Abstract
Pathological angiogenesis is a hallmark of various vascular diseases, including vascular eye disorders. Dysregulation of microRNAs (miRNAs), a group of small regulatory RNAs, has been implicated in the regulation of ocular neovascularization. This study investigated the specific role of microRNA-145 (miR-145) in regulating vascular endothelial cell (EC) function and pathological ocular angiogenesis in a mouse model of oxygen-induced retinopathy (OIR). Expression of miR-145 was significantly upregulated in OIR mouse retinas compared with room air controls. Treatment with synthetic miR-145 inhibitors drastically decreased levels of pathological neovascularization in OIR, without substantially affecting normal developmental angiogenesis. In cultured human retinal ECs, treatment with miR-145 mimics significantly increased the EC angiogenic function, including proliferation, migration, and tubular formation, whereas miR-145 inhibitors attenuated in vitro angiogenesis. Tropomodulin3 (TMOD3), an actin-capping protein, is a direct miR-145 target and is downregulated in OIR retinas. Treatment with miR-145 mimic led to TMOD3 inhibition, altered actin cytoskeletal architecture, and elongation of ECs. Moreover, inhibition of TMOD3 promoted EC angiogenic function and pathological neovascularization in OIR and abolished the vascular effects of miR-145 inhibitors in vitro and in vivo. Overall, our findings indicate that miR-145 is a novel regulator of TMOD3-dependent cytoskeletal architecture and pathological angiogenesis and a potential target for development of treatments for neovascular eye disorders.
Keywords: angiogenesis, endothelial cell, microRNA, miR-145, neovascularization, retinopathy, Tmod3
Graphical Abstract
Introduction
Angiogenesis, the formation of new blood vessels from existing ones, plays important roles in both physiological development and pathological conditions. The process of de novo vascular growth or neovascularization encompasses a series of morphogenic events, including sprouting, branching, lumen formation, anastomoses, and remodeling into a perfused vascular network, all of which require coordinated interaction of endothelial cells (ECs).1 Dysregulated angiogenesis is associated with many diseases, including cardiovascular diseases, tumorigenesis, proliferative retinopathies, and neurodegeneration.2 In retinopathy, pathological ocular neovascularization is characterized by a leaky, fragile, tuft-like appearance, which may cause retinal hemorrhage, leading to tractional retinal detachment and vision loss.3 ECs play critical roles during angiogenesis, with those cells in the vascular growth front exposed to numerous angiogenic stimulators and inhibitors and following balanced guidance from these factors.1, 4 One of the most important factors in pathological angiogenesis—vascular endothelial growth factor (VEGF)—has been established as a key drug target in current anti-angiogenic therapies for cancers and neovascular eye diseases.5 Yet anti-VEGF therapies are effective for some but not all patients and may affect normal vessel homeostasis.6, 7, 8 Therefore, in designing improved targeted therapies for neovascular eye diseases, it is critical to identify additional factors controlling pathological angiogenesis, particularly those factors essential for the maintenance of normal vessel quiescence and transformation to proliferative neovessels under pathological conditions.
MicroRNAs (miRNAs) are a group of small (∼22 nucleotides in length), endogenous, non-coding, regulatory RNA molecules.9 miRNAs are involved in many biological processes, such as cell proliferation, cell death, neuronal differentiation and development, and angiogenesis.10, 11, 12, 13, 14, 15, 16 miRNAs function through base pairing to the complementary sequence in the 3′ UTR of target mRNAs, to induce their cleavage and translational repression,9 and hence are recognized as key mediators of post-transcriptional regulation through fine tuning gene expression. Previous studies have demonstrated the emerging role of miRNAs in vascular growth and neovascular diseases, including cancer and ocular angiogenesis.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 In addition, work from several laboratories, including ours, has identified altered expression of multiple miRNAs in murine models of oxygen-induced retinopathy (OIR),10, 20, 27 an experimental model characterized by ischemia-induced neovascularization in the retina. Specifically, expression of miR-145, a potential tumor suppressor,28 is significantly upregulated in retinas in the OIR model.27 miR-145 is co-transcribed with miR-143 as a cluster. Systematic miR-145 knockout (KO) and miR-143/145 double-KO mice are viable and show no overt abnormalities in cardiac structure and vascular smooth muscle cell differentiation.29 Moreover, tumor-specific deletion of miR-143/145 in a mouse model of lung adenocarcinoma exhibits diminished neovascularization and increased apoptosis, whereas wild-type mice injected with miR-143/145-expressing lung tumor cell lines develop aggressive lung tumors that are caused by stimulated proliferation of ECs.30 In the eye, miR-143/145 cluster regulates corneal epithelium formation31 and also intraocular pressure by modulating actin dynamics and contractility of trabecular meshwork.32 However, the role of miR-145 in pathological ocular angiogenesis and vascular endothelial function remains unclear.
In this study we investigated the potential role of miR-145 as a novel post-transcriptional regulator controlling ocular neovascularization. Using a mouse model of OIR, we demonstrated that inhibition of miR-145 reduces pathological angiogenesis in mouse retinas in vivo. In vitro administration of miR-145 mimics increases vascular EC functions including proliferation, migration, and tubular formation, whereas miR-145 inhibitors attenuate EC angiogenic functions. In addition, we identified tropomodulin 3 (Tmod3) as a novel target gene of miR-145 and determined the targeting site of miR-145 on the 3′ UTR of Tmod3 by a luciferase reporter assay. We present evidence that miR-145 represses Tmod3 and that the miR-145/TMOD3 axis regulates EC morphology and cytoskeletal architecture in vitro and pathological vascular growth in vivo. Together, our findings indicate that inhibition of miR-145 may serve as a potential therapeutic approach in developing treatments for pathological retinal angiogenesis.
Results
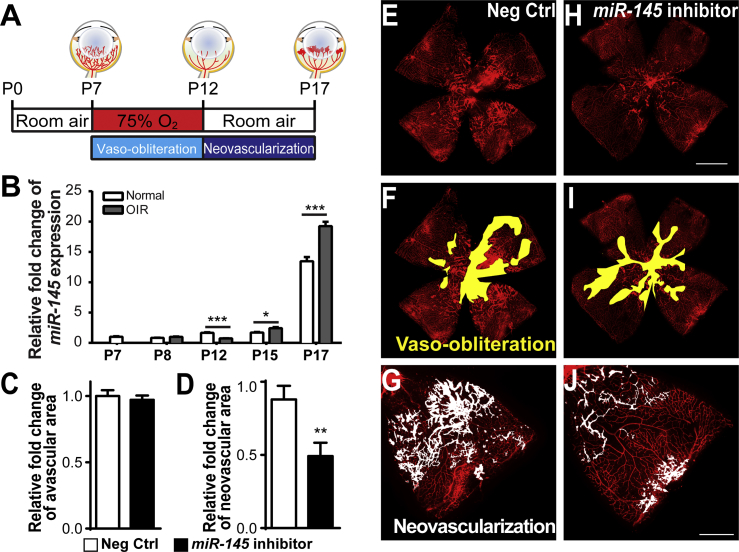
miR-145 Is Highly Expressed in Mouse Retinas with OIR
Our previous analysis of miRNA expression array profiles showed an increase in miR-145 levels by ∼60% in pathological retinas with OIR,27 a mouse model of proliferative retinopathy, by exposing neonatal mouse pups to 75% oxygen for 5 days from postnatal day 7 (P7) to P12, to induce maximal neovascularization at P17 (Figure 1A). We isolated total RNAs from mouse retinas with OIR and age-matched room air controls at various days (P7, P8, P12, P15, and P17) and evaluated miR-145 expression levels by qPCR. Expression of miR-145 revealed an increase during physiological development (Figure 1B, white bars). More importantly, in OIR retinas, miR-145 expression levels decreased at P12, but significantly increased at P15 and P17 (Figure 1B, gray bars), compared with the levels in age-matched normoxic retinas, with no significant change at P8. Together, these data are consistent with our previous array results in which miR-145 was upregulated in P17 retinas with pathological neovessels27 and further validate dysregulation of miR-145 in OIR, which may affect pathological vascular growth in retinopathy.
Figure 1.
miR-145 Suppresses Pathological Neovascularization in Mouse Retinas with Oxygen-Induced Retinopathy
(A) Schematic diagram of the oxygen-induced retinopathy (OIR) model. Neonatal mice were exposed to 75% of oxygen from postnatal day 7 (P7) to 12. Vessel loss was induced during oxygen exposure, and maximal neovascularization was induced at P17, 5 days after the mice were returned to room air. (B) Expression levels of miR-145 were analyzed in OIR retinas compared with age-matched room air control retinas at different time points. MiR-145 expression was significantly reduced in OIR retinas at P12, yet was upregulated at both P15 and P17 compared with normoxic retinas, normalized to U6 small nuclear RNA (snRNA) as the control (n = 6 per group). (C–J) Intravitreal injection of the miR-145 inhibitor or the non-targeting negative control was performed in OIR mouse eyes at P12, with the miR-145 inhibitor injected into one eye and the negative control in the contralateral eye, followed by analysis of the retinal vasculature at P17. Quantitative analysis of vaso-obliteration (C) and pathological neovascularization (D) showed that miR-145 inhibitor treatment significantly reduced neovascularization in OIR retinas compared with negative control treatment of contralateral retinas, with no significant difference in vaso-obliteration (n = 16 per group). Representative retinal whole mounts were stained with Griffonia simplicifolia isolectin B4 (IB4) from P17 OIR mice with injection of negative control (E) or miR-145 inhibitor (H); areas of vaso-obliteration (F, negative controls, and I, miR-145 inhibitor) and neovascular tufts (G, negative controls, and J, miR-145 inhibitor) are labeled in yellow and white, respectively. Scale bars represent 1 mm (B) and 500 μm (F). Neg Ctrl, negative control RNA oligomers. Data are presented as means ± SEM. *p ≤ 0.05; **p < 0.005; ***p ≤ 0.001.
Inhibition of miR-145 Suppresses Pathological Neovascular Growth
To determine whether miR-145 influences pathological neovascularization in the OIR retinas, we performed intravitreal injection of miR-145 inhibitors into OIR eyes at P12 after mice were removed from the oxygen. Vaso-obliteration (Figures 1C, 1F, and 1I) and neovascularization (Figures 1D, 1G, and 1J) were analyzed at P17. miR-145 inhibitor treatment in OIR retinas significantly decreased the pathological neovascular area (by ∼50%), compared with the contralateral eyes treated with negative controls (Figure 1D), without affecting vessel loss (Figure 1C). These results indicate that dysregulated miR-145 affects the formation of pathological neovascularization in the mouse OIR model, and inhibition of miR-145 alleviates the formation of neovessels.
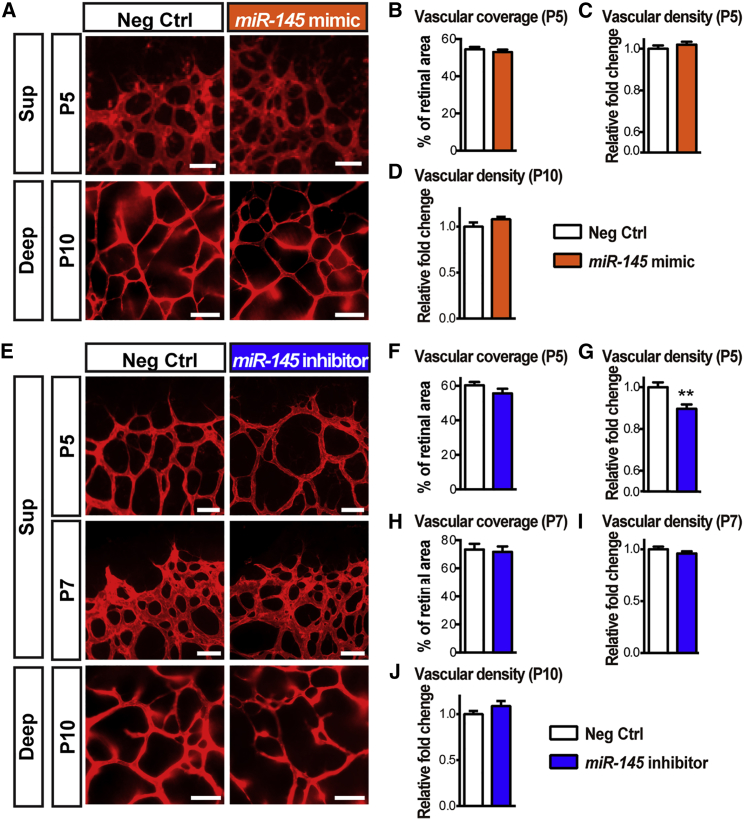
Given the increase in miR-145 expression during retinal development, we next examined the potential role of miR-145 in developing retinal vessels. miR-145 mimics or inhibitors were injected intravitreally into mouse eyes at P1, when mouse retinas are mostly devoid of blood vessels,33 and retinal flat mounts were collected at P5. Quantification of superficial vascular coverage and density showed no significant difference in the retinas treated with exogeneous miR-145 mimics compared with the contralateral eyes treated with negative controls (Figures 2A, top, 2B, and 2C). On the other hand, inhibition of miR-145 revealed a modest decrease (∼10%) in vascular density, with no significant difference in superficial layer vascular coverage (Figures 2E, top, 2F, and 2G). In addition, synthetic miR-145 inhibitors were also injected intravitreally into P5 mouse retinas, when the superficial retinal vessels had partially developed, and the retinal vascular areas were analyzed at P7. No significant vascular changes were observed with miR-145 inhibitor treatment at P7 (Figures 2E, center, 2H, and 2I). Moreover, to evaluate the effects of miR-145 during the development of the deep retinal vascular plexus, miR-145 mimics or inhibitors were injected intravitreally into P6 mouse eyes, and the deep-layer vasculature was observed at P10. Neither supplement of exogenous miR-145 mimics (Figures 2A, bottom, and 2D) nor inhibition of miR-145 (Figures 2E, bottom, and 2J) affected the development of deep retinal vessels significantly. These results suggest that, although miR-145 is critical for pathological neovascularization, it has little or minimal influence on normal retinal capillary development and vascular expansion.
Figure 2.
Minimal Effects of miR-145 on Developmental Retinal Angiogenesis
(A) Representative images of P5 and P10 mouse retinas treated with miR-145 mimics or negative control oligomers via intravitreal injection at P1 and P6, respectively, followed by staining with IB4 for observing the superficial (at P5, top) and deep (at P10, bottom) vascular layers. (B–D) Quantification of vascularized retinal areas (B, at P5) and vascular density (C, at P5, and D, at P10) in the negative control- and miR-145 mimic-treated groups show no significant difference. (E) Representative images of IB4-stained P5, P7, and P10 retinas treated with miR-145 inhibitors or negative controls at P1, P5, and P6, respectively. (F–J) Analyses of the vascularized areas of superficial retinas (F and H) and the vascular density of the superficial vasculature (G and I) or the deep vascular plexus (J) revealed no significant difference in the superficial retinal areas (P5, F, and P7, H) and vascular density (P7, I; P10, J) between the control- and miR-145 mimic-treated retinas (P5 and P7: n = 13 or 16 mice per group; P10: n = 7 or 8 mice per group), with very modest reduction of vascular density at P5 (G). Scale bars represent 50 μm (A and E), P5 and P7, and 100 μm (A and E), P10. Deep, deep retinal vascular layer; Neg Ctrl, negative control RNA oligomers; Sup, superficial retinal vascular layer. Data are presented as means ± SEM. **p ≤ 0.005.
Modulation of miR-145 Alters Vascular EC Angiogenic Functions
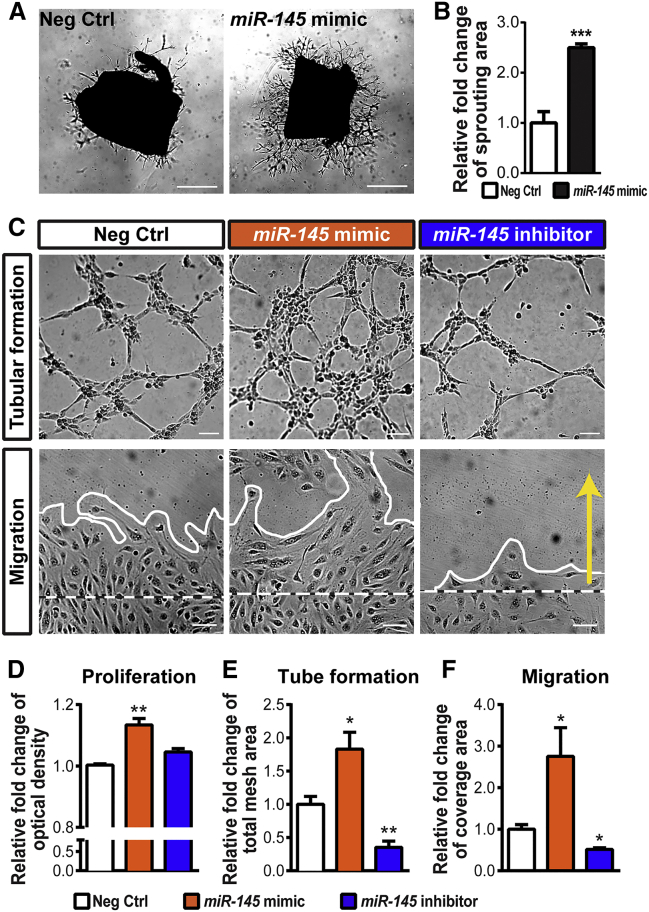
To further address the role of miR-145 in angiogenesis, we used ex vivo aortic ring culture treated with miR-145 mimics to investigate its effects on EC sprouting (Figures 3A and 3B). Aortic explants treated with miR-145 mimics revealed a significant increase in endothelial sprouting areas compared with the negative control oligomer-treated group (Figure 3B), suggesting that miR-145 promotes angiogenesis ex vivo.
Figure 3.
miR-145 Increases Endothelial Angiogenic Functions
(A) Representative images of vascular sprouting from aortic rings isolated from P20 mice. Tissue explants were treated with a non-targeting negative control (left) or the miR-145 mimic (right). (B) Quantitative analysis of the sprouting area in day 4 explants showed that the miR-145 mimic significantly increased sprouting ability ex vivo compared with rings treated with the negative control (n = 6–9 per group). (C–F) Human retinal microvascular endothelial cells (HRMECs) were treated with the miR-145 mimic, inhibitor, or negative control and analyzed for endothelial functions. Representative images (C) show tubular formation (top) and results of a wound-healing migration assay (bottom; white dashed lines indicate the initial boundaries of the scratches, white solid lines show the leading edges at 24 h after treatment, and the yellow arrow indicates the direction of cell migration). Quantitative analyses show that HRMECs treated with the miR-145 mimic displayed higher levels of proliferative activity in an MTT assay (D), mesh areas in a tube-formation assay (E), and cell coverage areas during migration (F), compared with cells treated with the negative control. Inhibition of miR-145 in HRMECs reveals significantly decreased levels of tube formation (E) and migration (F). (D) n = 3 per condition; (E and F) n = 6 per group. Scale bars represent 1 mm (A) and 100 μm (C). Neg Ctrl, negative control RNA oligomers. Data are presented as means ± SEM. *p ≤ 0.05; **p ≤ 0.005; ***p < 0.001.
We next examined whether miR-145 directly regulates EC angiogenic functions in human retinal microvascular endothelial cells (HRMECs). Treatment with miR-145 mimics substantially promoted HRMEC proliferation, tube formation, and migration (Figures 3C–3F). An approximately 14% increase in proliferation (Figure 3D), an 80% increase in tubular formation in mesh areas (Figure 3E), and a >2.7-fold increase in migration distance (Figure 3F) were observed with miR-145 mimic treatment. On the other hand, inhibition of miR-145 significantly decreased EC tube formation and migration (Figures 3C, 3E, and 3F), indicating direct pro-angiogenic effects of miR-145 on EC functions.
Tmod3 Is a Direct Target Gene of miR-145
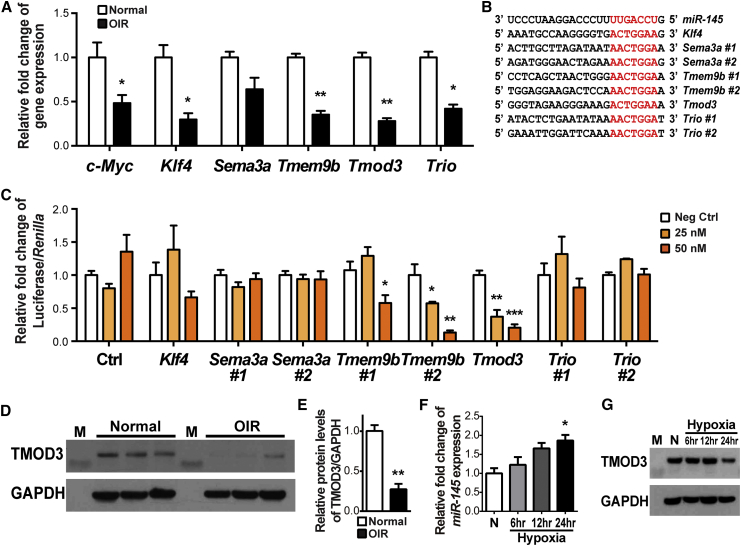
miRNA functions by regulating target gene expression post-transcriptionally by binding to the conserved seed sequences in the 3′ UTR of mRNA.34 We performed in silico analysis of putative miR-145 target genes based on the conservation of seed sequence matches across human and mouse, which identified Krüppel-like factor 4 (Klf4), semaphorin 3A (Sema3a), TMEM9 domain family member B (Tmem9b), Tmod3, and trio Rho guanine nucleotide exchange factor (Trio) as potential miR-145 targets with base pairing to the conserved seed sequences (UCCAGUUU). Consistent with miR-145 upregulation at P17 in OIR (Figure 1B), Klf4, Tmem9b, Tmod3, and Trio are all significantly decreased in the P17 OIR retinas, as was the known miR-145 target gene c-Myc (Figure 4A).
Figure 4.
Tmod3 Is a Target Gene of miR-145
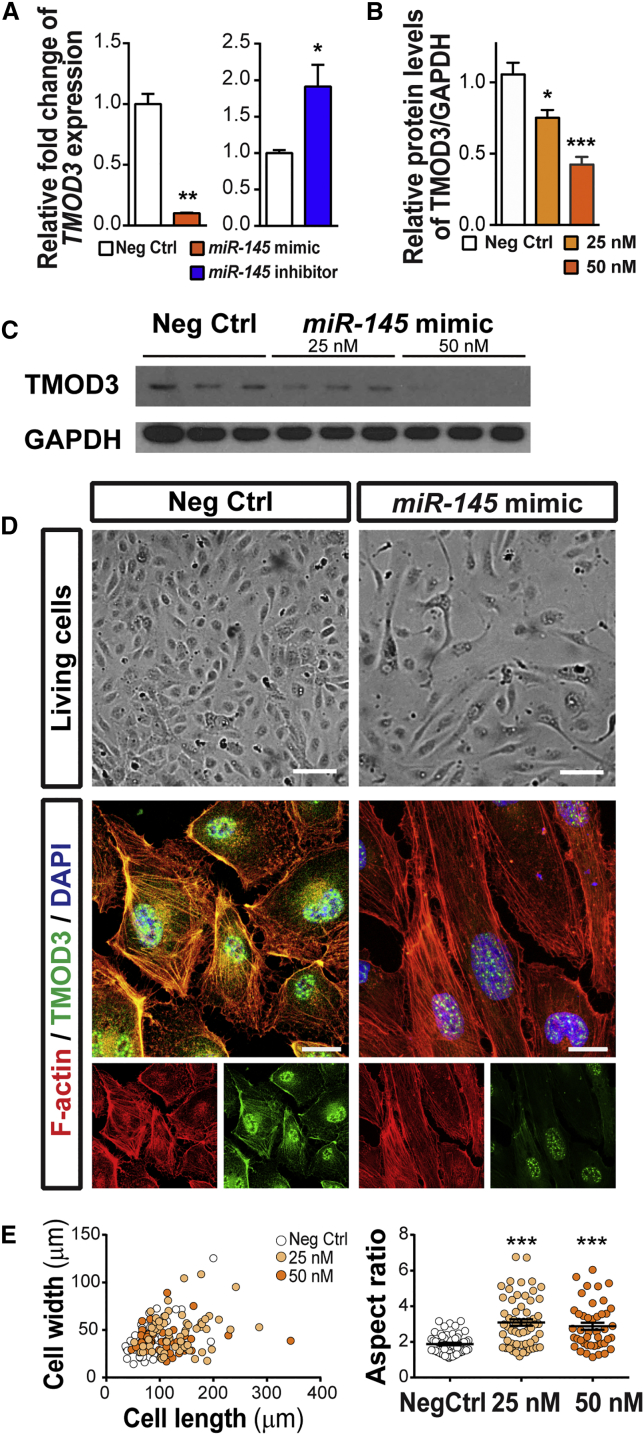
Candidate target genes of miR-145—Krüppel-like factor 4(Klf4), semaphorin 3A (Sema3a), TMEM9 domain family member B (Tmem9b), tropomodulin 3 (Tmod3), and trio Rho guanine nucleotide exchange factor (Trio)—were identified by analyzing the seed sequence of miR-145, conserved in both human and murine, for sequence complementarity to determine predicted target mRNAs. (A) Expression levels of Klf4, Tmem9b, Tmod3, and Trio were significantly downregulated in P17 OIR retinas compared with normoxic control retinas (n = 6 per group). c-Myc, a known miR-145 target gene, served as the positive control. Expression levels were normalized to 18S and then to the levels in normoxic retinas. (B) The sequence alignment between mouse miR-145 and the putative binding sites in the 3′ UTR of target genes, with sequences recognized by the miR-145 seed sequence shown in red. (C) Luciferase reporter assays of putative miR-145 target genes were performed in HEK293T cells. Cells were transfected with 25 or 50 nM of miR-145 mimics or the negative control, and each was co-transfected with constructs of luciferase reporter containing miR-145 target sequences of Klf4, Sema3a, Tmem9b, Tmod3, and Trio. Cells transfected with the Tmod3 target sequence resulted in significant repression of luciferase activity in a miR-145 dose-dependent pattern (n = 3–6 per group). Firefly luciferase activities were normalized to the Renilla luciferase and to the levels in the negative control. (D and E) Western blotting shows the expression of TMOD3 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in P17 OIR and normoxic control retinas. (E) Densitometric quantification shows that TMOD3 protein levels were substantially decreased in P17 OIR retinas compared with normoxic controls. GAPDH served as the internal loading control (n = 3 per group). (F) miR-145 expression levels were analyzed by real-time qPCR using total RNA obtained from HRMECs exposed to hypoxia for the indicated period of time. miR-145 expression was elevated over time in hypoxic HRMECs. miR-145 expressing levels were normalized to U6 snRNA and normoxia controls (n = 3 per group). (G) TMOD3 protein levels in HRMECs exposed to hypoxia for the indicated period of time were detected by western blot assay. GAPDH expression was used as a loading control. M, molecular weight markers; N, normoxia. Data are presented as means ± SEM. *p ≤ 0.05; **p ≤ 0.005; ***p ≤ 0.001.
To confirm whether these candidate genes are directly targeted by miR-145, we performed a luciferase reporter assay in HEK293T cells by cloning the 3′ UTR containing miR-145 seed sequences of Klf4, Sema3a, Tmem9b, Tmod3, and Trio into the pmirGLO vector (Figure 4B). The most significant reduction of luciferase activity by miR-145 mimic treatment was observed in cells transfected with Tmod3 constructs, revealing a >4-fold reduction in a dose-dependent manner (Figure 4C), with Tmem9b constructs also showing reduction to a lesser extent and no significant changes in the other gene constructs (Figure 4C). TMOD3 regulates EC migration by capping the pointed ends of actin filaments and blocking their elongation and depolymerization, thereby influencing actin cytoskeletal structure and dynamics in lamellipodia.35, 36 TMEM9B is a positive regulator of inflammatory cytokines.37 Our findings of miR-145 modulation of EC proliferation, migration, and tubular formation (Figure 3) are highly related to the known angiogenic functions of TMOD3 in ECs; hence, we focused on TMOD3.
We next investigated whether TMOD3 protein levels are altered in OIR and, by miR-145 modulation, in HRMECs. In P17 OIR retinas, TMOD3 protein levels were decreased by ∼70% compared with normoxic retinas (Figures 4D and 4E), confirming downregulated Tmod3 mRNA levels in OIR (Figure 4A) and consistent with OIR induction of miR-145 (Figure 1B). Moreover, hypoxia treatment of HRMECs significantly induced miR-145 expression and also decreased TMOD3 protein levels (Figures 4F and 4G), suggesting that their regulation in OIR was likely hypoxia responsive. In addition, HRMECs treated with miR-145 mimics showed drastically decreased TMOD3 expression (by up to 90%), whereas cells treated with miR-145 inhibitors had substantially increased expression levels of TMOD3 (by ∼2-fold; Figure 5A). Importantly, TMOD3 protein levels demonstrated significant dose-dependent reduction after treatment with miR-145 mimics (Figures 5B and 5C). Together, these data confirm that Tmod3 is a primary target gene suppressed by miR-145 in vascular ECs and may thereby mediate the vascular effects of miR-145.
Figure 5.
miR-145 Suppresses TMOD3 Protein Levels and Affects EC Actin Cytoskeleton and Morphology
(A) Treatment of miR-145 mimics significantly decreased expression of TMOD3 in HRMECs, whereas miR-145 inhibitors increased expression of TMOD3, confirming that the Tmod3 gene is a direct target of miR-145 in vascular ECs. Expression levels were normalized to 18S and to cells treated with negative control (n = 3 per group). (B and C) Western blot analysis of TMOD3 and GAPDH in HRMECs transfected with miR-145 mimics (25 or 50 nM) or negative controls for 72 h. (C) Densitometric analysis shows that TMOD3 protein levels were substantially decreased in HRMECs treated with miR-145 mimics dose-dependently. GAPDH serves as internal loading control (n = 6–9 per group). (D) Representative images of living cells (top) and immunocytochemical staining (bottom) of F-actin (red) and TMOD3 (green) in HRMECs treated with miR-145 mimics or negative controls. Live HREMCs treated with miR-145 mimics showed morphological change with an elongated appearance; moreover, immunofluorescence imaging revealed decreased TMOD3 staining, longer F-actin fibers and less cytoskeleton mesh, and elongation of cell shape in the mimic-treated group. (E) Quantification of cell morphology by measuring individual cell width and length. Each dot represents one cell. The left graph shows that HRMECs treated with miR-145 mimics had increased cell length. The right shows the aspect ratio (cell length/cell width) of HRMECs with the different treatments. Cells treated with miR-145 mimics had a significantly increased aspect ratio compared with negative control-treated cells (n = 45–70 cells/group). Scale bars represent 100 μm (D, top) and 20 μm (D, bottom with immunocytochemical staining). Neg Ctrl, negative control RNA oligomers. Data are presented as means ± SEM. ***p ≤ 0.001.
miR-145 Affects EC Morphology and Cytoskeletal Architecture through TMOD3
We next asked whether downregulation of Tmod3 by miR-145 alters EC morphology and functions. Because the canonical function of TMOD3 is as a regulator that caps actin filaments and modifies the dynamics and architecture of the actin network,35 we examined whether miR-145 induces alteration of EC functions via TMOD3-dependent morphological changes in the EC cytoskeleton. HRMECs were treated with negative controls or miR-145 mimics for 72 h, and the cells were live imaged prior to immunostaining with TMOD3 and F-actin. Significant morphological changes were observed in the miR-145 mimic-treated cells, showing decreased levels of TMOD3 staining, marked elongation of cell shape, and less organized actin meshwork (Figure 5D). In addition, measurement of both the length and width of ECs revealed a substantial increase in cell length and significantly higher aspect ratios (length versus width) in the miR-145 mimic-treated group compared with the negative controls (Figure 5E).
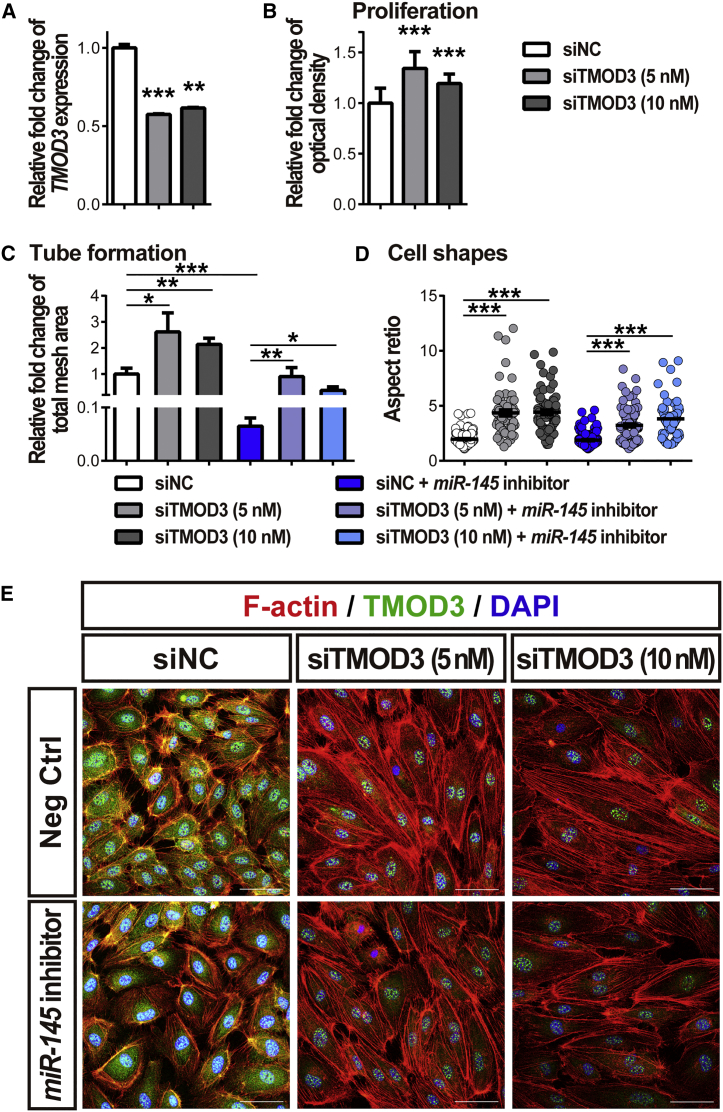
To evaluate the role of TMOD3 in mediating the effects of miR-145 on EC functions, we performed co-transfection of small interfering RNA (siRNA) targeting Tmod3 (siTMOD3) and miR-145 inhibitors to HRMECs. Treatment with siTMOD3 significantly suppressed (by ∼50%) Tmod3 expression in HRMECs, compared with negative control siRNA (siNC) (Figure 6A), confirming successful knockdown. Treatment with siTMOD3 significantly promoted HRMEC proliferation and angiogenic function (Figures 6B and 6C) and caused morphological cytoskeleton changes, including distinctly elongated cell shapes with increased aspect ratio and less organized actin mesh (Figures 6D and 6E), similar to the vascular phenotypes observed in miR-145 mimic-treated cells (Figures 3C–3F and 5). Moreover, co-treatment with siTMOD3 and miR-145 inhibitors significantly reversed the effects of miR-145 inhibitors on endothelial angiogenic function (Figure 6C). These data indicate that miR-145 influences the vascular EC cytoskeletal meshwork and morphologic changes important for EC sprouting and angiogenesis and that the vascular effects of miR-145 are mediated largely through TMOD3.
Figure 6.
TMOD3 Mediates EC Functions and Cytoskeletal Morphology
(A and B) HRMECs were transfected with small interfering RNA (siRNA) targeting TMOD3 (siTMOD3) (5 or 10 nM) or negative control siRNA (siNC) for 24 h. (A) Treatment of siTMOD3 significantly decreased expression of TMOD3 in HRMECs. Expression levels were normalized to 18S and to cells treated with siNC (n = 3 per group). (B) Quantitative analyses of MTT assays showed that HRMECs treated with siTMOD3 had significantly increased proliferative activity (n = 9–12 per condition). (C and E) HRMECs were transfected with miR-145 inhibitors (50 nM) or negative controls (Neg Ctrl) combined with siTMOD3 (5 or 10 nM) or siNC for 24 or 48 h and analyzed for tube formation (C) and cell shape (D and E). In the tube formation assay, HREMCs transfected with siTMOD3 had a significant increase in total mesh area, even in the miR-145 inhibitor co-transfected groups (C); n = 6 cells per group. Furthermore, knockdown of TMOD3 in HRMECs show a substantially increased aspect ratio (cell length/cell width) indicating elongated cell shape compared with cells treated with siNC (D); n = 60–130 cells per group. Immunocytochemical staining of F-actin (red) and TMOD3 (green) in HRMECs demonstrated decreased TMOD3 staining, longer F-actin fibers, less cytoskeleton mesh, and elongation of cell shape in the siTMOD3-treated group (E). Scale bars represent 50 μm (E). Data are presented as means ± SEM. *p ≤ 0.05; **p ≤ 0.005; ***p < 0.001.
Inhibition of TMOD3 Exacerbates Neovessel Formation and Reverses the Vascular Effects of miR-145 in OIR
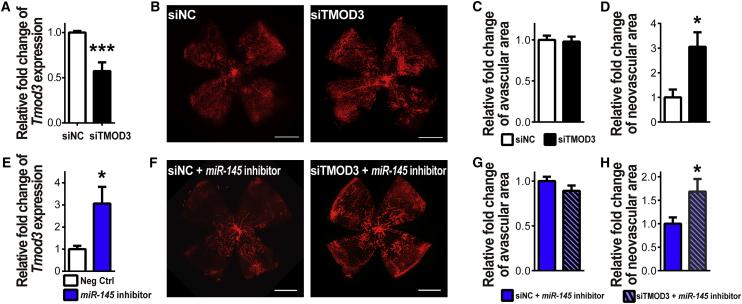
To further verify the neovascular effect of Tmod3 in vivo, we intravitreally injected siTMOD3, alone or in combination with miR-145 inhibitors, into OIR mouse eyes at P12 and analyzed the pathological vasculature at P17. siTMOD3 treatment suppressed the retinal expression of Tmod3 by ∼50% (Figure 7A) and led to a substantial increase of neovascularization in the OIR mouse eyes compared with contralateral siNC injection (Figures 7B–7D), supporting the anti-angiogenic effects of TMOD3 in vivo. Moreover, miR-145 inhibitor treatment increased the retinal expression of Tmod3 by ∼3-fold in vivo (Figure 7E), and siTMOD3 co-treatment with miR-145 inhibitors significantly induced neovascularization in OIR, hence reversing the anti-angiogenic effects of miR-145 inhibitors in OIR (Figures 7F–7H) and suggesting that the vascular effects of miR-145 on pathological neovascularization in OIR (Figure 1D) is mediated in part by its regulation of Tmod3. Overall, our results indicate that the miR-145/TMOD3 axis plays a significant role in regulating neovascularization in OIR.
Figure 7.
Inhibition of Tmod3 Increases Pathological Neovascularization in OIR
(A–D) Intravitreal injection of siTMOD3 or siNC was performed in OIR mouse eyes at P12, with siTMOD3 injected in one eye and siNC in the contralateral eye. Efficiency of siTMOD3 was verified by qPCR (A), showing an ∼50% reduction of Tmod3 expression after treating with siTMOD3 in the OIR retinas. Expression levels were normalized to Gapdh and to negative controls (n = 4 mice per group). Representative retinal whole mounts stained with IB4 from P17 OIR mice (B) were used for analysis of retinal pathologic vasculature. Quantification of vaso-obliteration (C) and pathological neovascularization (D) at P17 in OIR showed significantly increased neovascular area with siTMOD3 treatment compared with that in contralateral eyes treated with siNC (D), without a significant difference in vaso-obliteration (C) (n = 7 per group). (E) Retinal Tmod3 expression was analyzed in P17 OIR retinas with intravitreal injection of miR-145 inhibitors into one eye and non-targeting negative controls (Neg Ctrl) into the contralateral eye at P12. Tmod3 expression was significantly increased in OIR retinas treated with miR-145 inhibitors by ∼3-fold. Expression levels were normalized to Gapdh and to negative controls (n = 6 mice per group). (F–H) OIR mouse eyes were intravitreally injected with miR-145 inhibitors combined with siTMOD3 in one eye and siNC in the contralateral eye at P12 after removal from an oxygen chamber. Staining with IB4 in P17 mouse retinas (F) were used for quantification of vaso-obliteration (G) and neovascularization (H). miR-145 inhibitor combined with siTMOD3 treatment showed a significant increase in neovascular area in OIR retinas compared with contralateral eyes treated with the siNC-miR-145 inhibitor cocktail, but no significant effect on vaso-obliteration (n = 8 per group). Scale bars represent 1 mm (B and F). Data are presented as means ± SEM. *p < 0.05; ***p ≤ 0.001.
Discussion
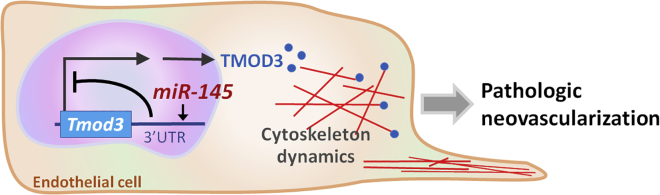
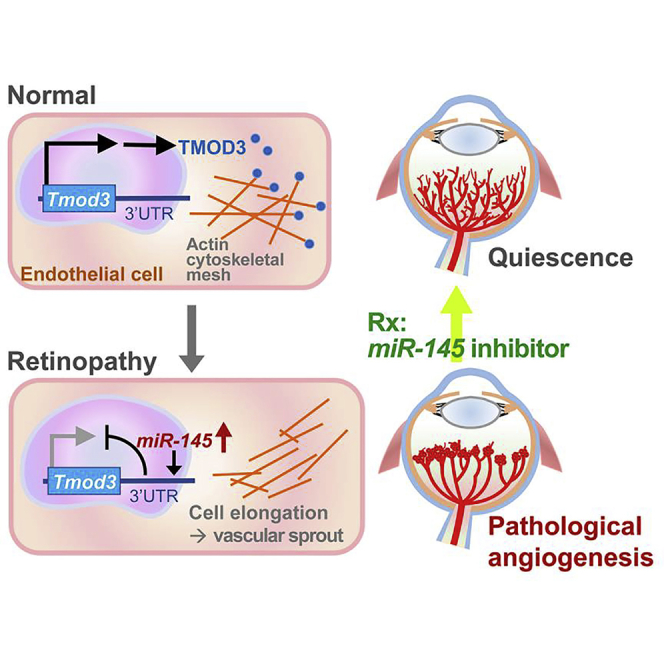
In this report, we present findings that retinal miR-145 is a key regulator of EC actin dynamics and cell structure during pathological ocular neovascularization through post-transcriptional control of Tmod3. In the ischemia-induced experimental retinopathy, upregulated miR-145 repressed the expression of Tmod3, causing alleviation of TMOD3 binding to the pointed ends of actin filaments that allowed its elongation and depolymerization, thereby leading to alterations in EC cytoskeletal architecture and dynamics, which may affect pathological angiogenic sprouting (Figure 8).
Figure 8.
Proposed Role of the miR-145-TMOD3 Axis in Proliferative Retinopathy
In normal retinal vessels, endothelial TMOD3 binds to the pointed ends of actin filaments, keeping the cytoskeletal structure steady and maintaining blood vessels in quiescence. In proliferative retinopathy, increased expression of endothelial miR-145 represses expression of Tmod3, resulting in changed dynamics and architecture of actin filaments, which may lead to increased EC sprouting and proliferation, which affect pathological angiogenesis. Inhibition of miR-145 suppresses neovessel formation in experimental retinopathy.
Our previous study in the mouse model of OIR identified a significant increase of retinal miR-145 during the neovascular phase,27 indicating that dysregulation of miR-145 may be linked to pathological retinal angiogenesis. Here, we found that miR-145 increased gradually in the neonatal mouse retinas during physiological development and was robustly upregulated in the OIR retinas during formation of neovascular tufts in the second hypoxia phase (Figure 1). Upregulation of miR-145 during the neovascular phase of OIR likely reflects its hypoxia-responsive nature, where in HRMECs miR-145 was upregulated in the hypoxic condition (Figure 4F and 4G). This observation is consistent with previous studies in bladder cancer, indicating that miR-145 is a hypoxia-regulated miRNA targeting by hypoxia-inducible factor-1 alpha subunit (HIF-1α),38 which also regulates transcription of angiogenic genes in the mouse OIR model and human retinopathy.39 On the other hand, the increasing expression of miR-145 during retinal development may correlate with the retinal maturation process and may reflect in part potential physiological hypoxia during development, in addition to induction by other signaling pathways, such as serum response factor (SRF)29 and Notch.40 Previous studies demonstrated that activation of miR-143/145 could be regulated in parallel by SRF-dependent networks and Notch1 intracellular domain-containing complexes.29, 40 Both signaling pathways have been shown to be important in retinal vascular development and association with the pathogenesis of neovascular eye diseases.29, 40, 41, 42, 43, 44, 45, 46
miR-145 is often co-transcribed with miR-143 and has been reported to be one of the most abundantly expressed miRNAs in vascular walls in arteries and in smooth muscle cells.28, 47 Expression of miR-145 is significantly downregulated, both in atherosclerosis in humans and in a rat model of arterial balloon injury.47, 48 In addition to smooth muscle cells, miR-143/145 is also expressed in vascular ECs and at low levels in normoxic cultured ECs,28 consistent with our finding of low miR-145 levels in normoxic retinas and suggesting that steady and low levels of miR-145 is sufficient in maintaining EC quiescence. On the other hand, miR-143/145-deficient mice show attenuated tumor angiogenesis and limited tumor expansion, suggesting that loss of miR-143/145 from the tumor microenvironment suppresses tumor neoangiogenesis.30 In line with this pro-angiogenic role of miR-143/145, we found that treatment with miR-145 inhibitors intravitreally injected into OIR mouse eyes significantly suppressed neovascularization (Figure 1), indicating that miR-145 induction in retinopathy may be a key pathogenic factor contributing to pathological retinal neovascularization. In addition, miR-145 mimic treatment promoted angiogenesis both in murine aortic ring explants and in cultured human ECs (Figure 3), further supporting the pro-angiogenic function of miR-145.
Our study identified Tmod3 as a novel direct target gene of miR-145, supported by the findings that modulation of miR-145 significantly altered Tmod3 levels in vitro and in vivo (Figures 4, 5, and 7). The TMOD family is a group of proteins that cap the slow-growing (pointed) ends of actin filaments, blocking their elongation and depolymerization, hence regulating the stability, length, and architecture of the actin cytoskeleton.35 Four TMOD isoforms are expressed in vertebrates in a tissue-specific and developmentally regulated manner. Vascular ECs contain only TMOD3, associated with the dynamic actin filament network in ECs.36 During angiogenesis, rapid remodeling of EC actin filament networks occurs, in response to signaling cascades elicited by growth stimuli or environmental cues.49 Fischer et al.36 reported that TMOD3 is a negative regulator of the EC motility in human microvascular ECs. Their study localized TMOD3 to the actin filament, found it to be specifically enriched in the leading lamellipodia of ECs, and showed that modulation of TMOD3 altered free pointed and barbed ends in the lamellipodial actin filaments, concomitant with altered EC motility.36 Moreover, a previous study in adipocytes showed that TMOD3 is an AKT2 effector, mediating insulin-induced cortical actin remodeling.50 In line with this evidence, we found that exogenous miR-145-treated human retinal ECs showed diminished Tmod3 expression in the actin filaments and altered EC actin dynamics and cell structure (Figure 5), associated with increased ability for cell proliferation, tubular formation, and cell migration (Figure 3). TMOD3 inhibition mimicked the vascular effects of miR-145 and reversed the effects of miR-145 inhibitors in vitro and in vivo, suggesting that the vascular effects of miR-145 on EC functions and in OIR are likely mediated via its transcriptional regulation of Tmod3.
Work in miR-145 KO and miR-143/145 double-KO mice showed notably thinner smooth muscle layers of the arteries, with a decreased number and prominence of stress fibers in the smooth muscle cells.29 It is suggested that the absence of miR-143/145 causes an imbalance of cytoskeletal homeostasis, thereby perturbing stress fiber formation and actin remodeling, resulting in altered cell morphology.29 Previous studies found expression of miR-143/145 in multiple cell types in the eyes, including vascular smooth muscle cells, pericytes, ciliary muscles, extraocular muscles, photoreceptors, and Müller glia.32, 51 miR-143/145 regulates actin dynamics and the contractility of cells in the trabecular meshwork to affect eye pressure.32 miR-145 also plays an important role in the TGF-β1-stimulated corneal myofibroblast activity and contractility that affects corneal scaring.52 In our study, we found that exogenous miR-145 influenced cytoskeletal architecture and caused elongation of cell shapes in cultured human retinal ECs (Figure 5). These findings suggest that miR-145 regulates its downstream targets, including Tmod3, in multiple cell types to induce actin cytoskeletal changes in diverse tissues.
Together, our data link miR-145 and Tmod3 with EC functional change, which may underlie the vascular effects of miR-145 on pathological ocular angiogenesis in OIR. In OIR retinas, hypoxia-induced miR-145 expression may enhance neovascular sprouting growth by repression of Tmod3 and thereby influence EC actin remodeling in the angiogenic front to enhance neovascular sprouting (Figure 8). Yet potential contribution from other target genes such as Tmem9b, an inflammatory regulator,37 cannot be excluded. Inflammatory changes also occur in retinopathy and contribute to neovascular response, and miR-145 may also exert anti-inflammatory effects via Tmem9b to dampen inflammation and suppress in part the neovascular response in OIR, an effect that awaits further investigation.
Dysregulated miRNAs have been associated with abnormal ocular angiogenesis in clinical retinopathy.10, 23, 27 This study presents evidence of a novel role of miR-145 as a regulator of EC morphology and angiogenesis in experimental retinopathy. These findings provide new insight into targeting miR-145 and the endothelial miR-145/TMOD3 axis as potential miRNA-based therapeutic strategies for retinopathy and are also relevant for other vascular diseases associated with pathological angiogenesis. Recently, intravitreal injection of PF-04523655, a synthetic siRNA targeting a stress-induced gene, RTP801, was well tolerated in a phase I/IIa study in patients with choroidal neovascularization and neovascular age-related macular degeneration,53, 54 as well as in diabetic macular edema.55 With respect to post-transcriptional gene repression, the biogenesis and mechanisms of miRNAs are similar to siRNAs,56 suggesting the future possibility of targeting dysregulated miRNAs via intravitreal injection of miRNA modulators as potential therapeutic options.
Materials and Methods
Animals
All animal studies were approved by the Institutional Animal Care and Use Committee at Boston Children’s Hospital and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6J mice (stock no. 000664) were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and used for this study.
OIR Model
The OIR was induced in mice as described previously.57, 58, 59 Briefly, neonatal mice with their nursing mother were exposed to 75% oxygen at P7 for 5 days and then returned to room air at P12. At the selected time points, the mice were sacrificed, followed by enucleation of the eyes for the following experiments.
Intravitreal Injection
For evaluating the effects of miR-145 and Tmod3 in pathological angiogenesis, intravitreal injections were performed in OIR mice at P12 after their removal from an oxygen chamber.23, 60 The mice were anesthetized with isoflurane in oxygen from a precision vaporizer for approximately 5 min until they became immobilized. The eyelids were separated, and the eyes were proptosed with gentle pressure. For each mouse, 1 μg of miR-145 inhibitors (mirVana; Ambion, Thermo Fisher Scientific, Waltham, MA, USA) and/or siRNA targeting TMOD3 (siTMOD3) (Integrated DNA Technologies, Skokie, IL, USA) dissolved in 0.5 μL of vehicle solution was injected behind the limbus of the eye, and the contralateral eye was injected with an equal amount of negative control RNAs or siRNAs with scrambled sequence (siNC), with or without 1 μg of miR-145 inhibitors. The eyes were lubricated with sterile saline after injection, and an antibiotic eye ointment was applied. After 5 days in room air, the mice were sacrificed at P17, for the study of retinal vasculature.
To verify the effects of miR-145 during physiological vascular development, anesthesia was induced in neonatal mice at P1, P5, and P6 by placing them indirectly on crushed ice for approximately 5 min until they became immobilized, and the eyes were gently proptosed with fine tweezers. One eye was intravitreally injected with 1 μg miR-145 inhibitors or mimics (mirVana; Ambion) dissolved in 0.5 μL of vehicle solution in one eye, and the contralateral eye was injected with an equal amount of negative control oligomers. As described previously, topical lubricating solution and occasional antibiotic ointment were applied to the eyes. At P5, P7, or P10, the mice were sacrificed for the study of superficial and deep layers of retinal vasculature.
Retinal Whole Mounts and Quantification of Vasculature
Both vaso-obliteration and pathological neovascularization in the OIR mouse retinas were quantified at P17, as previously described.57 Briefly, the eyes were fixed with 4% paraformaldehyde dissolved in PBS at room temperature for 1 hour, and the retinas were dissected for overnight incubation with fluorescent Griffonia simplicifolia isolectin B4 (Invitrogen, Thermo Fisher Scientific) and flat mounted. Images of retinal whole mounts were examined by fluorescence microscope (Zeiss AxioOberver.Z1; Carl Zeiss, Oberkochen, Germany). Vascular loss and neovascular areas in OIR were quantified by using Adobe Photoshop (Adobe Systems, San Jose, CA, USA) and ImageJ (NIH, Bethesda, MD, USA; https://imagej.nih.gov/ij/). Vascular density in the developing retinas was analyzed, using the Vessel Analysis plugin with Fiji.61 Researchers performing quantification of retinal vessels were masked to the identity of samples; n represents the number of eyes quantified.62
Aortic Ring Assay
Aortic ring assays were performed as described previously.63, 64 Twenty-day-old mice were euthanized and the aortae were dissected and cut into 1-mm-thick rings, followed by overnight incubation in serum-free medium, then embedded in 30 μL of growth factor-reduced Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) in 24-well tissue culture plates containing Complete Classic Medium (Cell Systems, Kirkland, WA, USA) and incubated at 37°C with 5% CO2. Medium was changed every other day in all groups. Images of individual explants were taken with a Zeiss AxioOberver.Z1 microscope beginning at 4 days after plating. The vascular sprouting area was quantified with a semi-automated macro plug-in for ImageJ.
Bioinformatics
The conserved seed sequences of miR-145 from different species, including human and murine, were obtained from miRBase (http://www.mirbase.org/).65, 66 Potential targets of miR-145 were predicted according to the algorithms of TargetScan v.7 (http://www.targetscan.org/)67 and microRNA.org (miRanada-mirSVR) (http://www.microrna.org/microrna/home.do/),68 with incorporation of the context score ++ <−0.2 and mirSVR score <−0.5, respectively.
Real-Time qRT-PCR
Total RNA was extracted from the homogenized retinas of mice or the cultured cells by PureLink RNA Mini Kit (Invitrogen), according to the manufacturer’s instructions, followed by synthesis of cDNA by reverse transcription with iScript Reverse Transcriptase (Bio-Rad, Hercules, CA, USA). MicroRNA was isolated from retinas of mice in OIR and normoxia with the miRNeasy Micro Kit (QIAGEN, Hilden, Germany), and the cDNA was generated using the TaqMan miR reverse transcription kit (Applied Biosystems, Thermo Fisher Scientific).
Quantitative analysis of gene expression was determined by qRT-PCR with a C1000 Thermal Cycler (Bio-Rad) and 2× SYBR Green qPCR Master Mix (Bimake.com, Houston, TX, USA) with specific primers. Each target gene cDNA copy number was normalized to the housekeeping gene S18, using the comparative CT (ΔΔCT) method. miR-145 expression level was also analyzed by qPCR with TaqMan miRNA Gene Expression Assays and TaqMan Universal PCR Master Mix (Applied Biosystems), according to the manufacturer’s instructions. Data were quantified by ΔΔCT method and normalized to U6 small nuclear RNA (snRNA) as the endogenous reference. A list of specific primers is summarized in Table S1.
Cell Culture
HRMECs (Cell Systems) were cultured at 37°C and 5% CO2 in a humid atmosphere in Complete Classic Medium (Cell Systems). HEK293T cells (ATCC, Manassas, VA, USA) were maintain in high-glucose DMEM (Invitrogen) supplemented with 10% fetal bovine serum in 5% CO2 at 37°C.
Transfection
miR-145 mimics, inhibitors, and non-targeting scrambled negative control RNAs (Ambion), as well as siTMOD3 and siNC (Integrated DNA Technologies, San Jose, CA, USA) were used. Cells or aortic explants were transfected with (1) 25 or 50 nM of miR-145 mimics, inhibitors, or negative control RNAs or (2) 50 nM of miR-145 inhibitors or negative control RNAs combined with 5 or 10 nM of siTMOD3 or siNC, using Lipofectamine 3000 (Invitrogen), according to the manufacturer’s instructions. Briefly, synthetic miRNA oligomers or siRNAs were complexed with the transfection reagent in serum-free culture medium and added to cells or aortic explants in fresh full-growth medium. Cells were then used for the following assays after a 24 h transfection.
Tube Formation Assay
HRMECs transfected with various modulators or controls were seeded in a 24-well plate coated with Matrigel (BD Bioscience), at a density of 1 × 105 cells/well. Cells were incubated at 37°C for 6 h and then imaged with a Zeiss AxioObserver.Z1 microscope to assess the formation of tube-like structures. The total mesh area was analyzed by the Angiogenesis Analyzer plug-in for ImageJ69 (n = 6 per group).
In Vitro Scratch Assay
HRMECs transfected with various modulators or controls were seeded at a concentration of 2 × 105 cells/well. After 24 h of transfection, the cells were treated with 10 μg/mL mitomycin C (Sigma-Aldrich, St. Louis, MO, USA) for 20 min at 37°C. Cell monolayers were wounded by scraping with a pipette tip and incubated at 37°C for 14–18 h. Images of the cells were taken immediately after scraping and again after cell migration into wounded areas. The area covered by the migrating cells was quantified and normalized to the original wound area (n = 6 per group).70
MTT Assay
HRMECs transfected with various modulators or controls were seeded at a concentration of 2 × 105 cells/mL into 96-well plates and incubated with DMEM at 37°C for 24 h. Cell proliferative activity was assayed using the Vybrant MTT Cell Proliferation kit (Invitrogen) according to the manufacturer’s protocol. Absorbance was read at 570 nm by using the xMark Microplate Spectrophotometer (Bio-Rad).
Luciferase Reporter Assay
The oligonucleotides comprising the miR-145 target sequences were synthesized by Integrated DNA Technologies and annealed and cloned into the pmirGLO vector (Promega, Madison, WI, USA) for luciferase reporter assays. The miR-145 target sequences within the 3′ UTR of each target gene were predicted by TargetScan v.7 and microRNA.org, as described previously.67, 68 Constructs containing sequences with the miR-145 seed-matched sites (Table S2) were confirmed by sequencing at Eton Bioscience (San Diego, CA, USA). HEK293T cells were plated into 96-well plates at 2 × 104 cells/well and transfected with the reporter plasmids containing target sequences or empty vectors as the control, using Lipofectamine 2000 (Invitrogen). Cells were co-transfected with 25 or 50 nM miR-145 mimics or negative controls (Ambion). After 24 h, luciferase activity was measured, using the Dual-Glo Luciferase Assay System (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity and to negative controls.
Hypoxia Treatment in HRMEC Culture
HRMECs were maintained as described in previous sections and allowed to grow until >90% confluence, as inspected visually. To achieve hypoxia, an air mixture (5% CO2/95% N2) was used to flush the hypoxia cell culture chamber containing cell culture plates to create a hypoxic condition with <2% O2, as measured by oxygen sensor. HRMECs were exposed for 6, 12, and 24 h to the hypoxic condition and harvested. Control HRMECs were maintained in normoxia and harvested at 24 h.
Immunofluorescence and Imaging
HRMECs growing on coverslips were fixed with 4% paraformaldehyde at room temperature for 10 min followed by washes in PBS and then blocked with blocking buffer (1× PBS, 5% normal goat serum, and 0.1% Triton X-100) for 30 min. Cells were incubated overnight at 4°C with anti-TMOD3 antibody (1:200 dilution; Aviva Systems Biology, San Diego, CA, USA). After washes in PBS, the cells were incubated with Alexa Fluor 488 goat anti-rabbit IgG antibody (1:200 dilution; Invitrogen), in parallel with rhodamine phalloidin (1:200 dilution; Invitrogen) for 1 hour at room temperature, followed by counterstaining with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen). Cells were then rinsed, mounted in mounting medium, and imaged with an inverted Zeiss LSM880 confocal microscope. Cell shape was determined by measuring individual cell length and width, using Adobe Photoshop and ImageJ. The aspect ratio of cell shape denoted the ratio of the maximum length to the maximum width.
Western Blot Analysis
Retinas or cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (Thermo Scientific) with protease and phosphatase inhibitors (Sigma-Aldrich). Lysates then were placed in SDS sample buffer and heated at 100°C for 5 min. Proteins were separated by SDS-PAGE, transferred to polyvinylidene fluoride membranes, and probed with an anti-TMOD3 antibody (Aviva Systems Biology) and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (Santa Cruz Biotechnology, Dallas, TX, USA). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG and anti-rabbit IgG secondary antibodies (GE Healthcare Life Sciences, Marlborough, MA, USA) were used. Signals were detected with the Novex ECL Chemiluminescent Substrate Reagent Kit (Invitrogen).
Statistical Analysis
Quantitative data are presented as means ± SEM, and asterisks in figures represent the p-value according to the two-tailed Student’s t test (two groups) or ANOVA (more than two groups): * p ≤ 0.05; ** p ≤ 0.005; *** p ≤ 0.001.
Author Contributions
C.-H.L. and J.C. conceived of and designed the study and wrote the manuscript. C.-H.L., Z.W., and S.H. collected and analyzed the data. Y.S. provided expert advice. All authors edited and approved the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the following funders in United States: Knights Templar Eye Foundation under the Pediatric Ophthalmology Career-Starter Research Grant (to C.-H.L.), NIH R01 grants (EY024963 and EY028100), BrightFocus Foundation, Massachusetts Lions Eye Research Fund, Inc., and a Research to Prevent Blindness Dolly Green Special Scholar Award (to J.C.). Z.W. was supported by a Knights Templar Eye Foundation Pediatric Ophthalmology Career-Starter Research Grant and Y.S. was supported by a Boston Children's Hospital Faculty Career Development Award. We thank Rubi Duran, Alexander Poblete, Steve Cho, and William Britton for their excellent technical assistance.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtn.2019.03.001.
Supplemental Information
References
- 1.Potente M., Gerhardt H., Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Afzal A., Shaw L.C., Ljubimov A.V., Boulton M.E., Segal M.S., Grant M.B. Retinal and choroidal microangiopathies: therapeutic opportunities. Microvasc. Res. 2007;74:131–144. doi: 10.1016/j.mvr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford Y., Ferrara N. VEGF inhibition: insights from preclinical and clinical studies. Cell Tissue Res. 2009;335:261–269. doi: 10.1007/s00441-008-0675-8. [DOI] [PubMed] [Google Scholar]
- 6.Darlow B.A., Ells A.L., Gilbert C.E., Gole G.A., Quinn G.E. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch. Dis. Child. Fetal Neonatal Ed. 2013;98:F170–F174. doi: 10.1136/archdischild-2011-301148. [DOI] [PubMed] [Google Scholar]
- 7.Hu J., Blair M.P., Shapiro M.J., Lichtenstein S.J., Galasso J.M., Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch. Ophthalmol. 2012;130:1000–1006. doi: 10.1001/archophthalmol.2012.592. [DOI] [PubMed] [Google Scholar]
- 8.Wallace D.K., Wu K.Y. Current and future trends in treatment of severe retinopathy of prematurity. Clin. Perinatol. 2013;40:297–310. doi: 10.1016/j.clp.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 9.He L., Hannon G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal S., Chaqour B. MicroRNA signature and function in retinal neovascularization. World J. Biol. Chem. 2014;5:1–11. doi: 10.4331/wjbc.v5.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennecke J., Hipfner D.R., Stark A., Russell R.B., Cohen S.M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 12.Chang T.C., Mendell J.T. microRNAs in vertebrate physiology and human disease. Annu. Rev. Genomics Hum. Genet. 2007;8:215–239. doi: 10.1146/annurev.genom.8.080706.092351. [DOI] [PubMed] [Google Scholar]
- 13.Im H.I., Kenny P.J. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35:325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krek A., Grün D., Poy M.N., Wolf R., Rosenberg L., Epstein E.J., MacMenamin P., da Piedade I., Gunsalus K.C., Stoffel M., Rajewsky N. Combinatorial microRNA target predictions. Nat. Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 15.Lim L.P., Lau N.C., Garrett-Engele P., Grimson A., Schelter J.M., Castle J., Bartel D.P., Linsley P.S., Johnson J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 16.Anand S., Cheresh D.A. Emerging Role of Micro-RNAs in the Regulation of Angiogenesis. Genes Cancer. 2011;2:1134–1138. doi: 10.1177/1947601911423032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand S., Majeti B.K., Acevedo L.M., Murphy E.A., Mukthavaram R., Scheppke L., Huang M., Shields D.J., Lindquist J.N., Lapinski P.E. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicoli S., Standley C., Walker P., Hurlstone A., Fogarty K.E., Lawson N.D. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Png K.J., Halberg N., Yoshida M., Tavazoie S.F. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2011;481:190–194. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- 20.Shen J., Yang X., Xie B., Chen Y., Swaim M., Hackett S.F., Campochiaro P.A. MicroRNAs regulate ocular neovascularization. Mol. Ther. 2008;16:1208–1216. doi: 10.1038/mt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poliseno L., Tuccoli A., Mariani L., Evangelista M., Citti L., Woods K., Mercatanti A., Hammond S., Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 22.Wang S., Olson E.N. AngiomiRs: key regulators of angiogenesis. Curr. Opin. Genet. Dev. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C.H., Sun Y., Li J., Gong Y., Tian K.T., Evans L.P., Morss P.C., Fredrick T.W., Saba N.J., Chen J. Endothelial microRNA-150 is an intrinsic suppressor of pathologic ocular neovascularization. Proc. Natl. Acad. Sci. USA. 2015;112:12163–12168. doi: 10.1073/pnas.1508426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westenskow P.D., Kurihara T., Aguilar E., Scheppke E.L., Moreno S.K., Wittgrove C., Marchetti V., Michael I.P., Anand S., Nagy A. Ras pathway inhibition prevents neovascularization by repressing endothelial cell sprouting. J. Clin. Invest. 2013;123:4900–4908. doi: 10.1172/JCI70230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A., Richardson J.A., Bassel-Duby R., Olson E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Q., Gallagher R., Ufret-Vincenty R., Li X., Olson E.N., Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23∼27∼24 clusters. Proc. Natl. Acad. Sci. USA. 2011;108:8287–8292. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C.H., Wang Z., Sun Y., SanGiovanni J.P., Chen J. Retinal expression of small non-coding RNAs in a murine model of proliferative retinopathy. Sci. Rep. 2016;6:33947. doi: 10.1038/srep33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent O.A., McCall M.N., Cornish T.C., Halushka M.K. Lessons from miR-143/145: the importance of cell-type localization of miRNAs. Nucleic Acids Res. 2014;42:7528–7538. doi: 10.1093/nar/gku461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xin M., Small E.M., Sutherland L.B., Qi X., McAnally J., Plato C.F., Richardson J.A., Bassel-Duby R., Olson E.N. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimitrova N., Gocheva V., Bhutkar A., Resnick R., Jong R.M., Miller K.M., Bendor J., Jacks T. Stromal Expression of miR-143/145 Promotes Neoangiogenesis in Lung Cancer Development. Cancer Discov. 2016;6:188–201. doi: 10.1158/2159-8290.CD-15-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S.K., Teng Y., Wong H.K., Ng T.K., Huang L., Lei P., Choy K.W., Liu Y., Zhang M., Lam D.S. MicroRNA-145 regulates human corneal epithelial differentiation. PLoS ONE. 2011;6:e21249. doi: 10.1371/journal.pone.0021249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X., Zhao F., Xin M., Li G., Luna C., Li G., Zhou Q., He Y., Yu B., Olson E. Regulation of intraocular pressure by microRNA cluster miR-143/145. Sci. Rep. 2017;7:915. doi: 10.1038/s41598-017-01003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stahl A., Connor K.M., Sapieha P., Chen J., Dennison R.J., Krah N.M., Seaward M.R., Willett K.L., Aderman C.M., Guerin K.I. The mouse retina as an angiogenesis model. Invest. Ophthalmol. Vis. Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 35.Yamashiro S., Gokhin D.S., Kimura S., Nowak R.B., Fowler V.M. Tropomodulins: pointed-end capping proteins that regulate actin filament architecture in diverse cell types. Cytoskeleton (Hoboken) 2012;69:337–370. doi: 10.1002/cm.21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer R.S., Fritz-Six K.L., Fowler V.M. Pointed-end capping by tropomodulin3 negatively regulates endothelial cell motility. J. Cell Biol. 2003;161:371–380. doi: 10.1083/jcb.200209057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dodeller F., Gottar M., Huesken D., Iourgenko V., Cenni B. The lysosomal transmembrane protein 9B regulates the activity of inflammatory signaling pathways. J. Biol. Chem. 2008;283:21487–21494. doi: 10.1074/jbc.M801908200. [DOI] [PubMed] [Google Scholar]
- 38.Blick C., Ramachandran A., McCormick R., Wigfield S., Cranston D., Catto J., Harris A.L. Identification of a hypoxia-regulated miRNA signature in bladder cancer and a role for miR-145 in hypoxia-dependent apoptosis. Br. J. Cancer. 2015;113:634–644. doi: 10.1038/bjc.2015.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartnett M.E., Penn J.S. Mechanisms and management of retinopathy of prematurity. N. Engl. J. Med. 2012;367:2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boucher J.M., Peterson S.M., Urs S., Zhang C., Liaw L. The miR-143/145 cluster is a novel transcriptional target of Jagged-1/Notch signaling in vascular smooth muscle cells. J. Biol. Chem. 2011;286:28312–28321. doi: 10.1074/jbc.M111.221945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lobov I.B., Cheung E., Wudali R., Cao J., Halasz G., Wei Y., Economides A., Lin H.C., Papadopoulos N., Yancopoulos G.D., Wiegand S.J. The Dll4/Notch pathway controls postangiogenic blood vessel remodeling and regression by modulating vasoconstriction and blood flow. Blood. 2011;117:6728–6737. doi: 10.1182/blood-2010-08-302067. [DOI] [PubMed] [Google Scholar]
- 42.Weinl C., Riehle H., Park D., Stritt C., Beck S., Huber G., Wolburg H., Olson E.N., Seeliger M.W., Adams R.H., Nordheim A. Endothelial SRF/MRTF ablation causes vascular disease phenotypes in murine retinae. J. Clin. Invest. 2013;123:2193–2206. doi: 10.1172/JCI64201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellström M., Phng L.K., Hofmann J.J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A.K., Karlsson L., Gaiano N. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 44.Franco C.A., Blanc J., Parlakian A., Blanco R., Aspalter I.M., Kazakova N., Diguet N., Mylonas E., Gao-Li J., Vaahtokari A. SRF selectively controls tip cell invasive behavior in angiogenesis. Development. 2013;140:2321–2333. doi: 10.1242/dev.091074. [DOI] [PubMed] [Google Scholar]
- 45.Bao Z.Z., Cepko C.L. The expression and function of Notch pathway genes in the developing rat eye. J. Neurosci. 1997;17:1425–1434. doi: 10.1523/JNEUROSCI.17-04-01425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benedito R., Roca C., Sörensen I., Adams S., Gossler A., Fruttiger M., Adams R.H. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 47.Cheng Y., Liu X., Yang J., Lin Y., Xu D.Z., Lu Q., Deitch E.A., Huo Y., Delphin E.S., Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji R., Cheng Y., Yue J., Yang J., Liu X., Chen H., Dean D.B., Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 49.Pollard T.D., Borisy G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 50.Lim C.Y., Bi X., Wu D., Kim J.B., Gunning P.W., Hong W., Han W. Tropomodulin3 is a novel Akt2 effector regulating insulin-stimulated GLUT4 exocytosis through cortical actin remodeling. Nat. Commun. 2015;6:5951. doi: 10.1038/ncomms6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quintero H., Gómez-Montalvo A.I., Lamas M. MicroRNA changes through Müller glia dedifferentiation and early/late rod photoreceptor differentiation. Neuroscience. 2016;316:109–121. doi: 10.1016/j.neuroscience.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 52.Ratuszny D., Gras C., Bajor A., Börger A.K., Pielen A., Börgel M., Framme C., Blasczyk R., Figueiredo C. miR-145 Is a Promising Therapeutic Target to Prevent Cornea Scarring. Hum. Gene Ther. 2015;26:698–707. doi: 10.1089/hum.2014.151. [DOI] [PubMed] [Google Scholar]
- 53.Hanout M., Ferraz D., Ansari M., Maqsood N., Kherani S., Sepah Y.J., Rajagopalan N., Ibrahim M., Do D.V., Nguyen Q.D. Therapies for neovascular age-related macular degeneration: current approaches and pharmacologic agents in development. BioMed Res. Int. 2013;2013:830837. doi: 10.1155/2013/830837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.PF-04523655 Study Group Phase 1 dose-escalation study of a siRNA targeting the RTP801 gene in age-related macular degeneration patients. Eye (Lond.) 2012;26:1099–1105. doi: 10.1038/eye.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DEGAS Clinical Study Group Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS study) Invest. Ophthalmol. Vis. Sci. 2012;53:7666–7674. doi: 10.1167/iovs.12-9961. [DOI] [PubMed] [Google Scholar]
- 56.Chakraborty C., Sharma A.R., Sharma G., Doss C.G.P., Lee S.S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol. Ther. Nucleic Acids. 2017;8:132–143. doi: 10.1016/j.omtn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connor K.M., Krah N.M., Dennison R.J., Aderman C.M., Chen J., Guerin K.I., Sapieha P., Stahl A., Willett K.L., Smith L.E. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat. Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith L.E., Wesolowski E., McLellan A., Kostyk S.K., D’Amato R., Sullivan R., D’Amore P.A. Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 59.Liu C.H., Wang Z., Sun Y., Chen J. Animal models of ocular angiogenesis: from development to pathologies. FASEB J. 2017;31:4665–4681. doi: 10.1096/fj.201700336R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J., Connor K.M., Aderman C.M., Willett K.L., Aspegren O.P., Smith L.E. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest. Ophthalmol. Vis. Sci. 2009;50:1329–1335. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stahl A., Connor K.M., Sapieha P., Willett K.L., Krah N.M., Dennison R.J., Chen J., Guerin K.I., Smith L.E. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12:297–301. doi: 10.1007/s10456-009-9155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker M., Robinson S.D., Lechertier T., Barber P.R., Tavora B., D’Amico G., Jones D.T., Vojnovic B., Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat. Protoc. 2011;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 64.Li J., Liu C.H., Sun Y., Gong Y., Fu Z., Evans L.P., Tian K.T., Juan A.M., Hurst C.G., Mammoto A., Chen J. Endothelial TWIST1 promotes pathological ocular angiogenesis. Invest. Ophthalmol. Vis. Sci. 2014;55:8267–8277. doi: 10.1167/iovs.14-15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griffiths-Jones S., Grocock R.J., van Dongen S., Bateman A., Enright A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kozomara A., Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agarwal V., Bell G.W., Nam J.W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4 doi: 10.7554/eLife.05005. 05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Betel D., Koppal A., Agius P., Sander C., Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carpentier G., Martinelli M., Courty J., Cascone I. Luxembourg Institute of Science and Technology; 2012. Angiogenesis Analyzer for ImageJ. Proceedings of the 4th ImageJ User and Developer Conference; pp. 198–201. [Google Scholar]
- 70.Gong Y., Yang X., He Q., Gower L., Prudovsky I., Vary C.P., Brooks P.C., Friesel R.E. Sprouty4 regulates endothelial cell migration via modulating integrin β3 stability through c-Src. Angiogenesis. 2013;16:861–875. doi: 10.1007/s10456-013-9361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.