Abstract
Fusion of cortical granules with the oocyte plasma membrane is the most significant event to prevent polyspermy. This particular exocytosis, also known as cortical reaction, is regulated by calcium and its molecular mechanism is still not known. Rab3A, a member of the small GTP-binding protein superfamily, has been implicated in calcium-dependent exocytosis and is not yet clear whether Rab3A participates in cortical granules exocytosis. Here, we examine the involvement of Rab3A in the physiology of cortical granules, particularly, in their distribution during oocyte maturation and activation, and their participation in membrane fusion during cortical granule exocytosis. Immunofluorescence and Western blot analysis showed that Rab3A and cortical granules have a similar migration pattern during oocyte maturation, and that Rab3A is no longer detected after cortical granule exocytosis. These results suggested that Rab3A might be a marker of cortical granules. Overexpression of EGFP-Rab3A colocalized with cortical granules with a Pearson correlation coefficient of +0.967, indicating that Rab3A and cortical granules have almost a perfect colocalization in the egg cortical region. Using a functional assay, we demonstrated that microinjection of recombinant, prenylated and active GST-Rab3A triggered cortical granule exocytosis, indicating that Rab3A has an active role in this secretory pathway. To confirm this active role, we inhibited the function of endogenous Rab3A by microinjecting a polyclonal antibody raised against Rab3A prior to parthenogenetic activation. Our results showed that Rab3A antibody microinjection abolished cortical granule exocytosis in parthenogenetically activated oocytes. Altogether, our findings confirm that Rab3A might function as a marker of cortical granules and participates in cortical granule exocytosis in mouse eggs.
Keywords: Rab3A, cortical granule exocytosis, cortical reaction, mouse oocyte, mouse egg
INTRODUCTION
Cortical granules derive from Golgi apparatus and are membrane-bound organelles. As their name indicates, these granules are concentrated in the cortex of the unfertilized eggs, the outermost few microns of the egg that are distinct both morphologically and biochemically from the inner cytoplasm [28]. Fertilization stimulates cortical granule movement toward the plasma membrane and fusion. In fact, mammalian cortical granules were first described by C.R. Austin studying fertilization in hamster oocytes [2]. Austin showed that these granules disappeared almost completely after spermatozoon penetration.
Fusion of cortical granules with the oocyte plasma membrane is the most significant event to prevent polyspermy. This particular secretory process, also known as cortical reaction, is regulated by calcium like other exocytoses. Cortical granule exocytosis is distinct from most other regulated secretory vesicles because cortical granules are not renewed after their fusion with plasma membrane [25] and despite being first described 60 years ago, its mechanistic basis remains a mystery [15].
Regulated exocytosis in eukaryotic cells is mediated through a series of protein interactions which are responsible for the correct trafficking, tethering/docking, and fusion of secretory vesicles with a target membrane. Different members of the Rab family of monomeric guanosine triphosphatases (GTPases) are involved in the interaction and fusion of intracellular membrane-bound compartments. Considerable amounts of research have shown that Rab3A participates in calcium-regulated exocytosis in neurons[41], chromaffin cells [20;22;27], pancreatic cells [35;50], melanotrophs [42], and mammalian sperm [5;6]. Nevertheless, the function of Rab3A in exocytosis still remains elusive.
In sea urchin oocytes, it is known that Rab3A mediates cortical granule exocytosis [9]; however, it is not clear whether or not Rab3A participates in this secretory process in mammalian oocytes. First, Masumoto and collaborators documented the expression and localization of Rab3A in mouse oocyte and suggested, based on the cortical localization of Rab3A observed by immunofluorescence staining, that this protein might be involved in cortical granules exocytosis [29]. In contrast and very recently, Dr. Sun’s group has reported that Rab3A is distributed in the cytoplasm of mouse oocytes and, using RNAi, they propose that Rab3A would have a role in cortical granule distribution and /or assimetric division during meiotic maturation of mouse oocytes [47]. Therefore, it is not clear if Rab3A is involved in cortical granule exocytosis and it has not been investigated carefully.
The aim of this work was to examine in deep the involvement of Rab3A in the physiology of cortical granules, particularly, in their distribution and secretion. We reported that Rab3A overlaps with cortical granules during their migration during oocyte maturation and egg activation, and, based on the colocalization of EGFP-Rab3A with cortical granules, we proposed Rab3A as a possible marker of these granules. Using a functional assay to measure cortical granule exocytosis, we also documented that the microinjection of active Rab3A triggers cortical reaction, and that this activation is not dependent on extracellular calcium. Finally, blocking endogenous Rab3A by microinjecting an anti-Rab3A antibody, we demonstrated that Rab3A participates in cortical granule exocytosis.
MATERIALS AND METHODS
Reagents
Purified equine chorionic gonadotrophin (PMSG) and human chorionic gonadotropin (hCG) were generously donated by Syntex SA (Buenos Aires, Argentina). Glutathione sepharose were from GE Healthcare (Buenos Aires, Argentina). Ni-NTA-agarose was from Qiagen (Hilden, Germany).Sephadex G-25 was from Pharmacia (Uppsala, Sweden). Vectashield mounting medium and rhodamine-conjugated Lens Culinaris Agglutinin (LCA) were from Vector Laboratories (CA, USA). Rabbit polyclonal IgG directed against Rab3A (anti-Rab3A) was purchased from Stressgen Biotechnologies Corporation (CA, USA). Goat anti-rabbit IgG conjugated to DyLigth488, and horseradish peroxidase were purchased from Jackson Immuno Research Laboratories (PA, USA). All other reagents were obtained from Sigma Chemical (MO, USA) or from ICN Biochemicals (Buenos Aires, Argentina).
Oocyte collection
All animal experiments were approved by the Institutional Animal Care and Use Committee of the School of Medicine (Protocol approval 25-2014). Six-to-twelve-week-old females CF-1 were superovulated with intraperitoneal (i.p.) injection of 5 IU pregnant mare’s serum gonadotropin (PMSG) followed 48 h later by i.p. injection of 5 IU of human chorionic gonadotropin (hCG) as previously described [33]. Germinal Vesical (GV)-intact oocytes were recovered 42–46 h after PMSG injection in HEPES-buffered culture medium (M2 medium). To prevent oocyte maturation, milrinone (final concentration 2.5 μM) was added. Ovulated (Metaphase II, MII) eggs were obtained by pulling the oviducts open with fine forceps into M2 medium 15–17 h after administration of hCG. Cumulus cells were dispersed with 0.3 % hyaluronidase and gentle aspiration through a pipette. After collection, both GV-intact oocytes and MII eggs were cultured in CZB medium [7] until use.
Recombinant proteins
Plasmid encoding Rab3A was generously provided by Dr. P. Stahl (Washington University, St. Louis, MO, USA). Plasmid fused to GST in pGEX-2T was transformed in BL21 (DE3) cells (Stratagene, La Jolla, CA, USA) and expression was induced overnight at 22 °C with 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). Recombinant proteins were purified by affinity chromatography on glutathione–sepharose beads as previously described [51]. Rab3A was always used prenylated and loaded with guanosine 5′-[γ-thio] triphosphate as described in [51]. Prior to microinjection, Rab3A protein was filtered on a column of Sephadex G-25 previously equilibrated with microinjection buffer (120 mM KCl, 20 mM Hepes, pH 7.4).
Plasmid Construction
The EGFP fragment was excised from the pEGFP-N1 vector (Clontech-Takara Bio Inc., Shiga, Japan) by SalI/NotI double digestion and inserted into the SalI/NotI site of the pGEM-T Easy vector (Promega, Madison, WI). Similarly, the EGFP-Rab3A fragment was excised from the pEGFP-C1-Rab3A vector [45] by NheI/SacII double digestion and inserted into the SpeI/SacII site of the pGEM-T Easy vector. The inserts of the resultant pGEM-T-EGFP and pGEM-T-EGFP-Rab3A vectors were verified with an automated sequencer.
Complementary RNA synthesis
Complementary RNA (cRNA) was synthesized from linearized plasmid DNA using SP6 RNA polymerase and the mMESSAGE mMACHINE kit (Ambion,) according to the manufacturer’s instructions. The cRNA was polyadenilated using Poly(A) tailing kit (Ambion) and purified by using MEGAclear (Ambion). The final cRNA concentration was determined by spectrophotometry, and cRNA integrity was confirmed by analyzing a sample on a formaldehyde gel.
Microinjection experiments
Eggs from CF-1 mice were microinjected using Narishige manipulators coupled to an Olimpus transmitted light microscope. Microinjection and holding pipettes were prepared using an automated capillary extruder P-97 puller (Sutter Instrument) and a Narishigue MF-830 microforge, respectively. Microinjection pipettes were filled by suction from a microdrop containing recombinant proteins, antibodies or cRNA and the solution was expelled into the cytoplasm of eggs by pneumatic pressure using a PLI-100 picoinjector (Harvard Apparatus, Cambridge, MA). Oocytes were microinjected into 30 μl drops of M2 medium placed in Petri dishes and covered with mineral oil. Twenty oocytes were microinjected in each condition with approximately the same volume (7–10 pl).
Immunocytochemistry
For immunocytochemistry analysis, anti-Rab3A and DyLigth488-conjugated goat anti-rabbit IgG antibodies were used at the final concentration of 0.85 and 4 μg/ml, respectively. Briefly, the zona pellucida was removed by exposure briefly in an acid Tyrode's solution (1min., pH 2.5). The cells were fixed in 3.7 % paraformaldehyde for 1 h, washed in blocking solution (BS, 0.1 % BSA, 0.01 % Tween-20 in PBS for 5 min) and permeabilized in 0.1 % Tritón X-100 in PBS. After washed again in BS, the cells were blocked in 3 mg/ml BSA, 0.1 M glycine in PBS for 1 h RT, and incubated with the primary antibody diluted in BS for 1 h at RT or overnight (ON) at 4 °C. The samples were then washed in BS and incubated in the appropriate secondary antibody for 1 h. The samples were finally washed and placed onto a slide in a drop of Vectashield mounting medium containing Hoescht 33342 for DNA staining. The preparations were stored in the dark at 4 °C until observation. To evaluate the colocalization with the cortical granules, 1 μM of LCA lectin (conjugated with rhodamine or FITC) was added in the last 30 minutes of the secondary antibody incubation. All samples were observed in a confocal microscope Olympus Fluoview FV1000 (Olympus, Japan).
Immunoblotting
Protein extracts from equal numbers of MII eggs (100 eggs) were separated on a 10 % SDS-PAGE gel, transferred to Immobilon-P, and immunoblotted according to standard procedures. The anti-Rab3A antibody was used at a final dilution of 1 μg/ml, and the secondary antibody was a horseradish peroxidase conjugated anti-rabbit IgG. Immunoreactive proteins were detected using an ECL ADVANCE Western blotting Detection Kit (GE Healthcare) and a Fuji Film LAS-4000 Luminescent Image Analyzer.
In vitro fertilization
IVF were performed 14–15 hours post hCG injection using HTF medium as previously described [4;12]. Briefly, MII eggs were obtained as described above and only eggs with visible polar body were collected. After capacitation, mouse sperm were diluted to 2–4 x 105 sperm/ml. Eggs and sperm were mixed in a 500 μl fertilization drop of HTF medium containing 0.5 % BSA, and then cultured at 37 °C in an atmosphere of 5 % CO2/95 % air. After 3 hours, eggs were washed six times to remove any loosely attached sperm, and then cultured for 5 h. Fertilization was evaluated by the presence of the second polar body and the formation of both the male and female pronuclei by epifluorescence microscopy. Finally, embryos were fixed in 3.7 % paraformaldehyde and mounted as indicated below for cortical granules’ quantification.
Artificial activation of mouse eggs
After microinjection, MII eggs were activated with either 5 μM A23187 or 10 mM SrCl2 in calcium-depleted CZB (or M16) medium as previously described [12]. All ovulated eggs were treated with the indicated reagent within 1 hr of their collection to avoid deleterious effects of egg aging.
Cortical Granule Staining and Quantification
LCA conjugated with FITC or rhodamine was used for cortical granule staining as previously described and cortical granules were quantified according to de Paola et al [12]. Briefly, oocytes were compressed under a coverslip in order to provide a large flat field of granules for quantification as well as rapid qualitative assessments of cortical granules distribution (in both coverslip and slide associated cortical fields). Cortical granules (CG) density was computed as the mean of four non-overlapping cortical areas per egg (CG/100 μm2) and included only CGs in the cortex. After a threshold was applied, cortical granules were quantified using the Image J program. To illustrate the results, cortical granules density in the control condition was considered as 100 %.
Statistical analyses
All experiments were conducted at least 3 times on independent samples. Each group analyzed contained a minimum of 15–20 cells and results were evaluated using one-way ANOVA and post hoc tests. Differences were considered significant at the p<0.05 level.
RESULTS
Rab3A distribution during mouse oocyte maturation and egg activation
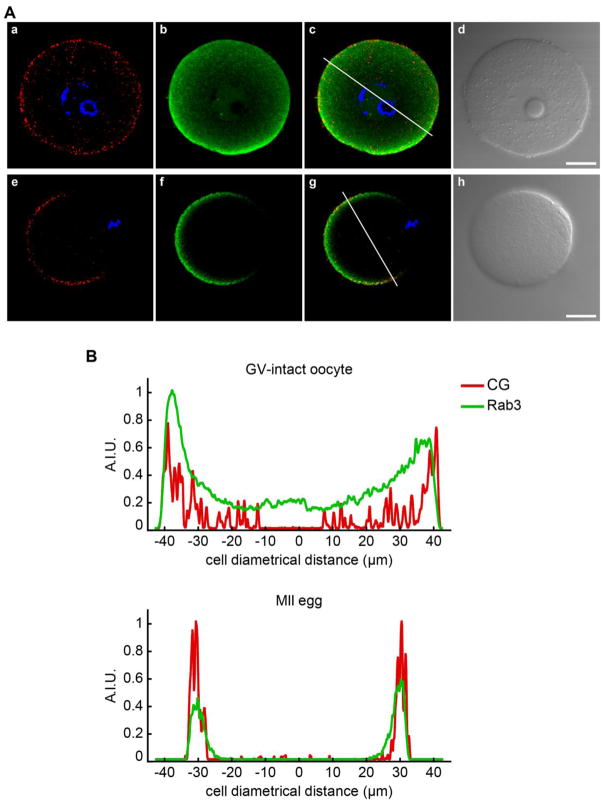
As mentioned previously, the localization of Rab3A in mouse oocytes is controversial since two different works have reported either cortical localization [29] or cytosolic distribution [47] associated to cortical granules. To resolve this discrepancy and having in mind that Rab3A has been involved in transport of synaptic vesicles to the active zone in mouse brain nerve terminals [26], we hypothesized that Rab3A is associated to cortical granules and, hence, Rab3A would have a similar pattern of distribution during oocyte maturation. Using indirect immunofluorescence, we analyzed the localization of Rab3A and cortical granules in two stages of mouse oocyte maturation: germinal vesicle (GV)-intact oocytes and metaphase II (MII) eggs. In GV-intact oocytes, Rab3A was present throughout the cytoplasm and in the cortical region (Fig 1A, b), following a similar pattern to cortical granules (Fig 1A, a). In MII eggs, Rab3A staining was enriched in the cortex region (Fig 1A, f), in coincidence with previous findings [29] . Furthermore, we observed that Rab3A was present in the cortical region enriched in cortical granules (Fig 1A, f and g).Fluorescence-intensity distribution analysis of Rab3A and cortical granules showed that Rab3A had a similar pattern to cortical granule distribution in GV-intact oocytes and MII eggs (Fig. 1B). From these results, we inferred that Rab3A and cortical granules have a similar distribution during oocyte maturation.
Figure 1.
Immunolocalization of Rab3A. A) GV-intact oocytes (a–d) and MII eggs (e–g) were immunostained using anti-Rab3A (green; b, f); cortical granules were stained with LCA conjugated with rhodamine (red; a, e); and DNA (blue) was stained with Hoechst 33342. Merged (c, g) and DIC images (d, h) are showed for each cell. Shown are representative images of three independent experiments. Scale bar, 20 μM. B) Analysis of fluorescence-intensity distribution (measured in arbitrary intensity units, A.I.U.) of Rab3A (green) and cortical granules (CG, red) in GV-intact oocytes (upper panel) and MII eggs (lower panel) using ImageJ. Fluorescence intensities were measured along lines traced in images c and g of panel A.
Then, because cortical granules are not renewed after their fusion with plasma membrane, we hypothesized that, if Rab3A is associated to cortical granules, it might no longer be detected after cortical granule exocytosis during egg activation. Mouse eggs can be parthenogenetically activated by different activators such as calcium ionophore and strontium, which induce cortical granule exocytosis [12]. By indirect immunofluorescence, we analyzed Rab3A distribution in MII eggs after parthenogenetic activation with strontium chloride (SrCl2, 10 mM) at different times. Cells were fixed after 10, 20, and 40 minutes in SrCl2 incubation. Interestingly, the fluorescence intensity of Rab3A decreased significantly after 40 minutes (Fig. 2A, top row), following the decrease of cortical granule staining (see Fig. 2A, middle row). Fluorescence-intensity distribution analysis showed that both Rab3A and cortical granule staining diminished over time (Fig. 2C). To corroborate the reduction of Rab3A expression, we performed western blot assays. As shown in Fig 2B, western blot results also showed that Rab3A expression was reduced after 40 minutes of parthenogenetic activation. To confirm the decline of cortical granules, we quantified cortical granules for each time analyzed as previously described in de Paola and collaborators [12]. As shown in Fig. 2C, cortical granules density measured as cortical granules per 100 μm2 (CG/100 μm2) decreased after 40 min. Altogether, these results confirm our hypothesis that Rab3A is associated to cortical granules and both have a similar pattern of distribution during oocyte maturation and egg activation.
Figure 2.
Rab3A distribution during egg activation. A) MII eggs were collected and activated with 10 mMSrCl2. After 0, 10, 20, and 40 min of activation, cells were fixed, permeabilized and immunostained with anti-Rab3A antibody (top row). Cortical granules were stained with LCA conjugated with rhodamine and DNA was stained with Hoechst 33342 (middle row). Bottom row shows merged images from top and middle row. Shown are representative images of three independent experiments. Scale bar, 50 μM. B) Immunoblot analysis of Rab3A protein in protein extracts derived from 100 non activated MII eggs (Ctrl) and 100 activated MII eggs (SrCl2). Proteins were resolved on 10 % SDS-PAGE gel and immunoblotted with anti-Rab3A as described in Materials and Methods. The experiment was performed 3 times and a representative blot is shown. C) Analysis of fluorescence-intensity distribution (measured in arbitrary intensity units, A.I.U.) of Rab3A and cortical granules (CG) for the MII egg showed in A. On the right, the graph also shows cortical granules density (CG/100 μm2) corresponding for each time of activation (for more details see text).
Overexpressed EGFP-Rab3A colocalizes with cortical granules
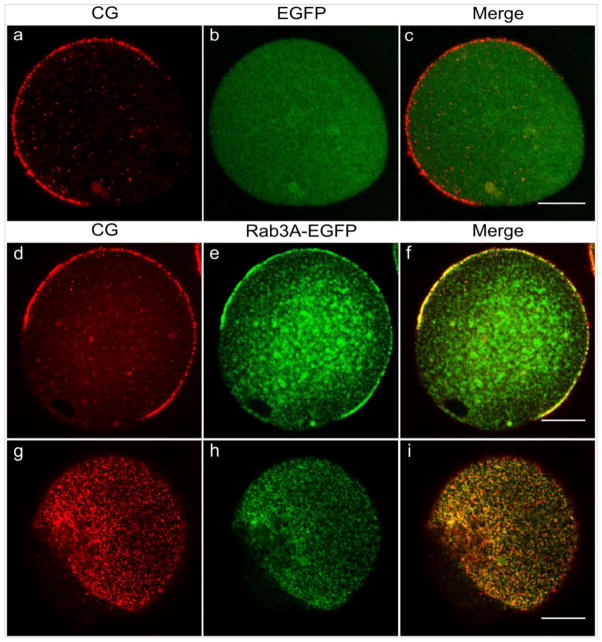
Based on the finding that Rab3A and cortical granules have a similar pattern of distribution during oocyte maturation and egg activation, we hypothesized that Rab3A might be a potential marker of cortical granules. To test this hypothesis we overexpressed EGFP-Rab3A and EGFP by microinjecting their cRNA in mouse oocytes to analyze the localization of the Rab3A- and EGFP- fluorescent protein. GV-intact oocytes were collected and microinjected with either EGFP cRNA or EGFP-Rab3A cRNA in presence of milrinone to allow cRNA translation. After 4 h of incubation, oocytes were washed out from milrinone and matured overnight. As shown in Figure 3, EGFP-Rab3A (green, middle row) colocalized with cortical granules stained with rhodamine (red, middle row) in the cortical region enriched in these granules and it was absent in the cortical granules free domain. In addition, overexpressed EGFP-Rab3A was also observed in the cytoplasm of the cells.To quantify the degree of colocalization between the two fluorophores, EGFP and rhodamine in the cortical region, we determined the Pearson correlation coefficient according to [1]. This coefficient has a range of +1 (perfect correlation) to -1 (perfect but negative correlation) with 0 (zero) denoting the absence of a relationship. The cortical region was operationally defined as all cytoplasm 2 μm below the plasma membrane [18]. The Pearson correlation coefficient for EGFP and rhodamine colocalization in the cortical region was +0.967 for the MII egg showed in the middle panel of Fig. 3. This indicates that Rab3A and cortical granules have almost a perfect correlation or colocalization. On the other hand, EGFP, used as a control, showed a uniform distribution all over the cytoplasm and did not colocalize with cortical granules (green, top row). In effect, Pearson correlation coefficient for EGFP and cortical granules was +0.149 for the image showed on the top panel of Fig 3. Similar results were obtained in all analyzed cells. Therefore, these findings confirm that Rab3A colocalizes with cortical granules in cortical region of mouse oocytes.
Figure 3.
EGFP-Rab3A colocalizes with cortical granules in MII eggs. GV- intact oocytes were microinjected with EGFP cRNA (top row) or EGFP-Rab3A cRNA (middle row) in presence of milrinone to allow cRNA translation. After 4 hours, milrinone was washed out and the microinjected GV-intact oocytes were matured overnight. Shown are equatorial planes of representative images of MII eggs overexpressing EGFP (b) and EGFP-Rab3A (e). Cortical granules were stained with LCA conjugated with rhodamine (a, d). Merged images (c, f, i) are shown at the end of each row. Bottom row shows a surface view to highlight the colocalization of EGFP-RAb3A and cortical granules. Scale bar, 20 μM.
Cortical granule exocytosis is a calcium-regulated secretion that represents a membrane fusion process essential for fertilization. Following sperm fusion, cortical granules undergo exocytosis to release their content into the perivitelline space. The secretion diffuses into the zona pellucida and transforms it into a physical barrier, avoiding polyspermy and ensuring normal embryonic development. The molecular mechanism of membrane fusion during cortical granule exocytosis is still poorly understood and finding a marker of cortical granules to analyze cortical reaction in vivo is a long-standing aim still not reached by reproductive biologists (Dr. Ducibella, Dr. Schultz, and Dr. Williams, personal communications). Our results show that Rab3A can be proposed as a marker of cortical granules.
Active Rab3A triggers cortical granule exocytosis
Rab3 is a small GTPase that has been conspicuously involved in calcium-dependent exocytosis as a membrane organizer [52]. Rab3 GTPase cycles between GTP-bound and GDP-bound state and undergoes membrane insertion through an isoprenyl lipid moiety. GTP-bound and GDP- bound Rab3 are known as the active and inactive Rabs protein, respectively [17]. To address the possibility that Rab3A was involved in cortical granule exocytosis, we attempted to perturb the endogenous Rab3A function by microinjecting both active or inactive recombinant Rab3A and analyzing its effect on cortical granule exocytosis.
Using our functional assay that measures cortical granule exocytosis in fixed cells [12], we first quantified this secretion process in metaphase II eggs activated by in vitro fertilization or by parthenogenetic activators such as calcium ionophore and strontium chloride. After each treatment, the zona pellucida was removed before fixation and cortical granules were stained with Lens Culinaris Agglutinin (LCA) conjugated with FITC. Cortical granule exocytosis was evaluated by cortical granules density as described in Materials and Methods. Figure 4A shows representative images for each condition mentioned above. We found that MII eggs in control conditions had 40 ±11 cortical granules per 100 μm2 (CG/100 μm2). After in vitro fertilization, the number of cortical granules remaining in the cell decreased approximately 80 % (IVF, 10 ± 5 CG/100 μm2) compared to control cells. Similarly, cortical granule exocytosis triggered by parthenogenetic activators, A23187 and strontium, decreased the number of cortical granules around 50 % (A23187, 18 ± 3 CG/100 μm2; SrCl2, 19 ±5 CG/100 μm2) compared to not activated MII eggs (Figure 4B). To analyze the effect of microinjected Rab3A on cortical granule exocytosis, Rab3A was produced in bacteria as a GST fusion protein, prenylated in vitro, and loaded with either GTP or GDP as described in Materials and Methods. Unbound nucleotides were eliminated by gel filtration. As shown in Figure 4, the microinjection of activated Rab3A (GTP, Fig. 4C) triggered the cortical reaction, whereas the GDP-bound form had no effect (GDP, Fig. 4C). Even more, Rab3A was able to activate cortical granule exocytosis in a similar magnitude to strontium chloride (positive control, SrCl2, Fig. 4C). Preparations in which Rab3A was omitted but received the same treatment with nucleotides had no effect (mock, Fig. 4C), indicating that residual GTPγS was not responsible for the stimulation. In addition, control microinjections of either GST (GST, Fig. 4C) or non-prenylated, unloaded Rab3A (none, Fig. 4C) were not able to activate cortical granule exocytosis.
Figure 4.
Active Rab3A triggers cortical granule exocytosis. A) MII eggs were collected and cortical granule exocytosis was not triggered (control) or triggered by in vitro fertilization (IVF), by calcium ionophore (A23187), and SrCl2 (SrCl2). After removing the zona pellucida, cells were fixed and mounted for cortical granules’ quantification. Shown are representative images for each condition. Cortical granules were stained with LCA conjugated with FITC (LCA-FITC) and DNA was stained with Hoechst 33342 (DIC-Hoechst). B) Quantification of cortical granules density (CG/100 μm2) in MII eggs activated by in vitro fertilization (IVF), calcium ionophore(A23187) and SrCl2(SrCl2). The graph shows raw data for each condition (not normalized). Data represent the means ± SEM of at least three independent experiments. The asterisks indicate significant differences from control condition (***, p< 0.001). C) Quantification of cortical granules density (% CG/100 μm2) in MII eggs activated by SrCl2 (SrCl2) and after microinjection of purified and prenylated GST-Rab3A loaded with GTPγS (GTP) or GDPβS (GDP). As negative controls, non-prenylated GST-Rab3A (none), mock preparations without recombinant Rab3A (mock) and glutathione-S-transferase (GST) were tested in the assay. Data were normalized as explained in Materials and Methods and represent the means ± SEM of at least three independent experiments. The asterisks indicate significant differences from control condition (***, p<0.001). D) Quantification of cortical granules density (% CG/100 μm2) in MII eggs activated by SrCl2 (SrCl2) and active Rab3A (microinjected Rab3A-GTP) in CZB prepared without (−Ca2+) or with calcium (+Ca2+). Data represent the means ± SEM of at least three independent experiments. The asterisks indicate significant differences from control condition (***, p< 0.001).
It has been reported that calcium influx across the plasma membrane is a requisite of egg activation signaling [32]. To determine whether extracellular calcium is required for Rab3A activation, we performed cortical granule exocytosis assay with active Rab3A in presence or absence of extracellular calcium. CZB medium was prepared with or without calcium and cells were microinjected and incubated in medium as indicated. As shown in Fig. 4D, prenylated and active Rab3A triggered cortical granule exocytosis in both conditions. These results show that prenylated and active Rab3A triggers cortical reaction, and that this activation is independent of extracellular calcium.
Rab3A participates in cortical granule exocytosis
Rab3A has been implicated in different calcium-regulated exocytoses and involved specifically in tethering and docking of secretory vesicles prior to fusion. The exact mechanism by which Rab3A participates in exocytosis is still not known. In fact, Rab3Ahas been shown to be inhibitory in several exocytotic events [14;21] and, on the contrary, other studies described Rab3A as a positive regulator in other secretion models [8;39].
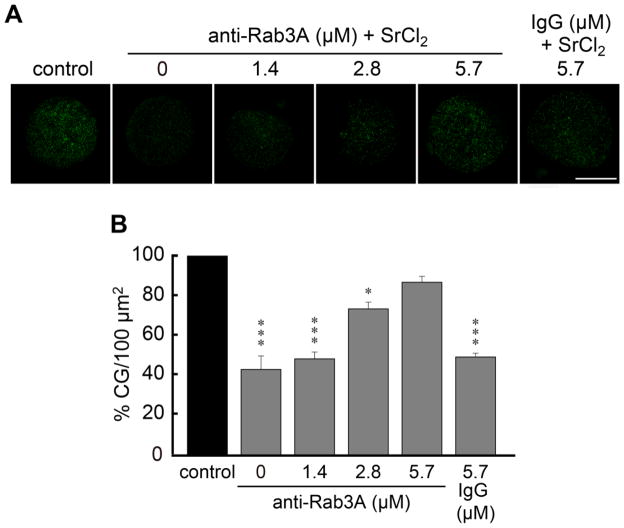
Based on our findings, we hypothesized that Rab3A participates in cortical granule exocytosis as a positive regulator. To test this hypothesis, we attempted to inhibit the function of the endogenous protein by microinjecting a polyclonal antibody raised against Rab3A. Increased concentrations of anti-Rab3A were microinjected into mouse eggs cytoplasm 15–30 min prior to activating cortical granule exocytosis by parthenogenetic activation with SrCl2. After 1 h of incubation with SrCl2, the zona pellucida of the treated eggs was removed before fixation. Cortical granule were stained with FITC-LCA and exocytosis was evaluated by cortical granules density as described in [12]. As shown in Fig. 5, anti-Rab3A antibody was able to inhibit cortical granule exocytosis in a concentration-dependent manner, when MII eggs were activated with SrCl2. The microinjection of a non-immune rabbit IgG protein showed no effect. Hence, these results confirm that Rab3A has an active role in cortical granule exocytosis.
Figure 5.
Microinjection of anti-Rab3A inhibits cortical granule exocytosis. A) Cortical granules of MII eggs microinjected with increasing concentrations of anti- Rab3A antibody (anti-Rab3A) or preimmune serum (IgG) prior to the activation of cortical granule exocytosis with SrCl2. After removing zona pellucida, cells were fixed, and mounted for cortical granules’ quantification. Shown are representative images for each condition in B. Scale bar, 50 μM. B) Quantification of the cortical granules’ density (% CG/100 μm2) in MII eggs microinjected with increasing anti-Rab3A concentrations or with IgG and activated with SrCl2. Data represent the means ± SEM of at least three independent experiments. The asterisks indicate significant differences from control condition (*, p<0.05; ***, p<0.001).
DISCUSSION
The purpose of this work was to examine the involvement of Rab3A in the physiology of cortical granules, particularly, in their distribution and cortical granule exocytosis. A previous report showed that Rab3A and Rab3D are expressed in mouse eggs and that Rab3A localizes at cortical region in unfertilized oocytes and two-cell embryos [29]. In contrast, it was reported very recently that Rab3A presents a cytoplasmic distribution in mouse eggs [47].
In this study, we described the localization and distribution of Rab3A in GV-intact oocytes, MII eggs and early activated MII eggs. Our results showed that in GV oocytes, Rab3A was present throughout the cytoplasm and in the cortical region following a similar pattern to cortical granule distribution. In MII eggs, Rab3A was mainly enriched in the cortex region. These results are coincident to those obtained previously by Masumoto and collaborators [29]; in addition, we report that Rab3A is not expressed in the cortical granule free domain around the meiotic spindle. Our findings do not support the exclusive cytosolic localization of Rab3A during oocyte maturation and egg activation published recently by Dr. Sun’s group [47]. Considering that localization studies are based on immunostaining methods, one possible explanation for this discrepancy might be given by the different antibodies used.
In mouse egg, actin microfilaments are important for the migration of mouse cortical granules to the cortex during oocyte maturation and it has been hypothesized that microfilament-based motor such as a myosin-like motor might be involved in this movement [10]. Rab GTPases have been identified as a link between Myosin V -a motor protein involved in vesicle movement on the actin cytoskeleton- and vesicle transport in different cell types [3;37;38]. In fact, Rab3A and Myosin Va are direct binding partners, interact on synaptic vesicles, and it has been shown that the Rab3A/Myosin Va complex is involved in transport of neuronal vesicles [49]. Even though the presence of Myosin Va and Myosin Vb hasbeen described in ovary and oocyte by immunocytochemistry [31], the function of Myosin V has not been explored in mouse eggs. According to our observations that Rab3A and cortical granules have a similar pattern of distribution during oocyte maturation, and a similar association between Rab3 and cortical granules migration throughout oogenesis has been postulated in sea urchin eggs [9], we proposed that Rab3A might be associated with Myosin V in cortical granules distribution and/or migration during mouse oocyte maturation. Nevertheless, this hypothesis needs to be tested. Because Rab3D is also expressed in mouse oocyte we cannot discard that Rab3D might participate in the migration and/or maturation of cortical granules. In fact, Rab3D has been involved in the maturation of secretory vesicles in pancreas [36], neurons [23], and PC12 cells [24]. These evidences support the idea that Rab3A might be involved in the migration of cortical granules during meiotic maturation probably through Myosin V. Although a role for Rab3A in cortical granule migration was recently suggested [47], more studies are needed to demonstrate a direct interaction between Rab3 isoforms and the maturation and migration of cortical granules in mouse eggs.
We also showed that Rab3A disappeared along with cortical granules after cortical granule exocytosis stimulated by strontium chloride. After exocytosis, the membrane that is incorporated into the plasma membrane needs to be retrieved by compensatory endocytosis [19]. Vogel and his colleagues have described in detail the retrieval of membranes by endocytosis after cortical reaction in sea urchin eggs [43;44;46]. Even more, they have described two independent forms of endocytosis in this model that maintain the cell surface homeostasis [11]. Even though the retrieval of membrane after cortical granules exocytosis has not been investigated in mammalian eggs, it is thought that such membrane recycling would also take place in human and mouse eggs [40]. Thus, considering that the retrieval of cortical granule membrane occurs in mouse egg, the disappearance of Rab3A means that Rab3A protein is being degraded after endocytosis. At least two possible mechanisms of protein degradation might be postulated: 1) degradation through a degradative pathway following the massive membrane retrieval after cortical granule exocytosis, and 2) ubiquitin degradation. Interestingly, Rab3A contains several lysine residues that can be ubiquitined for protein degradation when their sequence is analyzed in a predictor program of protein ubiquitination sites (http://www.ubpred.org). Further studies are needed to elucidate how Rab3A is degraded after cortical granule exocytosis.
Based on the observations that Rab3A and cortical granules have a similar pattern of distribution during oocyte maturation and that Rab3A expression diminished along with cortical granules after egg activation, we propose Rab3A as a possible marker of cortical granules. The fact that overexpressed EGFP-Rab3A and cortical granules have a Pearson correlation coefficient of +0.967 (very close to +1) means that the colocalization between Rab3A and cortical granules is almost perfect in the cortical region. Many attempts to generate a transgenic mouse with a fluorescent marker of cortical granules content have been tried and have failed (Dr. Ducibella, Dr. Schultz, and Dr. Williams, personal communications). Because of this, we propound that EGFP-Rab3A might function as a new marker of cortical granule membrane; nevertheless, more studies are needed.
The molecular mechanism by which Rab3 may regulate fusion in exocytosis is not clear yet [48]. In most fusion-dependent transport steps, the Rabs seem to promote fusion by inducing the formation of a multiprotein complex on the membranes that would be necessary for specific docking and SNARE activation [8;39]. However Rab3A, the Rab participating in synaptic vesicle fusion, one of the best characterized fusion processes, has been shown to be inhibitory in several exocytotic events [14;21]. The only evidence that Rab3 might mediate cortical granule exocytosis has been shown in sea urchin eggs [9], in which cortical granules are docked to plasma membrane. In these cells, the microinjection of a peptide corresponding to the effector domain of Rab3A inhibited cortical granule exocytosis; however, the microinjection of affinity-purified polyclonal antibodies, raised to a region encompassing the Rab3 effector domain had no effect on cortical granule exocytosis [9]. Therefore, these findings are not conclusive on the function of Rab3A in cortical reaction.
So far, Rab3A expression had been described having two different distributions, which suggest different functions of Rab3A in mouse oocytes. One, the cortical localization, in which it was thought to participate in cortical granules exocytosis [29], and, the other, the cytosolic localization, in which Rab3A was suggested to participate in cortical granule distribution [47]. It is worth to notice that no strong evidence was provided to conclude that Rab3A does not participate in cortical granule exocytosis in this last report. To determine whether Rab3A participates in cortical granule exocytosis, we used a functional assay that measures this secretory process quantifying cortical granules density after MII egg activation. We microinjected recombinant proteins and antibodies prior to parthenogenetic eggs activation. Our results show that: 1) the microinjection of recombinant Rab3A in MII eggs in its active form (GTP-bound) promoted cortical granule exocytosis, and 2) the microinjection of an anti-Rab3A antibody blocked cortical granule exocytosis in a concentration-dependent manner. Rab3 is a positive regulator of calcium-dependent exocytosis in human sperm [51] and pituitary melanotrophs [42]. Our findings strongly indicate that Rab3A acts as a positive regulator of cortical granule exocytosis in mouse eggs. Nevertheless, we cannot discard that Rab3D may also play a role in cortical reaction since it has been involved in non neuronal exocytosis [34]. Even more, mice with knockout mutation in Rab3A are fertile [16] and, even though the fertility rate for Rab3A-knockout female has not been reported, it is thought that Rab3D may function in CG exocytosis instead of Rab3A in these mutant mice.
How is active Rab3A able to trigger cortical granule exocytosis? It is known that Rabphilin 3A is an effector of Rab3A, and that only GTP-Rab3A (active form) is able to recruit Rabphilin 3A to vesicles in neurons [13;41]. Rabphilin 3A has been involved in cortical granule exocytosis in mouse eggs [30]. One possible answer to that question is that active Rab3A may recruit Rabphilin 3A to cortical granules facilitating the docking and fusion of cortical granules. More work will be necessary to better characterize how Rab3 is activated during cortical granule exocytosis, which Rab3-interacting factors are involved, and what is the function of each of them. In conclusion, the findings presented here propose that Rab3A might be used as a marker of cortical granules membrane and demonstrate that Rab3A has an active role in the release of cortical granules during cortical reaction.
HIGHLIGTHS.
Rab3A has a similar migration pattern to cortical granules in mouse oocytes
Rab3A can be a marker of cortical granules
Active Rab3A triggered cortical granule exocytosis
Blocking endogenous Rab3A inhibits cortical granule exocytosis
Rab3A participates in cortical reaction in mouse oocytes
Acknowledgments
FUNDING
This work was supported by Fogarty International Center (grant number RO1TW007571); Agencia Nacional de Promoción Científica y Tecnológica (grant number 2012-0218); CONICET (grant number PIP 11420110100402), and Universidad Nacional de Cuyo (grant number 06/M071).
We thank Alejandra Medero for technical assistance; MarianelaCarabajal for performing injections; and Veterinarian Julieta Scelta and Lucas Aldao for animal care. O.D.B., A.I.C, M.P., and M.N.Z. are thankful to CONICET, Argentina, for fellowships.
Footnotes
DECLARATION OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler J, Parmryd I. Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander's overlap coefficient. Cytometry A. 2010;77:733–742. doi: 10.1002/cyto.a.20896. [DOI] [PubMed] [Google Scholar]
- 2.AUSTIN CR. Cortical granules in hamster eggs. Exp Cell Res. 1956;10:533–540. doi: 10.1016/0014-4827(56)90025-8. [DOI] [PubMed] [Google Scholar]
- 3.Bahadoran P, Aberdam E, Mantoux F, Busca R, Bille K, Yalman N, de Saint-Basile G, Casaroli-Marano R, Ortonne JP, Ballotti R. Rab27a: A key to melanosome transport in human melanocytes. J Cell Biol. 2001;152:843–850. doi: 10.1083/jcb.152.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batiz LF, De Blas GA, Michaut MA, Ramirez AR, Rodriguez F, Ratto MH, Oliver C, Tomes CN, Rodriguez EM, Mayorga LS. Sperm from hyh mice carrying a point mutation in alphaSNAP have a defect in acrosome reaction. PLoS One. 2009;4:e4963. doi: 10.1371/journal.pone.0004963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bello OD, Zanetti MN, Mayorga LS, Michaut MA. RIM, Munc13, and Rab3A interplay in acrosomal exocytosis. Exp Cell Res. 2012;318:478–488. doi: 10.1016/j.yexcr.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustos MA, Roggero CM, De la Iglesia PX, Mayorga LS, Tomes CN. GTP-bound Rab3A exhibits consecutive positive and negative roles during human sperm dense-core granule exocytosis. J Mol Cell Biol. 2014;6:286–298. doi: 10.1093/jmcb/mju021. [DOI] [PubMed] [Google Scholar]
- 7.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 8.Coleman WL, Bill CA, Bykhovskaia M. Rab3a deletion reduces vesicle docking and transmitter release at the mouse diaphragm synapse. Neuroscience. 2007;148:1–6. doi: 10.1016/j.neuroscience.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Conner S, Wessel GM. rab3 mediates cortical granule exocytosis in the sea urchin egg. Dev Biol. 1998;203:334–344. doi: 10.1006/dbio.1998.9057. [DOI] [PubMed] [Google Scholar]
- 10.Connors SA, Kanatsu-Shinohara M, Schultz RM, Kopf GS. Involvement of the cytoskeleton in the movement of cortical granules during oocyte maturation, and cortical granule anchoring in mouse eggs. Dev Biol. 1998;200:103–115. doi: 10.1006/dbio.1998.8945. [DOI] [PubMed] [Google Scholar]
- 11.Covian-Nares JF, Smith RM, Vogel SS. Two independent forms of endocytosis maintain embryonic cell surface homeostasis during early development. Dev Biol. 2008;316:135–148. doi: 10.1016/j.ydbio.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Paola M, Bello OD, Michaut MA. Cortical Granule Exocytosis Is Mediated by Alpha-SNAP and N-Ethilmaleimide Sensitive Factor in Mouse Oocytes. PLoS One. 2015;10:e0135679. doi: 10.1371/journal.pone.0135679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deak F, Shin OH, Tang J, Hanson P, Ubach J, Jahn R, Rizo J, Kavalali ET, Sudhof TC. Rabphilin regulates SNARE-dependent re-priming of synaptic vesicles for fusion. EMBO J. 2006;25:2856–2866. doi: 10.1038/sj.emboj.7601165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominguez L, Yunes RM, Fornes MW, Burgos M, Mayorga LS. Calcium and phospholipase A2 are both required for the acrosome reaction mediated by G-proteins stimulation in human spermatozoa. Mol Reprod Dev. 1999;52:297–302. doi: 10.1002/(SICI)1098-2795(199903)52:3<297::AID-MRD7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Gardner AJ, Evans JP. Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reprod Fertil Dev. 2006;18:53–61. doi: 10.1071/rd05122. [DOI] [PubMed] [Google Scholar]
- 16.Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De CP, Hammer RE, Sudhof TC. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- 17.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulyas BJ. Cortical granules of mammalian eggs. Int Rev Cytol. 1980;63:357–392. doi: 10.1016/s0074-7696(08)61762-3. [DOI] [PubMed] [Google Scholar]
- 19.Gundelfinger ED, Kessels MM, Qualmann B. Temporal and spatial coordination of exocytosis and endocytosis. Nat Rev Mol Cell Biol. 2003;4:127–139. doi: 10.1038/nrm1016. [DOI] [PubMed] [Google Scholar]
- 20.Holz RW, Brondyk WH, Senter RA, Kuizon L, Macara IG. Evidence for the involvement of Rab3A in Ca(2+)-dependent exocytosis from adrenal chromaffin cells. J Biol Chem. 1994;269:10229–10234. [PubMed] [Google Scholar]
- 21.Iezzi M, Escher G, Meda P, Charollais A, Baldini G, Darchen F, Wollheim CB, Regazzi R. Subcellular distribution and function of Rab3A, B, C, and D isoforms in insulin-secreting cells. Mol Endocrinol. 1999;13:202–212. doi: 10.1210/mend.13.2.0228. [DOI] [PubMed] [Google Scholar]
- 22.Johannes L, Lledo PM, Roa M, Vincent JD, Henry JP, Darchen F. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 1994;13:2029–2037. doi: 10.1002/j.1460-2075.1994.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kogel T, Gerdes HH. Roles of myosin Va and Rab3D in membrane remodeling of immature secretory granules. Cell Mol Neurobiol. 2010;30:1303–1308. doi: 10.1007/s10571-010-9597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kogel T, Rudolf R, Hodneland E, Copier J, Regazzi R, Tooze SA, Gerdes HH. Rab3D is critical for secretory granule maturation in PC12 cells. PLoS One. 2013;8:e57321. doi: 10.1371/journal.pone.0057321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laidlaw M, Wessel GM. Cortical granule biogenesis is active throughout oogenesis in sea urchins. Development. 1994;120:1325–1333. doi: 10.1242/dev.120.5.1325. [DOI] [PubMed] [Google Scholar]
- 26.Leenders AG, Lopes da Silva FH, Ghijsen WE, Verhage M. Rab3a is involved in transport of synaptic vesicles to the active zone in mouse brain nerve terminals. Mol Biol Cell. 2001;12:3095–3102. doi: 10.1091/mbc.12.10.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CG, Pan CY, Kao LS. Rab3A delayed catecholamine secretion from bovine adrenal chromaffin cells. Biochem Biophys Res Commun. 1996;221:675–681. doi: 10.1006/bbrc.1996.0655. [DOI] [PubMed] [Google Scholar]
- 28.Liu M. The biology and dynamics of mammalian cortical granules. Reprod Biol Endocrinol. 2011;9:149. doi: 10.1186/1477-7827-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masumoto N, Ikebuchi Y, Tahara M, Yokoi T, Tasaka K, Miyake A, Murata Y. Expression of Rab3A in the cortical region in mouse metaphase II eggs. J Exp Zool. 1998;280:91–96. [PubMed] [Google Scholar]
- 30.Masumoto N, Sasaki T, Tahara M, Mammoto A, Ikebuchi Y, Tasaka K, Tokunaga M, Takai Y, Miyake A. Involvement of Rabphilin-3A in cortical granule exocytosis in mouse eggs. J Cell Biol. 1996;135:1741–1747. doi: 10.1083/jcb.135.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGurk L, Tzolovsky G, Spears N, Bownes M. The temporal and spatial expression pattern of myosin Va, Vb and VI in the mouse ovary. Gene Expr Patterns. 2006;6:900–907. doi: 10.1016/j.modgep.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci U S A. 2012;109:4169–4174. doi: 10.1073/pnas.1112333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michaut MA, Williams CJ, Schultz RM. Phosphorylated MARCKS: a novel centrosome component that also defines a peripheral subdomain of the cortical actin cap in mouse eggs. Dev Biol. 2005;280:26–37. doi: 10.1016/j.ydbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Millar AL, Pavios NJ, Xu J, Zheng MH. Rab3D: a regulator of exocytosis in non-neuronal cells. Histol Histopathol. 2002;17:929–936. doi: 10.14670/HH-17.929. [DOI] [PubMed] [Google Scholar]
- 35.Olszewski S, Deeney JT, Schuppin GT, Williams KP, Corkey BE, Rhodes CJ. Rab3A effector domain peptides induce insulin exocytosis via a specific interaction with a cytosolic protein doublet. J Biol Chem. 1994;269:27987–27991. [PubMed] [Google Scholar]
- 36.Riedel D, Antonin W, Fernandez-Chacon R, Alvarez de TG, Jo T, Geppert M, Valentijn JA, Valentijn K, Jamieson JD, Sudhof TC, Jahn R. Rab3D is not required for exocrine exocytosis but for maintenance of normally sized secretory granules. Mol Cell Biol. 2002;22:6487–6497. doi: 10.1128/MCB.22.18.6487-6497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez OC, Cheney RE. Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J Cell Sci. 2002;115:991–1004. doi: 10.1242/jcs.115.5.991. [DOI] [PubMed] [Google Scholar]
- 38.Roland JT, Kenworthy AK, Peranen J, Caplan S, Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell. 2007;18:2828–2837. doi: 10.1091/mbc.E07-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rupnik M, Kreft M, Nothias F, Grilc S, Bobanovic LK, Johannes L, Kiauta T, Vernier P, Darchen F, Zorec R. Distinct role of Rab3A and Rab3B in secretory activity of rat melanotrophs. Am J Physiol Cell Physiol. 2007;292:C98–105. doi: 10.1152/ajpcell.00005.2006. [DOI] [PubMed] [Google Scholar]
- 40.Schalkoff ME, Oskowitz SP, Powers RD. Ultrastructural observations of human and mouse oocytes treated with cryopreservatives. Biol Reprod. 1989;40:379–393. doi: 10.1095/biolreprod40.2.379. [DOI] [PubMed] [Google Scholar]
- 41.Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sedej S, Klemen MS, Schluter OM, Rupnik MS. Rab3a is critical for trapping alpha-MSH granules in the high Ca(2)(+)-affinity pool by preventing constitutive exocytosis. PLoS One. 2013;8:e78883. doi: 10.1371/journal.pone.0078883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith RM, Baibakov B, Ikebuchi Y, White BH, Lambert NA, Kaczmarek LK, Vogel SS. Exocytotic insertion of calcium channels constrains compensatory endocytosis to sites of exocytosis. J Cell Biol. 2000;148:755–767. doi: 10.1083/jcb.148.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith RM, Baibakov B, Lambert NA, Vogel SS. Low pH inhibits compensatory endocytosis at a step between depolarization and calcium influx. Traffic. 2002;3:397–406. doi: 10.1034/j.1600-0854.2002.30603.x. [DOI] [PubMed] [Google Scholar]
- 45.Tsuboi T, Fukuda M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J Cell Sci. 2006;119:2196–2203. doi: 10.1242/jcs.02962. [DOI] [PubMed] [Google Scholar]
- 46.Vogel SS, Smith RM, Baibakov B, Ikebuchi Y, Lambert NA. Calcium influx is required for endocytotic membrane retrieval. Proc Natl Acad Sci U S A. 1999;96:5019–5024. doi: 10.1073/pnas.96.9.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang HH, Cui Q, Zhang T, Wang ZB, Ouyang YC, Shen W, Ma JY, Schatten H, Sun QY. Rab3A, Rab27A, and Rab35 regulate different events during mouse oocyte meiotic maturation and activation. Histochem Cell Biol. 2016 doi: 10.1007/s00418-015-1404-5. [DOI] [PubMed] [Google Scholar]
- 48.Weimer RM, Jorgensen EM. Controversies in synaptic vesicle exocytosis. J Cell Sci. 2003;116:3661–3666. doi: 10.1242/jcs.00687. [DOI] [PubMed] [Google Scholar]
- 49.Wollert T, Patel A, Lee YL, Provance DW, Jr, Vought VE, Cosgrove MS, Mercer JA, Langford GM. Myosin5a tail associates directly with Rab3A-containing compartments in neurons. J Biol Chem. 2011;286:14352–14361. doi: 10.1074/jbc.M110.187286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaekura K, Julyan R, Wicksteed BL, Hays LB, Alarcon C, Sommers S, Poitout V, Baskin DG, Wang Y, Philipson LH, Rhodes CJ. Insulin secretory deficiency and glucose intolerance in Rab3A null mice. J Biol Chem. 2003;278:9715–9721. doi: 10.1074/jbc.M211352200. [DOI] [PubMed] [Google Scholar]
- 51.Yunes R, Michaut M, Tomes C, Mayorga LS. Rab3A triggers the acrosome reaction in permeabilized human spermatozoa. Biol Reprod. 2000;62:1084–1089. doi: 10.1095/biolreprod62.4.1084. [DOI] [PubMed] [Google Scholar]
- 52.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]