Abstract
Purpose
Previous studies suggest that stereotactic body radiation therapy (SBRT) is associated with higher toxicity rates for central lung tumors relative to peripheral tumors when using 3 fraction SBRT. The initial results from Radiation Therapy Oncology Group study 0813 suggest a safe toxicity profile of SBRT administered in 5 fractions for central non-small cell lung cancer (NSCLC). We reviewed our institutional data to evaluate the safety and efficacy of SBRT for central NSCLC.
Methods and materials
We reviewed our prospectively collected SBRT database for patients with central NSCLC who received SBRT between 2008 and 2014. The most frequent dose and fractionations were 50 Gy in 5 fractions (59%) and 48 Gy in 4 fraction (30%). Local control (LC), regional control, metastasis-free survival, and overall survival were calculated using Kaplan-Meier estimates. The National Cancer Institute Common Terminal Criteria for Adverse Events were used for toxicity grading.
Results
A total of 110 central lung tumors in 103 patients were included. The median age was 74 years (range, 40-95 years), and the median follow-up time of living patients was 50 months. The mean tumor size was 20 mm (range, 5-70 mm). The 5 year rate of LC, regional control, and distant control was 89%, 77%, and 82%, respectively. The median and 5-year overall survival were 3.5 years and 35%, respectively. No treatment variables were associated with tumor control or other clinical outcomes. A single patient experienced grade 3 radiation pneumonitis (0.97%). The rate of late toxicity grade ≥3 was 9.7% (grade 3, 7.7%; grade 4, 0.97%; grade 5, 0.97%) and included pneumonitis (3.9%), bronchial necrosis (2.9%), myocardial dysfunction (1.9%), and worsening heart failure (0.97%).
Conclusions
SBRT for central NSCLC provides high rates of LC. Despite excellent LC, patients remain at risk for regional and distant failure. The rate of grade 3 pneumonitis was consistent with that of prior reports. We observed low rates of grade 4-5 toxicity potentially attributable to SBRT. Our results contribute to the growing body of data in support of the safety of SBRT for central NSCLC.
Introduction
Stereotactic body radiation therapy (SBRT) is a standard-of-care definitive treatment modality for patients with medically inoperable non-small cell lung cancer (NSCLC) or those who are medically operable and decline surgery.1 SBRT is associated with excellent rates of local control (LC) and overall survival (OS) in a patient population that is often associated with significant comorbidities.2, 3, 4, 5, 6 The Radiation Therapy Oncology Group (RTOG) trial 0236 included patients with medically inoperable peripheral primary NSCLC and achieved LC and lobar control rates of 97.6% and 90.6%, respectively, at 3 years.6 The prospective study only included peripheral NSCLC because early prospective trials demonstrated excessive toxicity in central lung tumors treated with SBRT.7 Patients with perihilar/central tumors, defined as those within 2 cm of the proximal bronchial tree, experienced an 11-fold increased risk of severe toxicity, including 6 patients with grade 5 toxicity.
Despite caution from early evidence of 3-fraction regimens for central lesions, many centers use hypofractionated dose escalation with alternative dose fractionation regimens with a lower dose per fraction. Several institutions have reported outcomes with a variety of dose-fractionation schemes with acceptable toxicity and tumor control.8, 9, 10, 11, 12 The outcomes and toxicities of SBRT for central lung tumors are not well defined because the results of RTOG 0813, a prospective trial that evaluated SBRT for treatment of central NSCLC tumors, have not been published to date.
Many radiation therapy centers across the United States are implementing SBRT within their practices, especially to treat lung tumors.13 As such, there is important need to further evaluate the safety and efficacy of SBRT use for central tumors. To further aid this process, we investigated our institutional experience using SBRT for central lung tumors. To the best of our knowledge, this is the largest study to analyze the toxicity and outcomes of central lung NSCLC treated with SBRT.
Methods and Materials
Patients
This study was approved by our institutional review board. Our institution prospectively collects patient demographic and treatment-related data for all patients treated with SBRT. We retrospectively analyzed all patients with central NSCLC treated with SBRT between April 2008 and November 2014. Patients included in the study are those with central lung tumors, defined as gross tumor volume within 2 cm of the proximal bronchial tree or planning target volume (PTV) adjacent to mediastinal or pericardial pleura, as previously defined.7 Patients with metastasis to central lung locations from other primary sites were excluded.
All patients underwent a complete history, physical examination, and imaging with computed tomography (CT) and positron emission tomography (PET) for staging evaluation of the primary tumor and presence of distant metastasis. All patients either had histologic confirmation of NSCLC or evidence of tumor progression on serial CT imaging, with PET findings consistent with localized, node-negative NSCLC. All patients were deemed medically inoperable or declined surgery. Patient selection for SBRT was typically determined through multidisciplinary discussion. If patients were deemed intolerable of the potential risks of a biopsy, they were clinically diagnosed based on radiographic findings.
Mediastinal staging with endobronchial ultrasound was performed when there was a concern for nodal involvement based on size criteria and CT or fluorodeoxyglucose avidity on PET evaluation, per the discretion of the treating physician. Patients found to have biopsy or PET evidence of node positive or metastatic extrathoracic disease were excluded from the study.
SBRT technique
Patient simulation was performed using the Body-Fix whole-body immobilization system (Medical Intelligence, Schwabmunchen, Germany). Tumor motion was assessed with 4-dimensional CT imaging with Varian real-time position management (Varian Medical Systems, Palo Alto, CA). The breath-hold technique or abdominal compression were employed at the discretion of the treating radiation oncologist and were typically used when tumor motion was >1 cm or when treatment without would otherwise result in higher organ-at-risk irradiation. Gross tumor volume and internal target volume were segmented on a breath-hold or 4-dimensional CT scan, respectively. A 5-mm uniform margin was typically used for creation of the PTV. The dose and fractionation was at the discretion of the treating radiation oncologist.
Radiation therapy planning consisted of 3-dimensional conformal planning with multiple coplanar beams or intensity modulated radiation therapy with static gantry or volumetric modulated arc therapy–based techniques. Daily cone-beam CT was used for image guided radiation therapy localization. Treatment was delivered on consecutive weekdays. Dose constraints for organs at risk followed the recommendations of the American Association of Physicists in Medicine Task Group 101 report and protocol guidelines from RTOG 0813.14 The recommended maximal dose to the trachea and proximal bronchial tree was 30 Gy, 35 Gy, and 40 Gy for 3-, 4-, and 5-fraction regimens, respectively. The esophageal recommended maximal dose ranged from 25.2 to 35 Gy, and maximal heart dose ranged from 30 to 38 Gy, increasing with the number of fractions used. For 5-fraction regimens, mediastinal structures were permitted to have a maximal point dose of 105% of the prescription dose.
Statistical analysis
The primary end point of this study was assessment of toxicity attributable to radiation therapy at any time point after completion of radiation therapy and cancer-related outcomes. Toxicity was scored using the National Cancer Institute Common Terminal Criteria for Adverse Events, version 4.0. Acute toxicity was defined as toxicity that occurred within the first 3 months after SBRT, and late toxicity was considered at any time point beyond 3 months. Local failure was defined as primary tumor progression or recurrence based on serial CT/PET imaging, as well as marginal failures within 1 cm of the PTV. Regional failure was defined as lobar or mediastinal lymph node relapses. Metastatic failure was defined as nonregional nodal failure or contralateral lung or distant organ relapse. LC, regional control, OS, and metastasis-free survival (MFS) and progression were calculated using the Kaplan-Meier method and measured from the date of the last SBRT to the date of the event, last documented evaluation, or patient death.
Potential patient and treatment variables associated with clinical outcomes were analyzed using Cox proportional hazard models in both univariate and multivariate analyses. Multivariate analysis was performed using the backward selection method, incorporating prognostic factors with a P-value of < .2. A P-value of < .05 was considered statistically significant. Radiation dose and fractionation was normalized using a biologically effective dose (BED) of 10 Gy, which was calculated using the following equation:
where n is the number of fractions, d the dose per fraction, and was 10 for early responding tissue. All statistical analyses were performed using JMP software (SAS analytics), version 10.0.0.
Patient follow-up and toxicity evaluation
Patient follow-up typically involved serial thin-slice chest CT scans that included the adrenal glands with contrast every 3 to 6 months after SBRT. PET imaging was used when CT imaging suggested local relapse or metastatic disease. Tumor response was assessed and reported based on Response Evaluation Criteria in Solid Tumors, version 1.1. Percutaneous or endoscopic biopsies were performed for confirmation of local or regional relapse. Patients were assessed for toxicity on follow-up visits every 3 to 6 months with interim history and physical examination, which were documented prospectively.
Results
Patient and treatment characteristics
A total of 103 patients with 110 central NSCLC lesions were identified from our institutional SBRT database. Patient and treatment characteristics are reported in Table 1. The median follow-up time for all living patients is 50 months (range, 4.7-79.5 months). The median patient age at SBRT was 74 years (range, 40-95 years), and women composed the majority (n = 60; 54.5%). A total of 72.7% of patients (n = 80) had biopsy-proven malignancies, and the remaining patients were diagnosed on the basis of radiographic imaging.
Table 1.
Patient summary
| Characteristic | Value, n (%) | |
|---|---|---|
| Age | Median (range), y | 73 (40-95) |
| Sex | Male | 50 (45.5) |
| Female | 60 (54.5) | |
| T Stage | 1a | 56 (50.9) |
| 1b | 25 (22.7) | |
| 2a | 21 (19.1) | |
| 2b | 8 (7.3) | |
| Tumor size | Median (range), mm | 20 (5-70) |
| Laterality | Right | 56 (51) |
| Left | 54 (49) | |
| Lobe | Lower | 37 (33.6) |
| Middle | 11 (10) | |
| Upper | 62 (56.4) | |
| Biopsy proven | Yes | 80 (72.7) |
| Histology | Adenocarcinoma | 34 (30.9) |
| Squamous cell | 35 (31.8) | |
| Other | 11 (10) | |
| Unknown | 30 (27.3) | |
| Previous thoracic radiation | Stereotactic body radiation therapy | 8 (7.3) |
| External beam radiation therapy None |
7 (6.4) 95 (86.4) |
|
| Dose and fractionation | 60 Gy/5 fractions | 2 (1.8) |
| 54 Gy/3 fractions | 3 (2.7) | |
| 50 Gy/5 fractions | 65 (59.1) | |
| 48 Gy/4 fractions | 34 (30.1) | |
| Duration of stereotactic body radiation therapy, d | Other | 6 (5.4) |
| 3 | 3 (2.7) | |
| 4 | 29 (26.4) | |
| 5 | 52 (47.2) | |
| 6-10 | 26 (23.7) |
Squamous cell carcinoma was the most common histology (31.8%). The median tumor size was 2.0 cm (range, 0.5-7.0 cm). A total of 36 patients (32.7%) underwent previous pulmonary surgery, 7 patients (6.4%) received prior conventionally fractionated external beam radiation therapy, and 8 patients (7.3%) received prior thoracic SBRT. The most frequent doses and fractionations were 50 Gy in 5 fractions (59%) and 48 Gy in 4 fractions (30%), with a median BED10Gy of 100 (interquartile range, 100-105.6; range, 57.6-151.2).
Toxicity
Treatment was well tolerated with infrequent acute toxicity (Table 2). Overall, a total of 3 patients experienced grade 1 to 2 acute toxicity that was attributable to SBRT, including fatigue (n = 1; 0.97%) and cough (n = 2; 1.9%). A single patient (0.97%) experienced acute toxicity of grade 3 radiation pneumonitis that was treated with corticosteroids.
Table 2.
Grade ≥3 complications
| Toxicity | Type | Toxicity grade | n (%) |
|---|---|---|---|
| Acute toxicity | Radiation pneumonitis | 3 | 1 (0.97) |
| Late toxicity | Heart failure | 3 | 1 (0.97) |
| Myocardial infarction | 3 | 2 (1.9) | |
| Bronchial necrosis | 3 | 1 (0.97) | |
| Bronchial necrosis | 4 | 1 (0.97) | |
| Bronchial necrosis | 5 | 1 (0.97) | |
| Radiation pneumonitis | 3 | 4 (3.9) |
Overall, the rate of any grade 1 or 2 late toxicity that was potentially attributable to SBRT was 64.1% (n = 66; Table 2); this most frequently included fatigue, cough, chest wall pain, or mild worsening of subjective pulmonary function. A total of 4 patients experienced grade 2 pulmonary dysfunction that was empirically treated with corticosteroids. The rate of grade ≥3 late toxicity was 9.7% (grade 3, 8; grade 4, 1; and grade 5, 1) and included grade 3 radiation pneumonitis (n = 4; 3.9%), grade 3 myocardial infarction (n = 2; 1.9%), grade 3 heart failure (n = 1; 0.97%), grade 3 necrosis of proximal airway (n = 1; 0.97%), and grade 4 (n = 1; 0.97%) and grade 5 (n = 1; 0.97%) necrosis of the proximal airway with distal lung collapse and respiratory compromise. All patients who experienced grade 3 pneumonitis were prescribed 50 Gy in 5 fractions, and a single patient underwent treatment to 2 pulmonary lesions that were treated simultaneously. The average V20 Gy for these patients was 9.3% (range, 7.6-12.1%), and the mean dose to the total lung was 6.6 Gy (range, 6.1-7.3 Gy).
The patient who developed late grade 3 proximal airway necrosis presented with a 3.5 cm tumor in the right hilum. He was prescribed 57.5 Gy in 5 fractions, planned with volumetric modulated arc therapy. The maximal point dose to the proximal bronchial tree was 65.2 Gy. The dose to 1 cm3 was 60.05 Gy, and dose to 0.1 cm3 was 63.5 Gy.
In comparison, the patient who experienced late grade 5 proximal airway necrosis was a 60-year-old man who underwent right pneumonectomy approximately 13 years earlier with no adjuvant treatment. He developed a 4.75-cm tumor in the perihilar region of the remaining left lung at the level of the upper lobe bronchus. He was treated with SBRT in 2009 and prescribed to 48 Gy in 4 fractions with 3-dimensional conformal planning using 12 fields. The left upper bronchus received a maximum point dose of 59.9 Gy, with doses to 1 cm3 and 0.1 cm3 of 56.2 Gy and 59.2 Gy, respectively. Approximately 6 months after completion of SBRT, the patient developed diminished pulmonary capacity and was found to have granulation tissue completely obstructing the left upper lobe and lingual, as noted on endoscopy. Pulmonary function progressively declined, and the patient died 7 months after SBRT.
Clinical outcomes
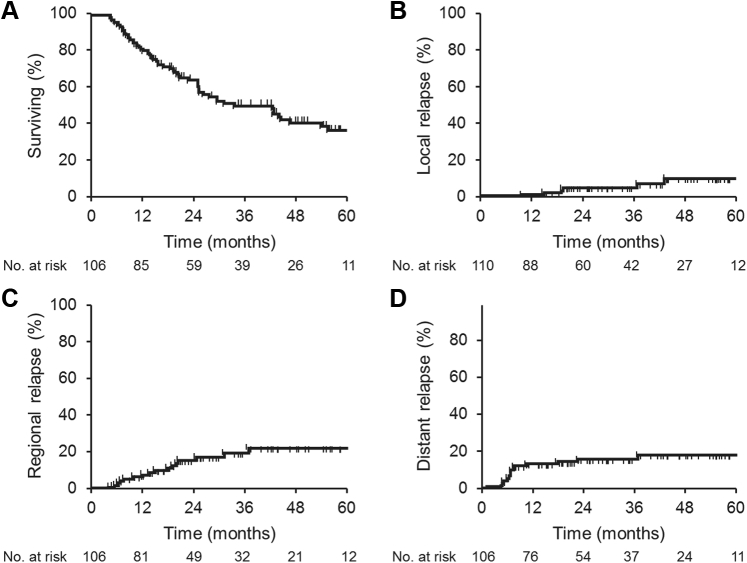
The median OS for all patients was 42.1 months, and the 1-, 3-, and 5-year rates of OS were 81.4%, 50.8%, and 35%, respectively (Fig 1A). Univariate predictors of OS only included stage T2 versus T1 (hazard ratio, 1.9; 95% confidence interval, 1.13-3.4; P = .019).
Fig. 1.
Kaplan Meyer survival estimates for patients treated with stereotactic body radiation therapy. (A) Overall survival; (B) local tumor control; (C) regional control; (D) metastasis-free survival based on dose.
Seven local recurrences were diagnosed at a median time period of 18.9 months (range, 9-62.4 months) after SBRT. The 1-, 3-, and 5-year rates of LC were 98.9%, 95%, and 89%, respectively (Fig 1B). Maximal tumor response was graded as complete response, partial response, or stable disease in 30 (27.2%), 63 (57.3%), and 17 (15.5%) patients, respectively, based on the Response Evaluation Criteria in Solid Tumors, version 1.1. Given the infrequent events of local recurrence, no prognostic association of univariates for the prediction of local failure was observed, including items such as histology, conformational biopsy, tumor size, dose and fractionation, or BED.
After SBRT, the median time to either regional or metastatic failure was 20.5 and 23.6 months, respectively. The 1-, 3-, and 5-year rates of regional control were 91.6%, 79.1%, and 77%, respectively, and the rates of MFS were 85.6, 82.7%, and 82.0%, respectively (Fig 1C, D). On Cox regression analysis, only tumor size of >2 cm was shown to be significantly associated with MFS (hazard ratio, 3.9; 95% confidence interval, 1.3-12.0; P < .02). No prognostic association of univariates was found for the prediction of regional control.
Discussion
SBRT has become widely implemented for the management of lung tumors in a variety of clinical settings. However, the optimal dose and fractionation schedule to achieve the ideal toxicity profile for the management of central lung tumors is unclear because the heterogeneity of treatment regimens, histologies, and patient populations reported in the current literature. In the present study, the patient population was restricted to patients with NSCLC and represents one of the largest studies on toxicity and outcomes of central lung tumors within this patient population.
We report excellent rates of 1-, 3-, and 5-year LC of 98.9%, 95%, and 89%, respectively, which was associated with an overall 9.7% rate of significant late toxicity (grade ≥3) and adds firm support for the safety and efficacy profile of SBRT for central lung irradiation.
Several single institutions have published their experiences and rates of toxicity with treatment of central tumors using SBRT and other hypofractionated regimens with 8 or 10 fractions. Chang et al reported on the MD Anderson Cancer Center experience for the treatment of centrally or superiorly located tumors.15 A total of 27 patients were treated and received either 40 Gy or 50 Gy in 4 fractions. The crude rate of LC was 100% with 50 Gy and 57% for 40 Gy in 4 fractions, which is consistent with the results from prior studies that show excellent rates of LC with BED10Gy > 100.16
Grade 2 radiation pneumonitis was observed in 28.6% of patients, and 1 patient developed brachial plexopathy. After this experience, Chang et al reported on an updated series of 100 patients who were treated with 50 Gy in 4 fractions or 70 Gy in 10 fractions.17 The actuarial LC rate was 96.5% for all patients, and centrally treated tumors demonstrated a safe toxicity profile with a 12% rate of radiation pneumonitis (grade 2, 11; grade 3, 1). No airway stenosis or narrowing was observed. Li et al further evaluated patients treated with 70 Gy in 10 fractions in a study of 82 patients.8 LC was excellent with a reported 2-year rate of 96.2%. Toxicity was low, but the radiation pneumonitis rate was slightly higher than that previously reported (grade 2, 2.4%).
In a study of 125 patients, Modh et al reported on the Memorial Sloan Kettering experience of SBRT for central lung tumors.18 Similar to the present study, the most frequent dose schedule was 45 to 50 Gy in 5 fractions or 48 Gy in 4 fractions, all of which had similar rates of LC regardless of dose. Toxicity was minimal, with 19 cases of radiation pneumonitis (grade 2, 18; grade 5, 1), 16 cases of esophagitis (grade 2, 14; grade 3, 2), and 3 patients with cardiac complications (grade 2, 2; grade 3, 1). The overall rate of severe complications was reported at 8%. We report a comparable rate of severe late toxicity (grade 3-5, 9.7%) in the present study, which is similar to what we previously reported for peripheral lung tumor SBRT.
With a dose and fractionation scheme most consistent with the present study, RTOG 0813 is a prospective phase 1/2 clinical trial designed to evaluate the maximum tolerated dose and efficacy of 5 fractions of SBRT for centrally located NSCLC, and the results have published only in abstract form to date.19, 20 From 2009 through 2013, a total of 120 patients were accrued. At the highest dose level (60 Gy in 5 fractions), dose-limiting toxicity was observed at 7.2% with a median follow-up of 26.6 months.20 With a dose per fraction ranging from 10 to 12 Gy, grade 3, 4, and 5 toxicities occurred in 10, 1, and 4 patients, respectively. Grade 5 toxicity was related to cardiac (n = 1), respiratory (n = 2), and pulmonary hemorrhage (n = 1).
In analysis of efficacy, the 2-year rates of LC for 11.5 Gy and 12 Gy per fraction were 89.4% and 87.7%, respectively.20 OS and progression-free survival were also similar between the doses. In a single institution, a prospective study evaluating 50 and 55 Gy in 5 fractions, Roach et al showed a higher rate of late grade 3 and 5 toxicities than the present study and RTOG 0813 (grade 3, 27%; grade 4, 12%; and grade 5, 2%). However, in this report, the attribution of these toxicities to radiation treatment was somewhat unclear because of the limited data on hospitalization at outside facilities and underscores the difficulty of attribution to radiation in generally unhealthy and high-risk patients.21
Our results compare favorably with those of the aforementioned studies, with 1- and 3-year LC rates of 98.9% and 95%, respectively, and a 9.7% rate of significant late toxicity (grade ≥3). Currently, whether SBRT with 5 fractions or a more prolonged hypofractionation with 8 to10 fractions is safer remains uncertain. However, the growing body of evidence suggests similar rates of both tumor control and toxicity with either regimen. Tekatli et al reported results of 80 patients with centrally located NSCLC who were treated at the VU Medical Center.22 The rate of grade 3 toxicity was 6.4% with no observation of grade 4 events, and OS was similar to that reported for 3 to 5 fractions of SBRT.
Previous studies report tumor size as a significant factor affecting LC. Dunlap et al reported significantly reduced LC at 2 years for T2 tumors versus T1 tumors (70% vs. 90%; P = .03).23 In the present study, we observed no difference in LC when tumor size is accounted for. Given that the vast majority of patients received a BED10Gy of ≥100 and with so few events, our sample size would be inadequate to detect a small difference in tumor control based on tumor size.
In addition, we observed an association between T2 tumors and OS, but no further clinical endpoints. Previous comparisons of SBRT and surgery show that clinical staging with PET/CT may underestimate patient stage in up to 35% of patients.24 Recent reports by Akthar et al demonstrated a lower negative predictive value of PET imaging of N1 disease in patients evaluated for SBRT with T2 tumors or centrally located tumors.25 Therefore, it is probable that some patients in our cohort with T2 tumors may have had more advanced subclinical disease, such as occult N1 or N2 disease, which could affect OS in this subset of patients.
Lack of histopathologic confirmation of malignancy, which is often omitted in high-risk patients, has been suggested to result in inflated reports of OS owing to the inclusion of benign pulmonary lesions. In the present study, a total of 30 patients (27.3%) did not have histologic confirmation of primary lung cancer but were treated on the basis of interval tumor growth on CT and PET/CT imaging that was consistent with malignancy, as well as other risk factors for the development of lung cancer. This can be considered a limitation to our study, but prior reports have shown that when PET imaging is used as a component of the work-up for NSCLC, the rate of benign disease diagnosis after surgery is low.26, 27 To address this concern, clinical outcomes were reviewed and dichotomized based on tissue confirmation versus no biopsy, and no differences in OS or other outcomes were observed.
Notably, all patients in the present study received radiation therapy on consecutive week days. Retrospective reports have found no detrimental impact on LC or OS for patients treated on consecutive versus nonconsecutive days.28 Concern regarding normal tissue repair between fractions have led to many trials mandating treatments on an every-2-day basis. RTOG 0236 and 0813 required a minimum of 40 hours between fractions. In contrast, 1 arm of RTOG 0915 delivered 48 Gy in 4 fractions over 4 consecutive days, but this trial only enrolled patients with peripheral tumors. Therefore, even among expert opinions and clinical trial designs, no consensus has been met with regard to this topic.
The favorable toxicity profile observed in this study provides further evidence of the safety profile of SBRT for central NSCLC even when delivered in a consecutive fashion, which suggests that extended treatment schedules may not be necessary.
Conclusions
SBRT for central lung tumors offers very high rates of LC and acceptable rates of severe toxicity comparable with peripheral tumors. Although extended hypofractionation schedules have been employed in previous studies, our data indicate that 4 to 5 fractions of SBRT administered on consecutive days is safe and effective, with the added benefit of a shortened treatment time for the patient. Our results, although limited because of their retrospective nature, lend further credence to the use of SBRT in the management of central NSCLC while awaiting results from prospective trials.
Footnotes
Sources of support: This work had no specific funding.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.National Comprehensive Cancer Network . Non-small cell lung cancer; 2017. NCCN guidelines version 3.2017. [Google Scholar]
- 2.Baschnagel A.M., Mangona V.S., Robertson J.M., Welsh R.J., Kestin L.L., Grills I.S. Lung metastases treated with image-guided stereotactic body radiation therapy. Clin Oncol (R Coll Radiol) 2013;25:236–241. doi: 10.1016/j.clon.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Fakiris A.J., McGarry R.C., Yiannoutsos C.T. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 4.Kelsey C.R., Salama J.K. Stereotactic body radiation therapy for treatment of primary and metastatic pulmonary malignancies. Surg Oncol Clin N Am. 2013;22:463–481. doi: 10.1016/j.soc.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Singh D., Chen Y., Hare M.Z. Local control rates with five-fraction stereotactic body radiotherapy for oligometastatic cancer to the lung. J Thorac Dis. 2014;6:369–374. doi: 10.3978/j.issn.2072-1439.2013.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timmerman R., Paulus R., Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmerman R., McGarry R., Yiannoutsos C. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 8.Li Q., Swanick C.W., Allen P.K. Stereotactic ablative radiotherapy (SABR) using 70 Gy in 10 fractions for non-small cell lung cancer: Exploration of clinical indications. Radiother Oncol. 2014;112:256–261. doi: 10.1016/j.radonc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Nuyttens J.J., van Zyp N.C.V., Praag J. Outcome of four-dimensional stereotactic radiotherapy for centrally located lung tumors. Radiother Oncol. 2012;102:383–387. doi: 10.1016/j.radonc.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Rowe B.P., Boffa D.J., Wilson L.D. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol. 2012;7:1394–1399. doi: 10.1097/JTO.0b013e3182614bf3. [DOI] [PubMed] [Google Scholar]
- 11.Schanne D.H., Nestle U., Allgauer M. Stereotactic body radiotherapy for centrally located stage I NSCLC: A multicenter analysis. Strahlenther Onkol. 2015;191:125–132. doi: 10.1007/s00066-014-0739-5. [DOI] [PubMed] [Google Scholar]
- 12.Song S.Y., Choi W., Shin S.S. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer. 2009;66:89–93. doi: 10.1016/j.lungcan.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Pan H., Simpson D.R., Mell L.K., Mundt A.J., Lawson J.D. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011;117:4566–4572. doi: 10.1002/cncr.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedict S.H., Yenice K.M., Followill D. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 15.Chang J.Y., Balter P.A., Dong L. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol. 2008;72:967–971. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onishi H., Araki T., Shirato H. Stereotactic hypofractionated high-dose irradiation for stage i nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101:1623–1631. doi: 10.1002/cncr.20539. [DOI] [PubMed] [Google Scholar]
- 17.Chang J.Y., Li Q.Q., Xu Q.Y. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: How to fly in a "no fly zone". Int J Radiat Oncol Biol Phys. 2014;88:1120–1128. doi: 10.1016/j.ijrobp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Modh A., Rimner A., Williams E. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2014;90:1168–1176. doi: 10.1016/j.ijrobp.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezjak A., Paulus R., Gaspar L. Efficacy and toxicity analysis of NRG Oncology/RTOG 0813 trial of stereotactic body radiation therapy (SBRT) for centrally located non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 2016;96:S8. [Google Scholar]
- 20.Bezjak A., Paulus R., Gaspar L. Primary study endpoint analysis for NRG Oncology/RTOG 0813 trial of stereotactic body radiation therapy (SBRT) for centrally located non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 2016;1:5–6. [Google Scholar]
- 21.Roach M.C., Robinson C.G., DeWees T.A. Stereotactic body radiation therapy for central early-stage NSCLC: Results of a prospective phase I/II trial. J Thorac Oncol. 2018;13:1727–1732. doi: 10.1016/j.jtho.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Tekatli H., Senan S., Dahele M., Slotman B.J., Verbakel W.F. Stereotactic ablative radiotherapy (SABR) for central lung tumors: Plan quality and long-term clinical outcomes. Radiother Oncol. 2015;117:64–70. doi: 10.1016/j.radonc.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Dunlap N.E., Larner J.M., Read P.W. Size matters: A comparison of T1 and T2 peripheral non-small-cell lung cancers treated with stereotactic body radiation therapy (SBRT) J Thorac Cardiovasc Surg. 2010;140:583–589. doi: 10.1016/j.jtcvs.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 24.Crabtree T.D., Denlinger C.E., Meyers B.F. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140:377–386. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 25.Akthar A.S., Ferguson M.K., Koshy M., Vigneswaran W.T., Malik R. Limitations of PET/CT in the detection of occult N1 metastasis in clinical stage I(T1-2an0) non-small cell lung cancer for staging prior to stereotactic body radiotherapy. Technol Cancer Res Treat. 2017;16:15–21. doi: 10.1177/1533034615624045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Tinteren H., Hoekstra O.S., Smit E.F. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: The plus multicentre randomised trial. Lancet. 2002;359:1388–1393. doi: 10.1016/s0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- 27.Herder G.J., Kramer H., Hoekstra O.S. Traditional versus up-front [18F] fluorodeoxyglucose-positron emission tomography staging of non-small-cell lung cancer: A Dutch cooperative randomized study. J Clin Oncol. 2006;24:1800–1806. doi: 10.1200/JCO.2005.02.4695. [DOI] [PubMed] [Google Scholar]
- 28.Samson P., Rehman S., Juloori A. Local control for clinical stage I non-small cell lung cancer treated with 5-fraction stereotactic body radiation therapy is not associated with treatment schedule. Pract Radiat Oncol. 2018;8:404–413. doi: 10.1016/j.prro.2018.04.004. [DOI] [PubMed] [Google Scholar]