Abstract
Purpose
Effective methods to ameliorate radiation enteropathy have not been developed. To address this issue, we investigated the reduced form of coenzyme Q10 (rCoQ10) as a potential radioprotector in a mouse model.
Methods and Materials
rCoQ10 was added to a standard laboratory mouse diet at a final concentration of 1.0% 9 days before irradiation and 30 days thereafter or dissolved in corn oil and administered transorally. Accumulated amounts of coenzyme Q10 (CoQ10) or coenzyme Q9 in the intestine were measured by high-performance liquid chromatography. Reactive oxygen species (ROS), apoptosis, and morphologic changes in the intestine were assessed by immunohistochemistry after administration of 13 Gy of x-ray to the mouse abdomen. Body weight and survival were monitored for 30 days after irradiation. Cytotoxicity using 3 human cancer cell lines and the tumor growth–inhibiting effect in a xenograft were investigated to determine whether rCoQ10 interferes with radiation-specific cytotoxic effects on tumor growth.
Results
CoQ10 was greatly accumulated in all sections of the intestine after both massive transoral dosing and dietary administration, whereas coenzyme Q9 was not. Administration of rCoQ10 suppressed ROS production and inhibited apoptosis in the crypts, resulting in preservation of villi structures after irradiation. Notably, 92% of mice fed the rCoQ10-supplemented diet were healthy and alive 30 days after irradiation, whereas 50% of control mice died (P < .05). Moreover, rCoQ10 did not interfere with radiation-specific cytotoxic effects on tumors either in vitro or in vivo.
Conclusions
Administration of rCoQ10 led to its accumulation in the intestine and induced radioprotective effects by inhibiting ROS-mediated apoptosis, thereby preserving intestinal structures. Our results indicated that rCoQ10 supplementation effectively ameliorated radiation enteropathy.
Introduction
During radiation therapy against tumors in the abdominal or pelvic cavity, sections of the small bowel, colon, or rectum are inevitably exposed to radiation, resulting in harmful adverse effects referred to as radiation enteropathy.1 Consequently, some patients can experience nutrient malabsorption, gut dysmotility, bowel obstruction, perforation, pain, bleeding, and fistula formation in later stages or after completion.2 Indeed, diarrhea often limits the dose in patients with uterine cervical cancer, rectal cancer, and other abdominal malignancies3; however, effective methods to ameliorate these symptoms have not been established. Ionizing radiation generates reactive oxygen species (ROS), which are key agents of both therapeutic effects and radiation-mediated damage, through radiolysis of H2O. In normal cells, endogenous antioxidant defense systems comprising superoxide dismutase, glutathione peroxidase, and catalase participate in clearance of intracellular ROS,4 whereas exogenous antioxidants can also prevent cellular damage by reacting with oxidizing free radicals and quenching ROS activity.5 By contrast, various cancers present increased levels of ROS6 and develop various endogenous antioxidant defense systems to maintain ROS homeostasis. Redox alterations in cancer cells are very complex as a result of the multiple factors involved in redox regulation and stress response.7 However, it remains unknown whether additional exogenous antioxidants can affect the antitumor effects of ionizing radiation beyond redox regulation.
Coenzyme Q (CoQ) is a redox-active quinone derivative harboring a variable number of isoprene units that range from 7 to 12 in different species.8 In human tissues, CoQ10 is the predominant homolog, whereas in the rodent, CoQ9 is predominant. CoQ10 is found in most tissues, with higher concentrations in the heart, liver, kidneys, and muscles relative to the intestine, colon, and lungs.9 The most recognized physiological function of CoQ is electron transfer in the mitochondrial respiratory chain.10 The reduced form of CoQ10 (rCoQ10) is also reportedly a potent antioxidant, with its antioxidant capacity dependent on not only its concentration but also its redox state. In the United States and Europe, CoQ10 has been widely used for >20 years as a dietary or food supplement for maintaining health; however, the potential effectiveness of rCoQ10 to reduce morbidities caused by radiation therapy remains unknown. This study investigated whether rCoQ10 exhibits radioprotective effects and determined which method of administration is more effective at ameliorating radiation enteropathy.
Methods and Materials
rCoQ10 preparation
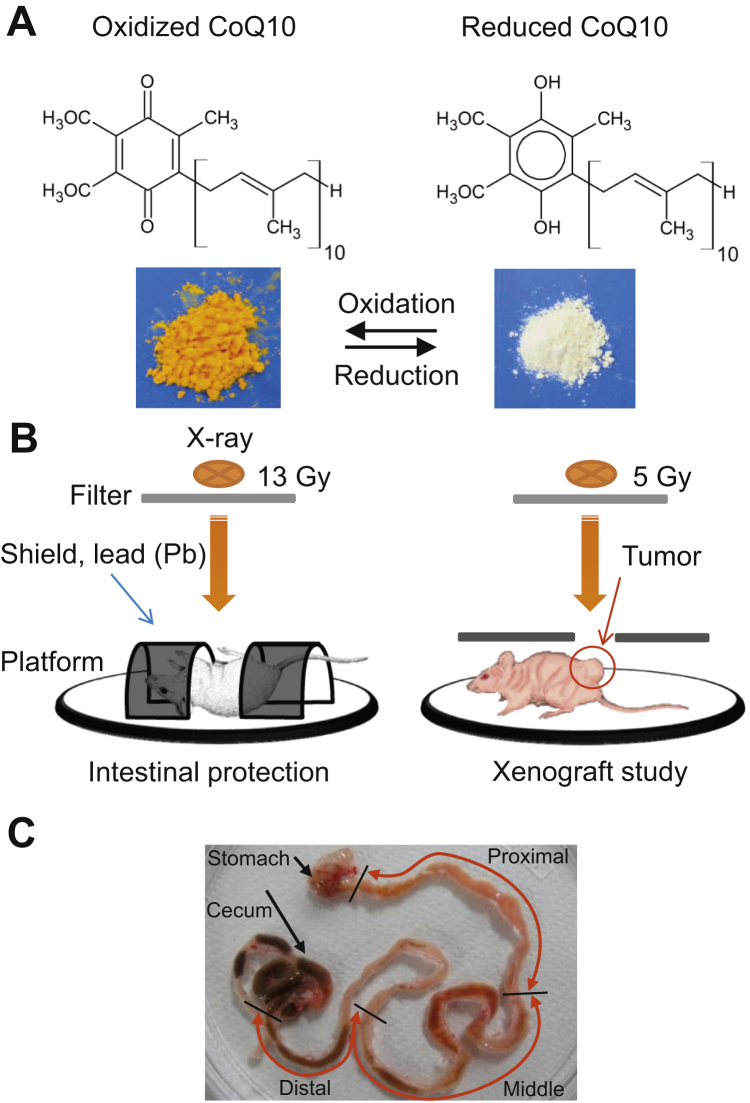
We obtained rCoQ10 from Kaneka Co (Osaka, Japan).11 Conversion between reduced and oxidized CoQ10 is shown in Figure 1A. rCoQ10 dissolved in corn oil at 60 mg/mL was administered transorally at 300 mg/kg per day over 2 consecutive days before and 1 hour before irradiation (rCoQ10 3 massive dose). For continuous administration, rCoQ10 was added to a standard laboratory mouse diet (powdered CE-2; CLEA Japan, Tokyo, Japan) at a final concentration of 1.0% using 1% (v/w) corn oil as a vehicle and the same dose for transoral administration 1 hour before irradiation (dietary rCoQ10 and single massive dose). The control diet was mixed with corn oil only. The diets were stored at −20°C and administered to animals for 9 days before irradiation and 30 days thereafter.
Fig. 1.
Structure, interaction, and features of oxidized and reduced coenzyme Q10 (CoQ10). (A) CoQ10 results. (B) Schemes for total abdominal irradiation and irradiation to xenograft (C) Sectioning of the intestine.
Mouse models
Animal experiments were approved by the Institutional Animal Care and Use Committee (no. P120606-R2) and were consistent with Kobe University regulations and Japanese regulations, including the Act on the Welfare and Management of Animals (Law No. 105; 1973, revised 2006), Standards Relating to the Care and Management of Laboratory Animals and Relief of Pain (Notification No. 88, 2006), and Fundamental Guidelines for the Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions (Notice No. 71, 2006). Male C57BL/6J mice aged 8 weeks and male BALB/cAJcl-nu/nu mice aged 4 weeks were purchased from CLEA Japan and housed at the animal facility, Kobe University, at 4 to 5 animals per cage and under standard laboratory conditions with 12-hour light/dark cycles, humidity from 50% to 60%, and temperature between 20°C and 24°C. Health was assessed daily, and body weights were recorded daily for 30 days after irradiation.
Irradiation
C57BL/6J mice were anesthetized and exposed in the supine position to a single 13-Gy dose of x-rays delivered at 0.57 Gy/min from an MBR-1505R2 generator (Hitachi Medical, Tokyo, Japan). The beam was filtered through a 1-mm aluminum board, and mice were rotated at 6 to 7.2 rpm during irradiation to achieve a uniform dose distribution, as described previously.12 For total abdominal irradiation (TAI), the head, upper body, and anal regions were shielded with custom lead blocks, and only the abdomen was exposed to the radiation beam in Figure 1B. For the xenograft study, the irradiation method differed to allow assessment of rCoQ10 effects on tumors. BALB/cAJcl-nu/nu mice were anesthetized and exposed in a prone position to a single 5-Gy dose of x-rays. The whole body, except for the area of the implanted tumor, was shielded with custom lead blocks, as illustrated in Figure 1B.
Measurement of CoQ homologs
Mice receiving continuous administration had rCoQ10 added to their diet 9 days before sacrifice and harvesting of the small intestine, whereas the group receiving the massive dose received rCoQ10 transorally over 2 consecutive days before and 1 hour before harvest. Mice receiving a combination of an rCoQ10 diet and a massive dose had rCoQ10 added to their diet 9 days before and 1 hour before sacrifice. Small intestines were harvested and divided into 3 segments (proximal, middle, and distal) from the pylorus to the cecum end (Fig. 1C). Each segment of intestine was carefully washed in physiological saline to remove contents possibly containing rCoQ10 in the diet; segments were then mixed with 2-propanol, homogenized, and centrifuged. Blood was collected from the abdominal aorta using heparin, and plasma was separated by centrifugation. The resulting supernatant was analyzed by high-performance liquid chromatography to quantify total CoQ10 or CoQ9, as described by Kubo et al.13
Measurement of ROS production
Dihydroethidium (DHE) was dissolved in dimethyl sulfoxide at 10 mg/mL and diluted immediately before use with phosphate-buffered saline warmed at 40°C to avoid precipitation, which occurs at <37°C. To detect ROS production in the intestine, 200 μL (30 mg/kg) DHE was intraperitoneally injected 1 hour before irradiation, as described previously.14 Segments of the intestine were collected 4 hours after irradiation and immediately frozen at −80°C; 4-μm sections of each specimen were assessed by fluorescence microscopy. ROS production was assessed using DHE, which reacts with O2− to form oxyethidium.15 Positive cells were counted in at least 20 crypt-villus units per specimen under a BZ-9000 fluorescence microscope (Keyence, Osaka, Japan).
TUNEL assay
To evaluate apoptosis, mice were sacrificed 8 hours after irradiation. Segments from the same portion of the intestine were harvested, fixed in 10% formalin, embedded in paraffin, sectioned at 5 μm, and assayed by TUNEL (terminal deoxynucleotidyl transferase dUTP nick-end labeling) staining using an in situ cell death detection kit (Roche, Indianapolis, IN) according to manufacturer instructions. Apoptotic cells were counted in at least 40 crypt-villus units per specimen under a BZ-9000 all-in-one fluorescence microscope (Keyence), as described previously.16
Histochemistry
The small intestine was harvested and its length was measured 4 days after irradiation after or in the absence of rCoQ10 administration. Segments of the intestine were embedded vertically in paraffin, cross sectioned at 5 μm, stained with hematoxylin and eosin, and visualized on a BZ-9000 light microscope (Keyence) to qualitatively assess villus shape, epithelial alignment, and crypt abundance.17
Cell viability assay
Crystal violet staining was performed using 3 human cancer cell lines (MIA PaCa-2 [pancreas], HCT116 [colon], and HeLa [uterine]) in vitro. Briefly, cells were added along with varying concentrations of rCoQ10 (10, 20, and 50 μg/mL) or control for 1 hour before 4- or 8-Gy irradiation. The cells were incubated for 72 hours with the rCoQ10-containing medium, washed with phosphate-buffered saline, and stained with 0.5% crystal violet/methanol solution at room temperature for 10 minutes. The stained cells were rinsed with tap water. For quantitative measurement, stained cells were dissolved in 50% ethanol at room temperature for 20 minutes, followed by measurement of absorbance at 570 nm.
Tumor growth inhibition
To generate xenograft models, male BALB/cAJcl-nu/nu were subcutaneously implanted with 2 × 106 human pancreatic cancer MIA PaCa-2 cells resuspended in Matrigel (BD Biosciences, Tokyo, Japan), as described previously.18 Mice were then randomly assigned to receive no further treatment (control), 5-Gy radiation with a normal diet, or 5-Gy radiation with 1% rCoQ10 dietary supplementation from 11 days before irradiation and without additional administration. Tumor size was measured 2 or 3 times weekly as follows: L × W 2 × (π/6), where L and W are the longest and shortest tumor diameters, respectively.
Statistical analysis
Differences between means were compared by 1-way analysis of variance, with a Dunnett test for post hoc comparisons. Survival rate was estimated by the Kaplan-Meier method using the log-rank test. A P < .05 was considered statistically significant. Data were analyzed using SAS software (Version 9.4; SAS Institute, Inc, Tokyo, Japan).
Results
CoQ10 accumulation after rCoQ10 administration
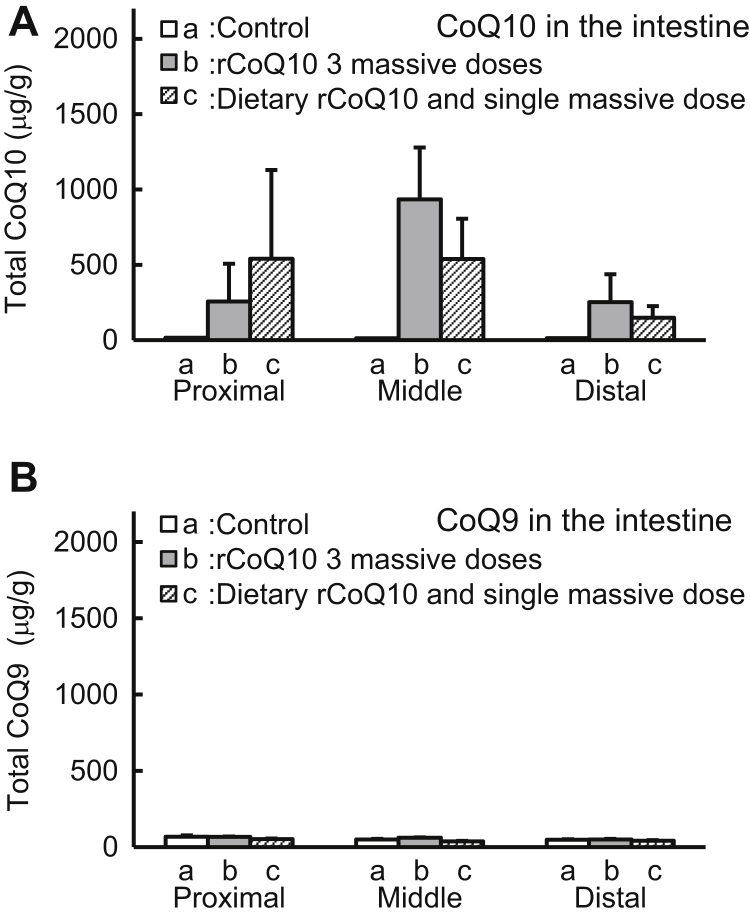
In control mice, basal concentrations of coenzyme homologs in the proximal, middle, and distal portions of the intestine were 14.7 ± 1.3 μg/g, 13.8 ± 4.7 μg/g, and 11.2 ± 2.0 μg/g (mean ± standard deviation; n = 5) for total CoQ10, respectively. After administration of massive doses of rCoQ10, these increased to 256 ± 251 μg/g, 934 ± 344 μg/g, and 252 ± 186 μg/g, respectively, whereas dietary rCoQ10 supplementation and massive single doses increased the concentrations to 540 ± 590 μg/g, 539 ± 267 μg/g, and 149 ± 67 μg/g, respectively (Fig. 2A). CoQ9 concentration remained unchanged by administration of rCoQ10 (Fig. 2B).
Fig. 2.
Evaluation of coenzyme Q (CoQ) 10 and CoQ9 accumulation. (A) Amounts of CoQ10 and (B) CoQ9 in the intestine according to high-performance liquid chromatography. Bars indicate amounts of CoQ10 or CoQ9 from (a) the intestines of control mice fed a normal diet, (b) mice fed a normal diet and administered transorally 3 massive doses (over 2 consecutive days and 1 hour before sacrifice), and (c) mice fed a diet with rCoQ10 for 9 days and single massive dose 1 hour before sacrifice. Error bars represent the mean ± standard deviation (n = 5).
rCoQ10-mediated suppression of radiation-induced ROS production
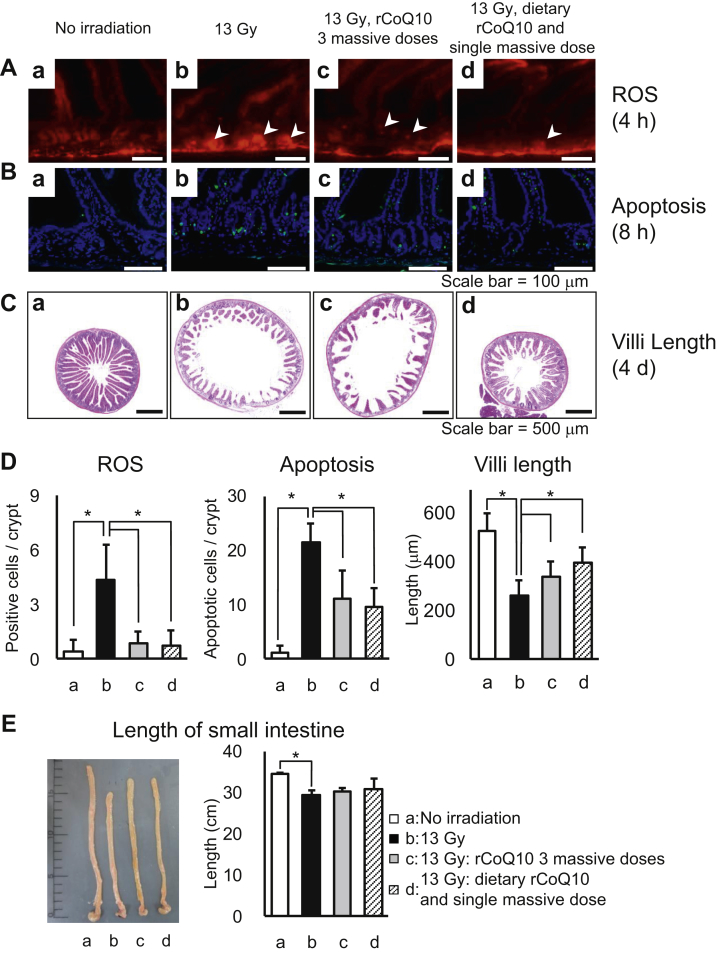
As shown in Figure 3A, compared with the no-irradiation control, bright-red fluorescence increased in the bottom of intestinal crypts in mice receiving 13-Gy radiation (Figs. 3Aa and Ab). These signals were diminished in mice administered rCoQ10, indicating that rCoQ10 effectively inhibited ROS production in intestinal crypts (Figs. 3Ac, 3Ad, and 3D, P < .05).
Fig. 3.
Radioprotective effects of reduced coenzyme Q10 (rCoQ10). Effects were measured according to (A) ROS in crypts at 4 hours after irradiation; (B) apoptosis after 8 hours; (C) villi length after 4 days; and (D) quantitative evaluations. (A-C) Images of intestines from (a) control mice, (b-d) mice receiving 13 Gy of total abdominal radiation, (c) mice receiving 3 massive doses of rCoQ10, and (d) mice receiving dietary rCoQ10 and a single massive dose of rCoQ10. (E) Measurement of intestinal lengths according to each treatment (a-d). *P < .05. Error bars represent the mean ± standard deviation (n = 3). Abbreviation: ROS = reactive oxygen species.
rCoQ10-mediated inhibition of radiation-induced apoptosis
Apoptotic cells were identified at 21.4 ± 3.5 cells/crypt-villus in specimens collected 8 hours after irradiation (Fig. 3Bb), whereas 1.2 ± 1.3 apoptotic cells/crypt-villus were identified in control mice (Figs. 3Ba and 3D). Three massive doses of rCoQ10 significantly reduced the number of apoptotic cells to 11.0 ± 5.2, and administration of dietary rCoQ10 and a single massive dose reduced the number to 9.5 ± 3.6 (Figs. 3Bc, 3Bd, and 3D, P < .05). These results indicated that rCoQ10 inhibited apoptosis in intestinal crypts.
The radioprotective effects of rCoQ10 in the intestine
As shown in Figure 3Ca, the intestine exhibited normal morphologic characteristics and contained long and narrow mucosal villi that were tightly aligned and extended into the intestinal lumen in control mice, whereas irradiated mice had severe mucosal damage (Fig. 3Cb) and shortening of the intestine (Fig. 3E). Notably, villi structures were well preserved in mice administered dietary rCoQ10 and a single massive dose (Fig. 3Cd). These results indicated that rCoQ10 had radioprotective effects in the intestine (Fig. 3D).
Weight change and survival after rCoQ10-mediated improvement of TAI-related effects
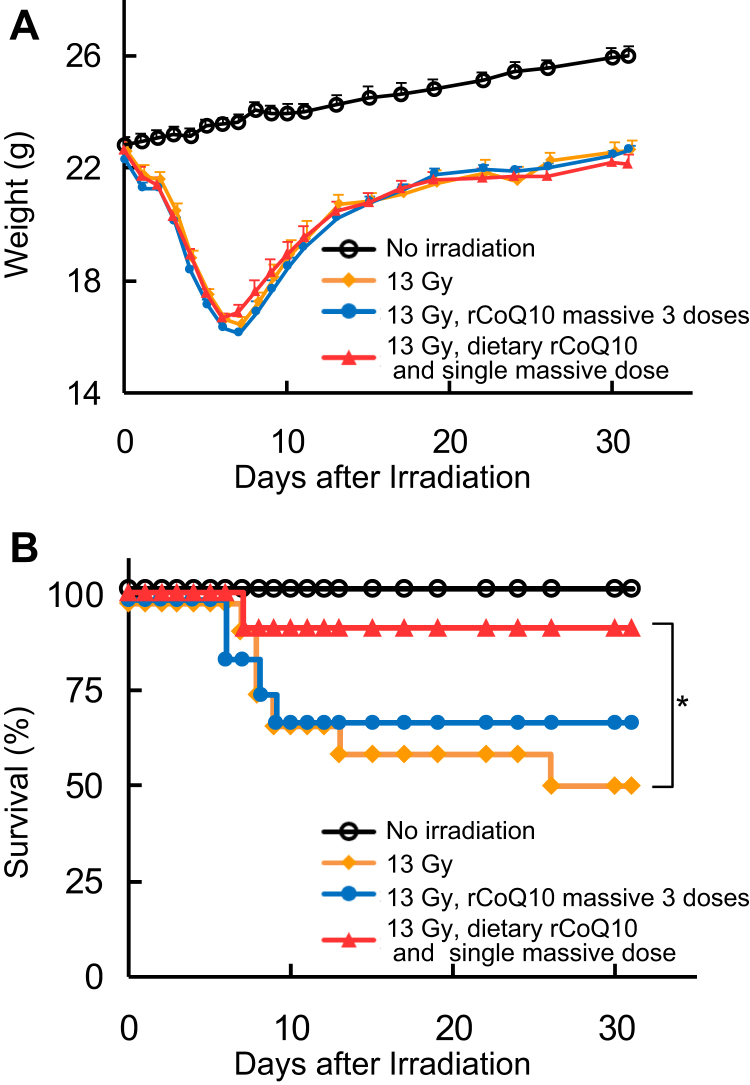
All irradiated mice lost weight over 7 days, beginning on the day after irradiation; however, all groups regained some weight beginning on day 8 (Fig. 4A). Irradiated mice not administered rCoQ10 started to die after 7 days, with 50% of mice eventually dying. By contrast, 33% mice administered 3 massive doses of rCoQ10 died at 6 to 9 days after irradiation, although the remaining 66% mice survived thereafter until the end of the experiment at 30 days. Notably, 92% of irradiated mice administered dietary rCoQ10 and a single massive dose were healthy and alive at 30 days after irradiation, indicating significantly enhanced survival (Fig. 4B; P < .05).
Fig. 4.
Weight change and survival. (A) Weight curves indicating nonirradiated mice (black), mice receiving 13 Gy radiation (yellow), mice receiving total abdominal irradiation (TAI) and 3 massive doses of reduced coenzyme Q10 (rCoQ10) and a normal diet (blue), and mice receiving TAI and dietary rCoQ10 along with a single massive dose of rCoQ10 (red) (n = 12). Error bars represent the mean ± standard deviation (n = 12). (B) Survival curves according to Kaplan-Meier analysis under the same conditions.
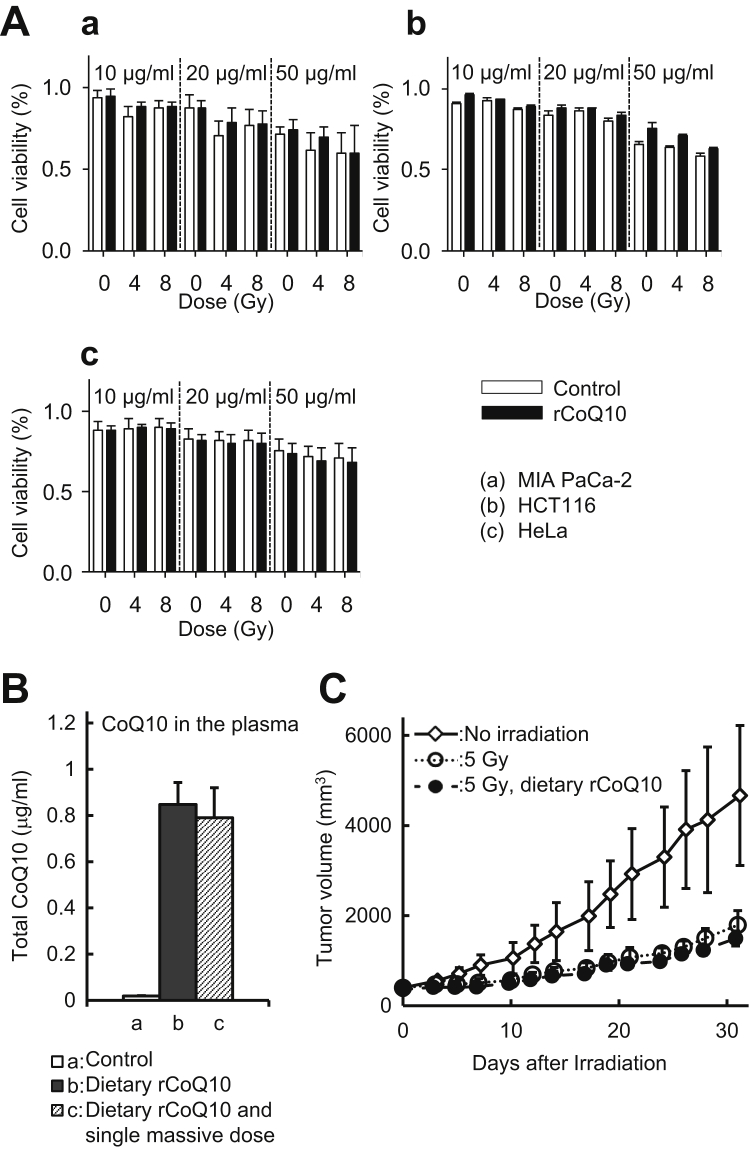
rCoQ10 does not interfere with radiation-specific effects
Three human cancer cell lines cultured in media supplemented with rCoQ10 displayed similar cytotoxic effects from irradiation as those cultured in the absence of rCoQ10 (Fig. 5A). Before in vivo study, CoQ10 concentration was evaluated. Although the administration method used for the xenograft study differed from that for the intestine-protection study, the plasma CoQ10 concentration remained similar between diet administration and administration via both diet and a single massive dose (0.84 ± 0.10 μg/mL vs 0.79 ± 0.13 μg/mL, respectively, and relative to control [0.02 ± 0.00 μg/mL; n = 5]) (Fig. 5B). The tumor growth rate at 30 days was similar in both groups of mice receiving 5 Gy radiation, regardless of rCoQ10 administration (Fig. 5C). These data suggest that rCoQ10 supplementation did not inhibit the antitumor effects of irradiation in vivo.
Fig. 5.
Reduced coenzyme Q10 (rCoQ10) does not interfere with radiation effect on cancer cells. (A) Radiation-specific cytotoxic effects in (a) MIA PaCa-2, (b) HCT116, and (c) HeLa cells with or without rCoQ10. (B) Plasma CoQ10 concentration by high-performance liquid chromatography. Bars indicate the CoQ10 concentration in (a) the intestines of control mice receiving a normal diet, (b) mice receiving dietary rCoQ10 for 14 days, and (c) mice receiving dietary rCoQ10 for 9 days and a single massive dose of rCoQ10 1 hour before sacrifice. Error bars represent the mean standard deviation (n = 5). (C) Open bars: cells in the absence of rCoQ10 treatment; closed bars: cells treated with rCoQ10. Growth of pancreatic cancer xenografts treated with 5 Gy of radiation in the presence or absence of dietary intake of rCoQ10 (n = 5). Open diamonds: nonirradiated mice; open circle: mice receiving 5 Gy radiation and a normal diet; and closed circles: mice receiving 5 Gy radiation supplemented with dietary CoQ10. Error bars represent the mean ± standard deviation.
Discussion
Herein, we report that rCoQ10 administration alone ameliorated radiation enteropathy, mainly through suppression of radiation-induced ROS production and apoptosis. A significant point emerging from this study is that administered rCoQ10 accumulated in the intestine and decreased severe radiation damage, thereby promoting survival.
Although radiation enteropathy develops via multiple mechanisms, the severity of the resulting damage depends on the radiation dose and delivery method. The condition is usually triggered by ROS-mediated apoptosis in crypt cells, followed by insufficient regeneration of villus epithelium, breakdown of the mucosal barrier, and mucosal inflammation.15, 19, 20 Accordingly, the structure of villi is considered a marker of gastrointestinal damage.17 In the present study, radiation-induced apoptosis in intestinal crypt cells was identified and led to villi degradation (Fig. 3). A previous report noted that a single high dose (≥13 Gy) of radiation induces lethal gastrointestinal (GI) and bone marrow (BM) damage in rodent models.21 Additionally, previous studies found that GI syndrome progressed rapidly during days 6 through 10 after whole-body radiation and was concomitant with BM damage.22 Moreover, they found that lethality occurred in these BM-protected mice after ≥19 Gy radiation, with >90% of the animals succumbing presumably to “pure” GI syndrome in ≤10 days, suggestive of the presence of evolving BM damage facilitating lethality via the GI syndrome. Paris et al16 reported that ionizing radiation induced lethal GI and BM toxicity in C57BL/6 mice after administration of 12- to 15-Gy whole-body radiation, with mice receiving 12 Gy able to be rescued by autologous BM transplantation; in contrast, mice receiving 15 Gy could not be rescued, suggesting that 15 Gy induced irreversibly lethal damage.
Compared with whole-body radiation, several factors, including radiation source, shielding method, mouse strain, body thickness, and age, might influence the effects of TAI. To determine the most appropriate dose for the purpose of our experiments, we performed preliminary experiments (n = 3) at between 10 Gy and 15 Gy in the absence of CoQ10 administration and found that doses >13 Gy were 100% lethal (Fig. E1; available online at https://doi.org/10.1016/j.adro.2019.01.006). Therefore, we used a dose of 13 Gy for intestine protection studies involving CoQ10. Our results indicated that rCoQ10 supplementation mitigated radiation-induced lethality (Fig. 4B), suggesting potential efficacy in relation to avoidance of radiation enteropathy. Specifically, we found that administration of dietary rCoQ10 along with a single massive dose provided enhanced protection according to intestinal histologic characteristics, thereby promoting improved overall survival of mice. This result suggested that the improved intestinal protection might be induced by systemic circulation of CoQ10. Furthermore, continuous administration of dietary rCoQ10 after irradiation might protect the intestines from ROS produced by mitochondria after radiation exposure.23 These findings indicated that elevated levels of rCoQ10 administration enhanced villi preservation and promoted survival.
The role and efficacy of each CoQ homolog in ameliorating radiation toxicity in the rodent intestine has not been elucidated. A brief literature review indicated the existence of few reports demonstrating roles for CoQ9 in the rodent intestine similar to that of CoQ10 in the human intestine or the effect of CoQ9 on antioxidant activity after irradiation. Lass et al24 reported that CoQ9 levels in tissue homogenates from serum and various organs (heart, muscle, liver, and kidney) are elevated after CoQ10 supplementation; however, there are no reports regarding whether supplementation results in CoQ9 or CoQ10 accumulation in the intestine. Our results indicated that continuous administration of rCoQ10 led to accumulation of CoQ10, but not CoQ9, in all portions of the small intestine (Fig. 2). Moreover, rCoQ10 suppressed radiation-induced apoptosis in crypt cells and in the lamina propria, ultimately avoiding villi degradation (Fig. 3) and suggesting that exogenous rCoQ10 might contribute to the suppression of radiation-induced damage in the intestine.
Useful strategies to reduce radiation toxicity in clinical or experimental settings have not been established. Because radiation morbidities are reportedly initiated by ROS, antioxidants and free-radical scavengers are thought to represent potentially radioprotective or therapeutic compounds.25, 26 Among these compounds, sulfhydryls are considered the most promising, but only amifostine (WR-2721; Ethyol) has been approved by the US Food and Drug Administration for radiotherapeutic treatment of head and neck cancer.27 Superoxide dismutase,28 the vitamin E analog γ-tocotrienol,29 and their combined use30 have been identified as potent, nontoxic, and natural radioprotective compounds. Additionally, mixtures containing CoQ10, tocopherol (vitamin E), and ascorbic acid (vitamin C)31 are efficacious antioxidants against radiation damage. Moreover, a review by Yasueda et al32 reported that antioxidants, such as CoQ10, might provide protection against chemotherapy-related toxicity and side effects without apparent adverse effects. Published data associated with preclinical and clinical safety studies indicate that CoQ10 does not cause serious adverse effects in humans and that it is well tolerated for use as a dietary supplement.33 On the other hand, in a previous toxicity study of rodents orally administered CoQ10 for 90 days, rats had tolerance for up to 3000 mg/kg per day.34 In the present study, the longest period of rCoQ10 administration was ∼40 days at a dose of ∼1200 mg/kg per day rCoQ10 in the diet, equating to 1% rCoQ10 and 300 mg/kg per day in a massive dose. Our results suggested an absence of toxicity in mice; however, our pilot experiment (Fig. E2; available online at https://doi.org/10.1016/j.adro.2019.01.006) finding 100% mortality in mice administered a high dose of radiation was repeated here, revealing that 300 mg/kg of a single massive rCoQ10 dose increased survival longer than a single 100 mg/kg dose. In the present study, we used rCoQ10 doses (1% rCoQ10 and 300 mg/kg). A previous study found that CoQ10 protects normal cells, including neuronal cells, astrocytes, retinal cells, and lens epithelial cells, from ROS-induced damage,35 although the underlying mechanisms have not been elucidated. Therefore, further investigation is warranted.
The efficacy of concurrent administration of CoQ10 during cancer treatment remains controversial. Lamson et al5 reported that exogenous antioxidants alone are beneficial during cancer treatment and do not reduce the efficacy of chemotherapy or radiation when administered concurrently. Similarly, Roffe et al36 surveyed several clinical trials of CoQ10 supplements and found no evidence of adverse effects or interference with standard chemotherapies. Consistent with these reports, our in vitro and in vivo experiments indicated no inhibition of radiation effects on malignant cells after rCoQ10 administration (Fig. 5). Importantly, we found that increased plasma CoQ10 concentrations were similar after both dietary administration and a single massive dose; therefore, in vivo experiments focused on dietary administration of rCoQ10 without an accompanying massive dose of rCoQ10, with this resulting in no changes in tumor growth or decreases in the antitumor effects of radiation therapy.
Conclusions
Our results indicated that rCoQ10 confers radioprotection by reducing ROS-mediated apoptosis in the intestine. Moreover, continuous administration of rCoQ10 as a dietary supplement was more effective than short-term administration alone. These findings promote rCoQ10 as a potentially functional dietary supplement for amelioration of radiation enteropathy.
Acknowledgments
The authors thank Hideyuki Kishida, Hiroshi Kubo, Mineko Ogura, and Mitsuaki Kitano from Kaneka Corporation, Japan. The authors also thank Izumi Takayama (Division of Radiation Oncology, Kobe University) for technical assistance.
Footnotes
Sources of support: This work was supported by Grants-in-Aid for Exploratory Research (grant nos. 16H05391 to R.S.; 15K09996 to Y.O.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Supplementary material for this article can be found at https://doi.org/10.1016/j.adro.2019.01.006.
Supplementary data
Fig. E1.
Survival curve in TAI without rCoQ10 administration. The survival curve indicate mice irradiated 10 Gy (closed squire), 12 Gy (closed circle), 13 Gy(closed triangle), 14 Gy (open diamond), and 15 Gy (open circle). (n = 3).
Fig. E2.
Survival curve in TAI for rCoQ10 concentration-dependent effect. The mice were added rCoQ10 100 mg/kg (closed triangle) or 300 mg/kg (closed circle) with a single massive dose 1 h before irradiation except with control mice (closed squire). All mice were derived 17 Gy to abdomen.
References
- 1.Jones R.M., Sloane V.M., Wu H. Flagellin administration protects gut mucosal tissue from irradiation-induced apoptosis via MKP-7 activity. Gut. 2011;60:648–657. doi: 10.1136/gut.2010.223891. [DOI] [PubMed] [Google Scholar]
- 2.Carr K.E. Effects of radiation damage on intestinal morphology. Int Rev Cytol. 2001;208:1–119. doi: 10.1016/s0074-7696(01)08002-0. [DOI] [PubMed] [Google Scholar]
- 3.Bismar M.M., Sinicrope F.A. Radiation enteritis. Curr Gastroenterol Rep. 2002;4:361–365. doi: 10.1007/s11894-002-0005-3. [DOI] [PubMed] [Google Scholar]
- 4.De Bont R., van Larebeke N. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 5.Lamson D.W., Brignall M.S. Antioxidants in cancer therapy; their actions and interactions with oncologic therapies. Altern Med Rev. 1999;4:304–329. [PubMed] [Google Scholar]
- 6.Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 7.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 8.Battino M., Ferri E., Gorini A. Natural distribution and occurrence of coenzyme Q homologues. Membr Biochem. 1990;9:179–190. doi: 10.3109/09687689009025839. [DOI] [PubMed] [Google Scholar]
- 9.Aberg F., Appelkvist E.L., Dallner G. Distribution and redox state of ubiquinones in rat and human tissues. Arch Biochem Biophys. 1992;295:230–234. doi: 10.1016/0003-9861(92)90511-t. [DOI] [PubMed] [Google Scholar]
- 10.Ernster L., Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 11.Kitano M., Mizuhashi F., Kubo H. Evaluation of the mutagenic and genotoxic potential of ubiquinol. Int J Toxicol. 2007;26:533–544. doi: 10.1080/10915810701707460. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu Y., Akasaka H., Miyawaki D. Evaluation of a small animal irradiation system for animal Experiments using EBT3 Model GAFCHROMICTM Film. Kobe J Med Sci. 2018;63:e84–e91. [PMC free article] [PubMed] [Google Scholar]
- 13.Kubo H., Fujii K., Kawabe T. Food content of ubiquinol-10 and ubiquinone-10 in the Japanese diet. J Food Compos Anal. 2008;21:199–210. [Google Scholar]
- 14.Brown S.L., Kolozsvary A., Liu J. Antioxidant diet supplementation starting 24 hours after exposure reduces radiation lethality. Radiat Res. 2010;173:462–468. doi: 10.1667/RR1716.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarpey M.M., Wink D.A., Grisham M.B. Methods for detection of reactive metabolites of oxygen and nitrogen: In vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:R431–R444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- 16.Paris F., Fuks Z., Kang A. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 17.Hall E.J. Radiobiology for the Radiologist. 5th ed. Lippincott William & Wilkins; Philadelphia, PA: 2000. Dose-response relationships for model normal tissues; pp. 317–318. [Google Scholar]
- 18.Akasaka H., Mizushina Y., Yoshida K. MGDG extracted from spinach enhances the cytotoxicity of radiation in pancreatic cancer cells. Radiat Oncol. 2016;11:153. doi: 10.1186/s13014-016-0729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauer-Jensen M., Denham J.W., Andreyev H.J. Radiation enteropathy—pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11:470–479. doi: 10.1038/nrgastro.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauer-Jensen M., Wang J., Denham J.W. Bowel injury: Current and evolving management strategies. Semin Radiat Oncol. 2003;13:357–371. doi: 10.1016/s1053-4296(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 21.Rotolo J.A., Kolesnick R., Fuks Z. Timing of lethality from gastrointestinal syndrome in mice revisited. Int J Radiat Oncol Biol Phys. 2009;73:6–8. doi: 10.1016/j.ijrobp.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Mason K.A., Withers H.R., Davis C.A. Dose dependent latency of fatal gastrointestinal and bone marrow syndromes. Int J Radiat Biol. 1989;55:1–5. doi: 10.1080/09553008914550011. [DOI] [PubMed] [Google Scholar]
- 23.Ogura A., Oowada S., Kon Y. Redox regulation in radiation-induced cytochrome C release from mitochondria of human lung carcinoma A549 cells. Cancer Letters. 2009;277:64–71. doi: 10.1016/j.canlet.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Lass A., Forster M.J., Sohal R.S. Effects of CoQ10 and a-tocopherol administration on their tissue levels in the mouse: Elevation of mitochondrial a-tocopherol by CoQ10. Free Radic Biol Med. 1999;26:1375–1382. doi: 10.1016/s0891-5849(98)00330-x. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y., Okunieff P. Radiation and third-generation chemotherapy. Hematol Oncol Clin North Am. 2004;18:55–80. doi: 10.1016/s0889-8588(03)00145-x. [DOI] [PubMed] [Google Scholar]
- 26.Kim W., Seong K.M., Youn B. Phenylpropanoids in radioregulation: Double edged sword. Exp Mol Med. 2011;43:323–333. doi: 10.3858/emm.2011.43.6.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brizel D.M., Wasserman T.H., Henke M. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 28.Salvemini D., Riley D.P., Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- 29.Berbée M., Fu Q., Boerma M. Gamma-tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad K.N., Kumar B., Yan X.D. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: A review. J Am Coll Nutr. 2003;22:108–117. doi: 10.1080/07315724.2003.10719283. [DOI] [PubMed] [Google Scholar]
- 31.Crane F.L. Biochemical functions of CoQ10. J Am Coll Nutr. 2001;20:591–598. doi: 10.1080/07315724.2001.10719063. [DOI] [PubMed] [Google Scholar]
- 32.Yasueda A., Urushima H., Ito T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: A systematic review. Integr Cancer Ther. 2016;15:17–39. doi: 10.1177/1534735415610427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hidaka T., Fujii K., Funahashi I. Safety assessment of CoQ10 (CoQ10) Biofactors. 2008;32:199–208. doi: 10.1002/biof.5520320124. [DOI] [PubMed] [Google Scholar]
- 34.Zhipeng W., Mingkai L., Shuyu C. Toxicity of coenzyme Q10: A report of 90-day repeated dose toxicity study in rats. J Toxicol Sci. 2007;32:505–514. doi: 10.2131/jts.32.505. [DOI] [PubMed] [Google Scholar]
- 35.Sikorska M., Lanthier P., Miller H. Nanomicellar formulation of CoQ10 (Ubisol-Q10) effectively blocks ongoing neurodegeneration in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model: Potential use as an adjuvant treatment in Parkinson's disease. Neurobiol Aging. 2014;35:2329–2346. doi: 10.1016/j.neurobiolaging.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roffe L., Schmidt K., Ernst E. Efficacy of CoQ10 for improved tolerability of cancer treatments: A systematic review. J Clin Oncol. 2004;22:4418–4424. doi: 10.1200/JCO.2004.02.034. [DOI] [PubMed] [Google Scholar]