Abstract
Intensive efforts have been made to describe the human microbiome and its involvement in health and disease. Culturomics has been recently adapted to target formerly uncultured bacteria and other unclassified bacterial species. This approach enabled us to isolate in the current study a new bacterial species, Parabacteroides timonensis strain Marseille‐P3236T, from a stool sample of a healthy 39‐year‐old pygmy male. This strain, is an anaerobic, gram‐negative, nonspore‐forming motile rod. Its genome is made up of 6,483,434 bp with 43.41% G+C content, 5046 protein‐encoding genes, and 84 RNA genes. We herein provide the full description of Parabacteroides timonensis strain Marseille‐P3236T through the taxonogenomic approach.
Keywords: culturomics, human, microbiome, new species, Parabacteroides timonensis
1. INTRODUCTION
The gut microbiota is well‐known for its microbial diversity and its role in health as well as in diseases. Even though scientific technologies have been greatly developed over the past years and have drastically facilitated the description of the gut microbiota, it still remains a challenging task (Turnbaugh et al., 2007) as the massive data generated over the last decade do not yet allow the clear depiction of the gut microbiota composition (Lagier et al., 2012). Nevertheless, the fact that 1 g of human stool might contain up to 1012 bacteria drives us to pursue our efforts in describing the human gut microbiota for which only around 2,776 species have been reported (Bilen et al., 2018; Hugon et al., 2015). Consequently, our laboratory has developed a new approach called culturomics which aims to isolate previously uncultured bacteria using sophisticated culture methods (Lagier et al., 2012). In doing so, culturomics has expanded our capabilities in human gut microbiota description and therefore lead to the isolation of a significant number of new genera and species (Lagier et al., 2016). The process begins with the cultivation of stool samples under varying conditions and bacterial growth is assessed over 30 days. At this point, Matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS) is primarily used for colony identification and 16S rRNA sequencing is adapted in case of MALDI‐TOF MS's identification failure. Subsequently, unidentified species are subjected to taxonogenomics description (Fournier & Drancourt, 2015). The genome of the concerned species is then sequenced for a genomic description, followed by a phenotypic and biochemical analysis (Fournier & Drancourt, 2015; Fournier, Lagier, Dubourg, & Raoult, 2015; Lagier et al., 2012). By adapting this procedure, we isolated a new species known as Parabacteroides timonensis (P. timonensis), a member of the Parabacteroides genus known to be gram‐negative, obligate anaerobic, nonmotile, rod‐shaped, and nonspore‐forming (Sakamoto & Benno, 2006). To date, eight Parabacteroides species have been isolated, out of which six were isolated from the human gut (www.bacterio.net). We demonstrate here the description of P. timonensis strain Marseille‐P3236T (=Culture Collection University Gothenburg (CCUG) 71183, =Collection de Souches de l'Unité des Rickettsies (CSUR) P3236) using the taxonogenomic approach.
2. MATERIALS AND METHODS
2.1. Ethics and sample collection
Before stool sample collection in Congo, the sample's donor has signed an informed consent. The donor is a healthy 39‐year‐old pygmy male and the collected stool samples were stored at −80°C for further analysis. In addition, an approval from the ethic committee of the Institut Fédératif de Recherche IFR48 (Marseille, France) carrying the number 09‐022 was obtained before launching the study.
2.2. Strain isolation
A loop of stool sample was diluted in phosphate‐buffered saline (Life Technologies, Carlsbad, CA, USA) prior to incubation in a blood culture bottle (BD BACTEC®, Plus Anaerobic/F Media, Le Pont de Claix, France), supplemented with 5% sheep blood and 5% filtered rumen, at 37°C under anaerobic conditions. Bacterial growth and isolation was done by subculturing samples after 5 days on 5% sheep's blood–enriched Columbia agar solid medium (bioMérieux, Marcy l'Etoile, France).
2.3. Colony identification
Isolated bacterial colonies identification trials were done first by using MALDI‐TOF MS analysis as previously described (Elsawi et al., 2017). In case of MALDI‐TOF MS identification failure, complete 16S rRNA sequencing was performed for further analysis with the same protocol used in our previous studies (Elsawi et al., 2017). Complete 16S rRNA nucleotide sequence are assembled and manipulated using CodonCode Aligner software (http://www.codoncode.com) and blasted in the online PubMed National Center for Biotechnology Information (NCBI) database for phylogenetic analysis. According to Kim, Oh, Park, & Chun (2014), a threshold of 98.65% 16S rRNA gene sequence similarity was used to classify a new species, whereas a threshold of 95% 16S rRNA gene sequence was used for new genus classification. Generated mass spectrum of the concerned species was added to our custom database and its 16S rRNA gene sequence was submitted to EMBL‐EBI database.
2.4. Growth conditions
In order to determine the optimal growth environment, strain Marseille‐P3236T was cultured using different conditions such as temperature, pH, atmosphere, and salinity. To begin with, this strain was cultured under anaerobic, aerobic, and microaerophilic conditions on 5% sheep's blood–enriched Colombia agar (bioMérieux) at 28, 45, 37, and 55°C. GENbag anaer and GENbag microaer systems (bioMérieux) were used for anaerobic and microaerophilic environment establishment, respectively. Furthermore, salt and acidity tolerance were evaluated using concentration of 0%, 5%, 15%, and 45% NaCl and pH values of 6, 6.5, 7, and 8.5.
2.5. Morphological and biochemical assays
Biochemical characteristics of Marseille‐P3236T strain were determined using different API galleries (20A, ZYM, and 50CH, bioMérieux) according to the manufacturer's instructions. Not to mention, sporulation ability was tested by culturing this strain after exposing a bacterial suspension to a thermic shock of 80°C for 10 min. Strain Marseille‐P3236T morphology was determined as previously described (Elsawi et al., 2017). Additionally, DM1000 photonic microscope (Leica Microsystems, Nanterre, France) was used to observe the motility of strain Marseille‐P3236T from a fresh culture with a 100× objective lens. Cellular fatty acid methyl ester (FAME) analysis was performed using around 43 mg of bacterial biomass per tube as formerly described (Elsawi et al., 2017).
2.6. Antibiotic susceptibility
Antibiotics susceptibility testing (AST) of Marseille P‐3236T strain was evaluated by performing minimum inhibitory concentrations (MICs) using the E‐test strip method (Biomérieux) on Columbia agar + 5% sheep blood (Biomérieux) with the following agents: rifampicin, imipenem, amikacin, cefotaxime, benzylpenicillin, vancomycin, erythromycin, amoxicillin, ceftriaxone, minocycline, metronidazole, teicoplanin, and daptomycin.
2.7. Genome sequencing and analysis
Genomic DNA (gDNA) of Marseille‐P3236T strain was extracted as previously described with 50 μL being eluted (Elsawi et al., 2017). Quantification was done using the Qubit assay with the high sensitivity kit (Life technologies, Carlsbad, CA, USA) and determined to be 134 ng/μl.
gDNA sequencing, library preparation, fragmentation, and tagmentation were performed as previously described with an optimal DNA fragment size of 8.675 kb (Lagier et al., 2016). Circularization was done using 511.4 ng of tagmented fragments with no size selection. The circularized DNA was mechanically sheared to small fragments with optima at 1059 bp on the Covaris device S2 in T6 tubes (Covaris, Woburn, MA, USA). Library profile visualization was done on a High Sensitivity Bioanalyzer LabChip (Agilent Technologies Inc, Santa Clara, CA, USA) and the final library concentration was determined at 27.8 nmol/L. After that, libraries were normalized at 2 nM and pooled. Dilution at 15 pM was done after a denaturation step and pooled libraries were loaded in the sequencer for automated cluster generation and a single sequencing run of 39 h in a 2 × 151‐bp was performed. Total information of 5.1 Gb were generated from a 542 K/mm2 cluster density with a quality control filters threshold of 95.7% (10,171,000 passing filter paired reads). Within this run, index representation for Marseille‐P3236T strain was determined to 7.69%. The 782,587 paired reads were trimmed and then assembled. Genome assembly, annotation, and comparison were done using the same pipeline and tools as previously described (Elsawi et al., 2017).
3. RESULTS AND DISCUSSION
3.1. Strain identification and phylogenetic analysis
The identification of P3236T strain using MALDI‐TOF MS failed due to the absence of its mass spectrum in the current databases. However, a typical spectrum was added to our custom database (Figure 1) after performing a complete 16s rRNA gene sequencing. Strain Marseille‐P3236T exhibited a 97.05% sequence similarity with Parabacteroides gordonii strain MS‐1 (NR_112835.1), the phylogenetically closest species with standing nomenclature (Figure 2). Accordingly, with more than 1.3% sequence divergence from its phylogenetically closest species with standing in nomenclature, we propose the classification of Marseille‐P3236T strain as a new species within the Parabacteroides genus. The protein spectrum of Marseille‐P3236T strain was compared with those of other close species under the same family. This comparison revealed its uniqueness with specific and different peaks compared to others (Figure 3).
Figure 1.
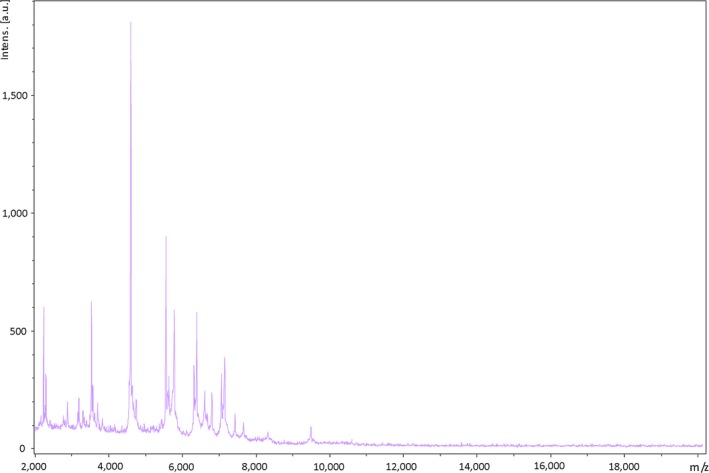
Reference mass spectrum representing Parabacteroides timonensis strain Marseille‐P3236T
Figure 2.
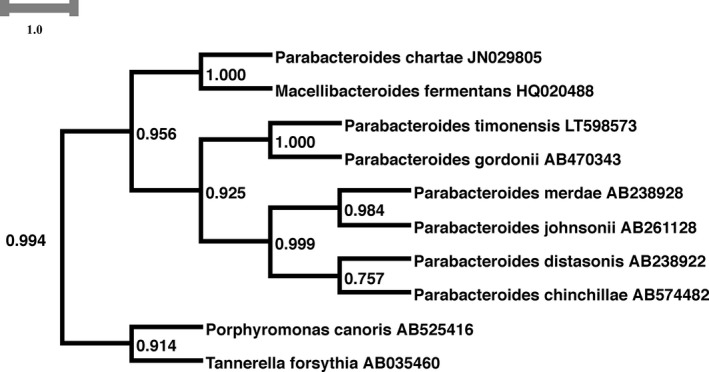
Phylogenetic subtree highlighting the position of Parabacteroides timonensis strain Marseille‐P3236T relative to other close species
Figure 3.
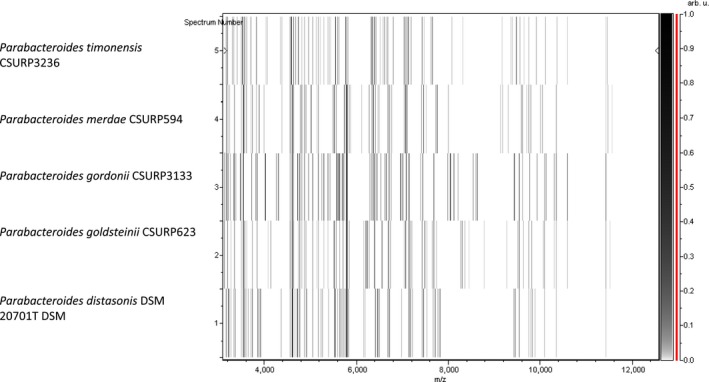
Gel view comparing mass the mass spectrum of Parabacteroides timonensis strain Marseille‐P3236T to other species. The gel view displays the raw spectra of loaded spectrum files arranged in a pseudo‐gel like look. The x‐axis records the m/z value. The left y‐axis displays the running spectrum number originating from subsequent spectra loading. The peak intensity is expressed by a Gray scale scheme code. The right y‐axis indicates the relation between the color of a peak and its intensity, in arbitrary units. Displayed species are indicated on the left
3.2. Phenotypic and biochemical characterization
Strain Marseille‐P3236T is a gram‐negative rod, motile, unable to sporulate, and grows anaerobically between 25 and 42°C but optimally at 37° (Table 1). It is able to endure a range of pH between 6 and 8.5 and can sustain only a 5% salinity concentration. Strain Marseille‐P3236T is catalase positive, oxidase negative, and can be seen as smooth colonies with a diameter of 0.9–1 mm. Under electron microscopy, each bacterial cell has an average length of 1.4–2.7 μm and an average diameter of 0.5 μm (Figure 4).
Table 1.
Classification and general features of Parabacteroides timonensis strain Marseille‐P3236T
| Property | Term |
|---|---|
| Current classification | Domain: Bacteria |
| Phylum: Bacteroidetes | |
| Class: Bacteroidia | |
| Order: Bacteroidales | |
| Family: Porphyromonadaceae | |
| Genus: Parabacteroides | |
| Species: Parabacteroides timonensis | |
| Type strain: P3236 | |
| Gram strain | Negative |
| Cell shape | Rod |
| Motility | Motile |
| Sporulation | Negative |
| Temperature range | 30‐42°C |
| Optimum temperature | 37°C |
Figure 4.
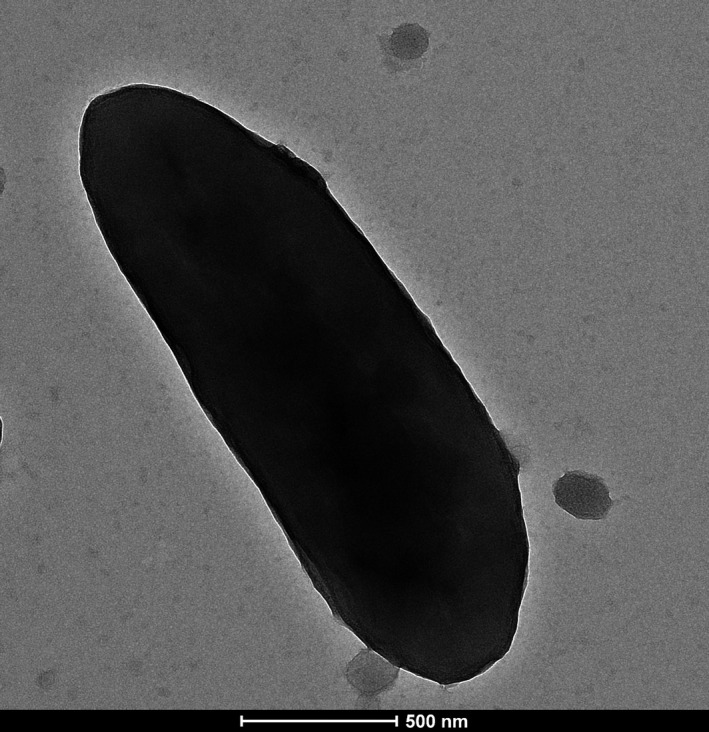
Electron micrographs of Parabacteroides timonensis strain Marseille‐P3236T using a Tecnai G20, at an operating voltage of 200 keV. Scale bar = 200 nm
MICs of strain Marseille‐P3236T for various antimicrobial agents were as follows: rifampicin (8 μg/ml), Imipenem (8 μg/ml), amikacin (>256 μg/ml), cefotaxime (6 μg/ml), benzylpenicillin (16 μg/ml), vancomycin (4 μg/ml), erythromycin (0.016 μg/ml), amoxicillin (1.5 μg/ml), ceftriaxone (4 μg/ml), minocycline (0.25 μg/ml), metronidazole (>256 μg/ml), teicoplanin (2 μg/ml), and daptomycin (12 μg/ml).
The main biochemical features obtained by API tests are summarized in Table 2. Additionally, the general features of strain Marseille‐P3236T have been listed in Table 3.
Table 2.
Main biochemical features of strain Parabacteroides timonensis strain Marseille‐P3236T obtained by API tests (20A, 50CH, and ZYM)
| Test | Results | Test | Results | Test | Results |
|---|---|---|---|---|---|
| Alkaline phosphatase | + | Fermentation (d‐glucose) | + | Fermentation (d‐turanose) | + |
| Esterase (C4) | + | Fermentation (d‐fructose) | + | Fermentation (d‐lyxose) | − |
| Esterase Lipase (C8) | + | Fermentation (d‐mannose) | + | Fermentation (d‐tagatose) | + |
| Lipase (C14) | − | Fermentation (l‐sorbose) | − | Fermentation (d‐fucose) | − |
| Leucine arylamidase | + | Fermentation (l‐rhamnose) | + | Fermentation (l‐fucose) | − |
| Valine arylamidase | + | Fermentation (d‐ulcitol) | − | Fermentation (d‐arabitol) | − |
| Cystine arylamidase | + | Fermentation (Inositol) | − | Fermentation (l‐arabitol) | − |
| Trypsin | − | Fermentation (d‐mannitol) | + | Fermentation (potassium gluconate) | + |
| α‐chymotrypsin | − | Fermentation (d‐sorbitol) | + | Fermentation (potassium 2‐Ketogluconate) | − |
| Acid phosphatase | + | Fermentation (Methyl‐αd‐mannopyranoside) | + | Fermentation (potassium 5‐Ketogluconate) | − |
| Naphthol‐AS‐BI‐phosphohydrolase | + | (Fermentation (Methyl‐αd‐glucosamine)) | + | Indole formation | − |
| α‐galactosidase | + | Fermentation (n‐acetylglucosamine) | + | Urease | − |
| β‐galactosidase | + | Fermentation (Amygdaline) | + | Acidification (Glucose) | + |
| β‐glucuronidase | + | Fermentation (Arbutin) | + | Acidification (Mannitol) | − |
| α‐glucosidase | + | Fermentation (Esculin ferric citrate) | + | Acidification (Lactose) | + |
| β‐glucosidase | + | Fermentation (Salicin) | + | Acidification (Saccharose) | + |
| N‐acetyl‐β‐glucosaminidase | + | Fermentation (d‐cellobiose) | + | Acidification (Maltose) | + |
| α‐mannosidase | − | Fermentation (d‐Maltose) | + | Acidification (Salicin) | − |
| α‐fucosidase | − | Fermentation (d‐lactose) | + | Acidification (Xylose) | + |
| Fermentation (Glycerol) | − | Fermentation (d‐melibiose) | + | Acidification (Arabinose) | + |
| Fermentation (Erythritol) | + | Fermentation (d‐saccharose/sucrose) | + | Hydrolysis (protease) (Gelatin) | − |
| Fermentation (d‐arabinose) | + | Fermentation (d‐trehalose) | + | Hydrolysis β‐ glucosidase (Esculin) | − |
| Fermentation (l‐arabinose) | + | Fermentation (Inuline) | + | Acidification (Glycerol) | − |
| Fermentation (d‐ribose) | + | Fermentation (d‐melezitose) | + | Acidification (Cellobiose) | − |
| Fermentation (d‐xylose) | + | Fermentation (d‐raffinose) | + | Acidification (Mannose) | + |
| Fermentation (l‐xylose) | − | Fermentation (Starch) | + | Acidification (Melezitose) | − |
| Fermentation (d‐adonitol) | − | Fermentation (Glycogen) | − | Acidification (Raffinose) | + |
| Fermentation (Methyl‐β‐d‐xylopyranoside) | − | Fermentation (xylitol) | − | Acidification (Sorbitol) | − |
| Fermentation (d‐galactose) | + | Fermentation (Gentiobiose) | + | Acidification (Rhamnose) | + |
| Acidification (Trehalose) | + |
Table 3.
General characteristics of Parabacteroides timonensis strain Marseille‐P3236T
| Properties | Parabacteroides timonensis |
|---|---|
| Cell length (μm) | 1.4‐2.7 |
| Oxygen requirement | Strictly anaerobic |
| Gram stain | Negative |
| Salt requirement | − |
| Motility | + |
| Endospore formation | − |
| Indole | − |
| Production of | |
| Alkaline phosphatase | + |
| Catalase | + |
| Oxidase | − |
| Urease | − |
| β‐galactosidase | + |
| n‐acetyl‐glucosamine | + |
| Acid from | |
| l‐Arabinose | + |
| d‐Ribose | + |
| d‐Mannose | + |
| d‐Mannitol | + |
| d‐glucose | + |
| d‐fructose | + |
| d‐maltose | + |
| d‐lactose | + |
| G+C content (mol%) | 43.41 |
| Habitat | Human gut |
The major fatty acid found in this strain was 12‐methyl‐tetradecanoic acid (46%). Several specific 3‐hydroxy branched structures were described. Minor amounts of unsaturated, branched, and other saturated fatty acids were also detected (Table 4).
Table 4.
Cellular fatty acids composition of Parabacteroides timonensis strain Marseille‐P3236T
| Fatty acids | Name | Mean relative % (a) |
|---|---|---|
| 15:0 anteiso | 12‐methyl‐tetradecanoic acid | 46.0 ± 0.5 |
| 15:0 | Pentadecanoic acid | 8.6 ± 0.4 |
| 18:1n9 | 9‐Octadecenoic acid | 8.4 ± 0.4 |
| 17:0 3‐OH iso | 3‐hydroxy‐15‐methyl‐Hexadecanoic acid | 7.6 ± 0.4 |
| 16:0 | Hexadecanoic acid | 6.1 ± 0.1 |
| 18:2n6 | 9,12‐Octadecadienoic acid | 5.8 ± 0.5 |
| 15:0 iso | 13‐methyl‐tetradecanoic acid | 4.7 ± 0.1 |
| 16:0 3‐OH | 3‐hydroxy‐Hexadecanoic acid | 3.9 ± 0.3 |
| 17:0 3‐OH anteiso | 3‐hydroxy‐14‐methyl‐Hexadecanoic acid | 1.4 ± 0.6 |
| 14:0 iso | 12‐methyl‐Tridecanoic acid | 1.2 ± 0.1 |
| 5:0 iso | 3‐methyl‐butanoic acid | 1.0 ± 0.1 |
| 14:0 | Tetradecanoic acid | 1.0 ± 0.1 |
| 18:0 | Octadecanoic acid | TR |
| 16:1n7 | 9‐Hexadecenoic acid | TR |
| 15:0 3‐OH | 3‐hydroxy‐Pentadecanoic acid | TR |
| 17:0 3‐OH | 3‐hydroxy‐Heptadecanoic acid | TR |
| 17:1n7 | 10‐Heptadecenoic acid | TR |
| 20:4n6 | 5,8,11,14‐Eicosatetraenoic acid | TR |
| 13:0 iso | 11‐methyl‐Dodecanoic acid | TR |
| 18:1n7 | 11‐Octadecenoic acid | TR |
| 16:0 3‐OH iso | 3‐hydroxy‐14‐methyl‐Pentadecanoic acid | TR |
| 16:0 9,10‐methylene | 2‐hexyl‐Cyclopropaneoctanoic acid | TR |
| 13:0 anteiso | 10‐methyl‐Dodecanoic acid | TR |
| 17:0 | Heptadecanoic acid | TR |
| 13:0 | Tridecanoic acid | TR |
Mean peak area percentage; TR = trace amounts <1%.
3.3. Genome properties
Strain Marseille‐P3236T had a genome of 6,483,434 bp long with 43.41 mol% of G+C content. It is composed of eight scaffolds (composed of 13 contigs). Of the 5,130 predicted genes, 5,046 were protein‐coding genes and 84 were RNAs (6 genes are 5S rRNA, 1 gene is 16S rRNA, 5 genes are 23S rRNA, and 72 genes are tRNA genes). A total of 3,253 genes (64.47%) were assigned as putative function (by cogs or by NR blast) and 202 genes were identified as ORFans (4%). The remaining genes were annotated as hypothetical proteins (1,483 genes; 29.39%) (Table 5). The representations of strain Marseille‐P3236T’ genome and its genes repartition into COG functional categories were done in Figure 5 and Table 6, respectively.
Table 5.
General genomic characteristics of strain Marseille‐P3236T
| Number | Percenta | |
|---|---|---|
| Size (bp) | 6,483,434 | 100 |
| Number of G+C | 2,813,492 | 43.41 |
| Number total of genes | 5,130 | 100 |
| Number total of protein genes | 5,046 | 98.36 |
| Number total of RNA genes | 84 | 1.64 |
| Number total of tRNA genes | 72 | 1.40 |
| Number total of RNA (5S, 16S, 23S) genes | 12 | 0.23 |
| Coding sequence size | 5,884,794 | 90.77 |
| Coding sequence gene protein size | 5,858,682 | 90.36 |
| Coding sequence tRNA gene size | 5,555 | 0.09 |
| Coding sequence (5S, 16S, 23S) gene size | 20,557 | 0.32 |
| Number of protein‐coding gene | 5,046 | 100 |
| Number of protein associated to COGs | 2,513 | 49.80 |
| Number of protein associated to orfan | 202 | 4.00 |
| Number of protein with peptide sigNAl | 1,641 | 32.52 |
| Number of gene associated to resistance genes | 0 | 0 |
| Number of gene associated to PKS or NRPS | 11 | 0.22 |
| Number of genes associated to virulence | 705 | 13.97 |
The total is based on either the size of the genome in base pairs or the total number of protein‐coding genes in the annotated genome.
Figure 5.

Graphical circular map of the genome of Parabacteroides timonensis strain Marseille‐P3236T. From outside to the center: Contigs (red/gray), COG category of genes on the forward strand (three circles), genes on forward strand (blue circle), genes on the reverse strand (red circle), COG category on the reverse strand (three circles), G+C content
Table 6.
Number of genes associated with the 25 general COG functional categories
| Code | Value | % of total | Description |
|---|---|---|---|
| [J] | 194 | 3.8446293 | Translation |
| [A] | 0 | 0 | RNA processing and modification |
| [K] | 194 | 3.8446293 | Transcription |
| [L] | 165 | 3.2699168 | Replication, recombination and repair |
| [B] | 0 | 0 | Chromatin structure and dynamics |
| [D] | 25 | 0.49544194 | Cell cycle control, mitosis, and meiosis |
| [Y] | 0 | 0 | Nuclear structure |
| [V] | 128 | 2.5366626 | Defense mechanisms |
| [T] | 183 | 3.6266348 | Signal transduction mechanisms |
| [M] | 233 | 4.617519 | Cell wall/membrane biogenesis |
| [N] | 22 | 0.43598887 | Cell motility |
| [Z] | 1 | 0.019817676 | Cytoskeleton |
| [W] | 0 | 0 | Extracellular structures |
| [U] | 37 | 0.7332541 | Intracellular trafficking and secretion |
| [O] | 96 | 1.902497 | Posttranslational modification, protein turnover, chaperones |
| [X] | 25 | 0.49544194 | Mobilome: prophages, transposons |
| [C] | 142 | 2.8141103 | Energy production and conversion |
| [G] | 248 | 4.914784 | Carbohydrate transport and metabolism |
| [E] | 180 | 3.5671818 | Amino acid transport and metabolism |
| [F] | 67 | 1.3277843 | Nucleotide transport and metabolism |
| [H] | 131 | 2.5961156 | Coenzyme transport and metabolism |
| [I] | 83 | 1.6448673 | Lipid transport and metabolism |
| [P] | 297 | 5.8858504 | Inorganic ion transport and metabolism |
| [Q] | 36 | 0.7134364 | Secondary metabolites biosynthesis, transport and catabolism |
| [R] | 229 | 4.538248 | General function prediction only |
| [S] | 108 | 2.140309 | Function unknown |
| _ | 2,533 | 50.19818 | Not in COGs |
3.4. Comparison of genome properties
The draft genome sequence of Marseille‐P3236T strain was compared to those of Parabacteroides gordonii (P. gordonii) (AUAE00000000), Parabacteroides distasonis (P. distasonis) (JNHP00000000), Porphyromonas canoris (P. canoris) (JQZV00000000), Parabacteroides johnsonii (P. johnsonii) (ABYH00000000), Parabacteroides merdae (P. merdae) (AJPU00000000), and Tannerella forsythia (T. forsythia) (CP003191).
Genome of Marseille‐P3236T strain was smaller than those of P. gordonii (6,483 and 6,677 MB, respectively), but was nevertheless larger than those of P. distasonis, P. canoris, P. johnsonii, P. merdae, and T. forsythia (5,316, 2,203, 4,787, 4,459, and 3,282 MB, respectively). The G+C content of Marseille‐P3236T strain was smaller than those of P. distasonis, P. canoris, P. johnsonii, P. merdae, T. forsythia, and P. gordonii (43.41%, 44.85%, 44.75%, 45.2%, 45.25%, 47.14%, and 44.43%, respectively). The gene content of Marseille‐P3236T strain was smaller than those of P. gordonii (5,046 and 5,326, respectively), but was larger than those of P. distasonis, P. canoris, P. johnsonii, P. merdae, and T. forsythia (4,800, 1,612, 4,515, 3,703, and 2,492, respectively).
Distribution of functional classes of predicted genes according to the COGs database regarding strain Marseille‐P3236T is presented in Figure 6. The distribution was similar in all the studied genomes. Strain Marseille‐P3236T shared the highest number of orthologous proteins with P. gordonii (2,614 with 84.62% similarity at the nucleotide level, Table 7).
Figure 6.
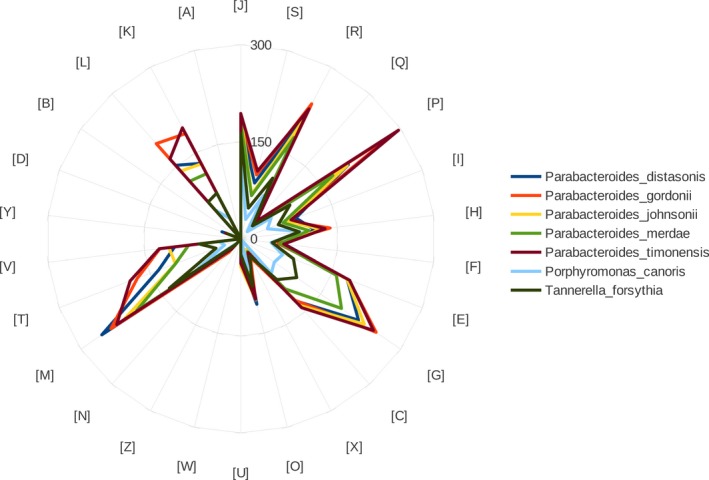
Distribution of functional classes of predicted genes according to the clusters of orthologous groups of proteins for Parabacteroides timonensis strain Marseille‐P3236T
Table 7.
The numbers of orthologous proteins shared between strain Marseille‐P3236T genomes and others closely related species genomes (upper right), average percentage similarity of nucleotides of shared orthologous proteins between genomes (lower left) and numbers of proteins per genome (bold)
| TF | PM | PC | PT | PJ | PG | PD | |
|---|---|---|---|---|---|---|---|
| TF | 2,492 | 1,287 | 822 | 1,388 | 1,207 | 1,379 | 1,248 |
| PM | 59.79 | 3,703 | 905 | 2,279 | 2,147 | 2,224 | 1,991 |
| PC | 56.21 | 55.97 | 1,612 | 935 | 860 | 922 | 871 |
| PT | 58.35 | 61.94 | 56.9 | 5,046 | 2,222 | 2,614 | 2,237 |
| PJ | 59.7 | 76.79 | 56.11 | 61.54 | 4,515 | 2,176 | 1,943 |
| PG | 58.45 | 62.12 | 56.94 | 84.62 | 61.71 | 5,326 | 2,216 |
| PD | 59.15 | 64.48 | 55.61 | 61.98 | 63.46 | 62 | 4,800 |
Note. TF, Tannerella forsythia; PM, Parabacteroides merdae; PC, PorphyromoNAs canoris; PT, Parabacteroides timonensis; PJ, Parabacteroides johnsonii; PG, Parabacteroides gordonii; PD, Parabacteroides distasonis.
As for digital DNA‐DNA hybridization (dDDH) values between strain Marseille‐P3236T and its phylogenetic closest species, it was 21.6 [19.3–24], 31 [28.6–33.5], 21.5 [19.2–23.9], 28 [25.7–30.5], and 23.5 [21.2–25.9] with P. johnsonii, P. canoris, P. distasonis, P. gordonii, and T. forsythia, respectively. In fact, 70% dDDH value is considered as a threshold to define a new bacterial species (Tindall, Rosselló‐Móra, Busse, Ludwig, & Kämpfer, 2010; Wayne et al., 1987). According to our results, strain Marseille‐P3236T shared with all its phylogenetically closest species with standing in nomenclature dDDH value of less than 70% and thus confirming it as a new species (Table 8).
Table 8.
Pairwise comparison of Parabacteroides timonensis strain Marseille‐P3236Twith other species using GGDC, formula 2 (DDH estimates based on identities/HSP length)a upper right
| PT | PJ | PC | PD | PG | TF | |
|---|---|---|---|---|---|---|
| PT | 100% | 21.6 [19.3–24%] | 31 [28.6–33.5%] | 21.5 [19.2–23.9%] | 28 [25.7–30.5%] | 23.5 [21.2–25.9%] |
| PJ | 100% | 30.8 [28.4–33.3%] | 22.5 [20.2–24.9%] | 21.3 [19.1–23.8%] | 28.8 [26.4–31.3%] | |
| PC | 100% | 28 [25.7–30.5%] | 33.3 [25.7–30.5%] | 32.9 [25.7–30.5%] | ||
| PD | 100% | 20.5 [18.3–23%] | 23.2 [20.9–25.6%] | |||
| PG | 100% | 17.1 [15–19.5%] | ||||
| TF | 100% |
Note. TF, Tannerella forsythia; PM, Parabacteroides merdae; PC, PorphyromoNAs canoris; PT, Parabacteroides timonensis; PJ, Parabacteroides johnsonii; PG, Parabacteroides gordonii; PD, Parabacteroides distasonis.
The confidence intervals indicate the inherent uncertainty in estimating DDH values from intergenomic distances based on models derived from empirical test data sets.
4. DISCUSSION
The human gut microbiota has been extensively studied by the scientific community and it has already been correlated with several health conditions such as obesity (Million et al., 2016), gastrointestinal diseases (Guinane & Cotter, 2013), or nonalcoholic fatty acid liver disease (Abu‐Shanab & Quigley, 2010). Profiling the bacterial content and its ratio in the human gut have led to the development of several therapeutic strategies such as probiotics, and also therapeutic improvements such as the case of CTLA‐4‐based cancer immunotherapy (Vétizou et al., 2015). Hence, describing the human gut microbiota without neglecting a group of its population is essential. Culturomics was developed for the purpose of isolating previously uncultured organisms along with attempting to correlate its sequences to operational taxonomic units (Lagier et al., 2012, 2016). This work adds on the previously performed descriptive studies on the human gut microbiota via culturomics (Lagier et al., 2016) by isolating a new bacterial species belonging to the Parabacteroides genus (Sakamoto & Benno, 2006). However, Parabacteroides has been previously isolated from clinical cases (Kierzkowska et al., 2017) and was the causative agent of bacteremia (Awadel‐Kariem, Patel, Kapoor, Brazier, & Goldstein, 2010). This fact renders the isolation and description of a new Parbacteroides species important as it can now be more easily identified if isolated in the future during a clinical case and thus facilitating medical diagnoses.
5. CONCLUSION
In conclusion, describing the human microbiota remains a challenging task requiring intensive efforts. Even though sequencing approaches proved to be efficient in this field, culturomics reemphasized the importance of culture in deciphering the dark matter of the human microbiome by shedding the light on previously unidentified and uncultured species (Lagier et al., 2016). Herein, we report the isolation of a new bacterial species from the human gut, Parabacteroides timonensis strain P3236T which represents the ninth Parabacteroides species and the seventh found in the human gut.
5.1. Description of Parabacteroides timonensis sp. nov
Parabacteroides timonensis (ti.mo.nen′sis. N.L. masc. adj. timonensis pertaining to La Timone, Marseilles’ hospital name, France, where the strain was isolated).
P. timonensis is motile, gram‐negative rod, catalase positive, oxidase negative, nonspore‐forming, strictly anaerobic, and has a cell length ranging between 1.4 and 2.7 μm. Colonies of P. timonensis are smooth and exhibit a diameter of 0.8–1 mm. The optimal growth temperature of P. timonensis is 37°C.
Positive reactions are observed with alkaline phosphatase, α‐glucosidase, esterase lipase C8, valine arylamidase, acid phosphatase, cystine arylamidase, α –galactosidase, naphthol‐AS‐BI‐phosphohydrolase, β‐galactosidase, leucine arylamidase, β‐glucuronidase, β‐glucosidase, esterase C4, N‐acetyl‐β‐glucosaminidase but negative with lipase (C14), trypsin, α‐chymotrypsin, α‐mannosidase, and α‐fucosidase. Acidification reactions are positive for saccharose, glucose, arabinose, lactose, maltose, raffinose, xylose, trehalose, and rhamnose but negative for glycerol, cellobiose, melezitose, sorbitol, mannitol, and salicin. This strain fails to form indole, is urease negative, and cannot hydrolyze gelatin or esculin. Strain Marseille‐P3236T is able to ferment d‐saccharose, d‐arabinose, d‐xylose, d‐glucose, d‐fructose, d‐ribose, d‐mannose, d‐glucose, l‐rhamnose, erythritol, salicin, methyl‐αd‐mannopyranoside, l‐arabinose, d‐cellobiose, d‐fructose, d‐galactose, d‐raffinose, d‐mannitol, d‐tagatose, d‐sorbitol, d‐maltose, arbutin, d‐lactose, methyl‐αd‐glucosamine, amygdaline, n‐acetylglucosamine, esculin ferric citrate, d‐trehalose, d‐melibiose, inulin, d‐melezitose, d‐turanose, starch, and potassium gluconate but not xylitol, potassium 5‐ketogluconate, potassium 2‐ketogluconate, methyl‐βd‐xylopyranoside, l‐xylose, l‐sorbose, l‐fucose, l‐arabitol, inositol, glycerol, glycogen, d‐ulcitol, d‐lyxose, d‐fucose, d‐arabitol, and d‐adonitol. Its major fatty acid is 12‐methyl‐tetradecanoic acid (46%).
Strain Marseille‐P3236T genome is 6,483,434 bp long with 43.41 mol% of G+C content. The 16S rRNA and genome sequences of P. timonensis were deposited in EMBL‐EBI under accession number LT598573 and FQSE01000000, respectively [32]. The type strain is Marseille‐P3236T (=CSUR P3236 = CCUG 71183), and was isolated from the stool sample of a healthy 39‐year‐old pygmy male from Congo.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors acknowledge Xegen (http://www.xegen.fr/) for genomic analyses performance and Claudia Andrieu for administrative assistance.
Bilen M, Mbogning Fonkou MD, Khelaifia S, et al. Taxonogenomics description of Parabacteroides timonensis sp. nov. isolated from a human stool sample. MicrobiologyOpen. 2019;8:e702 10.1002/mbo3.702
Funding information
This work has benefited from the French State support, managed by the ‘Agence Nationale pour la Recherche’ including the “Programme d'Investissement d'avenir” under the reference Méditerranée Infection 10‐IAHU‐03. This work was supported by Région Provence Alpes Côte d'Azur and European funding FEDER PRIMI.
DATA ACCESSIBILITY
16S rRNA gene sequence was deposited under the accession number: LT598573. The genome bioproject was deposited under the accession number: PRJEB18032. The strain was deposited under the following strain deposit numbers: CSUR P3236 and CCUG 71183.
REFERENCES
- Abu‐Shanab, A. , & Quigley, E. M. M. (2010). The role of the gut microbiota in nonalcoholic fatty liver disease. Nature Reviews Gastroenterology & Hepatology, 7(12), 691–701. 10.1038/nrgastro.2010.172 [DOI] [PubMed] [Google Scholar]
- Awadel‐Kariem, F. M. , Patel, P. , Kapoor, J. , Brazier, J. S. , & Goldstein, E. J. C. (2010). First report of Parabacteroides goldsteinii bacteraemia in a patient with complicated intra‐abdominal infection. Anaerobe, 16(3), 223–225. 10.1016/j.anaerobe.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Bilen, M. , Dufour, J.‐C. , Lagier, J.‐C. , Cadoret, F. , Daoud, Z. , Dubourg, G. , & Raoult, D. (2018). The contribution of culturomics to the repertoire of isolated human bacterial and archaeal species | Microbiome | Full Text [Internet]. Retrieved from https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-018-0485-5 (cited 2018 May 28). [DOI] [PMC free article] [PubMed]
- Elsawi, Z. , Togo, A. H. , Beye, M. , Dubourg, G. , Andrieu, C. , Armsrtong, N. , … Fournier, P. E. (2017). Hugonella massiliensis gen. nov., sp. nov., genome sequence, and description of a new strictly anaerobic bacterium isolated from the human gut. MicrobiologyOpen, 6(4), e00458 10.1002/mbo3.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier, P.‐E. , & Drancourt, M. (2015). New microbes new infections promotes modern prokaryotic taxonomy: A new section “TaxonoGenomics: New genomes of microorganisms in humans”. New Microbes New Infect, 7, 48–49. 10.1016/j.nmni.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier, P.‐E. , Lagier, J.‐C. , Dubourg, G. , & Raoult, D. (2015). From culturomics to taxonomogenomics: A need to change the taxonomy of prokaryotes in clinical microbiology. Anaerobe, 36 (Suppl C), 73–78. 10.1016/j.anaerobe.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Guinane, C. M. , & Cotter, P. D. (2013). Role of the gut microbiota in health and chronic gastrointestinal disease: Understanding a hidden metabolic organ. Ther Adv Gastroenterol, 6(4), 295–308. 10.1177/1756283X13482996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugon, P. , Dufour, J.‐C. , Colson, P. , Fournier, P.‐E. , Sallah, K. , & Raoult, D. (2015). A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect Dis, 15(10), 1211–1219. 10.1016/S1473-3099(15)00293-5 [DOI] [PubMed] [Google Scholar]
- Kierzkowska, M. , Majewska, A. , Szymanek‐Majchrzak, K. , Sawicka‐Grzelak, A. , Mlynarczyk, A. , & Mlynarczyk, G. (2017). The in vitro effect of clindamycin against Bacteroides and Parabacteroides isolates in Poland. J Glob Antimicrob Resist, 9, P49–52. [DOI] [PubMed] [Google Scholar]
- Kim, M. , Oh, H.‐S. , Park, S.‐C. , & Chun, J. (2014). Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. International Journal of Systematic and Evolutionary Microbiology, 64(Pt 2), 346–351. 10.1099/ijs.0.059774-0 [DOI] [PubMed] [Google Scholar]
- Lagier, J. C. , Armougom, F. , Million, M. , Hugon, P. , Pagnier, I. , Robert, C. , … Trape, J. F. (2012). Microbial culturomics: Paradigm shift in the human gut microbiome study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis, 18(12), 1185–1193. [DOI] [PubMed] [Google Scholar]
- Lagier, J. C. , Khelaifia, S. , Alou, M. T. , Ndongo, S. , Dione, N. , Hugon, P. , … Durand, G. (2016). Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol, 1, 16203 10.1038/nmicrobiol.2016.203 [DOI] [PubMed] [Google Scholar]
- Million, M. , Alou, M. T. , Khelaifia, S. , Bachar, D. , Lagier, J. C. , Dione, N. , … Fromonot, J. (2016). Increased gut redox and depletion of anaerobic and methanogenic prokaryotes in severe acute malnutrition. Scientific Reports, 6(1), 26051 Retrieved from http://www.nature.com/articles/srep26051 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sakamoto, M. , & Benno, Y. (2006). Reclassification of Bacteroides distasonis, Bacteroides goldsteinii and Bacteroides merdae as Parabacteroides distasonis gen. nov., comb. nov., Parabacteroides goldsteinii comb. nov. and Parabacteroides merdae comb. nov. International Journal of Systematic and Evolutionary Microbiology, 56(7), 1599–1605. 10.1099/ijs.0.64192-0 [DOI] [PubMed] [Google Scholar]
- Tindall, B. J. , Rosselló‐Móra, R. , Busse, H.‐J. , Ludwig, W. , & Kämpfer, P. (2010). Notes on the characterization of prokaryote strains for taxonomic purposes. International Journal of Systematic and Evolutionary Microbiology, 60(Pt 1), 249–266. 10.1099/ijs.0.016949-0 [DOI] [PubMed] [Google Scholar]
- Turnbaugh, P. J. , Ley, R. E. , Hamady, M. , Fraser‐Liggett, C. M. , Knight, R. , & Gordon, J. I. (2007). The human microbiome project. Nature, 449(7164), 804–810. 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vétizou, M. , Pitt, J. M. , Daillère, R. , Lepage, P. , Waldschmitt, N. , Flament, C. , … Poirier‐Colame, V. (2015). Anticancer immunotherapy by CTLA‐4 blockade relies on the gut microbiota. Science, 350(6264), 1079–1084. 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne, L. G. , Brenner, D. J. , Colwell, R. R. , Grimont, P. A. D. , Kandler, O. , Krichevsky, M. I. , … Starr, M. P. (1987). Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. International Journal of Systematic and Evolutionary Microbiology, 37(4), 463–464. 10.1099/00207713-37-4-463 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
16S rRNA gene sequence was deposited under the accession number: LT598573. The genome bioproject was deposited under the accession number: PRJEB18032. The strain was deposited under the following strain deposit numbers: CSUR P3236 and CCUG 71183.


