Abstract
Purpose
This study aimed to evaluate the toxicity of prostate and pelvic lymph node stereotactic body radiation therapy (SBRT) for high-risk prostate cancer.
Methods and Materials
Twenty-three patients with high-risk or lymph node-positive prostate cancer were treated with SBRT that delivered 37.5 to 40 Gy in 5 fractions to the prostate and seminal vesicles, with concomitant treatment of the pelvic nodes to 25 Gy. In general, patients received neoadjuvant, concurrent, and adjuvant androgen deprivation therapy for a duration of 18 months. Toxicities were evaluated with the Common Terminology Criteria for Adverse Events, version 3.0. The median follow-up was 19 months (range, 3-48 months).
Results
Acute grade 1 gastrointestinal (GI) toxicities were noted in 2 patients (9.1%). No patient experienced acute grade ≥2 GI toxicity. Acute genitourinary (GU) grade 1, 2, and 3 toxicities were observed in 7 patients (31.8%), 8 patients (36.4%), and 1 patient (4.5%), respectively. Late grade 2 GI and GU toxicities were observed in 2 patients (9.1%) and 6 patients (27.3%), respectively. No late grade ≥3 GI toxicity was noted. Late grade ≥3 GU (hemorrhagic cystitis) was noted in 1 patient (4.5%), which responded to laser fulguration.
Conclusions
SBRT with pelvic lymph node radiation therapy was feasible and well tolerated. The incidence of grade ≥3 GU and GI toxicities was uncommon. Continued follow-up will be required to determine the long-term safety and efficacy of this approach for high-risk patients.
Introduction
A standard approach for the radiotherapeutic management of high-risk prostate cancer is the use of high-dose external beam radiation therapy delivered with conventional fractionation in conjunction with androgen deprivation therapy (ADT). Recently, the results of 6 randomized trials have demonstrated that employing a moderate hypofractionated schedule of 60 to 70 Gy with 2.5 to 3.4 Gy per fraction was associated with efficacy comparable to what was achieved with conventional fractionation techniques.1, 2, 3, 4, 5, 6 However, the radiation field in these trials included only the prostate or the prostate and seminal vesicles.
There has been increasing interest in the use of stereotactic body radiation therapy (SBRT) in prostate cancer during the last 5 to 10 years. Most patients who are treated with this approach have low or intermediate risk, and the use of SBRT for high-risk disease is still being evaluated. Yet, in a recent review, Gonzalez-Motta and Roach identified 20 studies with a relatively small number of patients with short median follow-up times in which high-risk patients were treated with SBRT monotherapy or SBRT was used as a boost to the prostate after the delivery of whole pelvis/prostate treatment.7 Although the preferred radiotherapeutic intervention for high-risk disease at our institution is brachytherapy combined with external beam radiation therapy, in selected patients, SBRT had been used to treat the prostate while simultaneously delivering a lower dose of SBRT to the pelvic lymph nodes. Most of these patients were not candidates for a brachytherapy boost because of medical comorbidities, the presence of baseline urinary symptoms, or logistic considerations for patients who were eager to undergo only short-course therapy. Herein, we report on the treatment technique employed and early tolerance outcomes experienced in this cohort.
Methods and Materials
Patient characteristics
From April 2009 to April 2018, 80 patients who were treated with SBRT monotherapy and classified as having high-risk disease, including radiographically node-positive disease, were identified. All initial prostate biopsies were reviewed, and the Gleason score was confirmed by an institutional pathologist. Among these 80 patients, 23 received SBRT to the prostate with simultaneous dose painting to the pelvic nodes and represent the subjects of this report. The remaining patients received SBRT to the prostate and seminal vesicles only; the lymph nodes were excluded because these patients were either treated on an SBRT trial in which nodal radiation fields were not included, had coexisting significant medical comorbidities, or were >80 years of age. Table 1 shows the patient characteristics of these 23 patients.
Table 1.
Patient characteristics
| Characteristics | Value |
|---|---|
| Age (y), mean (SD) | 74 (6.0) |
| Clinical T stage, n (%) | |
| T1 | 13 (56.5) |
| T2 | 3 (13.0) |
| T3 | 6 (26.1) |
| T4 | 1 (4.35) |
| Gleason score, n (%) | |
| 7 | 2 (8.7) |
| 8 | 10 (43.5) |
| 9 | 10 (43.5) |
| 10 | 1 (4.3) |
| Initial PSA level (ng/mL) | |
| Median (range) | 8.7 (3.3-170.4) |
| <10, n (%) | 13 (56.6) |
| 10-20, n (%) | 5 (21.7) |
| >20, n (%) | 5 (21.7) |
| Risk group, n (%) | |
| High risk without nodal involvement | 20 (87.0) |
| High risk with positive node | 3 (13.0) |
| Baseline prostate volume (cm3) | |
| Median (range) | 42 (14-214) |
| Baseline international prostate symptom score | |
| Mean (SD) | 8.7 (6.7) |
| Baseline international index of erectile dysfunction; n = 14 | |
| Median (range) | 18.5 (1-30) |
| Duration of ADT (mo) | |
| Mean (SD) | 13.0 (7.0) |
| <6, n (%) | 3 (13.0) |
| 6-12, n (%) | 10 (43.5) |
| >12, n (%) | 10 (43.5) |
| Radiation dose to prostate and pelvic node, n (%) | |
| 37.5 Gy and 25 Gy | 2 (8.7) |
| 40.0 Gy and 25 Gy | 21 (91.3) |
| Rectal spacer, n (%) | |
| Yes | 5 (21.7) |
| No | 18 (78.3) |
Abbreviations: ADT = androgen deprivation therapy; PSA = prostate-specific antigen; SD = standard deviation.
Treatment technique and schedules
Initially, all patients underwent fiducial marker placement. In general, 3 fiducial markers of 3 mm length and 1.2 mm diameter were transperineally placed into the prostate under transrectal ultrasound guidance. These markers were used to confirm and monitor the prostate position before and during each SBRT treatment. The operative technique was slightly modified after the hydrogel rectal spacer (SpaceOAR, Augmenix Inc., Waltham, MA) was introduced in November 2015. Five patients (21.7%) who opted for hydrogel spacer placement underwent insertion of 10 mL of the hydrogel into the perirectal space posterior to Denonvilliers' fascia (rectoprostatic fascia) after the fiducial markers were placed.
Patients were simulated with an empty rectum and full bladder per the institutional guidelines 1 week after fiducial marker placement. For the rectum preparation, patients were instructed to take an enema the day before and the day of the simulation and on the day of each treatment. During simulation, patients underwent Foley catheter insertion for urethral visualization. Patients were instructed to drink 8 ounces of water 45 minutes before imaging to distend the bladder; and this was repeated prior to each treatment. Patients were immobilized in the supine position with a custom thermoplastic mold (Aquaplast) that extended from the abdomen to the mid-thigh and ankle support. Computed tomography (CT) simulation was performed with 2-mm slice thickness extending from L1 to mid-femur. CT simulation was followed by magnetic resonance (MR) simulation on a 3T scanner in the treatment position to incorporate patients' immobilization with the use of an indexed, flat tabletop. Since June 2016, MR-only simulation and planning for prostate SBRT has been routinely used at our institution. A commercial synthetic CT software called MRCAT (MR for Calculating Attenuation) was used to generate the synthetic CT. The details of the algorithm, dosimetric validation, and clinical implementation have been described previously.8, 9 The algorithm uses a single mDIXON MR sequence along with a constrained shape model to estimate body contour and to segment bone structures. Five tissue types are classified, and a bulk electron density is assigned to these tissues to generate a synthetic CT. MR-based contouring and planning was performed in 16 patients (69.6%). The remaining patients were simulated with CT only due to MR contraindication, including cardiac pacemaker, prosthetic heart valve, or multiple vascular stent.
The clinical target volume (CTV) for the prostate included the entire prostate, involved extraprostatic tissue, and bilateral seminal vesicles. To create a planning target volume (PTV), 5-mm anterior and lateral, 3-mm posterior, and 2-mm superior-inferior margins were applied to the CTV. The CTV for the lymph nodes (CTV_NODES) was delineated along the bilateral external iliac vessels and internal iliac vessels, as seen on MR, in accordance with the Radiation Therapy Oncology Group guidelines. The PTV of the lymph nodes (PTV_NODES) was variable depending on the bowel and bladder volume presentation. Generally, PTV_NODES were defined as CTV_NODES plus a 5 to 8 mm margin in all directions. PTV margins around the nodes were tightened when overlap was noted in the bowel and bladder. The summation of the CTV_NODES plus PTV_NODES margin around the pelvic vessels was approximately 10 mm (range, 6-15 mm), and MR-based contouring facilitated the use of tighter margins than CT-based contouring. Organs at risk, including the rectum, bladder, femoral heads, large bowel, small bowel, bladder trigone, and urethra, were also outlined.
A total dose of 37.5 to 40 Gy in 5 fractions delivered on alternating days was prescribed to the PTV, with a concomitant reduced dose to the PTV_NODES, which received 25 Gy in 5 fractions. There was no additional dose to gross pelvic nodes in positive-node patients because gross pelvic nodes disappeared after neoadjuvant ADT or underwent pelvic lymphadenectomy before simulation. Dose–volume tissue constraints for treatment planning are shown in Table 2. The D95 of the PTV achieved 90% to 100% of the prescription dose with a mean dose that ranged from 99% to 104%. On average, 85% of the PTV received the prescription dose with a range of 67% to 95%. The D95 of the PTV_NODES achieved 95% to 103% of the prescription dose, with a mean dose that ranged from 101% to 111%. The average nodal PTV volume that received the prescription was 96% (range, 88%-100%). All normal tissue constraints were met.
Table 2.
Treatment planning dose-volume constraints for normal tissues
| Structure | Parameter | Dose-volume constraint |
|
|---|---|---|---|
| PTV 40 Gy | PTV 37.5 Gy | ||
| Rectum | Max point dose, Gy | 41.2 | 38.6 |
| D1cc, Gy | 38.5 | NA | |
| Mean dose, Gy | 13 (16.4∗) | 12.2 (15.4∗) | |
| V24 Gy, % | <25 | <25 | |
| V30.15 Gy, cm3 | ≤8 | ≤8 | |
| Bladder | Max point dose, Gy | 42 | 39.4 |
| D53%, Gy | 24 | 24 | |
| D1cc | <103% of prescription dose | ||
| Femoral heads | Max point dose, Gy | 31 | 31 |
| Large bowel | Max point dose, Gy | 29 | 29 |
| Small bowel | Max point dose, Gy | 26.5 | 26.5 |
| Bladder trigone | Max point dose, Gy | 38 | 38 |
| Urethra | Max point dose, Gy | 42 | 39.4 |
| D1cc, Gy | 40 | NA | |
Abbreviations: Max = maximum; NA = not applicable; PTV = planning target volume.
This criteria was used if the first criterion is not achieved.
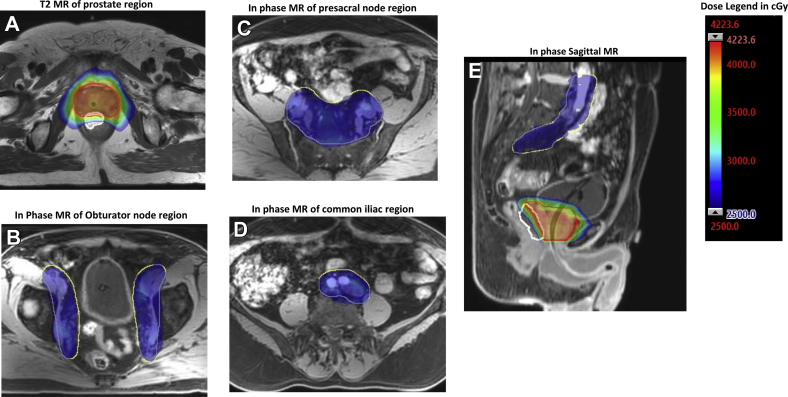
Treatment plans were generated with the Analytical Anisotropic Algorithm using the Eclipse treatment planning system (Varian Medical Systems, Inc.), versions 11.0 and 13.6, in 12 and 10 patients, respectively. The in-house treatment planning system (top module) was used for 1 patient. The maximum dose rate was 600 MU/min. Patients were either planned with static 9-field intensity modulated radiation therapy (IMRT; n = 3) or 3- or 4-arc volumetric arc therapy (VMAT; n = 20). Figure 1 shows the dose distribution from a 3-arc VMAT plan, overlaid on a T2w MR imaging scan.
Fig. 1.
Example of volumetric arc dose distribution of prostate concomitant with pelvic node radiation overlay on (A) axial T2-weighted, (B-D) axial mDIXON, and (E) sagittal mDIXON water contrast magnetic resonance image. Images are in transverse level of (A) prostate, (B) obturator region, (C) presacral region, and (D) common iliac region. Planning target volume of the prostate plus seminal vesicles, pelvic nodes and rectal spacer are contoured red, yellow, and white, respectively. Highlighted red/pink and blue areas correspond to 40 Gy and 25 Gy, respectively.
Patients initiated SBRT treatment within 2 weeks after simulation. Full-bladder and empty-rectum protocols were carried out before each treatment day. At our institution, daily 2-dimensional orthogonal KVs are used to position patients based on implanted fiducial markers. Two-dimensional KVs are followed by cone beam CT acquisition. Our physicians evaluate the bladder and rectum filling on daily cone beam CT scans to ensure that the change in filling did not cause a significant shift in prostate motion and the seminal vesicles have not moved out of the PTV. Patients are often repositioned (tilt is adjusted) if the bones are off by >0.5 cm when cone beam CT scans are matched to the planning CT based on implanted fiducials. Cone beam CTs are done before treatment. Intrafraction movement is monitored using Elipse intrafraction monitoring software, called IMR, which is based on a single 2-dimensional KV image acquired during treatment, either after a set MU for IMRT plans or preset gantry intervals for VMAT.
All patients in this cohort were treated with neoadjuvant, concurrent, and adjuvant ADT. ADT was planned in general for a total duration of 18 months, using luteinizing hormone–releasing hormone agonist via intramuscular injection.
Assessment of treatment outcome and toxicities
The follow-up schedule was 3 months after completion of radiation therapy, and subsequently every 3 to 6 months for the first 2 years and annually thereafter. For each visit, patients were evaluated for rectal and urinary toxicities in accordance with the Common Terminology Criteria for Adverse Events, version 3.0, grading system. Acute toxicity was defined as any toxicities that occurred within 3 months after radiation, and late toxicity was defined as any toxicities that occurred thereafter. All patients were available for gastrointestinal (GI) and genitourinary (GU) toxicity assessment. However, only 22 patients were evaluable for late GI/GU toxicity. Prostate-specific antigen was obtained at 3 months after the end of radiation therapy, and then 6 months thereafter. Patients who reached 2 years of follow-up after treatment were encouraged to undergo a posttreatment biopsy, which is our institutional standard of practice after SBRT. Biochemical failure was assessed using the Phoenix definition (prostate-specific antigen nadir + 2 ng/mL).
Statistical analysis
These retrospective data were locked at the end of July 2018. The follow-up time was calculated between the last follow-up date and the end date of radiation therapy. Descriptive analyses were summarized as means with standard deviations (SD), and medians with ranges for normally and nonnormally distributed continuous characteristics. Frequencies and proportions were reported for categorical characteristics. The Shapiro-Wilk normality test was used for distributed testing.
Results
The maximal toxicity incidence rates are summarized in Table 3. The median follow-up time was 19 months (range, 3-48 months) The maximum acute grade 1 GI toxicities were observed in 2 patients (9.1%). No grade ≥2 acute GI toxicities were noted. Patients who had acute GI toxicity experienced diarrhea, which resolved after treatment completion. The maximum acute GU toxicities were grade 1 in 7 patients (31.8%), grade 2 in 8 patients (36.4%), and grade 3 in 1 patient (4.5%). The most common GU toxicity was urinary frequency and urgency (70%). In almost all patients, symptoms were ameliorated after taking alpha blocker medications, except in 1 patient who experienced grade 3 GU toxicity of acute urinary retention and frequency that required catheterization.
Table 3.
Summary of acute and late GI and GU toxicities
| Grade 0 (%) | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | |
|---|---|---|---|---|
| Acute GI toxicity | 20 (90.9) | 2 (9.1) | 0 | 0 |
| Acute GU toxicity | 6 (27.3) | 7 (31.8) | 8 (36.4) | 1 (4.5) |
| Late GI toxicity | 18 (81.8) | 2 (9.1) | 2 (9.1) | 0 |
| Late GU toxicity | 7 (31.8) | 8 (36.4) | 6 (27.3) | 1 (4.5) |
Abbreviations: GI = gastrointestinal; GU = genitourinary.
Late GI toxicity was grade 1 in 2 patients (9.1%) and grade 2 in 2 patients (9.1%). No patient experienced grade ≥3 late GI toxicity. Patients who had grade 2 late GI toxicity presented with rectal bleeding. In the first patient, a colonoscopy diagnosed hemorrhoidal disease without active bleeding. The second patient had underlying atrial fibrillation and was treated with an oral anticoagulant; this patients received a diagnosis of radiation proctitis at 7 months from completion of SBRT and required argon plasma laser cauterization via sigmoidoscopy. Late grade 1 and grade 2 GU toxicities were observed in 8 (36.4%) and 6 (27.3%) patients, respectively. Grade ≥3 late GU toxicities was observed in 1 patient. Urinary frequency and urgency were the most common in grade 2 late GU toxicities and were noted in 4 of the 6 patients who experienced grade 2 toxicity. In contrast, the other 2 patients experienced grade 2 urinary retention and mild urge incontinence.
All symptoms fully resolved during the follow-up period, except in 1 patient who continued to require alpha blocker medications for chronic urinary frequency and urgency. The patient who experienced grade 3 late GU toxicity developed hematuria, which required a blood transfusion and intervention at 22 months after treatment. Cystoscopy revealed radiation cystitis. Of note, this patient had prior aortic valve replacement that required ongoing anticoagulation therapy. He had no further hematuria after focal cystoscopic fulguration. The incidence of each acute and late toxicity profile is shown in Table 4.
Table 4.
Acute and late toxicity profiles
| Acute | Grade 0 (%) | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) |
|---|---|---|---|---|
| Urinary frequency/urgency | 8 (36.4) | 7 (31.8) | 6 (27.3) | 1 (4.5) |
| Urinary retention | 17 (77.3) | 1 (4.5) | 4 (18.2) | 0 |
| Urinary incontinence | 22 (100) | 0 | 0 | 0 |
| Urinary hemorrhage | 22 (100) | 0 | 0 | 0 |
| Diarrhea | 20 (90.9) | 2 (9.1) | 0 | 0 |
| GI hemorrhage | 22 (100) | 0 | 0 | 0 |
| Late | ||||
| Urinary frequency/urgency | 7 (31.8) | 11 (50.0) | 4 (18.2) | 0 |
| Urinary retention | 16 (72.7) | 5 (22.7) | 1 (4.5) | 0 |
| Urinary incontinence | 20 (90.9) | 1 (4.55) | 1 (4.55) | 0 |
| Urinary hemorrhage | 20 (90.9) | 1 (4.55) | 0 | 1 (4.55) |
| Diarrhea | 21 (95.5) | 1 (4.5) | 0 | 0 |
| GI hemorrhage | 18 (81.8) | 2 (9.1) | 2 (9.1) | 0 |
Abbreviation: GI = gastrointestinal.
The mean duration of ADT was 13.0 ± 7.1 months. The major reasons for ADT discontinuation were intolerable side effects in 5 patients (21.7%), preferences of prescribed physician in 5 patients (21.7%), and patient refusal in 3 patients (13.0%). One patient (4.3%) with controlled prostate cancer died of recurrent lung cancer. The 2-year PSA relapse-free survival was 95.7%. A total of 9 of 23 patients (39.1%) reached the 2-year time point in their follow-up, and 6 underwent prostate rebiopsy. Five of 6 patients had negative biopsy results, and 1 patient had a positive biopsy result in 7 of 16 core biopsy tissues sampled. All rebiopsy patients were biochemically controlled at the time of the biopsy.
Discussion
This retrospective report highlights the feasibility of achieving the established target and normal tissues dose-volume constraints that were set, as well as early tolerance outcomes for concomitant treatment of high-dose SBRT to the prostate and a simultaneous lower dose to pelvic lymph nodes. In addition to the patient convenience of a short-course regimen, hypofractionated regimens may offer a superior radiobiologic advantage in delivering a higher biological dose to the lymph nodes. Using an alpha/beta ratio of 1.5 Gy, the biological effective dose is 108.3 Gy for a total dose of 25 Gy in 5 fractions versus 99 Gy for a total dose of 45 Gy in 25 fractions. A low incidence of acute toxicities was noted, and late toxicities to date have been comparable with the tolerance outcomes observed with whole pelvic IMRT-based treatment.10, 11 Longer follow-up will be required to confirm these findings.
The application of pelvic nodal SBRT concomitant with SBRT to the primary site for high-risk patients has been reported by others and is currently under evaluation in the ongoing prospective SATURN12 and SPORT trials.13 Murthy et al enrolled 68 high-risk and positive-node patients.14 In their analysis, 31 patients (45%) received a total dose of 35 to 37.5 Gy to the prostate concomitant with 25 Gy to the pelvic region. All patients had node-positive disease. At a median follow-up time of 18 months, pelvic nodal radiation therapy resulted in acceptable GI and GU toxicity. Acute grade 2 GI and GU toxicities were 3% and 12%, respectively, for the entire cohort. No acute grade ≥3 GI or GU toxicity was noted. Late grade 3 GI and GU toxicities were 0% and 3%, respectively.
The phase 1/2 SATURN study recently reported on the feasibility and tolerance of gantry-based SBRT, delivering 40 Gy to the prostate concurrent with 25 Gy to the pelvis and seminal vesicles in 5 weekly fractions.12 ADT was used for 12 to 18 months. Thirty patients were enrolled in this study. With a median follow-up of 25.7 months, grade 2 GU and GI toxicities were observed in 46.7% and 3.3%, respectively, at 3 months, and 52% and 32%, respectively, at ≥6 months. No grade ≥3 toxicities were noted.
Bauman et al reported on a phase 1/2 study of SBRT delivering 40 Gy to the prostate synchronously with 25 Gy to the pelvic nodes in 1 fraction per week over 5 weeks.15 Accrual to the trial was terminated early after 15 patients were treated because of the observed higher-than-anticipated late toxicities, which yielded a 60% incidence of grade ≥2 GI or GU toxicity and 26% of grade ≥3 GI or GU toxicity at 6 months. The authors indicated that the higher toxicity rates observed in the study at 6 months after completion of therapy are likely related to several factors, such as the larger margins of 5 mm circumferentially used for the PTV of the prostate, which was based on CT rather than MR imaging for target delineation. In addition, the use of cone beam CT without fiducial markers for image guidance and the inclusion of frail patients with significant comorbidities may have further contributed to the higher toxicity profile reported.
In the same study,15 3 patients developed grade 3 rectal bleeding that required argon plasma coagulation, and 1 patient developed a grade 4 small bowel obstruction. In contrast with these findings, we did not observe grade ≥3 acute/late GI toxicities and only noted 1 acute and late grade 3 GU toxicity each of urinary retention and GU hemorrhage, respectively, which subsequently resolved. Our well-tolerated profile may be associated with our tighter margins for the PTV of the prostate, the use of MR-based contouring and treatment planning, and possibly the use of a hydrogel spacer in selected patients (although only 20% of this cohort were treated with a hydrogel spacer). The use of a spacer has been associated with a reduction of the rectal dose, decreased toxicity, and improved quality of life among patients treated with conventionally fractionated external beam radiation therapy regimens, as reported by Hamstra et al.16 Of note, we currently do not recommend the placement of spacers in patients with gross posterior-located extracapsular disease.
The outcome of SBRT in the published literature for high-risk patients is summarized in Table 5. In all but 3 other studies, SBRT was directed to the prostate and seminal vesicles only. Three studies report on the long-term biochemical relapse-free survival outcomes of high-risk prostate cancer.17, 18, 19 With the fractionation scheme ranging from 32 to 40 Gy in 4 to 5 fractions, the 5-year biochemical relapse-free survival rate was 69% to 91%. The current study demonstrated biochemical control of 95.7%. Nevertheless, a comparison of outcomes is limited by the short follow-up time and residual suppressive effects of ADT in the current study. Although the incidence of grade ≥3 GI or GU toxicity was very low in this series, we recognize that small bowel and rectal toxicities are potential concerns for elective pelvic nodal irradiation, and the value and role of elective nodal radiation, especially in the setting of SBRT, is unknown. The Radiation Therapy Oncology Group study 09-24 is examining the impact of pelvic nodal radiation in a 2-arm design in the setting of patients treated with dose-escalated conventionally fractionated external beam radiation therapy. In this trial, patients undergo external beam radiation therapy using 45 Gy to the pelvis, followed by a boost of 79.2 Gy to the prostate or prostate radiation therapy alone. Long-term ADT is used in both arms.
Table 5.
Results of stereotactic body radiation therapy studies in high-risk prostate cancer
| Author | Total no. of patients (no. of HR) | Median FU (mo) | Dose (Gy) | Field Size | bRFS (%) | Toxicity |
|
|---|---|---|---|---|---|---|---|
| Grade 2, 3 GI (%) | Grade 2, 3 GU (%) | ||||||
| Kang17 | 44 (29) | 40 | 32-36/4 fx | P + SV | 90.9 | Acute: 25, 0 | Acute: 25, 0 |
| Late: 14, 0 | Late: 14, 0 | ||||||
| Bolzicco20 | 100 (17) | 36 | 35/5 fx | P + SV | 94∗ | Acute: 18, 0 | Acute: 12, 0 |
| Late: 1, 0 | Late: 3, 1 | ||||||
| Chen21 | 100 (8) | 27 | 35-36.35/5 fx | P + SV | 87.5 | Late gr ≥2; 1 | Late gr ≥2; 31 |
| King18 | 1100 (125) | 36 | 35-40/5 fx | P + SV | 81 | NR | NR |
| Lee22 | 45 (13) | 63 | 36/5 fx | P + SV | 89.7∗ | Acute: 4.4, 0 | Acute: 4.4, 0 |
| Late: 4.4, 0 | Late: 4.4, 4.4 | ||||||
| Berne-Tich23 | 142 (18) | 38 | 35-37.5/5 fx | P + SV | 83.9 | Acute: 4, 0 | Acute: 28, 2 |
| Late: 3, 0 | Late: 14, 2 | ||||||
| Davis24 | 437 (33) | 20 | 35-38/4-5 fx | P + SV | 89.8 | Acute: 1, 0 | Acute: 2, 0 |
| Late: 2, 0 | Late: 8, 0 | ||||||
| Ricco25 | 270 (32) | 50 | 35-37.5/5 fx | P + SV | 92 | Acute: NR | Acute: NR, 3.3 |
| Late: 2.7, 0 | Late: 16, 0 | ||||||
| Katz19 | 515 (38) | 72 | 35-36.25/5 fx | P + SV | 65 | Acute: < 5, 0 | Acute: < 5, 0 |
| Late: 4, 0 | Late: 9, 1.7 | ||||||
| Kotecha26 | 24 (13) | 25 | 36.25/5 fx | P + SV | 95.8∗ (boost 50 Gy) | Acute: 0, 0 | Acute: 38, 0 |
| Late: 8, 0 | Late: 4, 0 | ||||||
| Koskela27 | 218 (111) | 23 | 35-36.25/5 fx | P + SV | 92.8 | Acute: 0.4, 0 | Acute: 1.4, 0 |
| Late: NR, 0.9 | Late: NR, 1.8 | ||||||
| Murthy14 | 68 (31) | 18 | 35-37.5/5 fx | P + SV ± LN | 94∗ | Acute: 4, 0 | Acute: 12, 0 |
| Late: 4, 0 | Late: 4.5, 2.5 | ||||||
| Masunuru12 | 30 | 25 | 40/5 fx | P + SV ± LN | NR | Acute: 3.3, 0 | Acute: 46.7, 0 |
| Late: 32, 0 | Late: 52, 0 | ||||||
| Current study23 | (19) | 18 | 37.5-40/5 fx | P + SV + LN | 95.7 | Acute: 0, 0 | Acute: 36.4, 4.5 |
| Late: 9.1, 0 | Late: 27.3, 4.5 | ||||||
Abbreviations: bRFS = biochemical relapse-free survival; fx = fractions; FU = follow-up; GI = gastrointestinal; GU = genitourinary; HR = high risk; LN = pelvic lymph node; NR = not reported; P = prostate; SV = seminal vesicle.
All risk subgroup.
There are several limitations of this report, including its retrospective nature, the limited follow-up, and the fact that, to date, the role of SBRT to the prostate concomitant with pelvic radiation for high-risk prostate cancer is not well established. We note that although SBRT has been used for high-risk disease, its use for this cohort of patients is not established and is more routine for low- and intermediate-risk patients. Albeit with a short follow-up, SBRT in this report appears to yield results similar to those achieved with conventionally fractionated treatments.
The intent of this report is to present our technique for SBRT with concomitant treatment of the nodes. It is possible that late toxicity could be higher with a longer follow-up, and these patients will need to be followed carefully. Although a comparison with brachytherapy, followed by prostate/pelvic node radiation therapy, is outside the scope of this study, our initial report on SBRT with simultaneous pelvic node boost indicates that this approach is feasible and early toxicity outcomes are acceptable.
Although the intent for the patients reported here was to deliver a duration of 18 months of ADT, the median duration was 13 months because 50% of patients were elderly and experienced significant ADT symptoms. Finally, the potential tumor control advantage of adding nodal treatment in high-risk node-negative patients undergoing prostate SBRT is still unclear and will require further investigation.
Conclusions
Dose-painting SBRT to the prostate gland with concurrent pelvic node irradiation in 5 fractions was associated with a favorable toxicity profile and excellent early tumor control outcomes. This technique requires further prospective evaluation with a larger cohort of patients to confirm our findings.
Acknowledgments
The authors are grateful to Mr. James Keller for his help in editing this manuscript.
Footnotes
Sources of support: This research was partially supported by the National Institutes of Health, National Cancer Institute, Cancer Center Support Grant/Core Grant (P30 CA008748).
Conflicts of interest: Dr Zelefsky serves as a consultant for Augmenix. Dr McBride was given honoraria by Bristol Meyers in 2016 and receives research funding from Janssen.
References
- 1.Lee W.R., Dignam J.J., Amin M. NRG Oncology RTOG 0415: A randomized phase 3 noninferiority study comparing 2 fractionation schedules in patients with low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2016;94:3–4. [Google Scholar]
- 2.Aluwini S., Pos F., Schimmel E. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): Late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:464–474. doi: 10.1016/S1470-2045(15)00567-7. [DOI] [PubMed] [Google Scholar]
- 3.Dearnaley D., Syndikus I., Mossop H. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catton C.N., Lukka H., Gu C.S. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35:1884–1890. doi: 10.1200/JCO.2016.71.7397. [DOI] [PubMed] [Google Scholar]
- 5.Arcangeli G., Saracino B., Arcangeli S. Moderate hypofractionation in high-risk, organ-confined prostate cancer: Final results of a phase III randomized trial. J Clin Oncol. 2017;35:1891–1897. doi: 10.1200/JCO.2016.70.4189. [DOI] [PubMed] [Google Scholar]
- 6.Pollack A., Walker G., Horwitz E.M. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31:3860–3868. doi: 10.1200/JCO.2013.51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Motta A., Roach M., 3rd Stereotactic body radiation therapy (SBRT) for high-risk prostate cancer: Where are we now? Pract Radiat Oncol. 2018;8:185–202. doi: 10.1016/j.prro.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Tyagi N., Fontenla S., Zelefsky M. Clinical workflow for MR-only simulation and planning in prostate. Radiat Oncol. 2017;12:119. doi: 10.1186/s13014-017-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyagi N., Fontenla S., Zhang J. Dosimetric and workflow evaluation of first commercial synthetic CT software for clinical use in pelvis. Phys Med Biol. 2017;62:2961–2975. doi: 10.1088/1361-6560/aa5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashman J.B., Zelefsky M.J., Hunt M.S., Leibel S.A., Fuks Z. Whole pelvic radiotherapy for prostate cancer using 3D conformal and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:765–771. doi: 10.1016/j.ijrobp.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 11.Kaidar-Person O., Roach M., 3rd, Créhange G. Whole-pelvic nodal radiation therapy in the context of hypofractionation for high-risk prostate cancer patients: A step forward. Int J Radiat Oncol Biol Phys. 2013;86:600–605. doi: 10.1016/j.ijrobp.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Musunuru H.B., D'Alimonte L., Davidson M. Phase I/II study of stereotactic ablative radiotherapy including regional lymph node irradiation for patients with high-risk prostate cancer (SATURN): Early results. Int J Radiat Oncol Biol Phys. 2018 doi: 10.1016/j.ijrobp.2018.07.2005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Lyons C., McGarry C., Hounsell A. SPORT high-risk trial: A randomised feasibility study evaluating stereotactic prostate radiotherapy in high-risk localised prostate cancer with or without elective nodal irradiation. Eur J Surg Oncol. 2016;42:S235. [Google Scholar]
- 14.Murthy V., Gupta M., Mulye G. Early results of extreme hypofractionation using stereotactic body radiation therapy for high-risk, very high-risk and node-positive prostate cancer. Clin Oncol (R Coll Radiol) 2018;30:442–447. doi: 10.1016/j.clon.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Bauman G., Ferguson M., Lock M. A phase 1/2 trial of brief androgen suppression and stereotactic radiation therapy (FASTR) for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2015;92:856–862. doi: 10.1016/j.ijrobp.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Hamstra D.A., Mariados N., Sylvester J. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Kang J.K., Cho C.K., Choi C.W. Image-guided stereotactic body radiation therapy for localized prostate cancer. Tumori. 2011;97:43–48. doi: 10.1177/030089161109700109. [DOI] [PubMed] [Google Scholar]
- 18.King C.R., Freeman D., Kaplan I. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109:217–222. doi: 10.1016/j.radonc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Katz A., Formenti S.C., Kang J. Predicting biochemical disease-free survival after prostate stereotactic body radiotherapy: Risk-stratification and patterns of failure. Front Oncol. 2016;6:168. doi: 10.3389/fonc.2016.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolzicco G., Favretto M.S., Satariano N., Scremin E., Tambone C., Tasca A. A single-center study of 100 consecutive patients with localized prostate cancer treated with stereotactic body radiotherapy. BMC Urol. 2013;13:49. doi: 10.1186/1471-2490-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L.N., Suy S., Uhm S. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: The Georgetown University experience. Radiat Oncol. 2013;8:58. doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S.W., Jang H.S., Lee J.H., Kim S.H., Yoon S.C. Stereotactic body radiation therapy for prostate cancer patients with old age or medical comorbidity: A 5-year follow-up of an investigational study. Medicine (Baltimore) 2014;93:e290. doi: 10.1097/MD.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernetich M., Oliai C., Lanciano R. SBRT for the primary treatment of localized prostate cancer: The effect of gleason score, dose and heterogeneity of intermediate risk on outcome utilizing 2.2014 NCCN risk stratification guidelines. Front Oncol. 2014;4:312. doi: 10.3389/fonc.2014.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis J., Sharma S., Shumway R. Stereotactic body radiotherapy for clinically localized prostate cancer: Toxicity and biochemical disease-free outcomes from a multi-institutional patient registry. Cureus. 2015;79:e395. doi: 10.7759/cureus.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricco A., Manahan G., Lanciano R. The comparison of stereotactic body radiation therapy and intensity-modulated radiation therapy for prostate cancer by NCCN risk groups. Front Oncol. 2016;6:184. doi: 10.3389/fonc.2016.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotecha R., Djemil T., Tendulkar R.D. Dose-escalated stereotactic body radiation therapy for patients with intermediate- and high-risk prostate cancer: Initial dosimetry analysis and patient outcomes. Int J Radiat Oncol Biol Phys. 2016;95:960–964. doi: 10.1016/j.ijrobp.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Koskela K., Palmgren J.E., Heikkila J. Hypofractionated stereotactic body radiotherapy for localized prostate cancer - first Nordic clinical experience. Acta Oncol. 2017;56:978–983. doi: 10.1080/0284186X.2017.1288923. [DOI] [PubMed] [Google Scholar]