Summary
Recently, many super-resolution technologies have been demonstrated, significantly affecting biological studies by observation of cellular structures down to nanometer precision. However, current super-resolution techniques mostly rely on wavefront engineering or wide-field imaging of signal blinking or fluctuation, and thus imaging depths are limited due to tissue scattering or aberration. Here we present a technique that is capable of imaging through an intact Drosophila brain with 20-nm lateral resolution at ∼200 μm depth. The spatial resolution is provided by molecular localization of a photoconvertible fluorescent protein Kaede, whose red form is found to exhibit blinking state. The deep-tissue observation is enabled by optical sectioning of spinning disk microscopy, as well as reduced scattering from optical clearing. Together these techniques are readily available for many biologists, providing three-dimensional resolution of densely entangled dendritic fibers in a complete Drosophila brain. The method paves the way toward whole-brain neural network studies and is applicable to other high-resolution bioimaging.
Subject Areas: Optical Imaging, Neuroscience, Techniques in Neuroscience
Graphical Abstract

Highlights
-
•
Spatial resolution of 20 nm at ∼ 200 μm imaging depth (may be extended)
-
•
Super-resolution tracking of densely entangled neural fibers in an intact brain
-
•
All tools (FP, confocal, clearing, localization) are commercially available
Optical Imaging; Neuroscience; Techniques in Neuroscience
Introduction
Fluorescence microscopy is an indispensable tool in biomedical studies because it provides molecular information to be located within cells or tissues spatially and temporally. In the last decade, significant improvement of optical resolution beyond diffraction limit based on fluorescence microscopy has been realized (Huang et al., 2010), down to nanometer or even angstrom scales recently (Weisenburger et al., 2017). These techniques rely on either wavefront engineering or wide-field imaging of signal blinking or fluctuation (Huang et al., 2010), and thus are not able to enhance resolution inside thick tissue due to scattering, aberration, and out-of-focus signals. One particularly strong motivation for thick-tissue super-resolution is the urgent need to map the neuronal network and connectivity at dendritic level throughout a whole intact brain with high enough spatial resolution to understand brain functions.
There have been lots of recent efforts to enhance spatial resolution in deep tissue. For example, localization microscopy combined with temporal focusing (TF) (Vaziri et al., 2008) or (two-photon) light sheet (Chen et al., 2016, Zanacchi et al., 2011) provides optical sectioning for excitation, but the scattering-induced cross talk in space due to the wide-field detection scheme limits the thickness of the samples to be less than 100 μm. Another wide-field-based technique, structured illumination microscopy (SIM), exhibits similar issues on both excitation and detection. Even when combined with a line scan confocal scheme, the imaging depth of SIM is still limited to 20 μm (Schropp et al., 2017). On the other hand, stimulated emission depletion (STED), reversible saturable optical linear fluorescence transitions (RESOLFT), and saturated excitation (SAX) microscopies, which are based on a point scan confocal geometry and reduce the spatial cross talk, are demonstrated to achieve super-resolution at tens of micrometers imaging depth (Schnorrenberg et al., 2016, Takasaki et al., 2013, Yamanaka et al., 2013). The key is to maintain a consistent beam shape deep inside the sample. For example, the imaging depth of STED is enhanced to about 100 μm in a cell aggregate by using a correction collar to minimize spherical aberration (Urban et al., 2011). Recently, a hollow Bessel beam STED reported 100-nm resolution at imaging depth of 155 μm in an agarose sample, which is highly transparent (Yu et al., 2016). Although STED and RESOLFT seem to be more promising for tissue imaging, their resolutions are in general on the order of 60–100 nm, i.e., less resolving power than the localization approaches. One recent innovation is to expand the tissue, to achieve simultaneous clearing and resolution enhancement (Chang et al., 2017, Chen et al., 2015a, Ku et al., 2016). Nevertheless, the effective imaging depth is thus reduced, and the expansion techniques do not provide increasing ratio of depth and resolution. Thus, a better strategy is to combine localization with optical sectioned detection, such as line scan confocal microscopy (Lee et al., 2012). However, the reported resolution-depth performance of this combination is 60-nm spatial resolution at 80 μm depth, which is comparable to other methods.
In this work, we utilized spinning disk confocal microscopy, which is commercially available and widely used in biology, to reject out-of-focus fluorescence (Uno et al., 2014). The spatial resolution and penetration depth are simultaneously enhanced by combination of molecular localization and optical clearing (Liu and Chiang, 2003). With the aid of a blinking fluorescent protein, Kaede (Ando et al., 2002), which can be expressed in neurons of interests, we demonstrated that confocal localization deep imaging with optical clearing (COOL) can reach less than λ/20 lateral resolution at nearly 200 μm depth inside an intact Drosophila brain, which is the highest record of depth-over-resolution ratio reported to date in the literature. The combination of resolution and imaging depth provides a potential route to map the neural network among the densely packed 100,000 neurons in the whole brain at high resolution.
Results
Optical Sectioned Observation of Blinking Fluorescent Protein Kaede
Figures 1A and 1B show the comparison between conventional wide-field and spinning-disk confocal imaging on the same G0239-Gal4 neurons, which express Kaede fluorescent protein, in a Drosophila brain (see Supplemental Information for sample preparation) (Pai et al., 2013). As expected, the wide-field modality loses contrast within less than 50 μm depth, even if the brain was mounted in a clearing reagent, whereas confocal works well all the way into the brain. The images acquired by the spinning-disk confocal microscope demonstrate high quality of blinking fluorescence spots for localization, whose example is given in Figure 1C (see Video S1 for blinking fluorescence spots in deep tissue).
Figure 1.
Optical Sectioned Detection and Resolution Enhancement of COOL
(A) Wide-field image of a Drosophila brain at 45 μm depth. No detail is visible due to out-of-focus fluorescence.
(B) Spinning-disk confocal image of the same sample at 70 μm depth. The axial contrast is greatly improved.
(C) Localized fluorescence spots of (B).
(D–I) (D–F) Resolution enhancement of COOL at 5 μm depth and (G–I) at 160 μm depth. The insets are zoom-in views of white squares, and arrows mark the region corresponding to the profiles. In (F), the values of full-width-at-half-maximum of confocal, COOL, and localization uncertainty are 374, 77.8, and 25.2 nm, respectively. In (I), at the bottom of the brain, the corresponding numbers are 388, 78.4, and 21.6 nm, respectively, demonstrating that the super-resolution is maintained throughout the whole brain.
(J and K) (J) Zoom-in of the green squares in (G and H) and the corresponding profiles in (K) show clear separation of two dendrites by COOL.
(L) An extraordinary advantage of Kaede is its photoconvertibility. During the blinking image acquisition, the red form of Kaede gradually bleaches, as shown by the middle image and the temporal evolution curve in the bottom. The right-hand-side image shows that photoconversion of the abundant green form of Kaede by a 405-nm laser effectively replenishes the blinking red form for subsequent super-resolution imaging.
Cells for demonstration: (A–E, L) G0239-Gal4 cells and (G–J) krasavietz-Gal4 cells. Scale bars: 3 μm in (A–C); 5 μm in images and 500 nm in insets in (D, E, G, and H); 500 nm in (J); 500 nm, (L); and 5 μm in (I).
Kaede expressed on dendrites of G0239-Gal4 neurons blink at 65 μm depth.
As a photoconvertible fluorescent protein, it is no surprise that Kaede has been used in photoactivatable localization microscopy (PALM) (Betzig et al., 2006). However, please note that the blinking in Video S1 is not based on photoconversion. Here we found that the red form, or photoconverted form, of this photoconvertible fluorescent protein exhibits a blinking state, which has not been reported before. Its blinking properties are presented in Transparent Methods. The brightness of the blinking state is enough to enable ∼20-nm spatial resolution. Similar to all localization-based techniques, by accumulating images over time, a comprehensive map of fluorophore distribution is unraveled.
Resolution Enhancement in the Top and Bottom of an Intact Brain
This set of experiment is done in two different Gal4 lines, namely, G0239-Gal4 cells and krasavietz-Gal4 cells (see Figure S3 for these neuron morphologies and the areas for imaging). The former neuron is just below the surface of the brain, whereas the latter is located at the bottom of the brain. Therefore the selection of these two neurons provides a control group to examine resolution enhancement at different depths of an intact Drosophila brain.
Figures 1D and 1E demonstrate the significantly enhanced spatial resolution by COOL microscopy at the surface of brain. The cross-sectional profile of one dendrite is given in Figure 1F. Quantitatively, with the spinning disk confocal system, the full-width-at-half-maximum is about 408 ± 35 nm, whereas that of COOL is 80.8 ± 4.2 nm (statistics in Figure S1 and Table S1), which presents the real diameter of a dendrite inside the Drosophila brain (Pai et al., 2013). The spatial resolution of COOL is determined by the uncertainty of localization, i.e., 25.2 ± 9.8 nm (see Figure S2), which is 15 times better than the confocal resolution.
Please note that conventional wide-field localization techniques also provide resolution enhancement at the sample surface, thus the main impact of COOL microscopy is to achieve super-resolution inside a thick tissue. This capability is demonstrated in Figures 1G and 1H, which are acquired at the bottom of a Drosophila brain, namely, the dendrite network of krasavietz-Gal4 cells (Dubnau et al., 2003). As expected, the confocal microscopy shows a blurred image of the densely distributed neuropil, whereas the COOL modality provides high-contrast and high-resolution visualization of the dendrite network. The quantitative value in Figure 1I and the corresponding statistical analysis in Figure S2 indicate that the localization uncertainty is 21.6 ± 10.1 nm at this depth, manifesting that resolution enhancement down to less than λ/20 is consistently obtained throughout the whole brain. Figures 1J and 1K show that two adjacent dendrites can only be resolved by COOL microscopy. The ∼20-nm spatial resolution is necessary to distinguish tightly entangled neurites, whose minimal separation is two times the thickness of a cell membrane, i.e., 10 nm, when a fluorescent protein is expressed in cytosol.
Photoconversion to Replenish Blinking Fluorophores
An additional highlight of the technique lies in the photoconvertibility of Kaede, which allows replenishment of the blinking red form with the aid of a 405-nm laser, as demonstrated in Figure 1L (see Figure S4 for a control experiment showing that the red form of Kaede does not increase without 405-nm illumination). One major issue in most super-resolution microscopy is the bleaching of fluorophores under high-intensity illumination, resulting in structure discontinuity, and photoconvertibility potentially helps to solve this issue.
Volumetric Tracing of Densely Entangled Dendritic Fibers
The optical sectioned 20-nm resolution throughout a whole brain provides unprecedented opportunity to distinguish closely intertwined neurons in three dimensions. One nice example is the G0239-Gal4 neurons (Pai et al., 2013), which consists of two neurons in each side of the brain, and their dendritic fibers densely entangle into a complex network extending tens of micrometers. Here we show that COOL is able to unravel the neurite distribution, as shown in Figure 2. Figure 2A presents sequential images in the axial direction with COOL and confocal modalities. The arrowheads illustrate how to identify a dendrite twist in three-dimensions with COOL. The correspondingly reconstructed dendrite network is given in Figure 2B, with color marked on individual dendrites. It is apparent that only with the exceptional combination of spatial resolution, penetration depth, and optical sectioning of COOL the two three-dimensional (3D) intertwined dendrites can be discriminated without additional genetic manipulation, but this is not possible with either confocal or conventional localization microscopy. Figure 2C shows a large-scale separation of dendritic branches between the two cells of G0239-Gal4 (see Video S3 for the 3D view and Figure S5 for discussion with confocal and electron microscopy). These results demonstrate the potential of COOL toward constructing whole-brain neural networks even with the unusually thin dendrites in Drosophila.
Figure 2.
Three-Dimensional Imaging and Reconstruction to Trace Deep-Brain Neurites with Unprecedented Resolution
(A and B) (A) COOL (top) and confocal (bottom) images across two intertwined dendrites of V3 neurons. Numbers indicate the relative depth (μm) of image. The arrowheads indicate the endpoints of a specific neurite at different optical sections, enabling the subsequent 3D reconstruction of the green-colored dendrite in (B). Different colors indicate individual dendrites (see Video S2 for 3D view).
(C) 3D reconstruction of super-resolved complete dendritic network of G0239-Gal4 (see raw data in Video S3, segmentation in Video S4, and 3D view in Video S5). Two colors indicate that the fibers are from two different cells. The inset shows 3D confocal image from the same neuron, manifesting that the dense dendritic arbor cannot be resolved.
Scale bars: 3 μm in (A and B) and 5 μm in (C and its inset). The axial step is 0.75 μm, and the image thickness is 6 μm in (B) and 28 μm in (C).
Discussion
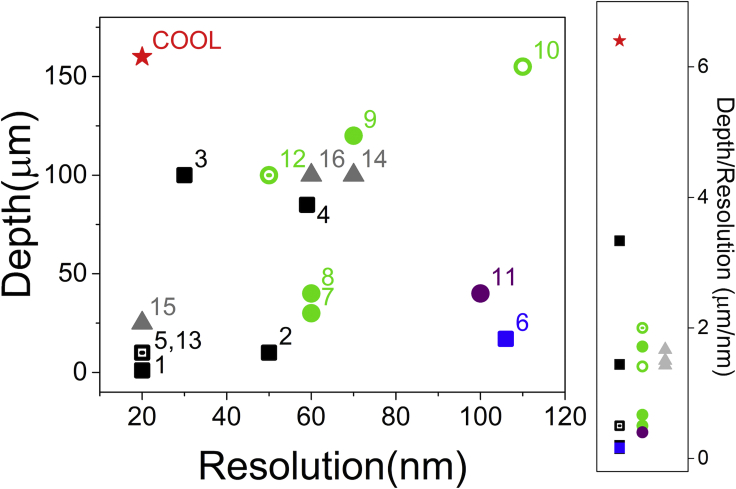
We have demonstrated localization microscopy at unprecedented depth in this study, with the aid of high-speed confocal setup, a blinking fluorescent protein, and optical clearing. The depth-resolution comparison of various state-of-the-art techniques are summarized in Figure 3. The COOL techniques are marked with a red star, which is located at the upper-left corner of the plot, i.e., the most wanted corner. To compare with other deep-tissue super-resolution techniques, the figure of merit can be defined as the ratio of imaging depth over spatial resolution. As shown in the right panel of Figure 3, COOL apparently provides the state-of-the-art performance over all other methods. Please note that the current 160-μm imaging depth represents the thickness of the Drosophila brain, not depth limit. As the super-resolution does not degrade throughout the whole brain, the COOL technique could be potentially applied to much thicker samples.
Figure 3.
Comparison of Reported Deep-Tissue Super-Resolution Techniques with 100-nm or Better Resolution
Wide-Field techniques are marked in squares (localization microscopy: 1–5 and 13 as black squares; SIM: 6 as blue square), scanning-based techniques are in circles (STED/RESOLFT: 7–10 and 12 as green circles; SAX: 11 as purple circle), sample expansion techniques are in gray triangles (14–16), and COOL is the star. In general, localization-based methods (black squares) provide better resolution, whereas scanning-based methods (circles) offer better imaging depth. Right panel: comparison of figures of merit (image depth over resolution) among various techniques. (1) Traditional localization microscope (Rust et al., 2006). (2) Combination of TF and localization microscope (Vaziri et al., 2008). (3) Combination of light sheet and PALM (Zanacchi et al., 2011). (4) Combination of line scan confocal and localization microscope (Lee et al., 2012). (5) Spinning disk confocal localization without clearing (the points 5 and 13 overlap together) (Schueder et al., 2017). (6) Hexagonal line-confocal SIM (Schropp et al., 2017). (7) Two-photon excitation with pulsed STED depletion (Takasaki et al., 2013). (8) RESOLFT (Schnorrenberg et al., 2016). (9) STED with aberration-reducing optics (Urban et al., 2011). (10) Hollow Bessel beam depletion STED in agarose (Yu et al., 2016). (11) SAX (Yamanaka et al., 2013). (12) Combination of tissue clearing and STED (Ke et al., 2016). (13) Combination of tissue clearing and localization microscopy (Ke et al., 2016). (14) Expansion microscopy (Chen et al., 2015a). (15) Iterative expansion microscopy (Chang et al., 2017). (16) Magnified analysis of the proteome (Ku et al., 2016).
One particular advantage of COOL comes from commercially available tools such as spinning disk confocal microscope and genetically controllable blinking proteins, thus facilitating easy access among biologists. The ability to resolve neuronal fine structures in intact animal brain below diffraction limit opens the possibility to unravel biological questions such as neuron morphological structure on a nanometer scale, as well as protein distribution on the synapses due to memory formation or loss, aging, social interactions, etc.
In summary, we have achieved an unprecedented combination of 20-nm lateral resolution in ∼200-μm-deep tissue samples, based on amalgamation of key technologies, including spinning disk confocal, localization microscopy, optical clearing, and a blinking fluorescent protein. The photoconvertible fluorescent protein enables sequential conversion into blinking forms, to facilitate neural tracing in a dense network. The technologies are readily available for many biologists without the need of upgrading hardware. This method can be widely used not only for brain neural network studies but also for other high-resolution imaging in biological tissues and cells.
Limitations of the Study
There are several limitations in the current design. First, the axial resolution is still diffraction-limited to about 1 μm due to the spinning disk confocal design. The axial resolutions of various super-resolution techniques have been well documented, and in our case (Sahl et al., 2017), they can be further enhanced by incorporating point-spread function engineering (Diezmann et al., 2016) into the spinning disk system. Second, the Kaede fluorescent protein requires a toxic chemical β-mercaptoethanol (βME) to stimulate its blinking (see sample preparation in the Transparent Methods), and FocusClear does not work for live tissue, so currently it is not possible for in vivo observation. However, we would like to point out that the spinning disk system is suitable for live brain studies, with reasonable penetration capability. Therefore finding a proper agent for live-tissue clearing (Zhu et al., 2013) as well as photoactivatable proteins that blink in live-cell conditions will enable 3D super-resolution in a live brain.
Third, the imaging speed is limited by the brightness and blinking rate of the fluorophores, and can be further improved by optimizing laser power and the density of activated fluorescent protein at each frame (Fox-Roberts et al., 2017). It is possible to achieve one super-resolved frame within a split second (Dertinger et al., 2009, Jones et al., 2011). Last but not least, the neurite fiber distribution is identified manually now. It will be a necessary next step to develop an auto-tracking program to find out dendritic tree divisions and locations of neurite terminals and synapses.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was financially supported by the Brain Research Center from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and Ministry of Science and Technology (MOST) in Taiwan. S.W.C. acknowledges the generous support from MOST under grant MOST-108-2321-B-002-004 and MOST-105-2628-M-002-010-MY4, as well as from the Foundation for the Advancement of Outstanding Scholarship. K.H.L. receives funding from Academia Sinica Career Award and MOST106-2112-M-001-029. L.A.C. acknowledges the funding from MOST 106-2321-B-007-008-MY3. We thank Dr. Shung-Ming Yang and Dr. Chieh-Han Lu for the initial test of Kaede protein.
Author Contributions
H.-Y.L. and L.-A.C. developed COOL technique, including finding of blinking Kaede. H.-Y.L. and K.-H.L. helped operation of spinning disk microscope. K.-J.H. and Y.-Y.L. assisted in sample preparation and system design. H.-Y.L. and L.-A.C. drafted the manuscript. K.-H.L., S.-W.C., and A.-S.C. designed the experiments, finalized the manuscript, and supervised the project.
Declaration of Interests
The authors declare no competing interests.
Published: April 26, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.03.025.
Contributor Information
Keng-Hui Lin, Email: kenghui@gate.sinica.edu.tw.
Shi-Wei Chu, Email: swchu@phys.ntu.edu.tw.
Ann-Shyn Chiang, Email: aschiang@life.nthu.edu.tw.
Supplemental Information
References
- Ando R., Hama H., Yamamoto-Hino M., Mizuno H., Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. U S A. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzig E., Patterson G.H., Sougrat R., Lindwasser O.W., Olenych S., Bonifacino J.S., Davidson M.W., Lippincott-Schwartz J., Hess H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- Chang J.B., Chen F., Yoon Y.G., Jung E.E., Babcock H., Kang J.S., Asano S., Suk H.J., Pak N., Tillberg P.W. Iterative expansion microscopy. Nat. Methods. 2017;14:593–599. doi: 10.1038/nmeth.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Tillberg P.W., Boyden E.S. Expansion microscopy. Science. 2015;347:543–548. doi: 10.1126/science.1260088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zong W., Li R., Zeng Z., Zhao J., Xi P., Chen L., Sun Y. Two-photon light-sheet nanoscopy by fluorescence fluctuation correlation. Nanoscale. 2016;8:9982–9987. doi: 10.1039/c6nr00324a. [DOI] [PubMed] [Google Scholar]
- Dertinger T., Colyer R., Iyer G., Weiss S., Enderlein J. Fast, background-free, 3D super-resolution optical fluctuation imaging (SOFI) Proc. Natl. Acad. Sci. U S A. 2009;106:22287–22292. doi: 10.1073/pnas.0907866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diezmann A.V., Shechtman Y., Moerner W.E. Three-dimensional localization of single molecules for super-resolution imaging and single-particle tracking. Chem. Rev. 2016;117:7244–7275. doi: 10.1021/acs.chemrev.6b00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J., Chiang A.S., Grady L., Barditch J., Gossweiler S., McNeil J., Smith P., Buldoc F., Scott R., Certa U. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr. Biol. 2003;13:286–296. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Fox-Roberts P., Marsh R., Pfisterer K., Jayo A., Parsons M., Cox S. Local dimensionality determines imaging speed in localization microscopy. Nat. Commun. 2017;8:13558. doi: 10.1038/ncomms13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Babcock H., Zhuang X. Breaking the diffraction barrier: super-resolution imaging of cells. Cell. 2010;143:1047–1058. doi: 10.1016/j.cell.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.A., Shim S.H., He J., Zhuang X.W. Fast, three-dimensional super-resolution imaging of live cells. Nat. Methods. 2011;8:499–505. doi: 10.1038/nmeth.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke M.-T., Nakai Y., Fujimoto S., Takayama R., Yoshida S., Kitajima T.S., Sato M., Imai T. Super-resolution mapping of neuronal circuitry with an index-optimized clearing agent. Cell Rep. 2016;14:2718–2732. doi: 10.1016/j.celrep.2016.02.057. [DOI] [PubMed] [Google Scholar]
- Ku T., Swaney J., Park J.Y., Albanese A., Murray E., Cho J.H., Park Y.G., Mangena V., Chen J.P., Chung K.H. Multiplexed and scalable super-resolution imaging of three-dimensional protein localization in size-adjustable tissues. Nat. Biotechnol. 2016;34:973–981. doi: 10.1038/nbt.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Miyanaga Y., Ueda M., Hohng S. Video-rate confocal microscopy for single-molecule imaging in live cells and superresolution fluorescence imaging. Biophys. J. 2012;103:1691–1697. doi: 10.1016/j.bpj.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.C., Chiang A.S. High-resolution confocal imaging and three-dimensional rendering. Methods. 2003;30:86–93. doi: 10.1016/s1046-2023(03)00010-0. [DOI] [PubMed] [Google Scholar]
- Pai T.P., Chen C.C., Lin H.H., Chin A.L., Lai J.S.Y., Lee P.T., Tully T., Chiang A.S. Drosophila ORB protein in two mushroom body output neurons is necessary for long-term memory formation. Proc. Natl. Acad. Sci. U S A. 2013;110:7898–7903. doi: 10.1073/pnas.1216336110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust M.J., Bates M., Zhuang X.W. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat. Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl S.J., Hell S.W., Jakobs S. Fluorescence nanoscopy in cell biology. Nat. Rev. Mol. Cell Biol. 2017;18:685–701. doi: 10.1038/nrm.2017.71. [DOI] [PubMed] [Google Scholar]
- Schnorrenberg S., Grotjohann T., Vorbruggen G., Herzig A., Hell S.W., Jakobs S. In vivo super-resolution RESOLFT microscopy of Drosophila melanogaster. Elife. 2016;5:e15567. doi: 10.7554/eLife.15567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schropp M., Seebacher C., Uhl R. XL-SIM: extending superresolution into deeper layers. Photonics. 2017;4:33. [Google Scholar]
- Schueder F., Lara-Gutierrez J., Beliveau B.J., Saka S.K., Sasaki H.M., Woehrstein J.B., Strauss M.T., Grabmayr H., Yin P., Jungmann R. Multiplexed 3D super-resolution imaging of whole cells using spinning disk confocal microscopy and DNA-PAINT. Nat. Commun. 2017;8:2090. doi: 10.1038/s41467-017-02028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki K.T., Ding J.B., Sabatini B.L. Live-cell superresolution imaging by pulsed STED two-photon excitation microscopy. Biophys. J. 2013;104:770–777. doi: 10.1016/j.bpj.2012.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno S.-n., Kamiya M., Yoshihara T., Sugawara K., Okabe K., Tarhan M.C., Fujita H., Funatsu T., Okada Y., Tobita S. A spontaneously blinking fluorophore based on intramolecular spirocyclization for live-cell super-resolution imaging. Nat. Chem. 2014;6:681–689. doi: 10.1038/nchem.2002. [DOI] [PubMed] [Google Scholar]
- Urban N.T., Willig K.I., Hell S.W., Nagerl U.V. STED nanoscopy of actin dynamics in synapses deep inside living brain slices. Biophys. J. 2011;101:1277–1284. doi: 10.1016/j.bpj.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri A., Tang J., Shroff H., Shank C.V. Multilayer three-dimensional super resolution imaging of thick biological samples. Proc. Natl. Acad. Sci. U S A. 2008;105:20221–20226. doi: 10.1073/pnas.0810636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenburger S., Boening D., Schomburg B., Giller K., Becker S., Griesinger C., Sandoghdar V. Cryogenic optical localization provides 3D protein structure data with Angstrom resolution. Nat. Methods. 2017;14:141–144. doi: 10.1038/nmeth.4141. [DOI] [PubMed] [Google Scholar]
- Yamanaka M., Yonemaru Y., Kawano S., Uegaki K., Smith N.I., Kawata S., Fujita K. Saturated excitation microscopy for sub-diffraction-limited imaging of cell clusters. J. Biomed. Opt. 2013;18:126002. doi: 10.1117/1.JBO.18.12.126002. [DOI] [PubMed] [Google Scholar]
- Yu W.T., Ji Z.H., Dong D.S., Yang X.S., Xiao Y.F., Gong Q.H., Xi P., Shi K.B. Super-resolution deep imaging with hollow Bessel beam STED microscopy. Laser Photon Rev. 2016;10:147–152. [Google Scholar]
- Zanacchi F.C., Lavagnino Z., Donnorso M.P., Del Bue A., Furia L., Faretta M., Diaspro A. Live-cell 3D super-resolution imaging in thick biological samples. Nat. Methods. 2011;8:1047–1049. doi: 10.1038/nmeth.1744. [DOI] [PubMed] [Google Scholar]
- Zhu D., Larin K.V., Luo Q.M., Tuchin V.V. Recent progress in tissue optical clearing. Laser Photon Rev. 2013;7:732–757. doi: 10.1002/lpor.201200056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaede expressed on dendrites of G0239-Gal4 neurons blink at 65 μm depth.