Abstract
Purpose
This study aimed to compare outcomes of patients with prostate cancer with bone metastases treated with stereotactic body radiation therapy (SBRT) versus conventionally fractionated radiation therapy (CFRT).
Methods and materials
An institutional, retrospective review was conducted of patients with prostate cancer receiving radiation therapy to bone metastases. In-field failure (IFF) was the primary outcome of the study, and distant failure (DF) and biochemical failure (BF) were secondary outcomes.
Results
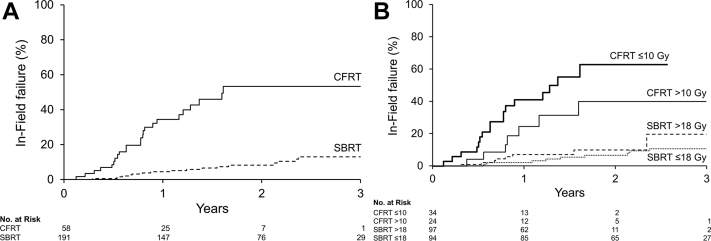
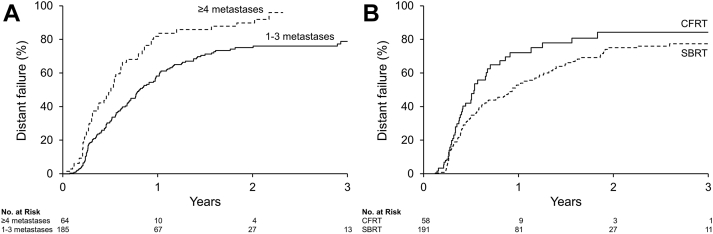
A total of 249 metastases (191 SBRT; 58 CFRT) in 201 patients with a median follow-up of 2.2 years were analyzed. The SBRT prescription dose was predominantly 18 Gy (45.5%) or 20 Gy (46.6%) in a single fraction. CFRT was given either as 8 Gy in 1 fraction (56.9%) or 20 Gy in 5 fractions (41.4%). Imaging follow up was performed most frequently with 11C-choline positron emission tomography/computed tomography (79%) or bone scan (10%). The median time to IFF was 1.6 years for CFRT-treated lesions and not met (>4.4 years) for SBRT. The 1- and 3-year IFF estimates were 34.4% (95% confidence interval [CI], 19.9-46.2) and 53.3% (95% CI, 34.3-66.8) for lesions treated with CFRT compared with 4.5% (95% CI, 1.4-7.5) and 12.9% (95% CI, 6.6-18-8) for those treated with SBRT (P < .01). On multivariate regression, the hazard ratio (HR) for IFF with CFRT compared with SBRT was 6.8 (95% CI, 3.7-12.5; P < .01). There were nonsignificant reduced rates of BF (HR, 1.4; 95% CI, 1.0-2.1; P = .05) and DF (HR, 1.3; 95% CI, 1.0-1.8; P = .08) in patients who received SBRT. The 3-year BF and DF estimates in these patients were 88.6% (95% CI, 82.0-92.8) and 82.2% (95% CI, 74.5-87.6), respectively.
Conclusions
SBRT for the management of prostate cancer bone metastases significantly reduces radiographic IFF. However, the high rate of subsequent DF and BF highlights the challenges in selecting patients who may benefit from aggressive radiation therapy.
Introduction
Prostate cancer (PCa) is the second leading cause of cancer-related mortality and the most common noncutaneous malignancy in men.1 The standard of care for men with metastatic PCa is systemic therapy (ST), primarily androgen deprivation therapy (ADT). Although survival is extended, these treatments are not curative and can be associated with significant morbidity and detriments to overall quality of life.2
Weichselbaum and Hellman hypothesized the existence of an intermediate state between localized and widespread dissemination, termed oligometastatic disease, in which malignant cells occupy a limited number of distant sites.3 In patients with oligometastases, often defined as ≤3 metastases, definitive local therapy has been proposed as a potential treatment option to delay disease progression and the need for ST.4
Stereotactic body radiation therapy (SBRT) uses precise immobilization and imaging to deliver highly conformal and ablative doses of radiation therapy (RT) in ≤5 fractions. Over the past decade, SBRT has emerged as a promising treatment technique for patients with oligometastatic disease.5 The safety and efficacy of SBRT for treating PCa bone metastases have been previously validated.6, 7, 8 Conventionally fractionated RT (CFRT) delivering moderate doses of RT has been demonstrated in multiple prospective studies to provide symptomatic relief of painful bone metastases.9 In the setting of bone metastases from renal cell carcinoma, SBRT has been associated with superior rates of radiographic and clinical control when compared with CFRT.10 However, data assessing local control for SBRT compared with CFRT in the treatment of PCa bone metastases are lacking.
In the current study, we compared the efficacy of SBRT versus CFRT in the treatment of PCa bone metastases and determine whether patient and treatment characteristics associated with improved outcomes can be identified.
Methods and Materials
Patients and follow up
After institutional review board approval, a retrospective evaluation was conducted of all patients with PCa receiving RT to bone metastases between January 2013 and May 2017 at a single institution. Patients were excluded from analysis if <6 months of clinical and imaging follow-up were available. Clinical follow up, although not mandated by a prespecified protocol, typically occurred at 3-month intervals in association with serum prostate-specific antigen (PSA) and radiographic surveillance. Imaging most commonly consisted of 11C-choline positron emission tomography (PET)/computed tomography (CT), technetium 99m-methyl diphosphonate bone scan, or CT at 3- or 6-month intervals.
Treatments
Patient RT and ST were administered at the discretion of the treating physicians. Pre-RT ST was defined as the receipt of ADT and/or chemotherapeutic agents that concluded more than 3 months before the start of RT, and peri-RT ST was the receipt of these agents within 3 months before, concurrent with, or after RT.
All RT treatments were administered with the use of a photon linear accelerator, and the RT dose, target volumes, and delivery techniques were selected by the treating radiation oncologist. CFRT was defined by the receipt of a prescription dose of either 8 to 10 Gy in 1 fraction or 20 Gy in 5 fractions. SBRT patients received a single fraction of 16 to 24 Gy, using target volumes as described previously.7 There was greater variation in treatment volumes for CFRT patients, but most involved a 1.5 to 2.5 cm expansion from gross tumor volume to planning target volume (PTV). All CFRT and SBRT patients received image guided target volume localization prior to each fraction, with CFRT patients typically aligned using paired orthogonal x-rays and SBRT patients with cone beam CT or ExacTrac (Brainlab, Munich, Germany) localization.
Biologically effective dose (BED) was calculated for each dose prescription using the following formula:
where n is the number of fractions, d the dose per fraction, and an α/β of 3.
Outcomes
In-field failure (IFF) was studied as the primary endpoint and was defined as any increase in size or radiotracer avidity of the treated lesion, subsequent use of a secondary local salvage therapy to the treated site, or the development of a new lesion within the initial 50% isodose line. Distant failure (DF) was defined as the appearance of new metastatic disease on follow-up imaging. As recommended by Scher et al, biochemical failure (BF) was defined as any of the following: (1) an initial decline from baseline PSA was observed, a PSA increase of ≥25% and ≥2 ng/mL above the nadir, or an increase of ≥25% and greater than the pretreatment PSA value, as confirmed by a second value ≥3 weeks later; (2) no initial decline from baseline if the baseline PSA was ≥2 ng/mL, a PSA increase of ≥25% and ≥2 ng/mL greater than baseline after 3 months, or a PSA increase of ≥2 ng/mL after 3 months if the baseline PSA was <2 ng/mL11; or (3) DF or initiation of ST occurring before a PSA increase meeting either of the 2 previous criteria.
Initiation or escalation of ST after RT was also evaluated. Escalation of ST was defined as any progression along the following continuum: ADT → second-generation antiandrogens (abiraterone or enzalutamide) → chemotherapy.
Post-RT toxicities were recorded in accordance with the Common Terminology Criteria for Adverse Events, version 4.03. All radiographic posttreatment in-field fractures within the treatment volume were also recorded.
Statistical analysis
Demographic and disease characteristics at diagnosis were compared between patients receiving SBRT and CFRT. Four patients received both SBRT and CFRT and were excluded from patient-based comparisons. Characteristics at the time of RT were compared between lesions treated with SBRT and CFRT. Comparisons were performed using the χ2 test for categorical data and the Wilcoxon rank sum test for continuous data.
All outcomes were calculated from the date of RT completion. Patients were censored from analyses at the date of last follow-up. The cumulative incidence of IFF, DF, BF, post-RT ST, and in-field fracture were estimated using the Fine-Gray method, with death as a competing risk and with each treated lesion considered individually. The Kaplan-Meier method was used to estimate overall survival (OS), and patients receiving multiple RT treatments to bone metastases were considered only once, beginning from the time of the first treatment. Hazard ratios (HRs) for single variable associations with outcomes were calculated using the Cox model.
Variables assessed for association with IFF included technique (SBRT or CFRT), age at treatment, Gleason score, castrate-resistant disease status, PTV, BED, anatomic site treated, and use and timing of ST. The effect of dose on IFF was assessed separately for SBRT (>18 Gy vs ≤18 Gy) and CFRT (≤10 Gy vs >10 Gy). The effect of number of metastases (1-3 vs ≥4) on DF was also assessed.
A multiple variable Fine-Gray analysis was performed to evaluate factors associated with IFF and included all variables with a univariate significance of ≤.20. This P-value criterion was chosen because of the low number of IFF events (n = 45). The variables included as candidates were technique, indication, PTV, and pre-RT ST. A backward selection method was used to select the final model. A P-value of .05 was set to determine significance.
Results
Patient and treatment characteristics
A total of 315 patients were initially identified. Of these, 201 patients with 249 metastases (191 SBRT, 58 CFRT) had sufficient follow-up and were included in the final analysis. The median follow-up was 2.2 years (interquartile range, 1.1-2.7). Patient and disease characteristics at diagnosis are shown in Table 1. Compared with patients treated with CFRT, those who received SBRT had a lower median PSA at diagnosis (7.0 vs 18.4; P < .01) and more frequently had N0 (78.9% vs 51.0%; P = .01) and M0 (80.3% vs 57.1%; P < .01) disease at initial diagnosis.
Table 1.
Patient and disease characteristics at diagnosis
| CFRT (n = 49) | SBRT (n = 152) | Total (n = 201) | P-value | |
|---|---|---|---|---|
| Age at diagnosis (y) | .01 | |||
| Median (range) | 63.4 (51.5-87.5) | 61.9 (41.2-87.1) | 62.7 (41.2-87.5) | |
| Gleason score, n (%) | .37 | |||
| 6 | 8 (18.2) | 15 (10.1) | 23 (12.0) | |
| 7 | 11 (25.0) | 58 (39.2) | 69 (35.9) | |
| 8 | 9 (20.5) | 23 (15.5) | 32 (16.7) | |
| 9 | 14 (31.8) | 45 (30.4) | 59 (30.7) | |
| 10 | 2 (4.5) | 7 (4.7) | 9 (4.7) | |
| Unknown | 5 (10.2) | 4 (2.6) | 9 (4.5) | |
| PSA at diagnosis (ng/mL) | 18.4 | 7.0 | 7.9 | <.01 |
| Median (range) | (0.3-1470.0) | (1.2-973.0) | (0.3-1470.0) | |
| T stage, n (%) | .08 | |||
| T1-2 | 23 (46.9) | 73 (48.0) | 96 (47.8) | |
| T3 | 17 (34.7) | 69 (45.4) | 86 (42.8) | |
| T4 | 1 (2.0) | 2 (1.3) | 3 (1.5) | |
| TX | 8 (16.3) | 8 (5.3) | 16 (8.0) | |
| N stage, n (%) | <.01 | |||
| N0 | 25 (51.0) | 120 (78.9) | 145 (72.1) | |
| N1 | 15 (30.6) | 27 (17.8) | 42 (20.9) | |
| NX | 9 (18.4) | 5 (3.3) | 14 (7.0) | |
| M stage, n (%) | <.01 | |||
| M0 | 28 (57.1) | 122 (80.3) | 150 (74.6) | |
| M1 | 16 (32.7) | 17 (11.2) | 33 (16.4) | |
| MX | 5 (10.2) | 13 (8.6) | 18 (9.0) | |
| Primary treatment, n (%) | <.01 | |||
| RP | 10 (20.4) | 31 (20.4) | 41 (20.4) | |
| RT | 5 (10.2) | 15 (9.9) | 20 (10.0) | |
| RP + adjuvant RT | 3 (6.1) | 9 (5.9) | 12 (6.0) | |
| RP + salvage RT | 10 (20.4) | 72 (47.4) | 82 (40.8) | |
| RT + salvage RP | 0 | 4 (2.6) | 4 (2.0) | |
| Systemic therapy | 19 (38.8) | 19 (12.5) | 38 (18.9) | |
| Cryotherapy | 1 (2.0) | 1 (0.7) | 2 (1.0) | |
| Elective surveillance | 1 (2.0) | 1 (0.7) | 2 (1.0) |
Abbreviations: CFRT = conventionally fractionated radiation therapy; PSA = prostate-specific antigen; RP = radical prostatectomy; RT = radiation therapy; SBRT = stereotactic body radiation therapy.
Treatment characteristics are shown in Table 2. Patients treated with SBRT more frequently had 1 to 3 metastases compared with those who received CFRT (84.8% vs 39.7%; P < .01) and less frequently received peri-RT ST (57.1% vs 79.3%; P = .02). Castrate-resistant disease status was not significantly different between the SBRT and CFRT groups (40.8% vs 34.5%; P = .39). The most common SBRT dose prescriptions were 18 Gy (45.5%) or 20 Gy (46.6%) in a single fraction. CFRT was most frequently given as either 8 Gy in 1 fraction (56.9%) or 20 Gy in 5 fractions (41.4%). Imaging follow up was performed with 11C-choline PET/CT (79%), bone scan (10%), CT (5%), magnetic resonance imaging (3%), or 18F-fluorodeoxyglucose PET/CT (3%).
Table 2.
Treatment characteristics
| CFRT (n = 58) | SBRT (n = 191) | Total (n = 249) | P-value | |
|---|---|---|---|---|
| Age at RT (y) | <.01 | |||
| Median (range) | 75.3 (52.7-92.3) | 70.6 (48.2-88.3) | 71.6 (48.2-92.3) | |
| PSA at RT (ng/mL) | <.01 | |||
| Median (range) | 6.1 (0.1-2794.0) | 1.7 (0.1-28.9) | 2.5 (0.1-2794.0) | |
| PSA value (ng/mL), n (%) | <.01 | |||
| 0-5 | 26 (44.8) | 140 (73.7) | 166 (66.9) | |
| 5-10 | 7 (12.1) | 31 (16.3) | 38 (15.3) | |
| 10-20 | 9 (15.5) | 13 (6.8) | 22 (8.9) | |
| >20 | 16 (27.6) | 6 (3.2) | 22 (8.9) | |
| Unknown | 0 | 1 (0.5) | 1 (0.4) | |
| Castrate-resistant, n (%) | .39 | |||
| No | 38 (65.5) | 113 (59.2) | 151 (60.6) | |
| Yes | 20 (34.5) | 78 (40.8) | 98 (39.4) | |
| Number of metastases at RT, n (%) | <.01 | |||
| 1 | 14 (24.1) | 120 (62.8) | 134 (53.8) | |
| 2 | 5 (8.6) | 27 (14.1) | 32 (12.9) | |
| 3 | 4 (6.9) | 15 (7.9) | 19 (7.6) | |
| 4 | 1 (1.7) | 6 (3.1) | 7 (2.8) | |
| ≥5 | 34 (58.6) | 23 (12.0) | 57 (22.9) | |
| Site treated, n (%) | .09 | |||
| Spine | 23 (39.7) | 82 (42.9) | 105 (42.2) | |
| Pelvis | 13 (22.4) | 64 (33.5) | 77 (30.9) | |
| Extremity | 15 (25.9) | 22 (11.5) | 37 (14.9) | |
| Sternum | 2 (3.4) | 7 (3.7) | 9 (3.6) | |
| Rib | 5 (8.6) | 16 (8.4) | 21 (8.4) | |
| Dose (Gy), n (%) | <.01 | |||
| 8 | 33 (56.9) | 0 | 33 (13.3) | |
| 10 | 1 (1.7) | 0 | 1 (0.4) | |
| 16 | 0 | 7 (3.7) | 7 (2.8) | |
| 18 | 0 | 87 (45.5) | 87 (34.9) | |
| 20 | 24 (41.4) | 89 (46.6) | 113 (45.4) | |
| 24 | 0 | 8 (4.2) | 8 (3.2) | |
| PTV (cm3) | <.01 | |||
| Median (range) | 228.3 (12.9-1665.3) | 33.0 (4.7-561.7) | 41.7 (4.7-1665.3) | |
| BED | <.01 | |||
| Median (range) | 29.3 (29.3-46.7) | 153.3 (101.3-216.0) | 126.0 (29.-216.0) | |
| Pre-RT systemic therapy, n (%) | .26 | |||
| H + C | 22 (37.9) | 62 (32.5) | 84 (33.7) | |
| H | 29 (50.0) | 85 (44.5) | 114 (45.8) | |
| C | 1 (1.7) | 2 (1.0) | 3 (1.2) | |
| None | 6 (10.3) | 42 (22.0) | 48 (19.3) | |
| Peri-RT systemic therapy, n (%) | .02 | |||
| H + C | 6 (10.3) | 10 (5.2) | 16 (6.4) | |
| H | 38 (65.5) | 94 (49.2) | 132 (53.0) | |
| C | 2 (3.4) | 5 (2.6) | 7 (2.8) | |
| None | 12 (20.7) | 82 (42.9) | 94 (37.8) |
Abbreviations: BED = biologically effective dose; C = chemotherapy; CFRT = conventionally fractionated radiation therapy; H = hormonal therapy; PSA = prostate-specific antigen; PTV = planning target volume; RT = radiation therapy; SBRT = stereotactic body radiation therapy.
In-field failure
The median time to IFF was 1.6 years and not met (>4.4 years) for CFRT- and SBRT-treated lesions, respectively. All documented IFF events involved an increase in size and/or radiotracer avidity within the initial PTV. The 1- and 3-year IFF estimates for SBRT-treated lesions were 4.5% (95% confidence interval [CI], 1.4-7.5) and 12.9% (95% CI, 6.6-18.8), respectively, compared with 34.4% (95% CI, 19.9-46.2) and 53.3% (95% CI, 34.3-66.8), respectively, for those receiving CFRT (Fig. 1A). On univariate regression analysis (Table 3), the HR for IFF with CFRT compared with SBRT was 6.8 (95% CI, 3.7-12.5; P < .01). Larger PTV (HR, 1.2; 95% CI, 1.1-1.2; P < .01), PSA >20 ng/mL (HR, 3.2; 95% CI, 1.4-7.3; P < .01), and presence of ≥4 metastases (HR, 2.9; 95% CI, 1.6-5.3; P < .01) were also predictive of IFF. IFF was decreased in lesions that received a higher BED (HR, 0.18; 95% CI, 0.1-0.4; P < .01) and pre-RT ADT (HR, 0.5; 95% CI, 0.3-0.9; P = .03). No other variables assessed, including Gleason score, castrate-resistant disease status, or anatomic location, were significantly associated with IFF.
Fig. 1.
In-field failure risk for lesions treated with (A) stereotactic body versus conventionally fractionated radiation therapy; and (B) high- and low-dose stereotactic body versus conventionally fractionated radiation therapy.
Table 3.
Univariate analysis of factors associated with in-field failure
| P-value | HR (95% CI) | |
|---|---|---|
| Technique | ||
| SBRT | 1.0 (Ref) | |
| CFRT | <.01 | 6.79 (3.69-12.49) |
| Age at RT (y) | .33 | 1.02 (.98-1.05) |
| Gleason | .63 | |
| 6 | 1.0 (Ref) | |
| 7 | .18 | .50 (.18-1.39) |
| 8 | .28 | .52 (.16-1.70) |
| 9 | .38 | .63 (.22-1.77) |
| 10 | .94 | .95 (.24-3.74) |
| PSA at RT (ng/mL) | .04 | |
| <5 | 1.0 (Ref) | |
| 5-10 | .64 | .80 (.30-2.09) |
| 11-20 | .31 | 1.66 (.62-4.41) |
| >20 | <.01 | 3.16 (1.36-7.32) |
| Castrate-resistant | ||
| No | 1.0 (Ref) | |
| Yes | .74 | .90 (.48-1.68) |
| No. of metastases at RT | ||
| 1-3 | 1.0 (Ref) | |
| ≥4 | <.01 | 2.92 (1.59-5.34) |
| PTV | <.01 | 1.15 (1.08-1.23) |
| BED | <.01 | .18 (.09-.37) |
| Site treated | ||
| Spine | .70 | 1.0 (Ref) |
| Pelvis | .99 | 1.00 (.49-2.04) |
| Extremity | .21 | 1.72 (.74-4.03) |
| Sternum | .69 | .66 (.08-5.17) |
| Rib | .91 | .93 (.28-3.10) |
| Pre-RT systemic therapy | ||
| H + C | .11 | 1.0 (Ref) |
| H | .03 | .49 (.25-.95) |
| C | .99 | 1.00 (.06-17.80) |
| None | .10 | .47 (.19-1.16) |
| Peri-RT systemic therapy | ||
| H + C | .84 | 1.0 (Ref) |
| H | .53 | 1.61 (.36-7.13) |
| C | .91 | 1.15 (.09-14.77) |
| None | .76 | 1.27 (.27-5.84) |
| SBRT dose (Gy) | ||
| ≤18 | 1.0 (Ref) | |
| >18 | .16 | 2.03 (.75-5.46) |
| CFRT dose (Gy) | ||
| ≤10 | 1.0 (Ref) | |
| >10 | .10 | .49 (.21-1.14) |
Abbreviations: BED = biologically effective dose; C = chemotherapy; CFRT = conventionally fractionated radiation therapy; CI = confidence interval; H = hormonal therapy; HR = hazard ratio; PTV = planning target volume; RT = radiation therapy; SBRT = stereotactic body radiation therapy.
On multiple variable analysis, RT technique was the only factor that retained significance, with CFRT-treated lesions demonstrating worse IFF (HR, 6.8; 95% CI, 3.7-12.5; P < .01). The cumulative incidence of IFF according to dichotomized dose levels within RT technique is shown in Figure 1B. IFF risk did not significantly differ between SBRT doses of >18 Gy versus ≤18 Gy (HR, 2.0; 95% CI, 0.8-5.4; P = .16). IFF also was not significantly different when comparing CFRT doses of ≤10 Gy with >10 Gy (HR, 0.49; 95% CI, 0.2-1.1; P = .10). Of the 45 patients with IFF events (27 CFRT, 18 SBRT), 3 events (2 CFRT, 1 SBRT) occurred before DF, and another 21 events (17 CFRT, 4 SBRT) were detected synchronously with DF. The remaining 21 recurrences occurred after DF was recorded.
Among the 50 lesions experiencing IFF, 15 received secondary local salvage therapy with percutaneous cryotherapy or reirradiation. Seven of the 40 failed CFRT-treated lesions were re-treated (2 cryotherapy, 5 reirradiation) compared with 8 of 10 failed SBRT-treated lesions (4 cryotherapy, 4 reirradiation).
Secondary oncologic outcomes
Patients with ≥4 metastases at the time of RT had a greater risk of DF than those with ≤3 metastases (HR, 1.9; 95% CI, 1.4-2.6; P < .01; Fig. 2A). There was no difference in BF (HR, 1.4; 95% CI, 1.0-2.1; P = .05) or DF risk (HR, 1.3; 95% CI, 1.0-1.8; P = .08) in patients who received SBRT compared with CFRT. BF estimates at 1 and 3 years were 81.6% (95% CI, 66.0-90.0) and 89.2% (95% CI, 71.8-95.9), respectively, for CFRT and 68.0% (95% CI, 60.5-74.1) and 88.6% (95% CI, 82.0-92.8), respectively, for SBRT. The 1- and 3-year DF estimates were 67.2% (95% CI, 51.7-77.8) and 94.0% (95% CI, 77.4-98.4), respectively, for CFRT and 63.1% (95% CI, 44.5-69.5) and 82.2% (95% CI, 74.5-87.6), respectively, for SBRT (Fig. 2B). The risk of ST escalation or initiation at 1 and 3 years were 23.7% (95% CI, 17.3-29.5) and 48.7% (95% CI, 39.5-56.5), respectively, for patients receiving SBRT versus 44.2% (95% CI, 29.1-56.0) and 59.0% (95% CI, 41.14-71.48), respectively, for CFRT (HR, 1.59; 95% CI, 1.03-2.46; P = .04). Patients who developed IFF had a significantly greater risk of escalation or initiation of ST compared with those who did not have IFF (HR, 9.77; 95% CI, 4.82-19-79; P < .0001).
Fig. 2.
Distant failure risk for lesions with (A) ≥4 versus 1 to 3 metastases at the time of treatment; and (B) lesions treated with stereotactic body versus conventionally fractionated radiation therapy.
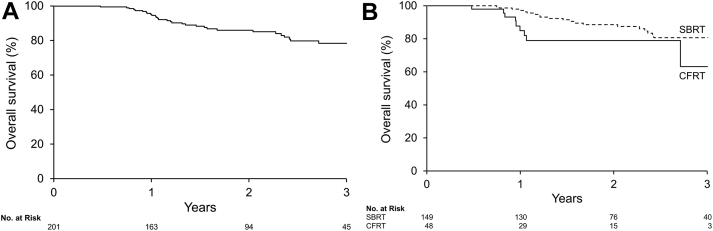
The estimated OS for the entire cohort was 95.1% (95% CI, 92.0-98.3) and 78.3% (95% CI, 70.5-86.0) at 1 and 3 years, respectively (Fig. 3A). Radiation technique did not significantly influence OS (P = .09) but favored SBRT patients (Fig. 3B).
Fig. 3.
Kaplan-Meier estimates of overall survival in (A) all patients and (B) patients treated with stereotactic body versus conventionally fractionated radiation therapy.
Toxicity
The incidence of radiographic fracture at the treated site was greater in lesions treated with CFRT (HR, 3.2; 95% CI, 1.2-8.3; P = .02). Risk of fracture at 1 and 3 years was 1.6% (95% CI, 0.0-3.3) and 6.6% (95% CI, 2.2-10.8), respectively, for lesions receiving SBRT compared with 9.1% (95% CI, 1.1-16.4) and 14.2% (95% CI, 3.6-23.5), respectively, in those receiving CFRT. Of the 8 CFRT fractures, 3 (37.5%) occurred in the context of IFF compared with 4 of 9 (44%) SBRT fractures.
A total of 16 patients experienced nonfracture toxicity in the post-RT period. Crude toxicity rates were 6.1% (3 of 49) in patients receiving CFRT and 8.6% (13 of 152) in those receiving SBRT. Acute grade 1 toxicities included pain flare (n = 8), esophagitis (n = 2), nausea (n = 2), cystitis (n = 1), and diarrhea (n = 1). There were only 2 acute grade 2 adverse events (pain flare and colitis), and each occurred in separate patients receiving SBRT. All RT-related toxicities resolved without long-term clinical consequences.
Discussion
The established role of RT for PCa with bone metastasis is symptom palliation, with a single fraction of 8 Gy endorsed as standard initial therapy.12 SBRT is a radiotherapeutic technique that delivers a higher BED and is undergoing controlled testing as an alternative strategy in this context (NCT00922974). SBRT is also a potential tumor-ablation method that has garnered interest as a means to alter the cascade of metastatic progression in the oligometastatic state and possibly delay ST in appropriately selected men with oligometastatic disease.13 This large observational series is the first to compare treatment outcomes for SBRT and CFRT in patients with PCa bone metastases. SBRT was associated with significantly reduced rates of IFF compared with CFRT, few treatment-related adverse events, and a delay in clinician-initiated ST (a subjective endpoint).
Prospective trials comparing conventional fractionation schemes for palliation of osseous metastases typically investigated single-fraction doses of 8 to 10 Gy and multifraction schedules of 20 to 24 Gy in 5 or 6 fractions or 30 Gy in 10 fractions.14, 15, 16, 17, 18 These studies have shown that CFRT dose fractionation does not affect palliation of pain symptoms, with a modest increase in the rate of same-site re-treatment for patients initially receiving lower-dose, single-fraction schedules. Randomized controlled trials have not yet provided comparative results between SBRT and CFRT as an early metastasis-directed therapy to formally test the Hellman-Weichselbaum hypothesis of a curable oligometastatic state in PCa. Prior uncontrolled studies using single-fraction SBRT for palliation of osseous metastases with doses of 15 to 24 Gy report high rates of in-field and symptomatic relief.19, 20, 21, 22 Our prior institutional report of patients with PCa with bone-only metastases demonstrated that ≥18 Gy in a single fraction resulted in superior metastasis control, with a potential increased benefit for lesions treated to >18 Gy (vs 18 Gy).7 A multi-institutional analysis of oligometastatic, treatment-naïve PCa treated with SBRT demonstrated improved local control in patients treated with a BED of >100 Gy.23 In the current study, we found that SBRT achieves superior local control compared with CFRT but did not identify greater local control with SBRT doses >18 Gy.
Previous studies have found an association between the number and anatomic location of metastatic sites with prognosis,24, 25 and we also noted that patients with oligometastatic disease at the time of treatment had a lesser rate of subsequent DF. Nearly 80% of patients treated with SBRT had BF, and >80% had subsequent DF at a previously untreated site by 3 years after treatment. This, taken with the high local control rates observed with SBRT, suggests that current radiologic imaging does not identify some other sites of metastatic disease present at the time of metastasis-directed therapy.
The efficacy and safety of SBRT has prompted significant interest in the use of local therapy to sites of oligometastatic disease as a means to delay disease progression or potentially achieve cure. A recent multicenter, randomized, phase 2 trial of 62 patients with oligorecurrent (≤3) PCa (55% nodal only) detected on 11C-choline PET/CT compared metastasis-directed therapy with active surveillance.13 The metastasis-directed therapy arm demonstrated delayed time to biochemical recurrence- and ADT-free survival, although the difference in ADT-free survival was not statistically significant. Our data also suggest that enhanced local control of bone metastases is associated with a lower risk of initiating or escalating ST. These data are provocative, although the clinical implications of an ADT-free survival or BF endpoint in patients with metastatic PCa are uncertain.
SBRT provided more robust local control, but it was not associated with improved DF or OS compared with CFRT, despite more favorable prognostic characteristics7, 24, 25 in the group treated with SBRT. Although 85% of patients treated with SBRT had ≤3 radiologically detected metastatic sites, the rates of DF remained high. This observation underscores the challenges in identifying truly oligometastatic patients who might benefit from aggressive metastasis-directed therapy. Most of our patients were initially imaged and subsequently followed with 11C-choline PET/CT, which has been shown to improve imaging detection of metastatic disease.26 Despite improvements in PET-based imaging for PCa, these studies have technical limitations in their ability to detect micrometastatic disease.27 Serum-based circulating tumor cells may represent an emerging technology to assist in identifying patients with a minimal systemic disease burden.28 Ultimately, improved methods will be needed to facilitate patient selection to optimize the benefits of local therapy for oligometastatic disease.
The cornerstone of therapy for castration-naïve metastatic PCa is early ADT with chemotherapy29, 30, 31 or an androgen biosynthesis inhibitor31, 32 in some patients. Treatment of castration-resistant disease follows a similar paradigm with consideration of a second-generation antiandrogen31, 33 or systemic radionuclide.34 Future work must focus on determining if the tumor ablation property of SBRT alters the natural history of metastatic PCa and whether SBRT either in conjunction with or in lieu of ST alters the current treatment paradigm. Multiple ongoing prospective trials (eg, PCS IX [NCT02685397], CORE [NCT02759783], ORIOLE [NCT03143322], and STORM) seek to further determine the role of RT as a metastasis-directed therapy.
CFRT-treated lesions demonstrated an increased risk of radiologic fracture despite prior evidence that single-fraction SBRT is associated with higher fracture rates, especially at doses >18 Gy.35, 36 The reported fracture risk for CFRT may be inflated owing to the increased risk of IFF; however, CFRT fractures had a lower rate of associated IFF (37.5%) compared with SBRT (44.4%). The significance of these findings is unclear and is limited by small event numbers; only 17 total fractures were observed throughout the post-RT period. The possibility remains that the more advanced disease state that was more prevalent in CFRT patients contributed to this difference in subsequent fractures. Ultimately, these radiographic fractures were rarely symptomatic and thus are of questionable clinical significance.
Our study is limited by its retrospective nature, which introduces the possibility that unrecognized confounders or selection biases influenced reported outcomes. The heterogeneity of patient characteristics and treatments presents challenges in reporting and interpreting outcomes related to disease progression. Another limitation is that the majority of patients in this study were imaged initially, and in follow-up, with frequent 11C-choline PET scans, which are not widely available for use in most clinical settings. Furthermore, not all patients were assessed by PET imaging, and a comparison of outcomes across various imaging modalities may potentially underestimate the risk of IFF in certain patient subsets. Patients were not followed in a uniform manner according to protocol specifications, and a possibility exists that not all adverse events were captured. Additionally, excluding patients with <6 months of follow-up may lead to an underestimation of treatment-related toxicity.
Finally, with non–protocol-based use of ST, it is challenging to determine patients in whom RT may have influenced outcomes based on its use in this population. Nonetheless, the magnitude of difference in IFF rates between SBRT and CFRT strongly signals a treatment effect that favors SBRT. The high rate of BF and DF subsequent to SBRT calls into question the validity of the Weichselbaum-Hellman hypothesis of an oligometastatic state in PCa. Our observations should fuel the need to test this hypothesis in a randomized controlled trial for patients with PCa, as currently being studied in breast cancer (NRG BR002 [NCT02364557]) and lung cancer (SARON [NCT02417662]).
Conclusions
SBRT provides superior in-field control for PCa bone metastases compared with CFRT with low rates of treatment-related toxicity. In SBRT-treated patients, BF and DF were common, and this finding highlights the crucial need to determine the optimal application of local and systemic therapies in patients with oligometastatic PCa.
Footnotes
Sources of support: This work had no specific funding.
Conflicts of interest: Drs Park and Kwon receive research funding from the National Cancer Institute.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Taylor L.G., Canfield S.E., Du X.L. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–2399. doi: 10.1002/cncr.24283. [DOI] [PubMed] [Google Scholar]
- 3.Weichselbaum R.R., Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 4.Milano M.T., Katz A.W., Zhang H., Okunieff P. Oligometastases treated with stereotactic body radiotherapy: Long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–886. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 5.Chang B.K., Timmerman R.D. Stereotactic body radiation therapy: A comprehensive review. Am J Clin Oncol. 2007;30:637–644. doi: 10.1097/COC.0b013e3180ca7cb1. [DOI] [PubMed] [Google Scholar]
- 6.Habl G., Straube C., Schiller K. Oligometastases from prostate cancer: local treatment with stereotactic body radiotherapy (SBRT) BMC Cancer. 2017;17:361. doi: 10.1186/s12885-017-3341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muldermans J.L., Romak L.B., Kwon E.D., Park S.S., Olivier K.R. Stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2016;95:696–702. doi: 10.1016/j.ijrobp.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed K.A., Barney B.M., Davis B.J., Park S.S., Kwon E.D., Olivier K.R. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol. 2012;2:215. doi: 10.3389/fonc.2012.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow E., Zeng L., Salvo N., Dennis K., Tsao M., Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol. 2012;24:112–124. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Amini A., Altoos B., Bourlon M.T. Local control rates of metastatic renal cell carcinoma (RCC) to the bone using stereotactic body radiation therapy: Is RCC truly radioresistant? Pract Radiat Oncol. 2015;5:e589–e596. doi: 10.1016/j.prro.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scher H.I., Halabi S., Tannock I. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutz S., Berk L., Chang E. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Ost P., Reynders D., Decaestecker K. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 14.Roos D.E., Turner S.L., O'Brien P.C. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05) Radiother Oncol. 2005;75:54–63. doi: 10.1016/j.radonc.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Yarnold J.R. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: Randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol. 1999;52:111–121. [PubMed] [Google Scholar]
- 16.Nielsen O.S., Bentzen S.M., Sandberg E., Gadeberg C.C., Timothy A.R. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother Oncol. 1998;47:233–240. doi: 10.1016/s0167-8140(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 17.Gaze M.N., Kelly C.G., Kerr G.R. Pain relief and quality of life following radiotherapy for bone metastases: a randomised trial of two fractionation schedules. Radiother Oncol. 1997;45:109–116. doi: 10.1016/s0167-8140(97)00101-1. [DOI] [PubMed] [Google Scholar]
- 18.Steenland E., Leer J., Van Houwelingen H. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 19.Amdur R.J., Bennett J., Olivier K. A prospective, phase II study demonstrating the potential value and limitation of radiosurgery for spine metastases. Am J Clin Oncol Cancer Clin Trials. 2009;32:515–520. doi: 10.1097/COC.0b013e318194f70f. [DOI] [PubMed] [Google Scholar]
- 20.Garg A.K., Shiu A.S., Yang J. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer. 2012;118:5069–5077. doi: 10.1002/cncr.27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Velden J.M., Verkooijen H.M., Seravalli E. Comparing conventional radiotherapy with stereotactic body radiotherapy in patients with spinal metastases: Study protocol for an randomized controlled trial following the cohort multiple randomized controlled trial design. BMC Cancer. 2016;16:909. doi: 10.1186/s12885-016-2947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braam P., Lambin P., Bussink J. Stereotactic versus conventional radiotherapy for pain reduction and quality of life in spinal metastases: Study protocol for a randomized controlled trial. Trials. 2016;17:61. doi: 10.1186/s13063-016-1178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ost P., Jereczek-Fossa B.A., As N.V. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: A multi-institutional analysis. Eur Urol. 2016;69:9–12. doi: 10.1016/j.eururo.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Schweizer M.T., Zhou X.C., Wang H. Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Ann Oncol. 2013;24:2881–2886. doi: 10.1093/annonc/mdt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ost P., Decaestecker K., Lambert B. Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate. 2014;74:297–305. doi: 10.1002/pros.22750. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell C.R., Lowe V.J., Rangel L.J., Hung J.C., Kwon E.D., Karnes R.J. Operational characteristics of (11)c-choline positron emission tomography/computerized tomography for prostate cancer with biochemical recurrence after initial treatment. J Urol. 2013;189:1308–1313. doi: 10.1016/j.juro.2012.10.069. [DOI] [PubMed] [Google Scholar]
- 27.Evans J.D., Jethwa K.R., Ost P. Prostate cancer–specific PET radiotracers: A review on the clinical utility in recurrent disease. Pract Radiat Oncol. 2018;8:28–39. doi: 10.1016/j.prro.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Lindsay D.P., Caster J.M., Wang K. Prospective characterization of circulating tumor cells using a nanotechnology-based capture system in oligometastatic patients undergoing definitive radiation therapy. J Clin Oncol. 2017;35:11533. [Google Scholar]
- 29.Kyriakopoulos C.E., Chen Y.H., Carducci M.A. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080–1087. doi: 10.1200/JCO.2017.75.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James N.D., Sydes M.R., Clarke N.W. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sartor O., de Bono J.S. Metastatic prostate cancer. N Engl J Med. 2018;378:645–657. doi: 10.1056/NEJMra1701695. [DOI] [PubMed] [Google Scholar]
- 32.James N.D., de Bono J.S., Spears M.R. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penson D.F., Armstrong A.J., Concepcion R. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: The STRIVE trial. J Clin Oncol. 2016;34:2098–2106. doi: 10.1200/JCO.2015.64.9285. [DOI] [PubMed] [Google Scholar]
- 34.Parker C., Nilsson S., Heinrich D. Alpha Emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 35.Rose P.S., Laufer I., Boland P.J. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moussazadeh N., Lis E., Katsoulakis E. Five-year outcomes of high-dose single-fraction spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2015;93:361–367. doi: 10.1016/j.ijrobp.2015.05.035. [DOI] [PubMed] [Google Scholar]