Abstract
Purpose
Stereotactic body radiation therapy (SBRT) is a common treatment option for patients with metastatic tumors of the spine. The optimal treatment-, tumor-, and patient-specific characteristics necessary to achieve durable outcomes remain less well understood given the heterogeneous nature of the patient population this modality typically serves. The objective of this analysis was to better understand the determinants underlying SBRT spine treatment outcomes.
Methods and Materials
A total of 127 patients with 287 spine tumors were treated between March 2010 and May 2015. The median total doses for single-fraction and hypofractionated courses of treatment were 16 Gy (range, 16-20 Gy) and 24 Gy (range, 16-40 Gy), respectively. Radiologic local control and numeric pain score data were measured, and univariate and multivariate analyses were done to determine factors predictive of treatment response.
Results
Median follow-up was 5.9 months (range, 1-61 months). Radiologic local control was achieved in 84.7% of patients at 6 months and in 74.7% of patients at 1 year. Local control was found to be affected by the Spinal Instability Neoplastic Score, and was worse in patients with scores ≥7 (hazard ratio [HR]: 4.25; 95% confidence interval [CI], 1.57-11.51). Patients who required upfront surgical intervention to alleviate spinal cord compression, address mechanical spinal instability, or both had worse local control than those who did not require surgery (HR: 2.32; 95% CI, 1.04-5.17). Patients treated with a hypofractionated course compared with a single fraction had worse radiologic local control (HR: 2.63; 95% CI, 1.27-5.45). No patients developed radiation-induced myelitis after treatment, and the vertebral compression fracture rate was 9.1% after SBRT.
Conclusions
Patients with potentially unstable spines or needing upfront spinal surgery before SBRT are less likely to achieve durable radiologic local control. Additionally, patients treated with single-fraction regimens have improved local control compared with those treated with hypofractionated radiation.
Introduction
Approximately 30% of all patients with cancer will develop osseous metastatic disease during the course of their disease, and approximately 50% of these will involve the spine, with 30% of patients presenting with symptomatic spinal metastases.1, 2, 3 Spine stereotactic body radiation therapy (SBRT) enables focused delivery of highly conformal ablative doses of radiation to achieve local tumor control within the targeted vertebral body. The goal of treatment is to provide optimal palliative relief from pain and prevent or delay neurologic and functional morbidity by maximizing conformal coverage of the tumor-containing vertebral body with high-dose radiation while limiting doses to critical normal structures such as the spinal cord.4, 5
Retrospective studies have shown that higher focal radiation doses than those typically used in conventionally fractionated radiation regimens offer excellent and more durable tumor control with less treatment time.6, 7, 8 Several accruing or actively maturing phase 2 and 3 clinical trials, including NCT02512965 and NCT00922974, have been designed to compare the clinical outcomes of patients treated with spine SBRT versus conventional fractionation.9, 10 While we await results from these trials, data gathered from institutional studies of SBRT in the palliation of metastatic spinal disease can help determine more precisely the factors that contribute to the efficacy of this treatment to futher optimize outcomes.
Methods and Materials
Study objectives and patient population
The purpose of this retrospective, single-institution study was to assess factors that affect pain and local outcomes of patients with metastatic spine tumors after treatment with spinal SBRT at our institution. Data from patients treated between March 2010 and May 2015 were reviewed. Basic patient demographic and tumor characteristics and patient- and treatment-related outcomes were extracted from electronic medical records into a secure institutional database. Before data collection, approval was obtained from our institutional scientific advisory committee. A retrospective chart review protocol was further reviewed and approved by the institutional review board.
A total of 127 patients with 287 spine tumors treated over 143 sessions of SBRT were included in this study, with a median follow-up of 5.9 months (range, 1-61 months). All patients were treated with linear accelerator–based SBRT, using step-and-shoot static image modulated radiation or volumetric modulated arc therapy via dynamic conformal arcs. Image guidance was employed, including daily cone beam computed tomography (CT), followed by ExacTrac stereoscopic x-ray imaging before each field or arc to confirm setup accuracy.
Radiation treatment planning
Treatment planning goals followed in-house institutional directives with regard to target volume contours, normal tissue constraints, and dosimetric coverage goals (see Table E2; available online at https://dx.doi.org/10.1016/j.adro.2018.10.007). Treatment planning directives evolved to include contouring guidelines for standard SBRT spine cases based on Cox et al and Redmond et al for postoperative cases.11, 12 However, of note, many cases were treated before these guidelines were published in 2013 and 2017, respectively.
Briefly, for standard cases the gross tumor volume (GTV) was contoured using all imaging available, such as gadolinium-enhanced spine magnetic resonance imaging (MRI), CT myelogram, diagnostic CT, and PET/CT. In delineating the clinical target volume (CTV) in addition to including the GTV, the entire involved vertebral body was included in cases in which the GTV occupied this region and extended to include unilateral posterior elements such as the transverse process, pedicle/lamina, and spinous process on the dominant side of tumor involvement, or threatened involvement including abnormal marrow signal suspected of harboring microscopic disease to account for subclinical spread.
CTVs that completely encircled the spinal cord were avoided unless the vertebral body, bilateral posterior elements, and the spinous process were all simultaneously involved, or if there was disease along the entire circumference of the epidural space. If the GTV only involved the spinous process, then the CTV would include only the involved spinous process and the bilateral laminae. A margin of ≤3 mm was added to contain the GTV and CTV to create the planning target volume (PTV) at the discretion of the treating physician. This was typically pulled away from the spinal cord plus 1 mm at the discretion of the treating physician as long as GTV coverage was not compromised. The PTV was never permitted to overlap with the spinal cord as delineated on MRI or CT myelogram.
For postoperative cases, the preoperative extent of gross epidural and osseous disease based on image fusion with preoperative imaging and adjacent involved bony segments were included. Hardware was included or excluded based on physician preference but was included in cases in which avoidance would be otherwise difficult given the proximity to pre- and postoperative GTVs.
During treatment planning, spinal cord constraints took first priority and are listed in Table E2 (available online at https://dx.doi.org/10.1016/j.adro.2018.10.007). Prescription coverage goals include at least 90% of the PTV to be covered by the prescription dose, which was typically prescribed to cover the 80% to 90% isodose line. A conformality index (CI) of ≤1.2 was considered acceptable.
Radiographic and clinical assessment after SBRT
Follow-up imaging after SBRT included MRI, PET/CT, or diagnostic CT scans to determine radiologic local control of each treated vertebral body. Local failure was defined as any tumor growth at the treated vertebral level. Pre- and posttreatment imaging was evaluated by one of several neuroradiologists, and each scan was also reviewed by the authors to confirm the location and characteristics of treatment failure with respect to the original plan, including the presence of progressive or persistent bony or epidural disease or new or worsening vertebral fracture subsequent to treatment. For radiologic assessment of local control, the median follow-up time was defined as spanning from the last day of treatment to the date of posttreatment imaging.
Pain control was determined by comparing baseline and post-SBRT recorded pain scores using the numeric rating scale (NRS-11), an 11-point scale ranging from 0 to 10 to indicate no pain present (score of 0) to worst pain imaginable (score of 10).13 Pain scores were also categorized as improved, worse, or no change if the posttreatment value had changed by an interval of at least 1 point and was less than, greater than, or the same as the pretreatment value, respectively. For assessment of pain control, the median follow-up time was defined as spanning from the last day of treatment to the date of follow-up in clinic.
The degree of vertebral body tumor involvement was quantified using the Bilsky scale, where a score of 0 represents the absence of any spinal cord impingement, and an increase from 1 to 3 signifies compression present with increasing severity.14, 15, 16, 17 Each involved vertebral body was also assessed for mechanical stability by assigning a Spinal Instability Neoplastic Score (SINS), dichotomized into either likely mechanically stable (SINS: 0-6 points) or potentially unstable with surgical consultation recommended (SINS: 7-18 points).18, 19 Patients were referred for consideration of separation surgery and stabilization, typically in the event of high-grade epidural cord compression (eventually classified by Bilsky score) or acute neurologic deterioration clinically, or at the discretion of the treating radiation oncologist if spinal cord/tumor abutment was thought to likely compromise achievement of dosimetric goals. Surgical referral for stabilization was also considered based on SINS criteria, especially in patients with SINS ≥7, indicating surgical consultation was warranted.
Statistical considerations
The primary outcome variable of interest was time-to-local failure. We employed robust methods to account for the hierarchical structure of the data and patients who had multiple courses of SBRT over time (for new or locally progressive lesions that required reirradiation) during which multiple spine segments could be treated simultaneously. A multivariate Cox (proportional hazards) regression with a robust variance estimator was used to model time-to-local failure. The robust sandwich estimate of the covariance matrix was used to account for the hierarchical structure of the data.
The following variables of interest were examined as potential explanatory variables for local failure: demographic and baseline characteristics (sex, age at diagnosis, age at SBRT, Bilsky score, SINS, and previous radiation therapy), and treatment characteristics (radiosensitivity to conventional fractionation, surgery, dose and fractionation, number of lesions, and vertebral body coverage). Some variables were dichotomized (eg, Bilsky scores and SINS) to facilitate comparison and clinical interpretation.
All candidate variables were entered into the multivariate model to assess their aggregate effect on radiologic local control. A backward selection algorithm was applied to remove factors that did not contribute significantly to the model. A univariate screening of each of the variables of interest was also done. Both unadjusted and adjusted hazard ratios (HRs) were determined, and adjusted survival curves were prepared. Continuous and categorical variables were summarized using descriptive statistics. Univariate comparisons between groups for continuous variables were performed using the t test or Mann-Whitney test, as appropriate. The χ2 test or Fisher's exact test was used, as appropriate, to examine associations between categorical variables. In addition, a mixed models approach was used to analyze time-to-local failure. All analyses were done using SAS, version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Patient characteristics
A total of 127 patients and 287 spinal target lesions were targeted (Table 1). Within the cohort, there was an approximately equal number of men and women. The median age at the time of diagnosis was 65.6 years, and the median age at treatment was 66.2 years. Upfront surgery to facilitate epidural separation of tumor from spinal cord and to stabilize the spine before SBRT was performed in 20% of patients (n = 25) on a total of 54 spine segments. At least 1 extraspinal metastasis was detected in 64% of patients (n = 80), but metastatic disease was limited only to the spine in 36% of patients (n = 46).
Table 1.
Patient demographics and treatment characteristics
| Sex | |
| Male | 62 (48.8%) |
| Female | 65 (51.2%) |
| Age at diagnosis (y) | Mean: 64.3 |
| Median: 65.6 | |
| (range, 16.8-90.0) | |
| Age at first SBRT (y) | Mean: 64.9 |
| Median: 66.2 | |
| (range, 16.9-90.0) | |
| Post-scan follow-up time (mo) | Mean: 9.3 |
| Median: 5.86 | |
| (range, 1.0-61.0) | |
| SBRT courses per patient (n = 127 visits) | |
| 1 course | 112 (88.2%) |
| 2 courses | 14 (11.0%) |
| 3 courses | 1 (0.8%) |
| Number of vertebral bodies per course per patient | |
| Single vertebral body | 52 (40.9%) |
| Multiple vertebral bodies | 75 (59.1%) |
| Number of vertebral bodies per patient | |
| 1 spine segment | 52 (40.9%) |
| 2 spine segments | 30 (23.6%) |
| 3 Spine Segments | 26 (20.5%) |
| ≥ 4 Spine Segments | 19 (15.1%) |
| Upfront surgery | |
| Yes | 25 (19.8%) |
| No | 101 (80.2%) |
| Treated vertebral bodies (segments) by spine location | |
| Cervical vertebra | 37 (12.9%) |
| Thoracic vertebra | 141 (49.1%) |
| Lumbar vertebra | 95 (33.1%) |
| Sacral vertebra | 14 (4.9%) |
| Radiosensitivity (no. spine segments) | |
| Radiosensitive | 166 (57.8%) |
| Radioresistant | 121 (42.2%) |
| Radiosensitive (no. spine segments) | |
| Carcinoma (breast, prostate) | 115 (69.3%) |
| Myeloma | 26 (15.7%) |
| Squamous cell carcinoma (cervical, head and neck, esophageal, pancreas, skin) | 16 (9.6%) |
| Hemangioma | 6 (3.6%) |
| Ovarian | 3 (1.8%) |
| Radioresistant | |
| Carcinoma (renal cell, colon, uterine, thyroid) | 61 (50.4%) |
| Non-small cell lung cancer | 28 (23.1%) |
| Sarcoma | 24 (19.8%) |
| Melanoma | 6 (5.0%) |
| Merkel | 2 (1.7%) |
| Surgery performed (287 spine segments) | |
| Yes | 54 (18.8%) |
| No | 233 (81.2%) |
| Fraction details (n = 287 segments) | |
| Single fraction | 148 (51.6%) |
| 3 fractions | 107 (37.3%) |
| 5 fractions | 32 (11.2%) |
| Vertebral body coverage | |
| Complete | 228 (81.1%) |
| Partial | 53 (18.9%) |
| Baseline SINS | |
| Unstable (SINS ≥7) | 50 (32.3%) |
| Stable (SINS <7) | 105 (67.7%) |
| Baseline Bilsky score | |
| 0 | 76 (28.9%) |
| 1 | 125 (47.5%) |
| 2 | 35 (13.3%) |
| 3 | 27 (10.3%) |
| Previous radiation therapy treatment failure | |
| Any (conventional radiation therapy/SBRT) | 41 (14.3%) |
| None | 246 (85.7%) |
| Previous conventional radiation therapy | |
| Yes | 23 (8.0%) |
| No | 264 (92.0%) |
| Previous SBRT | |
| Yes | 18 (6.3%) |
| No | 269 (93.7%) |
| New/worse spinal fracture during treatment course | |
| Yes | 26 (9.06%) |
| No | 261 (90.9%) |
Abbreviations: SBRT = stereotactic body radiation therapy; SINS = Spinal Instability Neoplastic Score.
The majority of patients (88%; n = 112) underwent a single course of treatment, and 12% were treated with multiple courses (11% had 2 SBRT courses; and 1% were treated with 3 SBRT courses). Forty-one percent of patients (n = 52) required treatment to a single vertebral body, and 59% of patients (n = 75) had 2 or more spine segments treated. A total of 41 spine segments (14.29%) had received previous radiation therapy. Of these, 23 segments (8.01%) had prior conventionally fractionated radiation, and 18 segments (6.27%) had prior SBRT. The majority of the targeted spine metastases were derived from primary breast tumors (20.47%), followed by lung primaries (19.69%). When stratified by histologic subtype, the majority were carcinomas (49.61%). Forty-nine percent of the metastases were located within the thoracic spine (n = 141), and 33% were confined to the lumbar spine (n = 95). Finally, 13% (n = 37) and 5% (n = 14) of the lesions were located in the cervical and sacral regions, respectively.
Tumor characteristics
When categorizing patients on the basis of Bilsky scores, of the 263 spine segments, 23.6% (n = 62) were classified as having a frank high-grade cord compression (Bilsky: 2-3), and 76.4% (n = 201) had a low-grade epidural sac compression or no epidural sac or cord compression (Bilsky: 0-1). Tumor-containing vertebral bodies were assessed for spinal stability using the SINS and were dichotomized to stable (SINS: 0-6 points) and potentially unstable/surgical consultation recommended (SINS: ≥7 points). There were 155 spine segments with both clinical and radiologic information available from the medical record to enable SINS scoring. Of these, 32.3% (n = 50) were considered unstable, and 67.7% (n = 105) were considered stable.
Dosimetry
More than half of all vertebral bodies within our cohort (n = 148; 51.6%) were treated with a single fraction, and 48.4% (n = 139) were treated with a hypofractionated approach. Specifically, among 139 segments treated with hypofractionation, 107 vertebral bodies had doses delivered in 3 fractions, and 32 vertebral bodies had doses delivered in 5 fractions, accounting for 37.2% and 11.2%, respectively, of the total 287 spine segments.
The median dose used in the single-fraction treatment was 16 Gy (range,16-20 Gy); and for hypofractionated courses, the median total dose was 24 Gy (range, 16-40 Gy). The PTV included the entire vertebral body, in accordance with International Spine Radiosurgery Consortium Consensus Guidelines,12 in 81.14% (n = 228) of vertebral bodies.
Clinical outcomes
Local tumor control
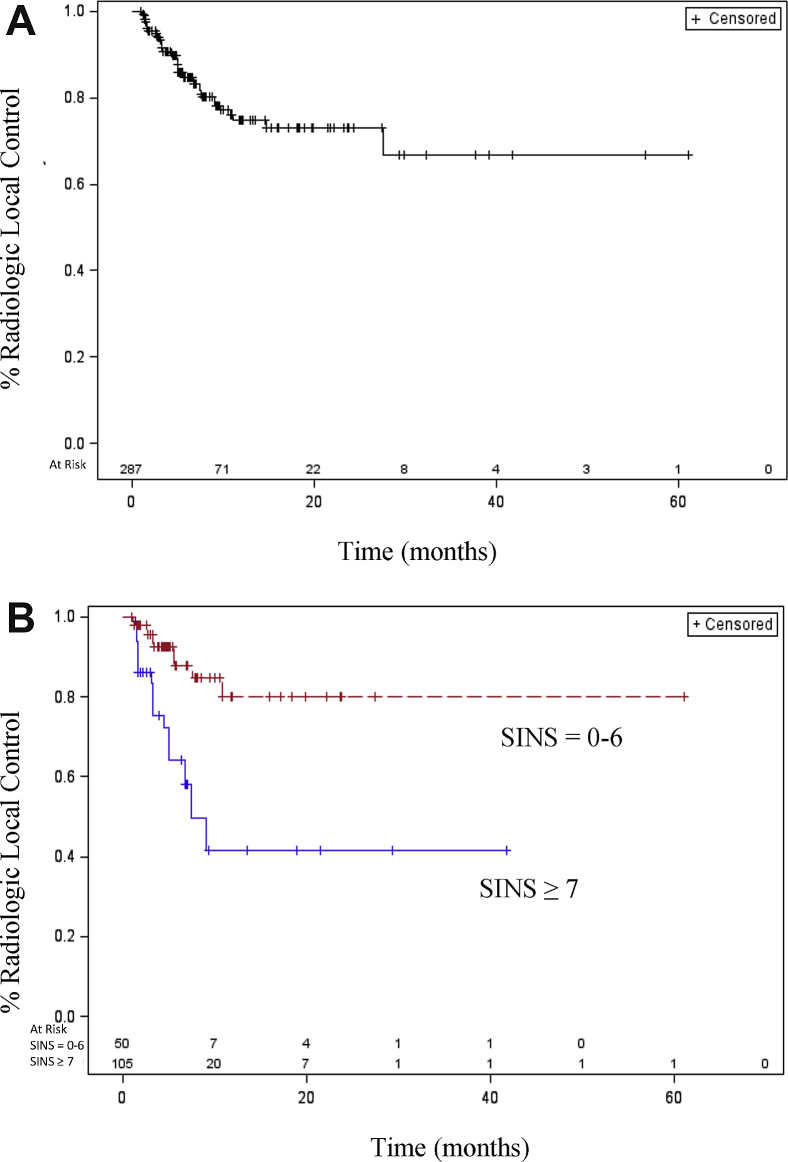
The median follow-up time was 5.9 months (range, 1-61 months). Radiologic local tumor control was achieved in 84.7% of patients at 6 months and in 74.7% at 1 year (Fig 1A). Univariate analyses were conducted to examine the patient-, tumor-, and treatment-specific variables for effect on radiologic local control (Table 2). A comparison of tumor- and spine-specific characteristics was carried out, including stratification by Epidural Spinal Cord Compression (Bilsky) score, SINS, and tumor histology as a function of radiosensitivity to conventional fractionation. Local control was only affected by SINS and was significantly worse in patients with scores ≥7, indicating the presence of at least potentially unstable spines in these patients (HR: 4.25; 95% confidence interval [CI], 1.57-11.51; Fig 1B).
Figure 1.
(A) With a median follow-up time of 5.9 months (range, 1-61 months), overall radiologic local control was achieved in 84.7% of patients at 6 months and 74.7% at 1 year. The median time to local failure was not reached. (B) Tumor-containing vertebral bodies were assessed for spinal stability using the Spinal Instability Neoplastic Score (SINS). Patients were dichotomized as stable (SINS: 0-6 points) or potentially unstable/surgical consultation recommended (SINS: ≥7 points). A total of 155 spine segments had both clinical and radiologic information available from the medical record to assign an SINS. Of these, 32.26% (n = 50) were considered unstable, and 67.74% (n = 105) were considered stable. Patients stratified by SINS had significantly worse radiologic local control at SINS ≥7 (hazard ratio: 4.25; 95% confidence interval, 1.570-11.514; P < .0044).
Table 2.
Results
| Risk factor | Unadjusted hazard ratio (95% confidence interval) | P-value |
|---|---|---|
| Age at diagnosis (y) | 0.988 (0.949-1.028) | <.5412 |
| Age at SBRT (y) | 0.988 (0.949-1.027) | <.5366 |
| Sex | <.5357 | |
| Male | 1.284 (0.582-2.836) | |
| Female | 1.00 | |
| Radiosensitivity | <.0795 | |
| Radioresistant | 1.982 (0.923-4.257) | |
| Radiosensitive | 1.00 | |
| Surgery | <.0396∗ | |
| Yes | 2.320 (1.041-5.173) | |
| No | 1.00 | |
| Fraction | ||
| Multifraction | 2.627 (1.266-5.450) | <.0095∗ |
| Single fraction | 1.00 | |
| No. extraspinal mets | <.1614 | |
| No mets | 1.773 (0.796-3.952) | |
| ≥1 Mets | 1.00 | |
| Pre-SINS group (N = 155) | <.0044∗ | |
| Potentially unstable/unstable: ≥7 | 4.252 (1.570-11.514) | |
| Stable: (0-6) | 1.00 | |
| Bilsky ESCC score (N = 263) | <.1571 | |
| + cord compression (= 1-3) | 1.983 (0.768-5.121) | |
| No cord compression = 0 | 1.00 | |
| Vertebral body coverage | <.6472 | |
| Partial | 0.814 (0.337-1.967) | |
| Complete | 1.00 |
Abbreviations: ESCC = epidural spinal cord compression; Mets = metastasis; SBRT = Stereotactic Body Radiation Therapy; SINS = Spinal Instability Neoplastic Score.
P < .05.
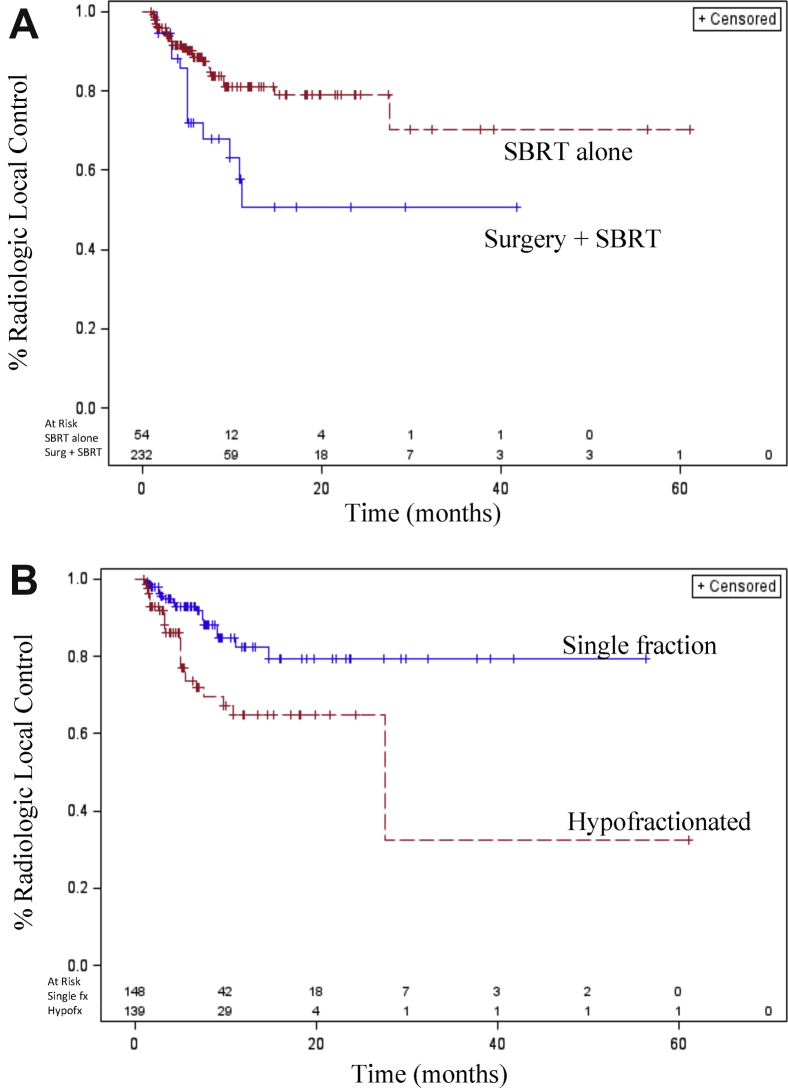
Treatment-specific variables were analyzed, including the degree of vertebral body target coverage, dose/fractionation, and the presence of upfront surgery before SBRT. There was no difference in ability to achieve local control based on the inclusion of the entire vertebral body within the PTV in accordance with International Spine Radiosurgery Consortium Consensus guidelines versus the inclusion of only MRI-delineated GTV with a margin. Patients who required upfront surgical intervention to alleviate spinal cord compression, address mechanical spinal instability, or both had worse local control than those who did not require surgery (HR: 2.32; 95% CI, 1.04-5.17; Fig 2A).
Figure 2.
(A) Patients who require upfront surgery before SBRT for the presence of high-grade epidural spinal cord compression, spinal column instability, or both had worse local control than those who did not require surgery (hazard ratio: 2.320; 95% confidence interval, 1.041-5.173; P < .0396). (B) Patients treated with hypofractionated stereotactic body radiation therapy had worse local control compared with those treated with a single fraction (hazard ratio: 2.627; 95% confidence interval, 1.266-5.450; P < .0095).
Additionally, patients treated with a hypofractionated course compared with a single fraction also had worse radiologic local control (HR: 2.63; 95% CI, 1.27-5.45; Fig 2B). However, when stratifying patients from the entire cohort by increasing total dose delivered within 1, 3, or 5 fractions or when converted to biologically effective dose, no significant correlations were found between increasing dose and local control on univariate or multivariate analyses (data not shown). A multivariate analysis showed only fractionation status (hypofractionated course vs single fraction) as significantly associated with worse radiologic local control.
In patients who required surgical intervention for either high-grade epidural spinal cord compression or architectural instability before SBRT, 23 of 54 vertebral bodies (43%) were treated with a single fraction of 16 to 18 Gy, and 21 of these 23 vertebral bodies (91%) achieved local control. Thirty-one of 54 vertebral bodies (57%) were treated with 3 to 5 fraction SBRT postoperatively, of which 15 segments (48%) that were targeted with a hypofractionated approach had worse local control worse compared with that in single-fraction patients (HR: 9.07; 95% CI, 2.03-40.5; see Table E1, available online at https://dx.doi.org/10.1016/j.adro.2018.10.007, for specific fractionation and doses used in postoperative patients).
Pain control
Numeric rating pain scale scores recorded pre- and post-SBRT were compared. Of the patients with both pre- and posttreatment follow-up scores available, those with a pretreatment score >0 were selected for further analysis. There were 51 follow-up visits at the time of the initial post-SBRT assessment that met these criteria, with a median follow-up time of 21 days posttreatment (8-196 days). At the initial follow-up, 53.8% of patients (n = 43) showed a decrease in pain after SBRT (Fig 3A).
Figure 3.
Numerical rating pain scale values (1-10) were collected at initial consultation and again at follow-up after the completion of stereotactic body radiation therapy (SBRT; median time to first follow-up visit: 21 days). The overall response rate in patients with a baseline level of pain present before SBRT (pre-SBRT pain score >0) are shown. (A) Of patients with both pre- and posttreatment pain scores available, 53.8% (n = 43) showed a decrease in score, 22.5% (n = 18) showed an increase, and 23.8% (n = 19) showed no change in pain score after SBRT. (B) Of the patients who had upfront surgery (n = 18), 38.9% of patients (n = 7) showed a decrease, 44.4% (n = 8) showed an increase, and 16.7% (n = 3) showed no change in pain score after SBRT. Of those who did not have upfront surgery before spine SBRT (n = 62), 58.1% (n = 36) had a decrease in pain score, 16.1% (n = 10) showed an increase, and 25.8% (n = 16) showed no change in pain score after SBRT.
When stratified by upfront spinal surgery before SBRT, of those who underwent surgery (n = 18), 38.9% of patients (n = 7) showed a decrease in pain. Of those who did not have upfront surgery before spine SBRT (n = 62), 58.1% (n = 36) had a decrease in pain (Fig 3B).
Toxicity
No grade ≥4 toxicities developed, and no patient developed radiation-induced myelitis after treatment. Radiologic assessment for new or worsening vertebral body fractures after treatment revealed a fracture rate of 9.1%, with 26 new or worse vertebral body fractures developing after SBRT out of 287 treated vertebral bodies.
Discussion
Herein, we report our initial institutional experience using radiosurgery to manage patients with metastatic spine disease. We included all patients treated at our institution since the initiation of the spine radiosurgical program to capture the diversity of disease burden, histology, and prior therapies typical at presentation of patients referred for spine SBRT.
Local control at 1 year for our cohort was 74.7%. Although direct comparisons are not feasible, this was numerically lower compared than the local control percentages described in the literature (Table 3). Heterogeneity in terms of histology, surgical status, and presence of spinal cord compression or history of prior radiation may be contributing factors. In fact, many studies that report better local outcomes excluded patients who received prior radiation therapy, had frank cord compression, or required upfront surgery to decompress or stabilize the spine before SBRT (Table 3).
Table 3.
Characteristics of published spine SBRT studies
| Author (y) | Local control | Total dose/fx | Included surgical patients | Excluded prior RT | Included patients with cord compression |
|---|---|---|---|---|---|
| Guckenberger et al (2014)40 | 89.9% at 1 y | 24 Gy (range, 8-60 Gy) in 3 fx (range, 1-20 Gy) | No | Yes | No |
| Gerszten et al (2007)41 | 90% without prior RT, and 88% with prior RT | 12.5-25 Gy (mean 20 Gy) | Yes | No | Yes |
| Yamada et al (2008)42 | 90% at 15-month median follow up | 24/1 fx | No | Yes | No |
| Garg et al (2012)43 | 88% at 18 months | 16-24 Gy/1 fx | No | Yes | No |
| Wang et al (2012)44 | 81% at 12 months | 27-40 Gy/3 fx | Yes | No | No |
Abbreviations: fx = fraction; RT = radiation therapy.
Additionally, local control rates are dependent on the total dose delivered, and higher doses yield improved local control.20 With this in mind, the median dose of the single-fraction SBRT course within this study was 16 Gy, and the median dose for hypofractionated courses was 24 Gy in 3 to 5 fractions. These doses are comparatively more conservative than those detailed in other institutional experiences. For instance, Yamada et al demonstrated an impressive improvement in local control with dose escalation. Using a cutoff of above or below 17.4 Gy delivered in a single fraction (range, 16-24 Gy to the 100% isodose line) to define a PTV D95 high dose and PTV D95 low dose, crude local progression was found to be 2.3% and 25%, respectively.20
With time, it became evident that the risk of toxicity including vertebral compression fracture (VCF) also increased proportionally with dose. As such, the ideal dose and fractionation regimen to strike a balance between tumor control and fracture risk remains unknown, and more data are needed to guide appropriate dose selection and predict which patients may be at higher risk of fracture and thus require prophylactic surgical stabilization or dose reduction.21, 22
Local control and fractionation
In terms of dose fractionation, improved local control was seen in patients treated with a single fraction compared with hypofractionated regimens in this study. Results from the literature have been mixed, with some finding more favorable outcomes with hypofractionation23 and others noting a benefit with single-fraction treatments.24, 25 The question remains whether larger single-fraction treatments contribute to a more favorable radiobiologic response leading to improved local control or, alternatively, if patients with larger, more complex tumors who are at greater risk of failing locally also have an increased likelihood of being selected for a hypofractionated approach, simply because of increased tumor burden or difficulty meeting normal tissue constraints.26, 27
Furthermore, a history of spine radiation or spinal cord compression may necessitate hypofractionation more often to respect spinal cord tolerance. Indeed, others have suggested that hypofractionated regimens may significantly mitigate the risk of myelopathy and VCF and lead to a reduction in the overall risk of long-term toxicity. However, more, preferably prospective, data are clearly needed to adequately address these questions.27
Histology
Of the 287 vertebral bodies treated, 166 (58%) contained tumors with histologies considered to be radiosensitive to conventional (ie, 2 Gy per fraction) fractionation, and 121 (42%) were considered radioresistant to conventional radiation therapy.7, 20, 28, 29, 30 Tumor characteristics, including histology and inherent susceptibility to radiation-induced cell death or radiosensitivity, have historically played a role in dictating local outcomes in response to conventionally fractionated radiation therapy.
Spine SBRT studies have shown varying results in terms of local control efficacy as a function of tumor histology. A recent study of patients treated with hypofractionated spine SBRT found that tumor histology remained a factor that predicted local failure on univariate analysis.31 Other studies revealed that perhaps ablative doses delivered during spine SBRT may be able to overcome the inherent radioresistance seen in some tumor histologies in response to conventional fractionation.20, 32 In agreement with these results, we saw no difference in local control when stratifying patients by conventionally radiosensitive and radioresistant histologies.
Vertebral body coverage
We had an opportunity to compare vertebral body coverage before and after formal SBRT spine consensus contouring guidelines12 were published. Local control was predicted to be worse if the MRI-based GTV alone was covered with a small margin—corresponding to partial vertebral body coverage—compared with complete inclusion of the entire affected vertebral body within the CTV. Of the 54 vertebral bodies in which the GTV plus margin alone was targeted, only 5 local failures were noted, all within the high-dose volume. However, there were no statistical differences in local control when comparing the degree of vertebral body inclusion within the target volume.
Surgery
Within the postoperative setting, patients within our cohort who required upfront surgery for spinal cord compression or spine instability did worse in terms of local control than those who did not require surgery. There are multiple possibilities to consider, including that patients who required upfront surgery simply have more advanced local disease in the form of either high-grade epidural spinal cord compression or spinal instability, and may accordingly do worse or fail locally more often because of this added disease burden. In fact, patients in our cohort with a SINS >7, indicating a potentially unstable spine and thus warranting surgical consultation, were found to have significantly worse local control as well.
Furthermore, postoperative SBRT target delineation and treatment planning is challenging because of the radiographic artifact generated from surgical hardware and the altered and distorted anatomy on postoperative imaging, which makes image fusion with preoperative scans and delineation of areas at risk for disease involvement more challenging. In fact, all patients in this series were contoured before formalized postoperative SBRT spine contouring consensus guidelines11 were published and available. These guidelines are now available to aid in standardizing postoperative SBRT target delineation, and it will be interesting in the future to see if this contributes to improved local outcomes.
In terms of dose and fractionation in the postoperative setting, Moulding et al analyzed high-dose single fraction (median: 24 Gy) versus lower-dose single fraction (18 or 21 Gy) after spine surgery. The researchers found that of the 4 of 20 patients who failed locally, 3 patients were in the lower-dose group.33 Laufer et al compared patients after separation surgery and a high-dose single-fraction treatment of 24 Gy, a high-dose hypofractionated course of 24 to 30 Gy in 3 fractions, or a low-dose hypofractionated course of 18 to 36 Gy in 5 to 6 fractions. The results showed that patients from the low-dose hypofractionated group had worse local control compared with those in the high-dose hypofractionated group, but no difference compared with the single-fraction group of patients. The latter comparison was thought to not be significant, perhaps because of the lower number of patients within the single-fraction cohort and reduced statistical power.34
Al-Omair et al compared postoperative spine SBRT patients treated with 1 to 2 fractions of 18 Gy to 26 Gy to those treated with 18 Gy to 40 Gy in 3 to 5 fractions. Patients had worse local control in the latter, more hypofractionated cohort.35 From these studies, Redmond et al concluded that patients treated with higher-dose single or hypofractionated regimens may have improved local control compared with patients treated with lower-dose multifractioned courses in the postoperative setting.26 Interestingly, when looking at dose and fractionation from within our postoperative cohort, we observed a similar trend, with apparent reduced local control as fractionation increases or dose decreases (Table E1; available online at https://dx.doi.org/10.1016/j.adro.2018.10.007).
Pain control
A numerically larger percentage of patients showed an improvement in pain scores in follow-up after SBRT. However, patients who required upfront surgery before SBRT had worse pain outcomes during follow up compared with those who did not require surgery.
A direct comparison of pain outcomes may be difficult because, in contrast to those undergoing radiation alone for uncomplicated spinal disease, patients who require upfront surgery again have more complex lesions at presentation, with significant neurologic compression or spinal instability.36 Additionally, high by Epidural Spinal Cord Compression (Bilsky) grade, an indication for upfront separation surgery, has been shown by others as a significant predictor of persistent pain after SBRT.37
Toxicity
Chang et al. performed a systematic review of the literature and determined from 24 studies that the crude VCF rate after spine SBRT was 13.7%.38 The VCF rate of this cohort was 9.1%, which is lower but consistent with the majority of patients within this study being treated with more conservative doses in fractionation regimens. Indeed, total dose and fractionation have been reported to correlate with an increased risk of fracture, with high-dose single-fraction treatment carrying the greatest risk.27 Fracture rates approaching 40% have occurred with doses of 24 Gy in a single fraction delivered to the 100% isodose line.39
In terms of avoidance of neurologic toxicity, higher-dose single-fraction regimens yield a risk of grade 3 myelopathy below 0.5% as long as the maximal point dose to the spinal cord is <14 Gy. In our cohort, no patients developed grade ≥3 myelopathy throughout follow-up. Therefore, neurologic toxicity was indeed acceptable, albeit with more conservative dose fraction schedules prescribed compared with those in other studies.27
Study limitations
These results are indeed hypothesis generating, although the retrospective nature of this analysis is a limiting factor. Ideally, prospective data are needed, and larger sample sizes will be helpful in the future to detect more robust and significant differences between treatment strata.
Conclusions
We present our institutional experience using SBRT to treat patients with metastatic tumors of the spine over an approximate 5-year period. An attempt was made to capture the heterogeneity that is typical of this population at the time of referral including the myriad tumor histologies with differing radiosensitivities,28 as well as the fact that these patients often present to a radiation oncology clinic with varying disease burdens after exposure to multiple prior treatment modalities.
From our analysis, we conclude that single-fraction treatment yields superior local control compared with hypofractionation, and patients with possible architectural spine instability or those who require upfront spine surgery have significantly worse local outcomes. Finally, our data support that treatment with conservative doses yields acceptable local disease and pain control with low rates of treatment-related toxicity.
Footnotes
Sources of support: This work had no specific funding.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Supplementary material for this article can be found at https://dx.doi.org/10.1016/j.adro.2018.10.007.
Supplementary Data
References
- 1.Sciubba D.M., Gokaslan Z.L. Diagnosis and management of metastatic spine disease. Surg Oncol. 2006;15:141–151. doi: 10.1016/j.suronc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Schneider F., Greineck F., Clausen S. Development of a novel method for intraoperative radiotherapy during kyphoplasty for spinal metastases (Kypho-IORT) Int J Radiat Oncol Biol Phys. 2011;81:1114–1119. doi: 10.1016/j.ijrobp.2010.07.1985. [DOI] [PubMed] [Google Scholar]
- 3.White A.P., Kwon B.K., Lindskog D.M., Friedlaender G.E., Grauer J.N. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14:587–598. doi: 10.5435/00124635-200610000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Chang B.K., Timmerman R.D. Stereotactic body radiation therapy: A comprehensive review. Am J Clin Oncol. 2007;30:637–644. doi: 10.1097/COC.0b013e3180ca7cb1. [DOI] [PubMed] [Google Scholar]
- 5.Martin A., Gaya A. Stereotactic body radiotherapy: A review. Clin Oncol. 2010;22:157–172. doi: 10.1016/j.clon.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Moraes F., Taunk N., Laufer I. Spine radiosurgery for the local treatment of spine metastases: Intensity-modulated radiotherapy, image guidance, clinical aspects and future directions. Clinics. 2016;70:101–109. doi: 10.6061/clinics/2016(02)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerszten P.C., Mendel E., Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: What are the options, indications, and outcomes? Spine (Phila Pa 1976) 2009;34:S78–S92. doi: 10.1097/BRS.0b013e3181b8b6f5. [DOI] [PubMed] [Google Scholar]
- 8.Chan N.K., Abdullah K.G., Lubelski D. Stereotactic radiosurgery for metastatic spine tumors. J Neurosurg Sci. 2014;58:37–44. [PubMed] [Google Scholar]
- 9.Ryu S., Pugh S.L., Gerszten P.C. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1-3) spine metastases: Phase 2 results. Pract Radiat Oncol. 2014;4:76–81. doi: 10.1016/j.prro.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng C.L., Soliman H., Myrehaug S. Imaging-based outcomes for 24 Gy in 2 daily fractions for patients with de novo spinal metastases treated with spine stereotactic body radiotherapy (SBRT) Int J Radiat Oncol Biol Phys. 2018;102:499–507. doi: 10.1016/j.ijrobp.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 11.Redmond K.J., Robertson S., Lo S.S. Consensus contouring guidelines for postoperative stereotactic body radiation therapy for metastatic solid tumor malignancies to the spine. Int J Radiat Oncol Biol Phys. 2017;97:64–74. doi: 10.1016/j.ijrobp.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox B.W., Spratt D.E., Lovelock M. International spine radiosurgery consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e597–e605. doi: 10.1016/j.ijrobp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira-Valente M.A., Pais-Ribeiro J.L., Jensen M.P. Validity of four pain intensity rating scales. Pain. 2011;152:2399–2404. doi: 10.1016/j.pain.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Bilsky M.H., Laufer I., Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine (Phila Pa 1976) 2009;34:S101–S107. doi: 10.1097/BRS.0b013e3181bac4b2. [DOI] [PubMed] [Google Scholar]
- 15.Bilsky M., Smith M. Surgical approach to epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20:1307–1317. doi: 10.1016/j.hoc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Quraishi N.A., Arealis G., Salem K.M.I., Purushothamdas S., Edwards K.L., Boszczyk B.M. The surgical management of mMetastatic spinal tumours based on an Epidural Spinal Cord Compression (ESCC) scale. Spine J. 2015;15:1738–1743. doi: 10.1016/j.spinee.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 17.Bilsky M.H., Laufer I., Fourney D.R. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13:324–328. doi: 10.3171/2010.3.SPINE09459. [DOI] [PubMed] [Google Scholar]
- 18.Huisman M., Van Der Velden J.M., Van Vulpen M. Spinal instability as defined by the spinal instability neoplastic score is associated with radiotherapy failure in metastatic spinal disease. Spine J. 2014;14:2835–2840. doi: 10.1016/j.spinee.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 19.Versteeg A.L., Verlaan J.J., Sahgal A. The spinal instability neoplastic score. Spine (Phila Pa 1976) 2016;41:S231–S237. doi: 10.1097/BRS.0000000000001822. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y., Katsoulakis E., Laufer I. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus. 2017;42:E6. doi: 10.3171/2016.9.FOCUS16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahgal A., Atenafu E.G., Chao S. Vertebral compression fracture after spine stereotactic body radiotherapy: A multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol. 2013;31:3426–3431. doi: 10.1200/JCO.2013.50.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahgal A., Whyne C.M., Ma L., Larson D.A., Fehlings M.G. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol. 2013;14:e310–e320. doi: 10.1016/S1470-2045(13)70101-3. [DOI] [PubMed] [Google Scholar]
- 23.Heron D.E., Rajagopalan M.S., Stone B. Single-session and multisession CyberKnife radiosurgery for spine metastases-University of Pittsburgh and Georgetown University experience. J Neurosurg Spine. 2012;17:11–18. doi: 10.3171/2012.4.SPINE11902. [DOI] [PubMed] [Google Scholar]
- 24.Ghia A.J., Chang E.L., Bishop A.J. Single-fraction versus multifraction spinal stereotactic radiosurgery for spinal metastases from renal cell carcinoma: Secondary analysis of phase I/II trials. J Neurosurg Spine. 2016;24:829–836. doi: 10.3171/2015.8.SPINE15844. [DOI] [PubMed] [Google Scholar]
- 25.Folkert M.R., Bilsky M.H., Tom A.K. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys. 2014;88:1085–1091. doi: 10.1016/j.ijrobp.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 26.Redmond K.J., Sahgal A., Foote M. Single versus multiple session stereotactic body radiotherapy for spinal metastasis: The risk-benefit ratio. Futur Oncol. 2015;11:2405–2415. doi: 10.2217/fon.15.160. [DOI] [PubMed] [Google Scholar]
- 27.Huo M., Sahgal A., Pryor D., Redmond K., Lo S., Foote M. Stereotactic spine radiosurgery: Review of safety and efficacy with respect to dose and fractionation. Surg Neurol Int. 2017;8:30. doi: 10.4103/2152-7806.200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katagiri H., Takahashi M., Inagaki J. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42:1127–1132. doi: 10.1016/s0360-3016(98)00288-0. [DOI] [PubMed] [Google Scholar]
- 29.Maranzano E., Bellavita R., Rossi R. Short-course versus split-course radiotherapy in metastatic spinal cord compression: Results of a phase III, randomized, multicenter trial. J Clin Oncol. 2005;23:3358–3365. doi: 10.1200/JCO.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 30.Mizumoto M., Harada H., Asakura H. Radiotherapy for patients with metastases to the spinal column: A review of 603 patients at Shizuoka Cancer Center Hospital. Int J Radiat Oncol Biol Phys. 2011;79:208–213. doi: 10.1016/j.ijrobp.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 31.Mehta N., Zavitsanos P.J., Moldovan K. Local failure and vertebral body fracture risk using multifraction stereotactic body radiation therapy for spine metastases. Adv Radiat Oncol. 2018;3:245–251. doi: 10.1016/j.adro.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanic S., Boike T., Rule W., Timmerman R. Is renal cell carcinoma really radioresistant? Experience with stereotactic body radiation therapy in patients for primary and metastatic renal cell carcinoma. Am J Clin Oncol Cancer Clin Trials. 2010;33:206. [Google Scholar]
- 33.Moulding H.D., Elder J.B., Lis E. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine. 2010;13:87–93. doi: 10.3171/2010.3.SPINE09639. [DOI] [PubMed] [Google Scholar]
- 34.Laufer I., Iorgulescu J.B., Chapman T. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: Outcome analysis in 186 patients. J Neurosurg Spine. 2013;18:207–214. doi: 10.3171/2012.11.SPINE12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Omair A., Masucci L., Masson-Cote L. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol. 2013;15:1413–1419. doi: 10.1093/neuonc/not101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaikova O., Fosså S.D., Bruland Ø.S., Giercksky K.E., Sandstad B., Skjeldal S. Radiotherapy or surgery for spine metastases? A population-based study of 903 patients in the south-eastern region of Norway. Acta Orthop. 2011;82:365–371. doi: 10.3109/17453674.2011.566142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puvanesarajah V., Lo S.F.L., Aygun N. Prognostic factors associated with pain palliation after spine stereotactic body radiation therapy. J Neurosurg Spine. 2015:1–10. doi: 10.3171/2015.2.SPINE14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang J.H., Shin J.H., Yamada Y.J. Stereotactic body radiotherapy for spinal metastases: What are the risks and how do we minimize them? Spine (Phila Pa 1976) 2016;41:S238–S245. doi: 10.1097/BRS.0000000000001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose P.S., Laufer I., Boland P.J. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guckenberger M., Mantel F., Gerszten P.C. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: a multi-institutional analysis. Radiat Oncol. 2014;9:226. doi: 10.1186/s13014-014-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerszten P.C., Burton S.A., Ozhasoglu C., Welch W.C. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 42.Yamada Y., Bilsky M.H., Lovelock D.M. High-Dose, Single-Fraction Image-Guided Intensity-Modulated Radiotherapy for Metastatic Spinal Lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 43.Garg A.K., Shiu A.S., Yang J. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer. 2012;118:5069–5077. doi: 10.1002/cncr.27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X.S., Rhines L.D., Shiu A.S. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol. 2012;13:395–402. doi: 10.1016/S1470-2045(11)70384-9. http://search.ebscohost.com/login.aspx?direct=true&db=c8h&AN=2011510340&site=ehost-live Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.