.653 | ||
| Lactate, mmol/L | 4.3 (1.5–7.0) | 2.7 (1.6–4.6) | 0.289 | 3.9 (1.3–6.9) | 2.8 (1.7–4.5) | 0.506 | ||
| Severity of illness | ||||||||
| APACHE II | 28 (17–38) | 21 (16–31) | 0.168 | 31 (20–32) | 20 (16–28) | 0.101 | ||
| SOFA score | 10 (5–14) | 9 (7–11) | 0.490 | 10 (7–11) | 9 (6–11) | 0.481 | ||
Data are presented as medians (interquartile ranges) or number (%).
ICU = intensive care unit, APACHE II = Acute Physiology and Chronic Health Evaluation II, SOFA = Sequential Organ Failure Assessment.
The rate of microbiologically confirmed sepsis did not differ significantly between the two groups (96% in the PCT group vs. 97% in the control group; P = 0.891). Blood cultures were positive in 71% of patients in the PCT group and 66% of patients in the control group (P = 0.680). Occurrence of multi-drug resistant pathogens was not significantly different between the two groups (17% in the PCT group vs. 3% in the control group; P = 0.164), and the majority of patients were administered adequate antibiotics in both groups (Table 1).
Median PCT levels on enrollment were similar in both groups (24.7 µg/L, IQR, 8.5–55.4 µg/L in the PCT group; 26.8 µg/L, IQR, 4.8–48.2 µg/L in the control group; P = 0.665). Other inflammatory markers, including leukocytosis and C-reactive protein level, did not differ between the two groups (Table 1).
Main outcomes
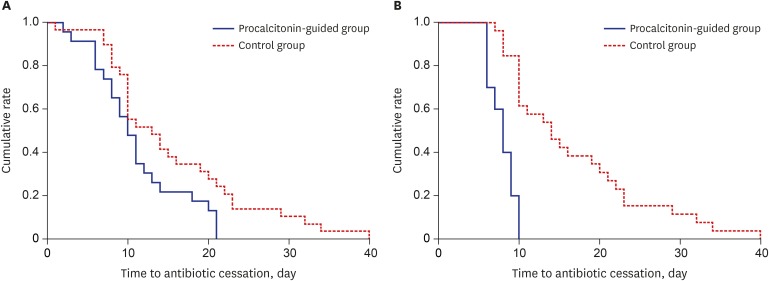
In the modified intention-to-treat population, the median antibiotic duration for the first episode of sepsis was 3 days shorter in the PCT group (10 days; IQR, 8–14 days) than in the control group (13 days; IQR, 10–21 days), although this difference did not achieve statistical significance (P = 0.078). However, cumulative rate of antibiotic cessation was significantly higher in the PCT group than in the control group (P = 0.029, log rank test) (Fig. 2A). The rate of clinical cure was not different between the two groups (83% in the PCT group vs. 79% in the control group; P = 0.858). Mortalities at Day 28 and in-hospital were also not significantly different between the two groups (Table 2). In addition, lengths of ICU and hospital stays were not different between the two groups.
Fig. 2. Probability of antibiotic cessation in patients with sepsis. Kaplan-Meier estimates of the probability of antibiotic cessation in the intention-to-treat population (A, P = 0.029 for log rank test) and the per-protocol population (B, P < 0.001 for log-rank test). Solid line represents the procalcitonin-guided group and the dotted line represents the control group.
Table 2. Primary and secondary outcome measures according to modified intention-to-treat analysis.
| Variables | Procalcitonin group (n = 23) | Control group (n = 29) | P value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Duration of antibiotic therapy, day | 10 (8–14) | 13 (10–21) | 0.078 | |
| Secondary outcomes | ||||
| Clinical cure | 20 (83) | 23 (79) | 0.858 | |
| Recurrence of the first episode of infection | 0 | 0 | - | |
| 28-day mortality | 4 (17) | 6 (21) | 0.709 | |
| In-hospital mortality | 6 (25) | 7 (24) | 0.942 | |
| Length of ICU stay, day | 4 (3–8) | 5 (3–8) | 0.588 | |
| Length of hospital stay, day | 21 (11–29) | 19 (10–29) | 0.796 | |
Data are presented as medians (interquartile ranges) or number (%).
ICU = intensive care unit.
In the PCT group, recommendations for antibiotic cessation were not followed in 10 patients. In addition, five patients died before a decision regarding antibiotic cessation could be taken (2 in the PCT group, 3 in the control group). The remaining 11 of 23 (48%) patients in the PCT group and 26 of 29 (90%) patients in the control group completed their antibiotic therapy: these two groups were compared as per-protocol analysis (Fig. 1). Similar to the modified intention-to-treat analysis, the two groups were balanced according to baseline characteristics except for the need for renal replacement therapy on ICU admission (Table 1). Patients strictly treated according to the PCT-guided protocol (excluding patients who had the algorithm overruled) had a significantly shorter median duration of antibiotic therapy than those in whom the protocol was not followed (8 days; IQR, 6–9 days vs. 16 days; IQR, 12–21 days; P < 0.001). Overall, the median duration of antibiotic therapy was significantly reduced in patients randomized to the PCT group compared with controls (8 days; IQR, 6–10 days vs. 14 days; IQR, 12–21 days; P = 0.001). Cumulative rate of antibiotic cessation was significantly higher in the PCT group than in the control group (P < 0.001, log-rank test) (Fig. 2B). Main secondary outcomes, such as clinical cure, 28-day mortality, hospital mortality, and ICU and hospital stays were comparable in the two groups (Table 3).
Table 3. Primary and secondary outcome measures according to per-protocol analysis.
| Variables | Procalcitonin group (n = 11) | Control group (n = 26) | P value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Duration of antibiotic therapy, day | 8 (6–9) | 14 (10–22) | < 0.001 | |
| Secondary outcomes | ||||
| Clinical cure | 10 (91) | 23 (85) | 0.829 | |
| Recurrence of the first episode of infection | 0 | 0 | - | |
| 28-day mortality | 1 (9) | 3 (12) | > 0.999 | |
| In-hospital mortality | 1 (9) | 4 (15) | > 0.999 | |
| Length of ICU stay, day | 4 (4–7) | 5 (3–8) | 0.523 | |
| Length of hospital stay, day | 17 (10–29) | 21 (12–33) | 0.454 | |
Data are presented as medians (interquartile ranges) or number (%).
ICU = intensive care unit.
Economic evaluation
In the modified intention-to-treat analysis, the mean daily costs per patient in the ICU were not significantly different; $1,128 (SD, $387; IQR, $947–$1,418) in the PCT group and $824 (SD, $589; IQR, $415–$824) in the control group (P = 0.134). Similarly, the mean daily costs per patient in the general ward were $410 (SD, $324; IQR, $205–$516) in the PCT group and $340 (SD, $333; IQR, $154–$491) in the control group (P = 0.660) (Table 4). The total cumulative estimated cost was higher in the PCT group than the control group by $2,842, where that of the PCT group and control group were $11,721 and $8,879, respectively. However, PCT-guided therapy reduced antibiotic costs by $30 ($241 in the PCT group vs. $270 in the control group) (Table 5).
Table 4. Mean daily costs per patient with sepsis.
| Variables | Modified intention-to-treat analysis | Per-protocol analysis | |||||
|---|---|---|---|---|---|---|---|
| Procalcitonin group (n = 23) | Control group (n = 29) | P value | Procalcitonin group (n = 11) | Control group (n = 26) | P value | ||
| Hospital costs | |||||||
| General ward | 410 ± 324 | 340 ± 333 | 0.660 | 506 ± 249 | 382 ± 328 | 0.461 | |
| ICU stay | 1,128 ± 387 | 824 ± 589 | 0.134 | 955 ± 421 | 700 ± 488 | 0.283 | |
| Treatment | |||||||
| Antibiotics | 24 ± 16 | 21 ± 25 | 0.177 | 26 ± 20 | 18 ± 22 | 0.358 | |
All medical costs are presented in U.S. dollars. Data are presented as mean ± standard deviation.
ICU = intensive care unit.
Table 5. Total cumulative costs per patient with sepsis.
| Variables | Modified intention-to-treat analysis | Per-protocol analysis | |||
|---|---|---|---|---|---|
| Procalcitonin group (n = 23) | Control group (n = 29) | Procalcitonin group (n = 11) | Control group (n = 26) | ||
| Hospital costs | |||||
| General ward | 6,968.1 | 4,758.1 | 6,572.9 | 6,117.5 | |
| ICU stay | 4,510.1 | 4,121.3 | 3,818.4 | 3,500.7 | |
| Treatment | |||||
| Antibiotics | 240.5 | 270.1 | 206.2 | 258.8 | |
| Laboratory test | |||||
| PCT | 242.8 | - | 207.3 | - | |
| Total cost | 11,721.0 | 8,879.4 | 10,598.6 | 9,618.2 | |
All medical costs are presented in U.S. dollars.
ICU = intensive care unit, PCT = procalcitonin.
The results from the per-protocol analysis showed similar trends to those obtained from the intention-to-treat analysis. The mean daily costs per patient in the ICU were $955 (SD, $421; IQR, $617–$1,252) in the PCT group and $700 (SD, $488; IQR, $402–$778) in the control group (P = 0.283), while the costs in the general ward were $506 (SD, $249; IQR, $418–$590) in the PCT group and $382 (SD, $328; IQR, $173–$543) in the control group (P = 0.461). Although the PCT group was not more cost-effective than the control group in the per-protocol analysis, the antibiotic cost was lower in the PCT group by $53 ($206 in the PCT group vs. $259 in the control group) (Table 4).
DISCUSSION
In the PRODA trial, we observed a significant reduction in antibiotic duration in critically ill patients with sepsis for whom a decision could be made to discontinue antibiotic use based on an analysis of serial PCT measurements using an algorithm (per-protocol analysis). Early discontinuation of antibiotics based on the PCT-guided protocol was non-inferior to standard care with respect to outcomes. In addition, PCT-guided antibiotic therapy decreased antibiotic costs by 20%.
PCT is the prohormone precursor of calcitonin that is expressed primarily by C cells of the thyroid gland. PCT is produced ubiquitously in response to endotoxins or mediators released in response to bacterial infections and is strongly correlated with the extent and severity of bacterial infections.21 Therefore, PCT is primarily used to establish the presence of bacterial infection.22 Furthermore, because serum PCT level halves daily when infection is controlled, PCT can be used to monitor disease course and response to treatment.23 The current Surviving Sepsis Campaign Guidelines also suggest that PCT level can be used to support shortening the duration of antibiotic therapy in sepsis patients, with a low quality of evidence.24 However, clinical experience with antimicrobial stewardship based on PCT level is limited25 and the potential for harm remains a concern, especially in critically ill patients.14
To the best of our knowledge, eight randomized controlled trials assessing the efficacy of PCT-guided discontinuation of antibiotics in ICU patients with sepsis have been published.6,7,8,9,10,11,12,13 These studies have addressed whether it is possible to stop antibiotic treatment based on a PCT-guided strategy in critically ill patients, although the strategies differed among the studies. A landmark study by Nobre et al.6 showed that a PCT-guided strategy was able to reduce the duration of antibiotic treatment for sepsis. This strategy was supported by subsequent studies conducted in surgical ICUs,7,8 although because these were single center studies with a relatively small sample of patients, the generalizability of their findings is limited. A subsequent large multi-center PRORATA study showed that a PCT-guided strategy to treat suspected bacterial infections in non-surgical patients in the ICU could reduce antibiotic exposure.9 However, this previous study evaluated a PCT-guided strategy that included decisions on when to initiate antibiotic treatment, in contrast to other recent trials, including our study. In addition, the PRORATA study was an open-label trial, which raises the possibility that treatment bias might have occurred. Subsequent RCTs were unable to confirm that a PCT-guided strategy may significantly reduce the duration of antibiotic treatment in patients with non-microbiologically proven apparent sepsis10 or in patients with proven bacterial infection.11 Furthermore, the relatively large multi-centre ProGUARD study conducted by the ANZICS clinical trials group also showed no significant reduction in duration of antibiotic treatment, antibiotic-free days, or overall antibiotic exposure between a standard care group and a PCT-guided group.12 Unlike previous studies, the majority of investigators in that study used a PCT cut-off of < 0.1 µg/L for antibiotic cessation. Evaluating PCT distribution over time, the majority of patients did not meet the cut-off of < 0.1 µg/L until they were beyond 7 days of antibiotic treatment. This is further supported by a reported adherence rate to the protocol of 97%. Hence, given the strict stopping rule of that study, it is conceivable that clinicians who were adherent to the protocol continued antibiotic therapy. Another confounding point may have been the availability of an antimicrobial stewardship strategy that was available for the standard therapy arm, which was not present in the other studies presented. The most recent and largest multi-center pragmatic SAPS study of 1,575 critically ill patients with suspected infection in which the algorithm to stop antibiotics was similar to PRORATA demonstrated a reduction in both antibiotic exposure and mortality.13 As in PRORATA, however, around 50% of physicians did not adhere to the recommendation to stop antibiotic treatment. Despite the strength of the evidence described above, it is unclear how generalizable the findings from Western countries are to Asian countries, including Korea. Our study provides additional evidence that a reduction in antibiotic exposure can be achieved without an increase in morbidity or mortality when using PCT-guided antibiotic therapy in a different environment to that in Western nations. However, the use of PCT as well as its impact outside of the clinical trial setting should be evaluated,26 because compliance with PCT algorithms is associated with decreased antibiotic duration without increased adverse events.27
Several studies have suggested that PCT-guided antibiotic therapy could result in a total costs saving due to a reduction in ICU and hospital length of stays, as well as a reduction in the duration of antibiotic therapy.28,29,30 However, cost-effectiveness was not evaluated in detail in the previously published randomized controlled trials. In the SAPS study,13 the costs for the first course of antibiotics were reduced due to a reduction in antibiotic duration and cost, but cost-effectiveness was not evaluated. In the present study, where we used a cost-minimization approach, PCT-guided antibiotic therapy did not reduce overall treatment-related costs but it did reduce antibiotic therapy-related costs. It is difficult to explain this result; however, this may be due to the different proportions of patients that required renal replacement therapy on ICU admission in the two groups. It was announced on June 1, 201731 that the PCT test for sepsis management will be reimbursed by the National Health Insurance Service of Korea based on the results of the present study and a budget impact analysis of reimbursements (data not shown).
This study is the first randomized controlled study conducted in a non-Western country to determine the efficacy and safety of PCT-guided antibiotic therapy in critically ill patients with sepsis. A strength of our study is that we performed cost-effectiveness analysis of PCT-guided antibiotic therapy in Korea, which has a compulsory national health insurance system. However, several limitations of the study should be acknowledged. First, our study population was representative of severely ill patients with sepsis with a higher severity of illness than in previous reports. In addition, no surgical patients were included in this study. Therefore our findings cannot be extrapolated to surgical patients in whom PCT might be elevated even in the absence of infection.32 In addition, we cannot exclude a potential harm of shortening antibiotic treatment based on PCT level given the relatively small number of patients enrolled, although we did not find any differences in secondary outcomes between the two groups. Second, cost data were available from only two hospitals. Even though the characteristics of the patients registered at each hospital were similar, clinicians’ treatment patterns could have differed among the hospitals. Finally, antibiotics were not stopped despite the recommendation to stop antibiotic treatment in 43% of patients in the PCT group, which is consistent with other major trials on PCT guided antibiotic treatment.9,13 Despite its limitation, the cumulative rate of antibiotic cessation was significantly reduced, indicating that especially inappropriate antibiotics were the first to be discontinued, which might turn out to be a major contributor to antibiotic stewardship.
In conclusion, PCT-guided antibiotic discontinuation in critically ill patients with sepsis could reduce the duration of antibiotic use and its costs with no apparent adverse outcomes in a country with a high prevalence of antimicrobial resistance and a national health insurance system.
Footnotes
Funding: This study was supported by a grant from the Ministry of Health and Welfare, Republic of Korea (NA14-003).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jeon K, Suh JK, Lee SM.
- Formal analysis: Jeon K, Suh JK.
- Investigation: Jeon K, Suh JK, Ryu HG, Na S, Hong SB, Lee HJ, Kim JY, Lee SM.
- Methodology: Jeon K, Suh JK, Jang EJ, Cho S, Ryu HG, Na S, Hong SB, Lee HJ, Kim JY, Lee SM.
- Writing - original draft: Jeon K, Suh JK.
- Writing - review & editing: Jeon K, Suh JK, Jang EJ, Cho S, Ryu HG, Na S, Hong SB, Lee HJ, Kim JY, Lee SM.
References
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Thomas Z, Bandali F, Sankaranarayanan J, Reardon T, Olsen KM Critical Care Pharmacotherapy Trials Network. A multicenter evaluation of prolonged empiric antibiotic therapy in adult ICUs in the United States. Crit Care Med. 2015;43(12):2527–2534. doi: 10.1097/CCM.0000000000001294. [DOI] [PubMed] [Google Scholar]
- 3.File TM., Jr Duration and cessation of antimicrobial treatment. J Hosp Med. 2012;7(Suppl 1):S22–S33. doi: 10.1002/jhm.988. [DOI] [PubMed] [Google Scholar]
- 4.Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen JA, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- 5.Svoboda P, Kantorová I, Scheer P, Radvanova J, Radvan M. Can procalcitonin help us in timing of re-intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology. 2007;54(74):359–363. [PubMed] [Google Scholar]
- 6.Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177(5):498–505. doi: 10.1164/rccm.200708-1238OC. [DOI] [PubMed] [Google Scholar]
- 7.Hochreiter M, Köhler T, Schweiger AM, Keck FS, Bein B, von Spiegel T, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13(3):R83. doi: 10.1186/cc7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroeder S, Hochreiter M, Koehler T, Schweiger AM, Bein B, Keck FS, et al. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg. 2009;394(2):221–226. doi: 10.1007/s00423-008-0432-1. [DOI] [PubMed] [Google Scholar]
- 9.Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. PRORATA trial group. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 10.Annane D, Maxime V, Faller JP, Mezher C, Clec'h C, Martel P, et al. Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ Open. 2013;3(2):e002186. doi: 10.1136/bmjopen-2012-002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deliberato RO, Marra AR, Sanches PR, Martino MD, Ferreira CE, Pasternak J, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76(3):266–271. doi: 10.1016/j.diagmicrobio.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Shehabi Y, Sterba M, Garrett PM, Rachakonda KS, Stephens D, Harrigan P, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med. 2014;190(10):1102–1110. doi: 10.1164/rccm.201408-1483OC. [DOI] [PubMed] [Google Scholar]
- 13.de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819–827. doi: 10.1016/S1473-3099(16)00053-0. [DOI] [PubMed] [Google Scholar]
- 14.Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39(9):2048–2058. doi: 10.1097/CCM.0b013e31821e8791. [DOI] [PubMed] [Google Scholar]
- 15.Hohn A, Schroeder S, Gehrt A, Bernhardt K, Bein B, Wegscheider K, et al. Procalcitonin-guided algorithm to reduce length of antibiotic therapy in patients with severe sepsis and septic shock. BMC Infect Dis. 2013;13(1):158. doi: 10.1186/1471-2334-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. Geneva: World Health Organization; 2014. [Google Scholar]
- 18.Yong D, Shin HB, Kim YK, Cho J, Lee WG, Ha GY, et al. Increase in the prevalence of carbapenem-resistant acinetobacter isolates and ampicillin-resistant non-typhoidal salmonella species in Korea: a KONSAR study conducted in 2011. Infect Chemother. 2014;46(2):84–93. doi: 10.3947/ic.2014.46.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christ-Crain M, Stolz D, Bingisser R, Müller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 20.Yoon YK, Park GC, An H, Chun BC, Sohn JW, Kim MJ. Trends of antibiotic consumption in Korea according to National Reimbursement Data (2008–2012): a population-based epidemiologic study. Medicine (Baltimore) 2015;94(46):e2100. doi: 10.1097/MD.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181(1):176–180. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 22.Schuetz P, Albrich W, Christ-Crain M, Chastre J, Mueller B. Procalcitonin for guidance of antibiotic therapy. Expert Rev Anti Infect Ther. 2010;8(5):575–587. doi: 10.1586/eri.10.25. [DOI] [PubMed] [Google Scholar]
- 23.Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171(15):1322–1331. doi: 10.1001/archinternmed.2011.318. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 25.Howard P, Pulcini C, Levy Hara G, West RM, Gould IM, Harbarth S, et al. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother. 2015;70(4):1245–1255. doi: 10.1093/jac/dku497. [DOI] [PubMed] [Google Scholar]
- 26.Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically Ill patients with sepsis. Clin Infect Dis. 2017;64(11):1509–1515. doi: 10.1093/cid/cix179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albrich WC, Dusemund F, Bucher B, Meyer S, Thomann R, Kühn F, et al. Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in “real life”: an international, multicenter poststudy survey (ProREAL) Arch Intern Med. 2012;172(9):715–722. doi: 10.1001/archinternmed.2012.770. [DOI] [PubMed] [Google Scholar]
- 28.Heyland DK, Johnson AP, Reynolds SC, Muscedere J. Procalcitonin for reduced antibiotic exposure in the critical care setting: a systematic review and an economic evaluation. Crit Care Med. 2011;39(7):1792–1799. doi: 10.1097/CCM.0b013e31821201a5. [DOI] [PubMed] [Google Scholar]
- 29.Harrison M, Collins CD. Is procalcitonin-guided antimicrobial use cost-effective in adult patients with suspected bacterial infection and sepsis? Infect Control Hosp Epidemiol. 2015;36(3):265–272. doi: 10.1017/ice.2014.60. [DOI] [PubMed] [Google Scholar]
- 30.Kip MM, Kusters R, IJzerman MJ, Steuten LM. A PCT algorithm for discontinuation of antibiotic therapy is a cost-effective way to reduce antibiotic exposure in adult intensive care patients with sepsis. J Med Econ. 2015;18(11):944–953. doi: 10.3111/13696998.2015.1064934. [DOI] [PubMed] [Google Scholar]
- 31.Ministry of Health and Welfare. Republic of Korea. [Updated 2017]. [Accessed July 1, 2017]. http://www.mohw.go.kr/front_new/jb/sjb0406vw.jsp?PAR_MENU_ID=03&MENU_ID=030406&CONT_SEQ=339838&page=1.
- 32.Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schüttler J. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med. 1998;24(7):680–684. doi: 10.1007/s001340050644. [DOI] [PubMed] [Google Scholar]