Abstract
Background:
Disease presentation and outcomes differ by race in a number of malignancies, but data in adult acute myeloid leukemia (AML) are limited.
Materials and Methods:
We conducted a retrospective analysis of pretreatment characteristics, referral and treatment patterns, and outcomes in 548 AML patients evaluated at the University of Maryland Greenebaum Cancer Center, a tertiary care referral center in Baltimore, MD, from 2000 through 2009. Cases were analyzed for time from diagnosis to referral, age, race, gender, socioeconomic status, antecedent hematologic disorder, cytotoxic or radiation therapy for prior malignancy, karyotype, FLT3 mutations, intensive chemotherapy, clinical trial participation, hematopoietic stem cell transplantation (HSCT) and overall survival (OS).
Results:
Black patients (n=105) were younger than white patients (n=396) (54 vs. 61 years, p<0.001), were more commonly female (55% vs. 45%, p<0.001), and had a lower estimated median household income ($42,677 vs. $53,534 per year, p<0.001). Black patients more frequently had complex karyotypes (26% vs. 12%, p=0.002) and less frequently normal karyotypes (27% vs. 42%, p=0.02). FLT3 mutation frequency was similar. Time to referral and proportion of patients receiving intensive chemotherapy did not differ, but both clinical trial participation (43% vs. 54%, p = 0.04) and HSCT (17% vs. 35% for patients <= 70 years old, p=0.001) were less frequent in blacks than whites. Nevertheless, OS was similar in all black and white patients (median 15 vs. 14 months, p=0.23), and when stratified by age, gender and karyotype risk classification.
Conclusion:
AML presentation and treatment differed in black and white patients, but OS was similar. Black patients appear to have barriers to clinical trial participation and HSCT, and there may be barriers to tertiary care referral for black males.
Keywords: Acute myeloid leukemia, Race, Racial Disparities
Introduction
Acute myeloid leukemia (AML) is characterized by maturation arrest of a malignant clone of myeloid cells, with inhibition of normal hematopoiesis. AML is the common type of acute leukemia affecting adults, and its incidence increases with age. The age-adjusted incidence rate of AML in the United States is 3.5 per 100,000 people per year. AML is more common in males, with a male to female ratio of 1.48, and is also more common in whites, with a white to black ratio of 1.2 [1].
Racial differences in clinical characteristics and outcome have been identified in a number of cancers, with blacks often having shorter overall survival (OS) than whites. This relationship has been identified in head and neck [2–4], skin [5], esophageal [6], colon [7], prostate [8–10], ovarian [10] and breast cancers [10–11]. The observed differences between populations are likely multifactorial, reflecting differences in access to care due to socioeconomic disparities, environmental exposures, cultural differences, tumor biology, and genetic mutations or polymorphisms influencing response to and/or toxicity of chemotherapeutic agents [12]. Socioeconomic disparities are generally thought to be the most important of these determinants and indeed many observed racial disparities are at least mitigated when these factors are taken into account.
Racial differences in disease presentation and treatment outcome have not been extensively studied in adult AML. Karyotype is the most significant predictor of outcome in AML, but only limited information is available on the distribution of AML karyotypes across races [13]. Additionally, molecular abnormalities with prognostic significance, most notably mutations of fms-like tyrosine kinase receptor-3 (FLT3), have been identified as having strong prognostic significance in AML [14], but there is little information available about their incidence in different races. Finally, data on outcomes by race are limited and inconsistent [10,13,15–16].
To assess racial differences in pretreatment characteristics, referral and treatment patterns and outcome in adult AML, we studied patients evaluated and treated from 2000 through 2009 at the University of Maryland Greenebaum Cancer Center (UMGCC) in Baltimore, Maryland, a tertiary care referral center with a relatively high proportion of minority patients.
Design and Methods
Study Population
We conducted a retrospective study of all patients with a diagnosis of AML evaluated at UMGCC from January 1, 2000 through December 31, 2009. This study was approved by the University of Maryland School of Medicine Institutional Review Board.
Data Collection
Data were obtained from retrospective electronic and paper chart review. Information collected included diagnosis date, date of referral to UMGCC, self-reported race, gender, date of birth, zip code of residence, presence or absence of an antecedent hematologic disorder (AHD) or chemotherapy and/or radiation therapy for a prior malignancy defining therapy-related AML (t-AML), karyotype at diagnosis, FLT3 mutation status at diagnosis recorded as wild-type (WT), internal tandem duplication (ITD) or D835 point mutation, whether intensive chemotherapy was administered, whether hematopoietic stem cell transplantation (HSCT) was performed, clinical trial participation at UMGCC at any point during AML care, date of last follow-up, disease status at last follow-up, and date of death. For patients without recent follow-up data or a recorded date of death in UMGCC records, a search of the Social Security Death Index was performed. Cytogenetic analysis was generally performed at our institution, but for patients not initially evaluated at UMGCC, cytogenetic results from another institution or laboratory at the time of diagnosis were used if available. Karyotype was classified as favorable, unfavorable or intermediate risk by Southwest Oncology Group (SWOG) criteria [17]. FLT3 testing was performed in several Clinical Laboratory Improvement Amendments (CLIA)-approved laboratories during approximately the last four years of the study period. Individual socioeconomic data were not collected, but were estimated using 1999 United States median household income census data based on the patient’s zip code.
Statistical Analysis
Univariate and bivariate analyses were used to describe the patient population and compare across subgroups defined by race. Significance was tested using the chi-square test, except for age distribution, which was tested using the student two-tailed t-test assuming unequal variances. Survival was analyzed by the method of Kaplan and Meier using GraphPad Prism software (La Jolla, CA), with equality of survivor functions assessed by the log-rank test. Statistical significance for all tests was defined as p <= 0.05. Multivariable analysis was performed using Cox proportional hazard regression with Stata SE 11.1 (StataCorp LP, College Station, TX).
Results
A total of 548 patients with a diagnosis of AML were identified, of whom 399 (73%) were white, 105 (19%) black, 22 (4%) Hispanic and 22 (4%) Asian. Karyotype data were available for 510 (94%) and FLT3 data for 177 (32%), with availability of karyotype and FLT3 data similar for all races. As the vast majority of our population was white or black, comparisons were limited to differences between these two groups.
Time from AML diagnosis to referral to UMGCC did not differ in blacks and whites (p=0.83), with the median time to referral of both groups 0 days, indicating that the majority of patients were referred prior to an established diagnosis. 19% of blacks and 22% of whites were referred more than 4 weeks after their initial AML diagnosis (p = 0.49). Of the patients referred more than 4 weeks after their initial diagnosis 75% of whites and 73% of blacks had received prior chemotherapy (p=0.41).
Patient and leukemia pretreatment characteristics are summarized in Table 1. Blacks presented at a significantly younger age than whites (54.4 vs. 60.8 years, p<0.001) and were more frequently female (55% vs. 47%, p<0.001). Complex karyotypes were markedly more common in blacks than in whites (26% vs. 12%; p=0.001), but the prevalence of both AHD and t-AML, in which complex karyotypes may be more common, was similar (26% vs. 28%, p=0.72). Additionally, normal karyotypes were less common in blacks than in whites (27% vs. 42%, p=0.02). The incidence of FLT3 mutations did not differ between blacks and whites, whether considering all patients or only those with normal or intermediate-risk karyotypes.
Table 1.
Pretreatment characteristics of white and black AML patients [numbers (%)]
| White N=399 | Black N=105 | p-value | ||
|---|---|---|---|---|
| Age | Median (years) | 61 | 54 | <0.001 |
| Sex | Male | 211 (53%) | 47 (45%) | <0.001 |
| Female | 188 (47%) | 58 (55%) | ||
| Onset | de novo | 278 (70%) | 76 (72%) | 0.11 |
| Secondary | 114 (29%) | 25 (24%) | 0.20 | |
| AHD | 74 (19%) | 13 (12%) | 0.11 | |
| t-AML | 40 (10%) | 12 (11%) | 0.47 | |
| Karyotype | t(15;17) | 24 (6%) | 9 (9%) | 0.15 |
| Other favorable | 23 (6%) | 9 (9%) | 0.31 | |
| t(8;21) | 7 (2%) | 5 (5%) | 0.08 | |
| inv(16), t(16;16) | 16 (4%) | 4 (4%) | 0.91 | |
| Intermediate | 210 (53%) | 41 (39%) | 0.02 | |
| Normal | 167 (42%) | 28 (27%) | 0.02 | |
| Unfavorable | 113 (29%) | 38 (36%) | 0.17 | |
| Complex | 49 (12%) | 27 (26%) | 0.001 | |
| Missing | 29 (7%) | 9 (8%) | 0.54 | |
| FLT3* | Wild-type | 45 | 6 | 0.58 |
| ITD | 27 | 6 | 0.54 | |
| D835 | 5 | 1 | 0.88 | |
| Missing | 138 | 13 | 0.75 | |
intermediate-risk karyotypes
AHD = antecedent hematologic disorder
t-AML= therapy-related AML
ITD= internal tandem duplication
Median household income, as estimated by patient’s zip code, was $42,667/year for blacks and $53,534/year for whites (p<0.001).
Intensive induction chemotherapy was delivered with similar frequency to black and white patients (79% of blacks and 75% of whites; p=0.19). Clinical trial participation was relatively high for both groups, but signficantly lower in blacks than in whites (43% vs. 54%, p = 0.04).
We also assessed the frequency of HSCT in the AML population (Table 2). With analysis limited to patients 70 years old or younger, a lower percentage of black patients underwent HSCT in the group as a whole (17% vs. 35%, p=0.001), and there was also a borderline lower frequency of HSCT in those with unfavorable karyotypes (19% vs. 39%, p=0.05). The types of transplants performed were similar in both races. Charts were reviewed to determine reasons for which HSCT was not considered or performed in black patients with intermediate- or high-risk karyotypes. Reasons included age (27%), refractory disease (21%), cormorbities or poor performance status (16%), death (9%), lack of available donor (6%), drug use or incarceration (6%), and offered but declined (3%). The remaining patients (12%) had either incomplete records or no clear documented reason for not pursuing HSCT.
Table 2.
Hematopoietic stem cell transplantation by race, age ≤70 years
| White | Black | p-value | ||
|---|---|---|---|---|
| Tranplants | All | 96 (35%) | 15 (17%) | 0.001 |
| Unfavorable karyotypes | 29 (39%) | 5 (19%) | 0.05 | |
| Transplant type | Allogeneic related donor | 58 (60%) | 8 (53%) | 0.30 |
| Allogeneic unrelated donor | 30 (31%) | 4 (27%) | 0.43 | |
| Autologous | 7 (7%) | 2 (13%) | 0.97 | |
| Syngeneic | 0 (0%) | 1 (6%) | * | |
| Haploidentical | 1 (1%) | 0 (0%) | * |
not calculated due to rarity
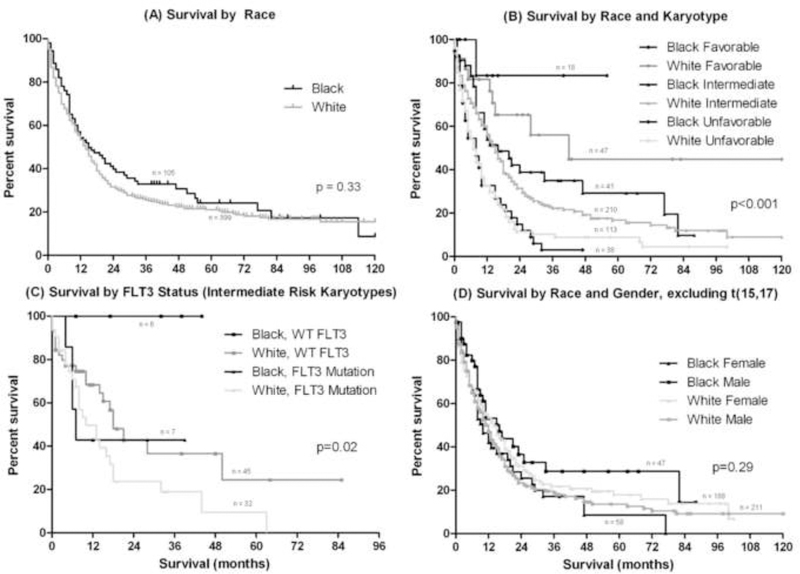
Median OS did not differ between black and white patients (15 vs. 14 months, p=0.23, Figure 1A, Table 3). As expected, OS differed significantly between low-, intermediate- and high-risk cytogenetic groups and by age at diagnosis. There was no difference in median OS between black and white patients within favorable, intermediate, and unfavorable risk groups (Figure 1B, Table 3). Similarly, there were no differences in median OS between black and white patients in the age groups under 40, 40 to 59 and 60 years old and above.
Figure 1 –
OS in (A) blacks vs. whites, (B) blacks vs. whites stratified by karyotype; comparisons of black and white patients within individual cytogenetic groups were not statistically significant and are detailed in Table 3, (C) blacks vs. whites with intermediate-risk karyotypes stratified by FLT3 status; FLT3 wild-type (WT) was a significant predictor of longer OS in black patients (p=0.03), but was of only borderline significance in whites (p=0.07), and (D) blacks vs. whites stratified by race and gender.
Table 3.
Overall survival (months) by karyotype
| White | Black | ||||
|---|---|---|---|---|---|
| n (%) | median OS | n (%) | median OS | p-value | |
| All patients | 370 | 14 | 97 | 15 | 0.33 |
| t(15;17) | 24 (6) | * | 9 (9) | 55 | 0.57 |
| Other favorable | 23 (6) | 42 | 9 (9) | * | 0.31 |
| Normal | 167 (45) | 15 | 28 (29) | 12 | 0.56 |
| All intermediate | 210 (57) | 15 | 41 (42) | 13 | 0.46 |
| Wild-type FLT3 | 45 | 18 | 6 | * | 0.04 |
| FLT3 mutation | 32 | 10 | 7 | 7 | 0.68 |
| Complex | 49 (13) | 7 | 27 (28) | 8 | 0.48 |
| All unfavorable | 113 (31) | 7 | 38 (39) | 8 | 0.81 |
not reached
Based on prior reported differences [13], additional analysis was performed on the favorable cytogenetic risk group. There was a trend toward more frequent t(8;21) in blacks compared to whites (5% vs. 2%, p=0.09), while the frequency of inv(16)/t(16;16) did not differ. Median OS for black patients with favorable karyotypes was not reached, compared to 42 months for white patients (p=0.31, Table 3).
When considering only patients with complex karyotypes, black and white patients had a similar age at presentation (58.3 vs. 61.4 years; p = 0.20), but the proportion of women remained significantly higher in blacks than in whites (62% vs. 41%, p<0.001). The incidence of AHD (15% vs. 16%) and t-AML (22% for both) was similar between black and white patients with complex karyotypes. Median OS was similarly short in black and white patients with complex karyotypes (8 vs. 7 months; p=0.48, Table 3).
When compared to patients with either FLT3-ITD or D835 mutations, FLT3 WT status was a significant predictor of longer OS in black patients with intermediate-risk karyotypes (not reached vs. 7 months; p=0.03), but there was only a trend toward significance in whites (18 vs. 13 months, p=0.07) (Figure 1C).
We also studied the interaction between race and gender in black and white patients (Figure 1D). While females did not differ in median age or OS by race, there was a much higher incidence of complex karyotypes in black females (30% vs. 12%, p=0.003), as well as a trend toward more total unfavorable karyotypes (45% vs. 28%, p=0.06) and a lower incidence of normal karyotypes (45% vs. 26%, p=0.04). Black males had significantly longer median OS compared to white males (21 months vs. 12 months p=0.02), but also had a younger median age (53 vs. 63 years, p<0.001) and a trend toward a higher incidence of t(15;17) (13 vs. 6%, p=0.09). When patients with t(15;17) were excluded, there was only a trend toward longer OS (16 vs. 12 months, p=0.08). Findings in black and white males were otherwise similar, including incidence of complex and of normal karyotypes. The trend toward longer OS for black males is likely due to the younger median age at presentation, since when black and white males within age groups were compared; there was no difference in OS.
For the entire population of patients age 70 years old or less and excluding those with t(15;17), undergoing HSCT was associated with longer median OS in unadjusted analysis (13 to 21 months, p = 0.007). The difference was particularly pronounced for blacks undergoing HSCT (n=15), with median OS of 77 vs. 12 months (p = 0.02), while median OS differed less in whites undergoing HSCT (n=93) (19 vs. 14 months; p = 0.05).
In a multivariable analysis using a Cox proportional hazard model, less favorable karyotypes, older age, secondary leukemia (AHD or t-AML), and FLT3 mutations were independent predictors of shorter OS, while HSCT and intensive induction chemotherapy were associated with longer OS. In contrast, race, gender, household income below the 25th percentile of our population (less than $41,375/year), FLT3 mutation, clinical trial participation, and referral delay of more than one month were not significant independent predictors (Table 4).
Table 4.
Multivariable analysis for risk of death
| HR | p-value | 95% CI | |
|---|---|---|---|
| Patient Characteristics | |||
| Black race (vs. white race) | 1.00 | 0.99 | 0.75–1.33 |
| Male gender (vs. female gender) | 1.00 | 0.97 | 0.80–1.23 |
| Age ≥ 60 years old (vs. <60 years old) | 1.65 | <0.001 | 1.29–2.11 |
| Income <25th percentile (vs. ≥25th percentile) | 1.03 | 0.834 | 0.80–1.32 |
| Disease Characteristics | |||
| Unfavorable karyotype risk group (vs. favorable risk) | 1.63 | <0.001 | 1.40–1.89 |
| FLT3 mutation (vs. wild-type FLT3) | 1.08 | 0.100 | 0.98–1.20 |
| Secondary AML (vs. de novo AML) | 1.40 | 0.01 | 1.07–1.82 |
| Treatment Characteristics | |||
| Intensive induction therapy (vs. non-intensive therapy) | 0.79 | 0.05 | 0.62–1.00 |
| Hematopoietic stem cell transplant (vs. no transplant) | 0.71 | 0.03 | 0.53–0.97 |
| Clinical trial (vs. no clinical trial) | 0.86 | 0.17 | 0.70–1.06 |
| >1-month delay in referral to UMGCC (vs. ≤1-month delay) | 0.96 | 0.77 | 0.77–1.21 |
UMGCC = University of Maryland Greenebaum Cancer Center
Discussion
In this study in a single institution with a 19% black AML population, we found that black adult AML patients were younger, were more commonly female and had a higher incidence of complex karyotypes as well as a lower incidence of normal karyotypes, but had similar survival when compared to whites. FLT3 mutations were equally frequent in black and white patients, but were a stronger predictor of OS in blacks than in whites with intermediate-risk karyotypes. To our knowledge, our study represents the largest single-institution series of black patients with AML and the second largest series reported to date.
Our results suggest some disparities in care between black and white patients. While time to referral did not differ and patients received intensive induction chemotherapy at equal rates, there was significantly less participation in clinical trials by black patients, and significantly fewer black patients received HSCT as compared to whites. Our study was not able to explain why these disparities exist, but we speculate that reasons may include socioeconomic factors, cultural issues, donor availability, comorbid health conditions, differences in performance status, and physician bias. The types of transplants performed were similar, suggesting that availability of unrelated donors for minorities may not have been a strong determinant, and our review of documented reasons for not proceeding to HSCT in black patients with intermediate- and high-risk karyotypes supports this. It was notable that blacks who did undergo HSCT had a particularly good OS, which is in contrast to some studies suggesting a higher level of transplant-related mortality in blacks due to an increased incidence of acute graft-versus-host disease [18–19].
Black and white AML patients have been compared in four previous studies. A study of 270 black and 2300 white patients with de novo AML enrolled on seven CALGB clinical trials described a higher incidence of both favorable- and unfavorable-risk karyotypes as well as a lower incidence of normal karyotypes in black patients, and short survival in black males in both unadjusted and adjusted analyses [13]. In an analysis of phase III SWOG trials, after adjusting for WBC count, age, sex, cytogenetics (classified as either favorable or unfavorable) and performance status, race was not a significant predictor of outcome in AML [10]. A study of a population-based cancer registry for the state of Florida observed black AML patients to be younger and to have higher levels of poverty and Medicaid use, and observed shorter survival for blacks, than whites in a multivariable analysis (HR 1.26, p = 0.001), but there was only a trend toward a difference in OS when the analysis was limited to patients under 60 years [15]. Finally, a single-institution study at the Cleveland Clinic reported a delay in post-remission therapy for black males, compared to white males, but not for black females in relation to white females, but did not find differences in the average number of cycles, the intensity of therapy delivered or survival [16].
It is important to note that our population was different from those in other published studies, particularly the CALGB study (Sekeres 2004), which also provided detailed information on karyotype differences between black and white patients. Ours was a single-institution study, and included all patients seen at our center, rather than being restricted to patients enrolled on clinical trials. The percentage of minority patients in our study was high (27%) in relation to other studies and the percentage of black patients with cytogenetic data available in our patient population (19%) was also high. Previous studies included approximately half the percentage of black patients, ranging from 8–11% [10,13,15–16]. Additionally, our median ages were approximately 5 years older than in the CALGB study for both black and white patients, presumably because we included older patients who would typically not be offered clinical trials. Our series also included patients with secondary leukemia and others who are usually excluded from clinical trials, such as those with poor performance status or central nervous system involvement. Our study is also the first to address possible racial differences in FLT3 mutation status and associated outcomes.
The racial composition of our patient population seems to accurately reflect that of our geographic area. According to census data, the State of Maryland is 60% white, 29% black, 6% Hispanic and 5% Asian. Thus, adjusting for the higher incidence of AML observed in whites, and considering that 20% of patients in our study lived outside the State of Maryland, in surrounding states with lower percentages of blacks, our AML population appears to approximate the expected racial distribution. When considering only the 68 patients who lived in the city of Baltimore, 53% were black, also approximating the expected racial distribution. In our AML population, black patients were younger than white patients. According to Surveillance, Epidemiology, and End Result (SEER) Program data [1], the median age at presentation of whites with AML is 68 years, with no difference between males and females, whereas the median age for blacks is is 59 years. Thus the age difference in our black and white patients appears to reflect a similar difference in the overall AML population. Of note, both racial groups presented at younger ages than observed in SEER, likely because older AML patients are not referred to tertiary care centers as frequently as younger patients.
Estimated median income was significantly lower in black than whites at $42,667/year for blacks and $53,534/year for whites (p<0.001). This was fairly comparable to median household incomes for the state of Maryland during the same time period, which was $41,600/year for blacks and $57,800/year for whites, suggesting that the socioeconomic makeup of our population was similar to that of the primary referral base.
While we found a significant female predominance in our black AML patient population, SEER data do not demonstrate a female predominance among black AML patients, with a median age of 59 years for black males and 58 years for black females.. It is thus possible that the female predominance in our population may be due to a referral bias, with fewer black males referred for treatment in relation to black females. Interestingly, a female predominance was also observed in the CALGB population described by Sekeres [13], offering some further support for the theory that black males may not be referred for tertiary care as often as black females.
Our findings that blacks have a younger age at presentation and a lower incidence of normal karyotypes are consistent with other reported data [13], but our other findings were surprising. In the CALGB population, blacks had a markedly higher incidence of t(8;21) compared to whites (17% vs. 5.8%), but this was not the case in our population. There was a trend toward a higher incidence of t(8;21) in blacks (5% vs. 2%, p=0.09), but the prevalence of this translocation was far lower in our population than in the CALGB study, possibly due to a referral bias selecting for higher-risk leukemia. Additionally the markedly higher incidence of complex karyotypes in blacks compared to whites in our series (26% versus 12%; p=0.002) was also not seen in the CALGB population (reported as 13% vs. 11%, p=0.30), again likely due to the lack of selection of our population, in relation to patients enrolling on phase III cooperative group clinical trials. The increased number of complex karyotypes in black females was striking. We can only speculate on reasons for this, but suspect that it is most likely due to differential environmental exposures, though it could also be due to different genetic susceptibility to leukemogenic processes.
FLT3 mutations have emerged as an important predictor of outcome in AML, particularly in patients with normal and other intermediate-risk karyotypes. FLT3 status was available for approximately one third of our patient population, including 80% of those diagnosed in 2006 or later. FLT3 WT status was a strong predictor of OS in black patients with intermediate-risk karyotypes, but not in whites. Interestingly, all 6 black patients with intermediate-risk karyotypes and WT FLT3 remained alive at the time of data analysis, with a median follow-up of 25 months, whereas OS for the 7 black patients with FLT3 mutations was 7 months. A larger number of patients will be required to further evaluate this intriguing observation.
We were also surprised to find a trend toward longer OS in black males, compared to white males, and the best unadjusted survival of the race/gender combinations after excluding t(15;17). This is in direct contrast to the findings of Sekeres et al., who reported that black men had significantly lower CR rates and shorter OS compared to other race/gender groups in both unadjusted and adjusted analyses. SEER reports age-adjusted mortality as 20.9% for white men, 23.9% for white women, 26.4% for black men and 21.5% for black women [1]. The favorable survival experienced by black males was presumably due to their younger median age at presentation, as there was no difference in survival examined within age groups.
Our study has several limitations. As multiple variables were examined, borderline significance should be interpreted with caution. This was a retrospective review and the data were dependent on accuracy of clinical documentation that was originally intended for patient care rather than for research. Some information previously identified of prognostic significance, including WBC count at diagnosis, performance status, and socioeconomic data, was not consistently available and therefore not analyzed. However socioencomomic status was estimated using patient zip code and 1999 U.S. census data. Our population has a significant referral bias, since we serve as a large transplant referral center, routinely drawing patients from not only the local Baltimore area but the entire State of Maryland, the District of Columbia, and parts of Virginia, Pennsylvania, and Delaware, thus likely selecting for higher-risk disease. Moreover, some patients included were referred to our Center with relapsed or refractory disease rather than at initial presentation, again selecting for higher-risk leukemia. This may explain, partially or in full, the low percentage of favorable karyotypes in our population compared to other reported series [17], and may also explain the relatively short survival observed in our favorable-risk white patients. Finally, patients were treated heterogeneously, some on clinical trials, some with standard regimens, and some with supportive care only. As noted previously, all patients had equal delivery of intensive chemotherapy, but the lower proportion of black patients undergoing HSCT suggests differences in access to care.
In conclusion, our study demonstrates important differences between racial populations with AML. We found that black adult patients with AML present at younger age, are more commonly female, and have a higher incidence of complex karyotypes and a lower incidence of normal karyotypes, but have similar survival when compared to whites, whether unstratified or stratified by age, karyotype or gender. It is not yet clear why these differences in presentation exist. Theories include different genetic susceptibility to leukemogenic processes, different environmental exposures, or some combination of both. Viewed in the context of other data, our research confirms that there are biologic differences in AML between blacks and whites. It also suggests that black males might not be referred to tertiary care centers as frequently as black females and white males and females, and that black patients may have barriers to obtaining HSCT.
Acknowledgements
Role of the funding source – This study was unfunded.
Footnotes
Conflict of Interest – There are no conflicts of interest to disclose.
References
- [1].Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK (eds). SEER Cancer Statistics Review, 1975–2008, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. [Google Scholar]
- [2].Settle K, Posner MR, Schumaker LM, Tan M, Suntharalingam M, Goloubeva O, et al. Racial survival disparity in head and neck cancer results from low prevalence of human papillomavirus infection in black oropharyngeal cancer patients. Cancer Prev Res 2009;2:776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Settle K, Taylor R, Wolf J, Kwok Y, Cullen K, Carter K, et al. Race impacts outcome in stage III/IV squamous cell carcinomas of the head and neck after concurrent chemoradiation therapy. Cancer 2009;115:1744–52. [DOI] [PubMed] [Google Scholar]
- [4].Molina MA, Cheung MC, Perez EA, Byrne MM, Franceschi D, Moffat FL, et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer 2008;113:2797–806. [DOI] [PubMed] [Google Scholar]
- [5].Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol 2007;6:10–6. [PubMed] [Google Scholar]
- [6].Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, McGinn TG, et al. Racial disparities in esophageal cancer treatment and outcomes. Ann Surg Oncol 2008;15:881–8. [DOI] [PubMed] [Google Scholar]
- [7].Dimou A, Syrigos KN, Saif MW. Disparities in colorectal cancer in African-Americans vs Whites: before and after diagnosis. World J Gastroenterol 2009;15:3734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schwartz K, Powell IJ, Underwood W 3rd, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology 2009;74:1296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Godley PA, Schenck AP, Amamoo MA, Schoenbach VJ, Peacock S, Manning M, Symons M, Talcott JA. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst 2003;95:1702–10. [DOI] [PubMed] [Google Scholar]
- [10].Albain KS, Unger JM, Crowley JJ, Coltman CA Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 2009;101:984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Morris GJ, Mitchell EP. Higher incidence of aggressive breast cancers in African-American women: a review. J Natl Med Assoc 2008;100:698–702. [DOI] [PubMed] [Google Scholar]
- [12].O’Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res 2009;15:4806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sekeres MA, Peterson B, Dodge RK, Mayer RJ, Moore JO, Lee EJ, et al. Differences in prognostic factors and outcomes in African Americans and whites with acute myeloid leukemia. Blood 2004;103:4036–42. [DOI] [PubMed] [Google Scholar]
- [14].Baldus CD, Mrózek K, Marcucci G, Bloomfield CD. Clinical outcome of de novo acute myeloid leukaemia patients with normal cytogenetics is affected by molecular genetic alterations: a concise review. Br J Haematol 2007;137:387–400. [DOI] [PubMed] [Google Scholar]
- [15].Byrne MM, Halman LJ, Koniaris LG, Cassileth PA, Rosenblatt JD, Cheung MC. Effects of Poverty and Race on Outcomes in Acute Myeloid Leukemia. Am J Clin Oncol 2011;34:297–304.. [DOI] [PubMed] [Google Scholar]
- [16].Brady AK, Fu AZ, Earl M, Kalaycio M, Advani A, Saunthararajah Y, et al. Race and intensity of post-remission therapy in acute myeloid leukemia. Leuk Res 2011;35:346–50. [DOI] [PubMed] [Google Scholar]
- [17].Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000;96:4075–83. [PubMed] [Google Scholar]
- [18].Karanth M, Begum G, Cook M, Lawson S, Porter C, Lister N, et al. Increased acute GvHD and higher transplant-related mortality in non-caucasians undergoing standard sibling allogeneic stem cell transplantation. Bone Marrow Transplant 2006;37:419–23. [DOI] [PubMed] [Google Scholar]
- [19].Mielcarek M, Gooley T, Martin PJ, Chauncey TR, Young BA, Storb R, et al. Effects of race on survival after stem cell transplantation. Biol Blood Marrow Transplant 2005;11:231–9. [DOI] [PubMed] [Google Scholar]