Abstract
L-Ascorbate (L-Asc), but not D-isoascorbate (D-Asc) and N-acetylcysteine (NAC) suppress HIF1 ODD-luc reporter activation induced by various inhibitors of HIF prolyl hydroxylase (PHD). The efficiency of suppression by L-Asc was sensitive to the nature of HIF PHD inhibitor chosen for reporter activation. In particular, the inhibitors developed to compete with alpha-ketoglutarate (αKG), were less sensitive to suppression by the physiological range of L-Asc (40–100 μM) than those having a strong iron chelation motif. Challenging those HIF activators in the reporter system with D-Asc demonstrated that the D-isomer, despite exhibiting the same reducing potency with respect to ferric iron, had almost no effect compared to L-Asc. Similarly, no effect on reporter activation was observed with cell-permeable reducing agent NAC up to 1 mM. Docking of L-Asc and D-Asc acid into the HIF PHD2 crystal structure showed interference of Tyr310 with respect to D-Asc. This suggests that L-Asc is not merely a reducing agent preventing enzyme inactivation. Rather, the overall results identify L-Asc as a co-substrate of HIF PHD that may compete for the binding site of αKG in the enzyme active center. This conclusion is in agreement with the results obtained recently in cell-based systems for TET enzymes and jumonji histone demethylases, where L-Asc has been proposed to act as a co-substrate and not as a reducing agent preventing enzyme inactivation.
Keywords: Catalytic cycle, HIF1 ODD-Luciferase reporter assay, Adaptaquin, HIF PHD inhibitor, TET enzyme, Jumonji demethylase
1. Introduction
L-ascorbic acid (L-Asc) is an established activator of non-heme iron α-ketoglutarate (αKG) dioxygenases, best exemplified by collagen and HIF (hypoxia inducible factor) prolyl hydroxylases (HIF PHD), and very recently by TET enzymes and jumonji histone demethylases. Hydroxylated prolines provide the necessary stability to collagen [1,2], whereas hydroxylation of Pro564 in HIF1α labels it for subsequent ubiquitinylation and proteasomal degradation [3]. Activation of HIF PHD inhibits the HIF-triggered anti-hypoxic program, which is known to significantly contribute to the resistance of malignant tumors. Such activation is obviously beneficial in promoting tumor killing, and L-ascorbic acid has been shown to be capable of lowering the level of HIF [4]. Cancer patients were shown to benefit from high doses of L-Asc delivered i.v. [5,6], but not orally [7]. Because of the latter finding, L-Asc as an anticancer treatment has been forgotten for decades. Interest in L-Asc has been renewed due to the recently demonstrated ability of millimolar concentrations to selectively kill cancer cells [8,9]; these concentrations are achievable by i.v. administration [10,11]. L-Asc administration significantly reduces tumor growth rates in mice, and in some of these studies, this effect was associated with the ability of L-Asc to inhibit the activity of the prosurvival HIF-1 [12,13]. Indeed, i.v. injections of high mM concentrations of L-Asc have been recently shown to be a promising treatment [14,15] and co-treatment [16] for cancer patients, despite the fact that the mechanism of this anticancer effect is still controversial [17].
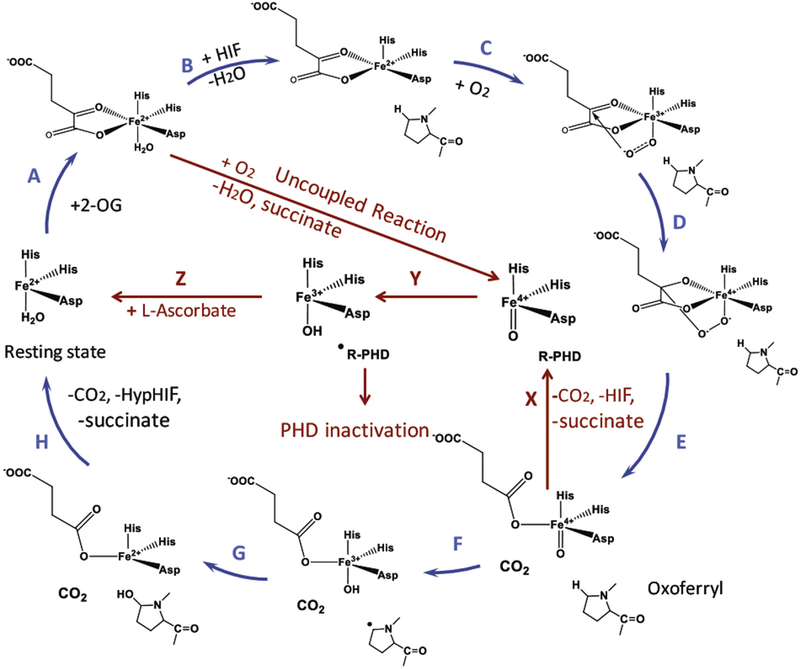
The catalytic cycle of HIF PHD (Scheme 1) is an example of a multi-substrate mechanism, launched by the binding of a specific protein substrate. Only upon HIF binding (step B), the water molecule is displaced from the 6th coordination position yielding penta-coordinated iron, which immediately binds and activates oxygen (step C). In the absence of a specific substrate this step is three to five orders of magnitude slower for the enzymes of the same catalytic mechanism [18]. Oxidative decarboxylation of αKG yields succinate (step E), which provides two ligands to the iron till the completion of the catalytic cycle. The oxo-iron formed in the first half-reaction then subtracts hydrogen atom from the HIF proline to form iron-bound hydroxyl radical (step F), which recombines with the proline radical, forming hydroxyl-proline (step G) [19]. Product dissociation is the rate-limiting step of the catalytic mechanism.
Scheme 1.
HIF prolyl hydroxylase catalytic cycle accounting for a plausible role of L-Asc preventing enzyme inactivation.
The major limitation in the homogeneous enzyme assay format for HIF PHD is the absence of a continuous assay of the enzyme activity [20,21]. The mechanistic studies on the L-Asc effect on the purified enzymes have been mainly performed with the use of high concentrations of the enzyme and iron, with a significant contribution of the so-called uncoupled reaction when the oxo-iron form of the enzyme is supposed to attack an aromatic residue inside the enzyme active center, and not HIF (step Y). Hence, the activating effect L-Asc is usually ascribed to enzyme stabilization in the so-called uncoupled reaction [22], by reducing the active center iron into the ferrous form (Scheme 1, step Z). In a typical homogeneous enzyme assay of HIF PHD activity, the assay mixture contains recombinant enzyme and HIF substrate (HIF peptide or recombinant HIF ODD), 200–500 μM αKG, 50–100 μM iron (II) sulfate or chloride, 2–4 mM L-Asc and catalase [21,22], with point by point measurement of a key substrate or product concentration. However, L-Asc is not strictly required for the in vitro reaction as has been shown in Ref. [23]. Free Fe2+ is limited and stringently controlled within the cell. Therefore, these in vitro enzymatic analyses do not reflect the whole scenario of HIF PHD in catalyzing HIF hydroxylation in the cell.
We have reported on an optimized cell-based HIF1 ODD-luc reporter assay for drug discovery purposes, which demonstrates a number of advantages for monitoring the activity of the endogenous HIF PHD [24]. The advantage of the HIF1 ODD-luc fusion reporter assay over the commonly used transcriptional HRE-luc reporters is that this assay is quantitative and permits real-time monitoring of drug action. The reporter background signal corresponds to the equilibrium between the HIF1 ODD fusion protein synthesis and degradation, which is controlled by HIF PHD activity (rate-limiting step) [24]. The treatment with HIF PHD inhibitors (or 1% O2 hypoxia [25]) results in immediate stabilization of HIF1 ODD-luc fusion protein. The steady-state luminescent signal, which corresponds to the system re-equilibration in the presence of a reversible inhibitor of HIF PHD is reached within 2–3 h incubation [24] This work presents the results of studying the effect of L-Asc versus D-isoascorbic acid (D-Asc) and another potent cell-permeable reducing agent, N-acetic cysteine (NAC), on the activation of HIF1 ODD-luc reporter by HIF PHD inhibitors of various chemical nature. The results obtained confirm the stereospecific effect of L-Asc on the enzyme activity, which cannot be ascribed to its reducing properties only.
2. Materials and methods
2.1. Materials
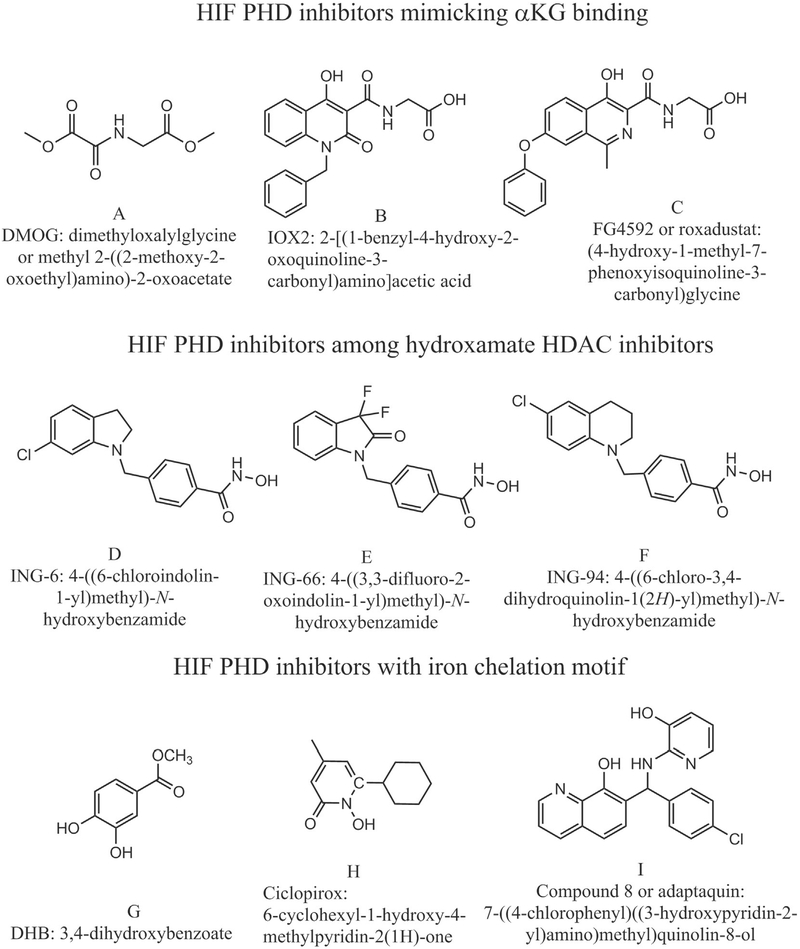
HIF PHD inhibitors FG4592 and IOX2, mimicking αKG binding mode, were purchased from Cayman Chemical Co (Ann Arbor, MI, USA). Compound 8 (adaptaquin), identified by us as a potent and specific HIF PHD inhibitor, and its improved version 8a (cat #4896–3212) was purchased from ChemDiv (Skolkovo, Russia). Ethyl 3,4-dihydroxybenzoate (DHB), iron chelator which is supposed to bind in the site of αKG in HIF PHD; dimethyloxalyl glycine (DMOG) an inhibitor-mimic of αKG, and ciclopirox, a HIF PHD inhibitor and a potent iron chelator were from Sigma-Aldrich. ING-6, ING-66, and ING-94, a group of phenyl hydroxamates inhibitors of HDAC6 exhibiting the properties of potent HIF activators both in vitro and in vivo, were synthesized by I.N.Gaisina (Gaisina et al., submitted). The structure of inhibitors used is shown in Scheme 2. 10 mM stock solutions of HIF PHD inhibitors were made in DMSO and used to prepare serial ×50 dilutions right before the addition to the cell media. L-Asc, as well as D-Asc and N-acetylcysteine (NAC) stock solutions were made just before the addition, in 50 mM Tris-HCl buffer, pH 7.4. Luciferase reagent was made by dissolving in 100 mM Tris-HCl buffer, pH 8.0, the following reagents: 6 mM MgSO4, 1 mM EDTA, 5 mM dithiothreitol, 1 mM ATP, 0.4 mM Co-enzyme A, 0.6 mM luciferin and 0.1 mM sodium pyrophosphate. The latter was made as 2 mM stock solution. The final pH was adjusted to 7.8 if necessary. Cell lysis reagent (×5) was from Promega. Cell culture media and reagents were from Gibco (USA). All other reagents used were from Sigma (St. Louis, MO).
Scheme 2.
Chemical formula of HIF PHD inhibitors used in the work.
2.2. Methods
HIF1 ODD-luc reporter assay:
SH-SY5Y cell lines stably expressing HIF1 ODD-luc reporter [24] were grown in the DMEM/F12 supplemented with GlutaMAX (Thermo Fisher Scientific) containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin and plated into 96–well white flat-bottom plates at 20,000 cell/well in 100 μL serum and incubated overnight at 37 °C, 5% CO2. After 20 h cultivation, L-Asc, or D-Asc, or NAC had been added to the wells (2 μL) to get the desired final concentrations, and then, the studied inhibitors were titrated within the desired range of concentrations depending on the strength of the enzyme inhibitor. The plates were incubated for various times (0–4 h) at 37 °C. The medium was removed, cells lysed with 20 μL Lysis buffer (Promega) for 7 min at RT, and luciferase activity was measured on a SpectraMax M5e platereader (Molecular Devices, Sunnyvale, CA) with 80 μL of luciferase reagent (made as described above). Based on the activation time-course, with the stabilization of luminescence level attributed to equilibration of the system, when the rate of luciferase fusion synthesis becomes equal to the rate of its degradation, 3 h incubation was used as optimal (optimization example is shown in Supplementary Fig. S1 for DHB). The reporter activation was normalized to the background luminescence and protein content as described in Ref. [24]. The experiments were performed in triplicate. EC50 values were calculated from a four-parameter sigmoid dose response using SigmaPlot non-linear regression module.
RT-PCR analysis:
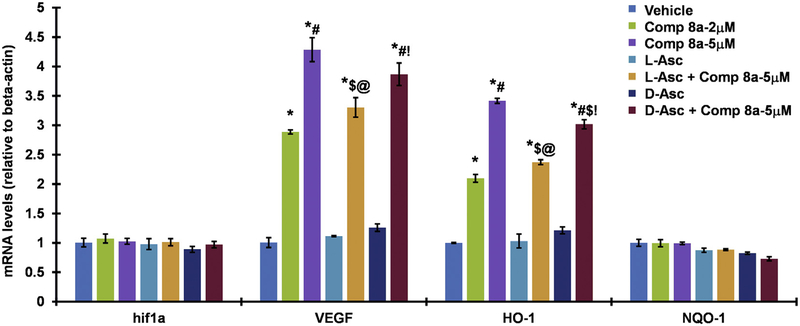
N2a mouse neuroblastoma cells were treated with either vehicle (DMSO + PBS), Compound 8a (at two different concentration 2 μM and 5 μM, prepared in DMSO), 100 μM L-Asc (prepared in 50 mM PBS), 100 μM D-Asc (prepared in 50 mM PBS), and the combinations of 5 μM Compound 8a with 100 μM L-Asc and D-Asc for 4 h. The lysates were used to isolate total mRNA using TRI reagent according to the manufacturer’s protocol (Sigma-Aldrich). Reverse transcription of total RNA was performed using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). The cDNA was diluted, and about 20 ng was used to amplify in an ABI prism 7900 HT Real-time PCR system (Applied Biosystems) for HIF1α, its target genes VEGF, HO-1, and Nrf2 target gene NQO-1 (as a negative control) using PowerUp SYBR Green Master Mix (Thermo Fisher Scientific). Cycling parameters were 95 °C for 10 s, followed by 60 °C for 1 min. Relative expression was calculated using the ΔΔCt method [26]. The experiment was performed in triplicate. One way ANOVA with post-hoc Tukey test was used for the determining the statistical significance among different groups. Values are expressed as a fold change from control reaction and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or β-actin expression. Mean ± SEM is plotted as bar graph.
Docking experiments
L-Asc were performed using the CDOCKER algorithm (Discovery studio 2.5, Accelrys, San Diego, CA), followed by force field minimization (CHARMm, [27]) and binding energy calculations using the PHD2 crystal structure with the bound inhibitor (2G19.pdb) as the starting template structure (there were amino acids missing on the N-terminus and C-terminus of the structural model, however these were not in close proximity to the binding site and therefore there was no need to add them to the structure). We applied docking restraints taking into account the results of the study on the structure-activity relationship for ascorbic acid analogs with respect to inhibition of collagen hydroxylase, which demonstrated a mandatory presence of two keto-groups in the analog structures for iron chelation [28] Manual docking was performed for D-Asc.
3. Results
3.1. L-ascorbic acid, but not D-isoascorbic acid or N-acetylcysteine, suppresses HIF1 ODD-luc reporter activation by Compound 8a, DHB and ING-6
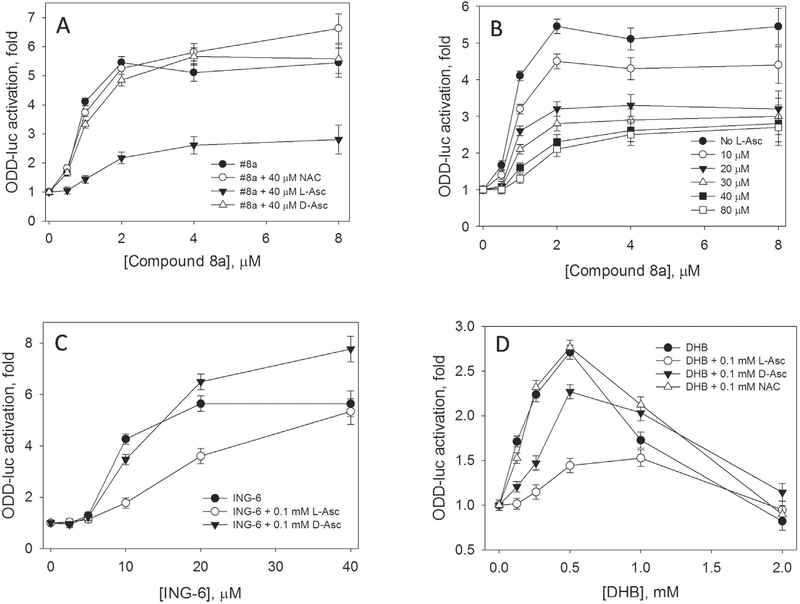
L-Asc potently suppresses reporter activation induced by Compound 8a, which specifically inhibits HIF PHD among other enzymes of this group and also is an iron chelator with the constant of 100 nM like all 8-hydroxyquinoline derivatives [24] (Fig. 1A and B). The effect is observed already at 10 μM L-Asc (a 20-fold excess over half-activation concentration, EC50). With an increase in L-Asc concentrations, EC50 increases ca. 1.5-fold, whereas the magnitude of activation drops 2.5-fold. The L-Asc effect shows saturation at concentrations above 40 μM (Fig. 1A). The L-Asc effect could be non-specific due to the iron reduction restoring the Resting state and preventing inactivation (Scheme 1, step Z); or it could be specific showing preference for L-Asc only. It is particularly note-worthy that neither D-Asc nor NAC applied in the equal concentration cause any change in reporter activation (Fig. 1A). An increase in NAC concentration up to 1 mM also has no suppressing effect on the reporter activation with either drug (not shown). In accord with the recent publication on in vivo suppressive effect of NAC on HIF stability, 10 mM NAC concentration in the blood is necessary to have an effect similar to 50 μM L-ascorbate [29]. Comparison of reducing power of L-Asc and D-Asc (1 mM) performed in the reaction of 1 mM potassium ferricyanide reduction in 50 mM Tris-HCl buffer, pH 7.4, showed no difference in reduction kinetics (results not shown). One may expect the same effect for the agents with the equal reducing potency. Thus, if the observed suppression effect is the result of preventing the enzyme inactivation in the uncoupled reaction with ascorbic acid, we have to assume that the reduction is stereospecific for L-Asc. The same preference for L-Asc is observed when competing with two other HIF PHD inhibitors tested, ethyl 3,4-dihydroxybenzoate (DHB) (Fig. 1C), and ING-6 (Fig. 1D). The suppressing effect in the case of ING-6 was clearly weaker than for Compound 8 and DHB, hence, the effect could depend on the structure/properties of HIF PHD inhibitors.
Fig. 1.
Inhibition of HIF1 ODD-luc reporter activation by L-ascorbate. A, Effect of N-acetyl cysteine (NAC), D- and L-Asc on reporter activation induced by Compound 8a upon 3 h incubation; B, Saturating character of L-ascorbate inhibition of reporter activation by Compound 8a; C, Effect of D- and L-Asc on reporter activation induced by ING-6; D, Effect of NAC, D- and L-Asc on reporter activation induced by 3,4-dihydroxybenzoate (DHB).
3.2. L-ascorbic acid suppression of reporter activation depends on the nature of HIF prolyl hydroxylase inhibitors
Three different groups of HIF prolyl hydroxylase inhibitors had been selected (Scheme 2) to study the suppression effect of L-Asc in the HIF1 ODD-luc reporter assay: the first group represent inhibitors mimicking the binding mode of αKG in the enzyme active center, namely dimethyloxalyl glycine (DMOG), FG-4592, and IOX2; the second group represent phenylhydroxamates with various “hanging tails” originally developed as HDAC6 inhibitors, which display appreciable side activity as HIF PHD inhibitors, and are successfully docked in the binding site of αKG providing two ligands for iron with their N-hydroxamate moiety (Gaisina et al., submitted); the third group represent iron chelators that can fit inside the PHD2 active center such as ethyl 3,4-dihydroxybenzoate (DHB), which inhibition constant in the HIF PHD enzyme assay equals to 5 μM [30]; ciclopirox, a potent iron chelator (with 50 nM constant) and “branched tail” oxyquinoline, Compound 8 (or adaptaquin), specific for HIF PHD (EC50 of ca. 2 μM) among other enzymes of this group [31].
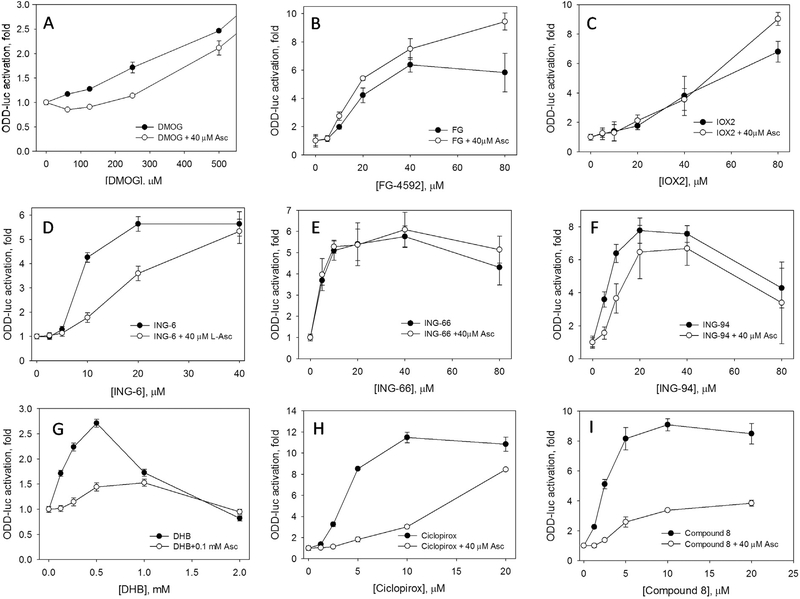
DMOG is widely used as a HIF activator working at ca. 1 mM concentration resulting in HIF1 and HIF2 stabilization after 2 h incubation, despite the fact that it is not specific for HIF PHD and targets all αKG-dependent enzymes, including that of mitochondria, - its inhibitory action on mithochondria respiration is immediate [32]. Taking into account the DMOG effect on cell metabolism, and Krebs cycle enzymes in particular, one may speculate that there is contribution of Krebs cycle inhibition into the observed HIF stabilization due to the accumulation of pyruvate and oxaloacetate, which are not very potent αKG mimics and whose effect on HIF stabilization is known to be suppressed by 100 μM L-ascorbate [33]. It can be seen that L-Asc suppression effect is pronounced for DMOG up to 250 μM (Fig. 2A), but at higher DMOG concentrations (mM range), comparable to the intracellular concentration of αKG in the neuroblastome cell line (2.3 mM [34]), L-Asc effect becomes negligible (not shown). This is in agreement with the reported absence of L-Asc effect on HIF stabilization induced by 1 mM monomethyloxalylglycine studied by immunoblotting in Ref. [35]. Our results suggest the importance of the concentration ratio of an inhibitor, αKG, and L-ascorbate, which indirectly indicates the competitive character of the observed effect.
Fig. 2.
Inhibition effect of L-ascorbate on activation of HIF1 ODD-luc reporter activation is sensitive to the nature of activator. 3 h incubation. Structure of activators used is shown in Scheme 2.
When we consider HIF PHD2 inhibitors specifically designed to bind the enzyme with high affinity, the suppressing effect of L-Asc on activation is minimal for FG-4592 (Fig. 2B), and IOX2 (Fig. 2C), if any. One may speculate that the excess of L-Asc over EC50 is ca. 1–2, which may be not sufficient. However, a better explanation is based on the fact that both inhibitors are specific for HIF PHD and competitive versus αKG. The inhibition constants reported for the homogeneous enzyme assay with PHD2 were in the nanomolar range (FG-4592 [36] and IOX2 [37]). Many orders of magnitude higher EC50 observed in the reporter assay for IOX2 and FG-4592 reflect significant competition from the intracellular αKG (2.3 mM), which is a well-known effect discussed before in Ref. [34]. Briefly, for a competitive inhibitor the dependence of the reaction rate on the substrate and inhibitor concentration can be expressed as follows:
V= Vm/(1 + Km/[S] + Km[I]/Ki[S]), where Vm is the maximal rate, Km is Michaelis constant for αKG, and Ki is the inhibition constant for a drug competing for the αKG binding site; [S] and [I] are αKG and drug concentrations, respectively. For a millimolar level of intracellular αKG, Km/[S] will be in the range of 0.001–0.01: the Km[I]/Ki[S] term will have an effect on the observed rate only at the inhibitor concentrations of 100–1000 fold higher than its true inhibition constant determined in the enzyme assay. As shown in Ref. [34], the EC50 value for Compound A (an αKG mimicking inhibitor developed by Amgen) in the neuroblastoma cell line is 18.5 μM, whereas the inhibition constant determined in the enzyme assay is 7 nM (a 3000-fold offset). If L-Asc competes with the reported Km of ca 50 μM [23] for the same αKG binding site with these inhibitors, it definitely fails since they being taken at micro-molar concentrations, which are three orders of magnitude higher than their true inhibition constant, had already outperformed 2.3 mM intracellular αKG. At higher concentrations of an inhibitor L-Asc seems to stimulate the activation (Fig. 2B and C). Titration of FG-4592 up 0.6 mM clearly demonstrates its toxicity above 100 μM, and D-Asc becomes protective and increases the activation effect, whereas L-Asc has no effect at all (Supplementary Fig. S2).
Phenyl hydroxamate inhibitors bind active center iron via the hydroxamate group. The three inhibitors chosen differ only in the structure of indoline rings, which are supposed to interact with the amino acid residues at the entrance to the active center. The EC50 values of these inhibitors are very close to each other (7–10 μM), and if L-Asc had been competing for the active site iron and not the enzyme, one may expect a similar suppressing effect for all three activators. However, the effect of L-Asc is sensitive to the indoline ring structure (Fig. 2D–F). ING-6 is most susceptible for L-Asc inhibition (Fig. 2D), whereas ING-66 is not sensitive at all (Fig. 2E). One of the possible explanations for the absence of the suppressing effect of L-Asc could be the pro-oxidative nature of the difluorooxindole ring in the “hanging tail” of ING-66, which may covalently modify cell components and exhibit enhanced toxicity. In this case, protective effect of L-Asc will mask its suppressing effect, similarly to the FG-4592 case.
The most pronounced suppression of reporter activation by physiological levels of L-Asc (0.04–0.1 mM) is observed for HIF PHD inhibitors with a chelating motif (Fig. 2G–I). DHB is a weak HIF PHD inhibitor (5 μM inhibition constant, EC50 0.25 mM), which binds to the αKG site and chelates the active site iron. The activation effect induced by DHB is very sensitive to L-Asc pointing to the strong competition from L-Asc for the active site iron (Fig. 2G). Ciclopirox is a potent iron chelator capable of binding iron inside HIF PHD active center and possibly to extract the iron from HIF PHD. L-Asc shifts EC50 for ciclopirox by an order of magnitude (Fig. 2H). For Compound 8, as we discussed above, there is a significant drop in the magnitude of activation, but a minor change in EC50 (Fig. 2I). The severity of suppression by L-Asc could originate from its significant excess over Compound 8 or ciclopirox, and thus, support the competing mode of its interaction with the enzyme against enzyme inhibitors. On the other hand, these inhibitors are iron chelators, and they do not compete for the αKG binding site protected by high intracellular concentrations of αKG, but they do compete for the active center iron binding.
3.3. Effect of L-ascorbic acid studied by RT-PCR (Fig. 3)
Fig. 3.
RT-PCR of L-Asc vs D-Asc (100 μM) effects on HIF-1 target genes induction by Compound 8a in N2a mouse neuroblastoma cells (4 h incubation with the inducer ± ascorbate). VEGF and HO-1 were used as HIF-1a and target genes, NQO-1, an Nrf2 target gene, was used as a negative control. Values are expressed as a fold change from control reaction and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or β-actin expression. Mean ± SEM is plotted as bar graph.(*p < .05 vs Vehicle control; #p < .05 vs Compound 8a 2 μM; $p,0.05 vs Compound 8a 5 μM; @p < .05 vs L-Asc (100 μM); !p < .05 vs D-Asc (100 μM); n = 3 per group).
Compound 8a is a potent HIF PHD inhibitor and displays a concentration-dependent activation of two major HIF1 target genes – VEGF and HO-1 (Fig. 3) at 4 h incubation, which makes the induction of this gene specific for HIF activation, whereas non-specific activation requires much longer time (for example, 16 h minimum are required for VEGF activation via PCG1α pathway [38]). Compound 8a is active even at 2 μM and 4 h incubation, which cannot be achieved with other known HIF PHD inhibitors. This activation originates from HIF1α stabilization and is not due to additional expression, since the level of HIF1α mRNA is unchanged (Fig. 3). The activation observed is specific for HIF1 targets: the well-known target of Nrf2 – NQO1 gene - shows no increased expression. Co-treatment with 100 μM L-Asc (the upper level of L-Asc in blood without additional supplementation) or D-Asc results in the inhibition of gene activation. The effect is more pronounced for L-Asc (20% inhibition for VEGF and 25% inhibition for HO-1) than for D-Asc (inhibition under 10%). Co-treatment of 5 μM Compound 8a with 100 μM L-Asc has almost the same effect on HIF1 target gene expression as lowering the activator concentration to 2 μM. Hence, the results obtained by RT-PCR prove the existence of a specific suppression by L-Asc, rather than D-Asc. This effect is less pronounced than that observed in the reporter assay (Fig. 1). We rule out the additional L-Asc effects on transcription of HIF1 target genes, since there are no changes in the presence of L-Asc in their expression within the experimental error (Fig. 3). HIF stabilization is the first step towards the activation of the transcriptional program that is dependent on some additional HIF modifications affecting its translocation to the nucleus. So, the less pronounced suppressing effects could reflect the difference between the integral nature of transcriptional response compared to HIF stabilization step monitored by the reporter the assay.
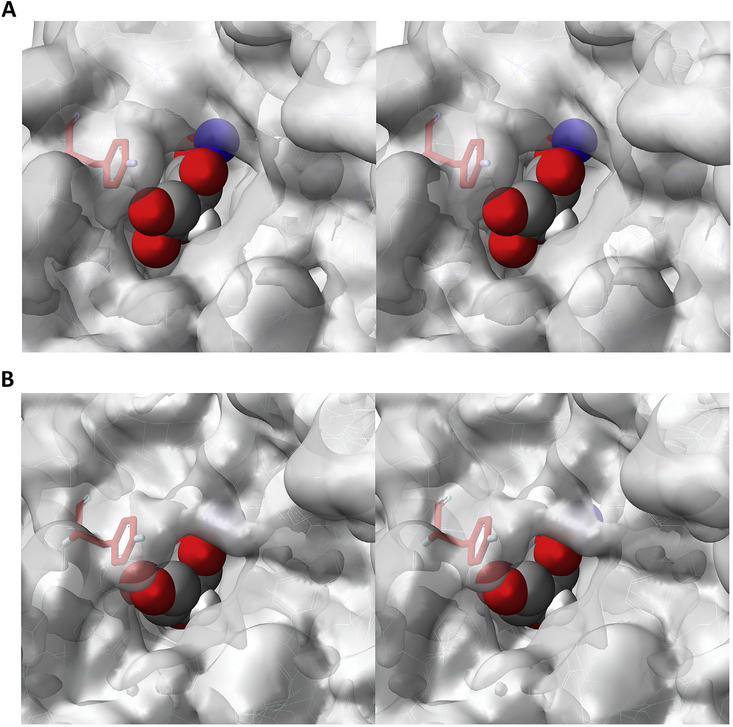
3.4. Docking
As shown in Fig. 4A, L-ascorbic acid can be successfully docked in the binding site of αKG, which is occupied by an inhibitor in the resolved crystal structure of HIF PHD2. In contrast, the attempts to dock its D-isomer, D-Asc, in the same binding site, were unsuccessful. As shown in Fig. 4B, there is a significant interference of Tyr310 in the enzyme active site with respect to the chiral hydroxyl-group distinguishing L- and D-conformations of ascorbic acid. Hence, L-Asc but not D-Asc, may occupy the αKG binding site and chelate the active center iron. The docking results are consistent with the experimentally observed stereospecificity of the observed suppressing effect, supporting a model for L-Asc binding to the HIF PHD active center.
Fig. 4.
Computer modeling of plausible L-Asc (A) and D-Asc (B) accommodation inside the active center of PHD2 (2G19.pdb). Tyr310 is shown in red. Iron atom in blue. Details in Materials and Methods.
4. Discussion
The data obtained suggest a competing nature of L-Asc for the mechanism of action of HIF PHD. The evidence for such conclusion is as follows: (1) L-Asc suppressing effect on reporter activation in the presence of HIF PHD inhibitors depends on the chemical nature of the inhibitor used; (2) cell treatment with cell-permeable reducing agent, NAC, (up to millimolar concentrations) has no effect of HIF1 ODD-luc reporter stabilization by HIF PHD inhibitors of any nature; (3) L-Asc suppressing effect on reporter activation shows a saturation behavior, indicative of the Michaelis-Menten type interaction; (4) D-Asc exerts a minor effect, if any, on reporter activation by various HIF PHD inhibitors. Exogeneous application of L-Asc is known to penetrate the cell preferentially via specific transporters [39,40] and reach the same intracellular concentration as the applied one [41]. In the absence of specific transporters for D-Asc, one may expect its weaker effect, but not the complete absence of such effect in the reporter assay. In addition, L-Asc, but not D-Asc, can be successfully docked in the αKG binding site of HIF PHD2.
Our observations and conclusions are in agreement with the recent research on other important enzymes of αKG Fe-dioxygenase group such as TET enzymes and jumonji histone demethylases. Both enzymes regulate the epigenome and their activity is directly linked to L-Asc availability [42].
TET enzyme catalyzes oxidative demethylation of cytosine in three consecutive steps, 5-methylcytosine (5 mC) into 5-hydroxymethylcytosine (5hmC), further to 5-formylcytosine (5 fC) and 5-carboxylcytosine (5caC), which are ultimately replaced by unmodified cytosine. The global loss of 5hmC changes the DNA methylation-demethylation dynamics and genome-wide gene expression, which eventually leads to malignant transformation. L-Asc plays the key role in the observed effects. In particular, genetic variation in L-Asc transporters (SVCT1 and SVCT2) is associated with the risk of certain types of cancer. Cosmics, a cancer somatic mutation database (http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/), shows SVCTs mutations identified in breast cancer, colorectal adenoma, brain tumors and other cancers.
The evidence for L-Asc working as a specific cofactor of TET, and not a general reducing agent, was obtained in a variety of experiments: (a) Cell treatment with reducers such as glutathione (GSH) do not change the level of 5hmC [43]; L-Asc directly enhances the activity of purified C-terminal catalytic domain of TET2 to oxidize 5 mC to 5hmC and 5 fC, while the reducers such as spermidine, vitamin B1, vitamin E, glutathione, NADPH, and L-cysteine do not [44]; (b) Iron removal from the culture medium did not affect the induction of 5hmC by L-Asc, hence, the effect of L-Asc on 5hmC generation is independent of cellular uptake of iron [43]; (c) L-Asc treatment of the cells cultured with different concentrations of glucose, a major precursor of αKG, exhibited similar levels of 5hmC generation, which is indirect proof of L-Asc acting as a true substrate, alternate to αKG [43]. These published observations for TET enzyme are consistent with those obtained in this study, that L-Asc is likely a true substrate and not just a reducing agent.
An open question is whether L-Asc can replace αKG as a PHD substrate completely. Degeneration of mitochondria in cancer cells may compromise the levels of available αKG, and in such scenario, one may speculate that L-Asc could play the role of αKG: L-Asc is known to reduce oxygen into hydrogen peroxide in the presence of trace metals such as iron, hence, the mechanism of HIF hydroxylation in the presence of L-Asc may first include generation of hydrogen peroxide from iron-bound oxygen right inside the HIF PHD2 active center, and then disproportionation of iron-bound hydrogen peroxide yielding oxoferryl and water. If such process occurs in reality, it also means that hydrogen peroxide itself can replace oxygen in the catalytic cycle of prolyl hydroxylase when catalytic iron is coordinated by any bi-ligand agent fitting into the αKG binding site. An indirect proof for such speculation can be found in the recent paper [45] on collagen 4-prolyl hydroxylase, where two enzyme inhibitors providing bi-ligand coordination of the active center iron were acting as enzyme activators in the presence of L-Asc.
5. Conclusions
The increasing interest in the L-Asc co-treatment of cancer dictates the need for re-examining the role of L-Asc in the catalytic cycle of many enzymes and in particular those directly relevant to cancer susceptibility and progression. These include the group of non-heme iron αKG dioxygenases such as HIF PHD and important epigenetic enzymes, as well as may others among this 60-member group in human genome which functions are still barely known.
Supplementary Material
Acknowledgement
The work was supported by Russian Science Foundation (grant No. 16–14-10226) and in part (RT-PCR experiments) by funding from Parkinson Support Group and NS101967 grant (BT).
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.biochi.2017.12.011.
References
- [1].Chopra RK, Ananthanarayanan VS, Conformational implications of enzymatic proline hydroxylation in collagen, Proc. Natl. Acad. Sci. U. S. A 79 (1982) 7180–7184, 10.1073/pnas.79.23.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bulleid NJ, Wilson R, Lees JF, Type-III procollagen assembly in semi-intact cells: chain association, nucleation and triple-helix folding do not require formation of inter-chain disulphide bonds but triple-helix nucleation does require hydroxylation, Biochem. J 317 (1996) 195–202. Pt 1, http://www.biochemj.org/bj/317/0195/bj3170195.htm%5Cnpapers3://publication/uuid/8525022E-F7C7-48E9-B8EE-6EB578C855F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bruick RK, McKnight SL, A conserved family of prolyl-4-hydroxylases that modify HIF, Science 294 (2001) 1337–1340, 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- [4].Kuiper C, Dachs GU, Currie MJ, Vissers MCM, Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response, Free Radic. Biol. Med 69 (2014) 308–317, 10.1016/j.freeradbiomed.2014.01.033. [DOI] [PubMed] [Google Scholar]
- [5].Cameron E, Pauling L, Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer, Proc. Natl. Acad. Sci. U. S. A 75 (1978) 4538–4542, 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cameron E, Pauling L, Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer, Proc. Natl. Acad. Sci. U. S. A 75 (1978) 4538–4542, 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moertel CG, Fleming TR, Creagan ET, Rubin J, O’Connell MJ, Ames MM, High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy. A randomized double-blind comparison, N. Engl. J. Med 312 (1985) 137–141, 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- [8].Carosio R, Zuccari G, Orienti I, Mangraviti S, Montaldo PG, Sodium ascorbate induces apoptosis in neuroblastoma cell lines by interfering with iron uptake, Mol. Canc 6 (2007) 55, 10.1186/1476-4598-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M, Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 13604–13609, 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M, Vitamin C pharmacokinetics: implications for oral and intravenous use, Ann. Intern. Med 140 (2004) 533–537, 10.1111/j.1445-5994.1980.tb03725.x. [DOI] [PubMed] [Google Scholar]
- [11].Duconge J, Miranda-Massari JR, Gonzalez MJ, Jackson JA, Warnock W, Riordan NH, Pharmacokinetics of vitamin C: insights into the oral and intravenous administration of ascorbate, P. R. Health Sci. J 27 (2008) 7–19. http://www.ncbi.nlm.nih.gov/pubmed/18450228. [PubMed] [Google Scholar]
- [12].Chen C, Sun J, Liu G, Chen J, Effect of small interference RNA targeting HIF-1alpha mediated by rAAV combined L: -ascorbate on pancreatic tumors in athymic mice, Pathol. Oncol. Res 15 (2009) 109–114, 10.1007/s12253-008-9063-7. [DOI] [PubMed] [Google Scholar]
- [13].Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, Lee LA, Semenza GL, V Dang C, HIF-dependent antitumorigenic effect of antioxidants in vivo, Canc. Cell 12 (2007) 230–238, 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vollbracht C, Schneider B, Leendert V, Weiss G, Auerbach L, Beuth J, Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany, In Vivo 25 (2011) 983–990. http://www.ncbi.nlm.nih.gov/pubmed/22021693. [PubMed] [Google Scholar]
- [15].Yeom CH, Jung GC, Song KJ, Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration, J. Kor. Med. Sci 22 (2007) 7–11, 10.3346/jkms.2007.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ma Y, Chapman J, Levine M, Polireddy K, Drisko J, Chen Q, High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy, Sci. Transl. Med 6 (2014), 10.1126/scitranslmed.3007154, 222ra18. [DOI] [PubMed] [Google Scholar]
- [17].Kuiper C, Vissers MCM, Ascorbate as a co-factor for fe- and 2-oxoglutarate dependent dioxygenases: physiological activity in tumor growth and progression, Front. Oncol 4 (2014) 359, 10.3389/fonc.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Price JC, Barr EW, Tirupati B, Bollinger JM, Krebs C, The first direct characterization of a high-valent iron intermediate in the reaction of an alpha-ketoglutarate-dependent dioxygenase: a high-spin FeIV complex in taurine/alpha-ketoglutarate dioxygenase (TauD) from Escherichia coli, Biochemistry 42 (2003) 7497–7508, 10.1021/bi030011f. [DOI] [PubMed] [Google Scholar]
- [19].Hoffart LM, Barr EW, Guyer RB, Bollinger JM, Krebs C, Direct spectroscopic detection of a C-H-cleaving high-spin Fe(IV) complex in a prolyl-4-hydroxylase, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 14738–14743, 10.1073/pnas.0604005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tuckerman JR, Zhao Y, Hewitson KS, Tian Y-M, Pugh CW, Ratcliffe PJ, Mole DR, Determination and comparison of specific activity of the HIF-prolyl hydroxylases, FE BS Lett. 576 (2004) 145–150, 10.1016/j.febslet.2004.09.005. [DOI] [PubMed] [Google Scholar]
- [21].Dao JH, Kurzeja RJM, Morachis JM, Veith H, Lewis J, Yu V, Tegley CM, Tagari P, Kinetic characterization and identification of a novel inhibitor of hypoxia-inducible factor prolyl hydroxylase 2 using a time-resolved fluorescence resonance energy transfer-based assay technology, Anal. Biochem 384 (2009) 213–223, 10.1016/j.ab.2008.09.052. [DOI] [PubMed] [Google Scholar]
- [22].Myllylä R, Majamaa K, Günzler V, Hanauske-Abel HM, Kivirikko KI, Ascorbate is consumed stoichiometrically in the uncoupled reactions catalyzed by prolyl 4-hydroxylase and lysyl hydroxylase, J. Biol. Chem 259 (1984) 5403–5405. http://www.ncbi.nlm.nih.gov/pubmed/6325436. [PubMed] [Google Scholar]
- [23].Flashman E, Davies SL, Yeoh KK, Schofield CJ, Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents, Biochem. J 427 (2010) 135–142, 10.1042/BJ20091609. [DOI] [PubMed] [Google Scholar]
- [24].Smirnova NA, Rakhman I, Moroz N, Basso M, Payappilly J, Kazakov S, Hernandez-Guzman F, Gaisina IN, Kozikowski AP, Ratan RR, Gazaryan IG, Utilization of an in vivo reporter for high throughput identification of branched small molecule regulators of hypoxic adaptation, Chem. Biol 17 (2010) 380–391, 10.1016/j.chembiol.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Karuppagounder SS, Basso M, Sleiman SF, Ma TC, Speer RE, Smirnova NA, Gazaryan IG, Ratan RR, In vitro ischemia suppresses hypoxic induction of hypoxia-inducible factor-1α by inhibition of synthesis and not enhanced degradation, J. Neurosci. Res 91 (2013) 1066–1075, 10.1002/jnr.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods 25 (2001) 402–408, 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [27].Brooks BR, Brooks CL, Mackerell AD, Nilsson L, Petrella RJ, Roux B, Won Y, Archontis G, Bartels C, Boresch S, Caflisch A, Caves L, Cui Q, Dinner AR, Feig M, Fischer S, Gao J, Hodoscek M, Im W, Kuczera K, Lazaridis T, Ma J, Ovchinnikov V, Paci E, Pastor RW, Post CB, Pu JZ, Schaefer M, Tidor B, Venable RM, Woodcock HL, Wu X, Yang W, York DM, Karplus M, CHARMM: the biomolecular simulation program, J. Comput. Chem 30 (2009) 1545–1614, 10.1002/jcc.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Majamaa K, Günzler V, Hanauske-Abel HM, Myllylä R, Kivirikko KI, Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase, J. Biol. Chem 261 (1986) 7819–7823. http://www.ncbi.nlm.nih.gov/pubmed/3011801. [PubMed] [Google Scholar]
- [29].Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, Lee LA, Semenza GL, Dang CV, HIF-dependent antitumorigenic effect of antioxidants in vivo, Canc. Cell 12 (2007) 230–238, 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang J, Buss JL, Chen G, Ponka P, Pantopoulos K, The prolyl 4-hydroxylase inhibitor ethyl-3,4-dihydroxybenzoate generates effective iron deficiency in cultured cells, FEBS Lett. 529 (2002) 309–312. http://www.ncbi.nlm.nih.gov/pubmed/12372619. [DOI] [PubMed] [Google Scholar]
- [31].Karuppagounder SS, Alim I, Khim SJ, Bourassa MW, Sleiman SF, John R, Thinnes CC, Yeh T-L, Demetriades M, Neitemeier S, Cruz D, Gazaryan I, Killilea DW, Morgenstern L, Xi G, Keep RF, Schallert T, Tappero RV, Zhong J, Cho S, Maxfield FR, Holman TR, Culmsee C, Fong G-H, Su Y, Ming G, Song H, Cave JW, Schofield CJ, Colbourne F, Coppola G, Ratan RR, Therapeutic targeting of oxygen-sensing prolyl hydroxylases abrogates ATF4-dependent neuronal death and improves outcomes after brain hemorrhage in several rodent models, Sci. Transl. Med 8 (2016), 10.1126/scitranslmed.aac6008, 328ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhdanov AV, Okkelman IA, Collins FWJ, Melgar S, Papkovsky DB, A novel effect of DMOG on cell metabolism: direct inhibition of mitochondrial function precedes HIF target gene expression, Biochim. Biophys. Acta 1847 (2015) 1254–1266, 10.1016/j.bbabio.2015.06.016. [DOI] [PubMed] [Google Scholar]
- [33].Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A, Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1, J. Biol. Chem 280 (2005) 41928–41939, 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- [34].Thirstrup K, Christensen S, Møller HA, Ritzén A, Bergström A-L, Sager TN, Jensen HS, Endogenous 2-oxoglutarate levels impact potencies of competitive HIF prolyl hydroxylase inhibitors, Pharmacol. Res 64 (2011) 268–273, 10.1016/j.phrs.2011.03.017. [DOI] [PubMed] [Google Scholar]
- [35].Knowles HJ, Raval RR, Harris AL, Ratcliffe PJ, Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells, Canc. Res 63 (2003) 1764–1768. http://www.ncbi.nlm.nih.gov/pubmed/12702559. [PubMed] [Google Scholar]
- [36].Flight MH, Deal watch: AstraZeneca bets on FibroGen’s anaemia drug, Nat. Rev. Drug Discov 12 (2013) 730, 10.1038/nrd4135. [DOI] [PubMed] [Google Scholar]
- [37].Murray JK, Balan C, Allgeier AM, Kasparian A, Viswanadhan V, Wilde C, Allen JR, Yoder SC, Biddlecome G, Hungate RW, Miranda LP, Dipeptidyl-quinolone derivatives inhibit hypoxia inducible factor-1α prolyl hydroxylases-1, −2, and −3 with altered selectivity, J. Comb. Chem 12 (2010) 676–686, 10.1021/cc100073a. [DOI] [PubMed] [Google Scholar]
- [38].Arany Z, Foo S-Y, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM, HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha, Nature 451 (2008) 1008–1012, 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- [39].Clark AG, Rohrbaugh AL, Otterness I, Kraus VB, The effects of ascorbic acid on cartilage metabolism in Guinea pig articular cartilage explants, Matrix Biol. 21 (2002) 175–184, 10.1016/S0945-053X(01)00193-7. [DOI] [PubMed] [Google Scholar]
- [40].Rivas CI, Zúñiga FA, Salas-Burgos A, Mardones L, Ormazabal V, Vera JC, Vitamin C transporters, J. Physiol. Biochem 64 (2008) 357–375, 10.1007/BF03174092. [DOI] [PubMed] [Google Scholar]
- [41].Kuiper C, Vissers MCM, Hicks KO, Pharmacokinetic modeling of ascorbate diffusion through normal and tumor tissue, Free Radic. Biol. Med 77 (2014) 340–352, 10.1016/j.freeradbiomed.2014.09.023. [DOI] [PubMed] [Google Scholar]
- [42].Young JI, Züchner S, Wang G, Regulation of the epigenome by vitamin C, Annu. Rev. Nutr 35 (2015) 545–564, 10.1146/annurev-nutr-071714-034228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Minor EA, Court BL, Young JI, Wang G, Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine, J. Biol. Chem 288 (2013) 13669–13674, 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yin R, Mao S-Q, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, Jiao Y, Li C, Liu S, Wu D, Gu W, Yang Y-G, Xu G-L, Wang H, Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals, J. Am. Chem. Soc 135 (2013) 10396–10403, 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- [45].Vasta JD, Raines RT, Human collagen prolyl 4-hydroxylase is activated by ligands for its iron center, Biochemistry 55 (2016) 3224–3233, 10.1021/acs.biochem.6b00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.