Abstract
Pyrimethanil (PYM) is a fungicide used pre- and post-harvest on many crops. It has a low acute toxicity but is of toxicological concern because of its antiandrogenic properties. The aim of the current work was to investigate some metabolism and estimate elimination kinetics of PYM in humans after experimental oral and dermal exposure. A liquid chromatography triple quadrupole mass spectrometry (LC–MS-MS) method was developed and validated for the analysis of PYM and its metabolite 4-hydroxypyrimethanil (OH–PYM) in human urine. The method was applied to analyze urine obtained from two volunteers experimentally exposed to PYM. The elimination of OH–PYM seemed to follow first-order kinetics and a two-phase excretion. After the oral exposure, the elimination half-life of OH–PYM in the rapid phase was 5 and 3 h for the female and male volunteer, respectively. In the slower phase, it was 15 h in both volunteers. After the dermal exposure, the half-life in the rapid phase was 8 h in both volunteers. In the slower phase, it was 30 and 20 h, respectively. About 80% of the oral dose was recovered as urinary OH–PYM in both volunteers. The dermal dose recovered as urinary OH–PYM was 9.4% and 19%, in the female and male volunteer, respectively. OH–PYM was mainly found as a conjugate of sulfonate and glucuronic acid. No free PYM was found. The analytical method showed good within-run, between-run and between-batch precision with a coefficient of variation between 6% and 12%. A limit of detection of 0.1 ng/mL and a limit of quantification of 0.4 ng/mL were achieved for both the analytes. The method was applied to biomonitor PYM exposure in populations in Sweden. OH–PYM was detected in nearly 50% and 96% of samples from the environmentally and occupationally exposed populations, respectively.
Introduction
Pyrimethanil (PYM) is a pre- and post-harvest fungicide, used on fruits and vegetables. PYM was first introduced in the 1990's and has a mode of action that is different from many other fungicides (1). It controls sporulation in the pathogens by inhibition of both the methionine biosynthesis and secretion of cell wall degrading enzymes.
Agricultural work involves a risk of exposure to pesticides like PYM. The general population may also be environmentally exposed, mainly via diet, although dermal exposure cannot be completely ruled out due to handling of foods treated with PYM. PYM has a low acute toxicity in humans but adverse effects have been reported in animal studies. These effects are primarily seen on the main target organs of PYM, the liver and the thyroid (2, 3). It is of toxicological concern as it has been found (in vitro) to be an endocrine disruptor and seems to disrupt the thyroid–pituitary homeostasis in rodents (4, 5).
In the risk assessment of pesticides, all routes of exposure, namely - oral, dermal and inhalation - should be considered (6). Biomarkers of exposure take all these routes into account and give an estimate of the internal exposure. However, a biomarker needs to be validated for its intended use (7). Studies on the uptake of PYM through these different routes in humans are lacking, a short coming in the view of safety control among agricultural workers. Further, there is a need for human studies of the exposure-response relationship. In animal studies, 4-hydroxypyrimethanil (OH–PYM) is reported as the major metabolite of PYM and is mainly excreted in urine. The parent PYM undergoes aromatic oxidation to form OH–PYM, which forms conjugates with sulfonate and glucuronic acid (3). Currently, there is no established exposure biomarker for PYM in humans.
The aim of this work was to estimate basic elimination kinetics and some metabolites of PYM in humans via the oral and dermal routes. The elimination kinetics of PYM was investigated by measuring one major metabolite in urine. A further objective was to develop and validate an LC/MS/MS method for the analysis of OH–PYM in human urine. In addition, our method was applied to determine the PYM exposure by measuring OH–PYM in environmentally and occupationally exposed populations in southern Sweden.
Experimental
Chemicals and materials
PYM, 4, 6-dimethyl-N-phenyl-2-pyrimidinamine, was purchased from Dr Ehrenstorfer (Augsburg, Germany). The metabolite OH–PYM, 4-[(4,6-dimethyl-2-pyrimidinyl) amino] phenol and the internal standard (IS) [2H4] OH–PYM were purchased from Toronto Research Chemicals (North York, ON, Canada). Acetic acid (glacial) was from Fisher Scientific (Loughborough, UK). Methanol and acetonitrile (hyper grade for LC–MS), ammonium acetate (EMSURE ACS, Reag. Ph Eur) and ammonia (25%) were from Merck (Darmstadt, Germany). β–glucuronidase/arylsulfatase from Helix pomatia and β–glucuronidase from E.Coli were both purchased from Roche Diagnostics Scandinavia AB (Bromma, Sweden). Formic acid (FA) was from Sigma-Aldrich Inc. (St. Louis, MO, USA). Solid phase extraction (SPE) columns, silica-based ISOLUTE®−96 ENV + 50 mg fixed-well plate were from Biotage (Uppsala, Sweden).
Instrumentation
The samples were analyzed using a triple quadrupole linear ion trap mass spectrometer, equipped with TurboIonSpray source (QTRAP 5500; AB Sciex, Foster City, CA, USA) coupled to a liquid chromatography system (UFLCRX, Shimadzu Corporation, Kyoto, Japan). The analysis was performed by selected reaction monitoring (SRM) in the positive ionization mode. The data acquisition and processing was performed using the Analyst software (AB Sciex). The LightSight Software 2.2.1 (AB Sciex) was used for prediction of metabolites of PYM in urine. The ion scans were acquired on a quadrupole time-of-flight mass spectrometer (QTOF; TripleTOF 5600, AB Sciex,) coupled to an LC system (UFLCRX, Shimadzu Corporation).
Preparation of samples
The sample preparation is described in detail in Supplementary data. The urine samples were thawed and aliquots of 500 μL were pipetted into 2 mL 96 well-plates. For calibration standards, PYM and OH–PYM were accurately weighed in 10-mL flasks in duplicates and dissolved in methanol. These stock solutions were diluted to final concentrations in the range of 10–4,000 ng/mL. A blank urine sample obtained from a healthy volunteer was spiked to prepare the calibration standards ranging from 0.25 to 100 ng/mL urine. For quality control (QC) samples, the blank urine sample was spiked with PYM and OH–PYM to give final concentrations of 1, 5 and 10 ng/mL. The QC samples were prepared in bulk (50 mL each) for the three concentrations and stored at −20°C as 2-mL aliquots to facilitate for a single use.
The urine samples, calibration standards, QCs and chemical blanks (prepared in milli-Q water) were added with 150 μL of 1-M ammonium acetate buffer (6.5 pH), 10 μL β-glucuronidase/arylsulfatase enzyme and 10 μL IS. The plates were covered with silicon mats (Sealing Mat, 96 square well, Kinesis, Cambridgeshire, UK), vortex-mixed and incubated overnight at 37°C with agitation. Further, the samples were applied on the 96-well SPE plate (silica-based ISOLUTE®−96 ENV + 50 mg fixed-well plate) which was preconditioned with methanol and water. The samples were applied thereafter and washed with water and 1% acetic acid in 40% methanol. The analytes were eluted into a 96-well plate using 1 mL of 5% ammonia (v/v) in acetonitrile. The 96-channel equipment CEREX 96 was used to aid extraction. The eluted samples were mixed and centrifuged at 3000 x g for 10 min before analysis.
Analysis
The chromatographic separation was carried out on a Poroshell 120EC-C18 column (4.6 × 100 mm, 2.7 μm, Agilent Technologies, Santa Clara, CA, USA). The chosen column provided a good separation of PYM and OH–PYM with stable retention times. The molecular ions for PYM (m/z 200.2) and OH–PYM (m/z 216.1) were formed in the positive mode with electro spray ionization (ESI). The transitions m/z 200.2/107.1, m/z 216.1/107.0 and m/z 220.1/111.0 for PYM, OH–PYM and [2H4] OH–PYM, respectively, at collision energy of 32 V, were selected as quantifier ions due to the best signal-noise ratio. The transition m/z 200.2/82.0 for PYM at collision energy 32 V, and transition m/z 216.1/159.2 for OH–PYM and m/z 220.1/163.2 for [2H4] OH–PYM at collision energy of 36 V, were selected as qualifier ions. The mobile-phase consisted of 0.1% formic acid in milli-Q water as (A) and 0.1% formic acid in methanol as (B). The sample injection volume was 3 μL and the flow rate through the column was 0.7 mL/min. The column temperature was maintained at 40°C. The mobile-phase gradient started with 30% mobile-phase B, followed by a linear gradient to 95% and held at 95% for 1.0 min before equilibration. The total analytical run time per sample, including equilibration, was 7.0 min. The analysis is described in detail in Supplementary data. All the samples were prepared and analyzed in duplicates. Each sample batch consisted of eight calibration standards, a urine blank and two sets each of QCs and chemical blanks.
Experimental exposure of volunteers
Two healthy non-smoking volunteers, one female (age: 69 years, weight: 57 kg) and one male (age: 45 years, weight: 75 kg) gave their written informed consent to participate. The volunteers were not under any medication during the study period and had minimized the intake of conventionally grown food.
Oral exposure
The volunteers received a single oral dose of PYM, which was equivalent to 50% of the accepted daily intake (ADI), which is 0.17 mg/kg/day (2). PYM was dissolved in 10 mL of ethanol to a concentration of 2 mg/mL, which was used as a stock solution. An organic cranberry juice (250 mL) was spiked with the appropriate amount of PYM to give a dose of 4.85 mg and 6.37 mg, for the female and male, respectively.
Dermal exposure
The volunteers received a single application of PYM on a 50-cm2 area on the inner side of the forearm. The dermal exposures were performed 12 weeks after the oral exposures. The dose of PYM was equivalent to 50% of the ADI as above. A volume of 658 μL and 866 μL, for the female and male volunteer respectively, was drawn from a 7.4 mg/mL stock solution of PYM and administered topically on the forearm. The application was administered in a vehicle of 75% (v/v) ethanol and 25% milli-Q water. After the application, the vehicle was evaporated to dryness and the skin was occluded with aluminum foil. After 8 h of exposure, the remainder of the dose was wiped off with cotton swabs moistened with the vehicle. Thereafter, the exposed area was tape-stripped using self-adhesive gauze (Fixomull®, BSN medical GmbH, Hamburg, Germany). Fifteen tapes cut in pieces of 10 × 5 cm, the size of the exposed area, were applied on the skin one after the other, and stripped off using clean forceps. The aluminum foil, swabs and tapes were stored separately. The aluminum foil was extracted in 50 mL and the swabs and tapes in 20 mL of methanol. The extracts were analyzed using a simplified method without using the enzyme hydrolysis step and SPE extraction, to quantify the amount of PYM in the aluminum foil, swabs and tapes.
In both experiments, a pre-exposure urine sample was collected from each volunteer immediately before the exposure. Urine was voided at 1-h intervals for the first 8-h after the exposure and henceforth, all voided urine was collected ad libitum up to 120 h. The time of voiding and the total volume of each sample were registered and all the samples were stored at −20°C until analysis.
Estimation of elimination half-life
The quantified concentrations of OH–PYM in the urine obtained from the oral and dermal exposure experiments were used to estimate the elimination half-life. The half-life of OH–PYM was calculated by the slope of the curve obtained from the plot of the natural log-linear concentration versus mid-time points between two sample collection times.
Optimization of enzyme hydrolysis
The suitability of enzyme for hydrolysis of urine was tested using urine obtained from the exposed volunteers. Ten samples each from the oral and dermal exposure, which contained high concentrations of OH–PYM, were selected from both volunteers. Two sets of aliquots (500 μL) were analyzed. One set was hydrolyzed using 20 μL β-glucuronidase and the other set was hydrolyzed with 10 μL β-glucuronidase/arylsulfatase. The samples were incubated overnight at 37°C and were extracted and analyzed using the method described. To test the enzyme efficiency, the concentrations of OH–PYM in the samples treated with β-glucuronidase were compared with those treated with β-glucuronidase/arylsulfatase.
To test the optimum time for enzyme hydrolysis, a urine sample obtained from one of the exposed volunteers containing high concentrations of OH–PYM was incubated with β-glucuronidase/arylsulfatase at four incubation time-points. At each time-point, an aliquot (500 μL) of the sample was prepared and incubated. The incubation experiment began in the reverse manner with the 48 h time-point as first incubation followed by 24, 12 and 6 h. The concentration of OH–PYM was determined in the samples and compared to select the optimum incubation time for the hydrolysis. The detailed descriptions of both experiments are presented in Supplementary data.
Urinary metabolites of PYM
The urine obtained from the exposed volunteers was used to study the biotransformation products of PYM. The samples without enzyme de-conjugation were injected into the LC–MS-MS and separation was performed as described in the analysis section. The LightSight® software was run in parallel with the system for mass spectrometric data acquisition. The software scanned for predicted metabolite mass ranges, based on the Phase I and Phase II biotransformation pathways, to create a list of possible metabolites. Based on the information dependent acquisition, the software created optimized SRMs for the detected metabolites. The metabolites were selected from this list by analyzing the peak intensity and detection of the peaks in urine obtained from both volunteers at different time-points.
A QTOF coupled to an LC system was used to acquire product ion scans (PIS) of the selected metabolites. The metabolites were separated using the method as described in the analysis section. The PIS were acquired in the positive mode with collision energy 30 V and a declustering potential of 80 V. The acquired PIS of the potential metabolites were compared with the PIS of the pure standards of PYM and OH–PYM for identification.
Correction for urinary dilution
The creatinine concentrations and density were determined in all the urine samples for dilution correction. The creatinine concentrations were determined using an enzymatic method (8), and were performed at the Department of Clinical Chemistry, University Hospital, Lund. The department has accreditation for creatinine analysis. Density was measured using a hand refractometer and the adjustment for urinary density, Cd, was calculated according to Cd = C(observed) × (1 − ρmean)/ (1 − ρsample density) where C(observed) is the concentration of the analyte in the urine sample, ρmean is the mean specific density of the samples from volunteers and ρsample density is the specific density of the sample.
Biomonitoring of PYM in Swedish populations
Environmentally exposed population
A morning void urine sample was collected in 2005–2011 from participants (n = 413) representing a general population in the south of Sweden, to assess the environmental exposure to PYM, likely mainly via diet. The population included participants (278 females and 135 males) residing in urban and rural areas, with a median age of 50 years (range 15–86 years).
Occupationally exposed population
A group of 20 apple growers and other horticulturists from southern Sweden were recruited in 2012–2015 and 109 urine samples were collected to assess occupational exposure. Spraying with PYM was performed repeatedly during each season.
The described analytical method was applied to determine OH–PYM in both populations. The studies will be published in detail elsewhere.
Results and discussion
Experimental exposure of volunteers
Oral experimental exposure
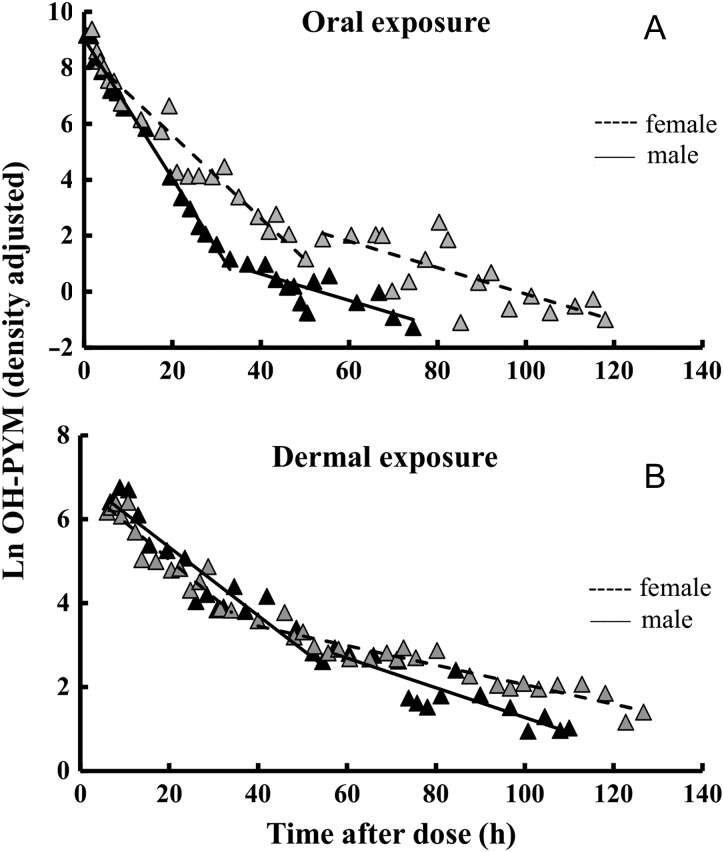
In the pre-exposure sample, the concentrations of OH–PYM were close to the LOD (0.1 ng/mL) in the urine from both volunteers. After the oral exposure to PYM, the urinary concentrations of OH–PYM increased rapidly and the Cmax was reached within 1–3 h (Table I). The maximum level of OH–PYM (density adjusted) was 12 μg/mL and 10 μg/mL, in the female and male volunteer, respectively. The urinary elimination of OH–PYM seemed to follow first-order kinetics and a two-phase excretion. Figure 1A shows the elimination curves (density adjusted) of OH–PYM after oral exposure. The creatinine adjusted, urinary volume adjusted and the raw elimination kinetics plots are presented in Supplementary Figures S1A, S2A and S3A, respectively, in Supplementary data. The estimated half-lives of OH–PYM for both volunteers are presented in Table I. A total of 80 and 77% of the dose were recovered as OH–PYM, in the urine of the female and male volunteer, respectively. Within the first 24 h, 79 and 77% of the dose was excreted in the female and male volunteer, respectively. The data indicate extensive metabolism of PYM to conjugated OH–PYM. No free PYM or free OH–PYM was detected in the urine samples.
Table I.
The estimated elimination half-life of OH–PYM in urine of two volunteers following oral and dermal exposure to PYM. The dose of PYM was 0.085 mg/kg of body weight which is equivalent to 50% of ADI.
| Volunteer | t1/2 unadjusted | r | t1/2 density adjusted | r | t1/2 creatinine adjusted | r | Cmax | Tmax | |
|---|---|---|---|---|---|---|---|---|---|
| (h) | (h) | (h) | (μg/mL) | (h) | |||||
| PYM (oral exposure) | |||||||||
| Female | Rapid phase | 5 | 0.96 | 5 | 0.94 | 4 | 0.94 | 12 | 3 |
| Slower phase | 15 | 0.54 | 15 | 0.55 | 14 | 0.50 | |||
| Male | Rapid phase | 3 | 0.98 | 3 | 0.99 | 3 | 0.98 | 10 | 1 |
| Slower phase | 32 | 0.15 | 15 | 0.60 | 15 | 0.69 | |||
| PYM (dermal exposure) | |||||||||
| Female | Rapid phase | 9 | 0.53 | 8 | 0.88 | 6 | 0.98 | 0.6 | 12 |
| Slower phase | 29 | 0.76 | 30 | 0.89 | 30 | 0.95 | |||
| Male | Rapid phase | 8 | 0.81 | 8 | 0.90 | 8 | 0.96 | 0.9 | 10 |
| Slower phase | 20 | 0.41 | 20 | 0.77 | 18 | 0.95 | |||
The half-life of elimination (t½) in urine was estimated from the slope of the plot of natural log-transformed concentration versus mid-time points and the correlation coefficient (r) is obtained from the curve. Cmax is the maximum urinary concentration of OH–PYM (density adjusted) at Tmax, which is the time of maximum excretion.
Figure 1.
Semi logarithmic plot of urinary elimination curves of OH–PYM for the female and male volunteer after oral and dermal exposure to PYM. The natural log-transformed density adjusted OH–PYM (ng/mL) concentrations were plotted versus the mid-time points. The half-life of OH–PYM was calculated by using the slope of the curves. For both volunteers, (A) represents the elimination curve after oral exposure and (B) represents the elimination curve after the dermal exposure.
Dermal exposure experiments
After exposure, the urinary concentrations of OH–PYM increased rather rapidly and Cmax was reached within 10–12 h after exposure (Table I). The maximum level of OH–PYM (density adjusted) was 0.6 μg/mL and 0.9 μg/mL, in the female and male volunteer, respectively. The urinary elimination of OH–PYM followed first-order kinetics and a two-phase excretion also here (Figure 1B). The estimated half-lives of OH–PYM are presented in Table I. The creatinine adjusted, urinary volume adjusted and the raw elimination kinetics plots are presented in Supplementary Figures S1B, S2B and S3B, respectively (Supplementary data). The recovery of OH–PYM in urine was 9.4% and 19%, in the female and male volunteer, respectively, of the total dose administered on the skin. A significant part of the dose was excreted within the first 24 h. No free PYM or free OH–PYM was found in any of the urine samples. Of the total administrated dose, 39% and 26% were recovered as parent PYM in the aluminum foil, swabs and tapes, from the female and male volunteer, respectively. Thus, after subtracting the dose found in the aluminum foil, swabs and tapes from the total applied dose (considering that the dose was not available for absorption), OH–PYM recovered in the urine was 16% and 26%, respectively, in the female and the male volunteer. The results are in agreement with the conclusions of the EFSA report that were based on an in vitro dermal absorption study performed on human skin samples and a mathematical skin permeation model (9). The concentrations of PYM in the tape extracts decreased serially from the first to the 15th tape (Suppementary Figure S4). The extracts from the 15th tape contained 4 and 2 μg/tape of PYM in the female and male, respectively. This indicates penetration of PYM into the skin.
The physicochemical characteristics of a substance, the choice of vehicle, the use of occlusion and the application area may affect the dermal absorption of compounds. The skin of the forearm is less permeable than the forehead and the neck (10). However, we chose the inner forearm as the site of exposure since it was convenient and is commonly used in dermal exposure studies (11). Washing of the skin after exposure (6) and the tape stripping, may also have affected the absorption of PYM.
Optimization of enzyme hydrolysis
β-glucuronidase/arylsulfatase was found to be more effective in de-conjugation of OH–PYM, and the quantified concentrations were much higher than those obtained by using β-glucuronidase only, Supplementary Table SIII. A hydrolysis period of 6 h was found to be sufficient to facilitate de-conjugation reaction. However, an overnight incubation was chosen, because it was practical for our work-flow and there was no difference in the concentrations between the two incubation times. The degradation of analytes during enzyme hydrolysis was tested using blank urine spiked with 25 ng/mL of OH–PYM. The samples were incubated continuously in triplicates at 37°C, from 0 to 144 h at five time points (0, 24, 48, 72 and 144 h). The OH–PYM seemed stable up to 72 h. The results are presented in Supplementary Figure S5.
Urinary metabolites of PYM
Our investigations suggested that the major biotransformation pathway of PYM was the formation of OH–PYM via aromatic oxidation, as reported in animal studies (3). In both the oral and dermal exposure experiments, OH–PYM was found as a conjugate of sulfonate (m/z = 296.07) and glucuronic acid (m/z = 392.15). The mass spectra of the conjugates are presented in Supplementary Figure S6. No free OH–PYM or PYM was detected.
The data indicate that the most common conjugation of OH–PYM was with sulfonates. This was tested by quantifying OH–PYM in the urine of the exposed volunteers after hydrolysis with β-glucuronidase and β-glucuronidase/arylsulfatase. The sulfonation was above 98% and 95% in the female and male volunteer, respectively, in both the exposure studies (Supplementary Table SIII). The inter-individual difference of sulfonation between the two volunteers, although small, may be related to various factors that can affect sulfotransferase activity (12).
Less than 10% of other metabolites have been reported in rat studies; they were formed by oxidation at different sites on the rings of PYM or by oxidation of the methyl group (3). In our work, such metabolites were not found. No parent PYM or its conjugates with sulfonate (expected m/z = 280) or glucuronic acid (expected m/z = 376) were found in our study, which is similar to reports from animal studies (3). Furthermore, our results did not indicate the presence of free di-oxidated metabolite (expected m/z = 232) of the parent PYM or conjugation of di-oxidated PYM with sulfonate (expected m/z = 312) or glucuronic acid (expected m/z = 408); such metabolites have been reported in animal studies.
Correction for urinary dilution
The correction for urinary dilution with density and creatinine gave a better correlation (r) of OH–PYM in the elimination half-life plots when compared to the unadjusted values. Hence, correction for urinary dilution is recommended; correction both with density and with creatinine may be applied. Factors such as age, sex, BMI and diet can affect density and creatinine values. Urinary creatinine excretion may be higher in young adults as compared to children and older age groups. Further, males often have higher creatinine excretion than females (13). These differences may be due to the body composition and muscle mass. However, studies suggest urinary creatinine to be more affected by these factors than urinary density (14). Hence, correction with urinary density may be the appropriate choice in population studies with mixed genders and diverse age groups (15); however, the density measurement is difficult to automate.
Method validation
Specificity
A chromatogram of urine obtained from a dermally exposed volunteer showing a peak of OH–PYM (transition m/z 216.1/107.0) quantified to 12 ng/mL is presented in Supplementary Figure S7A. The specificity of the analytical method was tested by analyzing blank urine obtained from 10 healthy volunteers. Blank urine was extracted by SPE and analyzed without IS to test for possible interferences caused by other compounds present in the matrix. No interfering signals with the analyte were observed. A chromatogram of a blank urine obtained from a healthy volunteer is presented in Supplementary Figure S7B. Also, the signal of OH–PYM in a blank urine spiked with 50 ng/mL did not seem to cause any interference with the IS, transition m/z 220.1/111.0 (Supplementary Figure S7C). An analyte-free urine spiked with 5 ng/mL of IS [2H4] OH–PYM did not seem to cause an interference with the signal of OH–PYM, transition m/z 216.1/107.0 (Supplementary Figure S7D).
Linearity
The linearity was determined by replicate analyzes of the calibration standards over a period of 18 months. The slopes of the regression lines were calculated using the mean slope and the coefficient of correlation (r2). For the calibration standards ranging from 0.25 to 100 ng/mL, excellent linearity was observed. The correlation coefficient (r2) observed for OH–PYM was above 0.995. The mean slope of the calibration lines (n = 8) for OH–PYM was 1.1 ± 0.1, and the intercept was 0.8 ± 0.3, at 95% confidence interval.
Limit of detection and quantification
Ten different blank urines collected from healthy volunteers were used to determine the LOD and LOQ. The LOD was calculated as three times and the LOQ as 10 times the standard deviation of the mean concentration of the peak at the analyte retention time. The LOD for both PYM and OH–PYM was 0.1 ng/mL and the LOQ was 0.4 ng/mL. The obtained LOD was low enough to detect PYM traces in the urine of general population (Supplementary Figure S8).
Precision
The precision of the method was determined as within-run, between-run and between-batch precision and is presented as coefficient of variation (CV) in Table II. The within-run precision was determined by analysis of 10 urines spiked each with 1, 5 and 10 ng/mL. The between-run precision was determined by duplicate analysis of QC samples, spiked with three concentrations of 1, 5 and 10 ng/mL with every analytical batch for a period of 18 months and expressed as CV. The between-batch precision was determined by comparing duplicate analyzes of 162 samples from the two exposure studies. The samples were prepared and analyzed in separate analytical batches. After analysis, the concentrations were grouped into three ranges, 0.1–10 ng/mL, 10–100 ng/mL and 100–9,300 ng/mL. The CV was determined as previously described (16). The determined within-run, between-run and between-batch precisions of OH–PYM were below 15% of the CV.
Table II.
The within-run, between-run and between-batch precision of the analytical method for OH–PYM determined at different concentrations. The precisions were determined using blank urine spiked with OH–PYM.
| Precision | n | OH–PYM | Mean measured | CV | Recovery |
|---|---|---|---|---|---|
| (ng/mL) | OH–PYM (ng/mL) | (%) | (%) | ||
| Within-run | 10 | 1 | 1.0 | 6.9 | 100 |
| 10 | 5 | 5.4 | 3.8 | 108 | |
| 10 | 10 | 12 | 3.6 | 120 | |
| Between-run | 23 | 1 | 1.0 | 8.0 | 100 |
| 23 | 5 | 5.4 | 6.6 | 108 | |
| 23 | 10 | 11 | 5.9 | 110 | |
| Between-batch | 69 | range 0.1–10 | 3.3 | 12 | |
| 50 | range 10–100 | 39 | 10 | ||
| 43 | range 100–9,300 | 1300 | 7.4 |
The within-run and between-run precisions at the three concentrations are expressed as a measure of coefficient of variation (CV) and recovery (%). The between-batch precision was calculated for the three concentration ranges, 0.1–10 ng/mL, 10–100 ng/mL and 100–9,300 ng/mL, using samples obtained from the experimental exposure of volunteers.
Matrix effects
A deuterium labeled IS was used in our method to compensate for the matrix effects. The matrix effects were studied at two concentrations of OH–PYM, by the post-extraction addition approach. The blank urine obtained from 10 healthy individuals were divided into two sets of aliquots and extracted by SPE. Then, one set containing 10 aliquots was spiked with 2.5 ng/mL of OH–PYM and a second set of 10 aliquots was spiked with 25 ng/mL of OH–PYM. IS was added to all the samples. After analysis, the peak area ratios of OH–PYM and IS were compared at both concentrations. The CV was below 15% at both concentrations, which was within our acceptable range. The results suggest a low matrix effect.
Recovery
The recovery of the method was evaluated at two concentrations of OH–PYM. Two sets containing 10 aliquots of blank urine were spiked with 2.5 ng/mL and 25 ng/mL of OH–PYM. Both the sets were extracted by SPE. Further, two sets containing 10 aliquots of blank urine each were first extracted and then spiked with 2.5 ng/mL and 25 ng/mL of OH–PYM. All four sets were spiked with IS after SPE extraction. After analysis, the quantified concentrations were compared at both concentrations and the sets of aliquots spiked after the SPE extraction were considered as equivalent to 100% recovery. The average recovery of OH–PYM at 2.5 ng/mL and 25 ng/mL was 93% and 94%, respectively.
Stability
The QC samples spiked with OH–PYM at the three concentrations of 1, 5 and 10 ng/mL were stable for 18 months under storage at −20°C. The CV determined at all three concentrations was between 6 and 8%, and an estimated recovery of the spiked OH–PYM was between 100 and 115%. Additionally, both PYM and OH–PYM were found to be stable in the standard solutions (in methanol) for a period of 18 months, when stored at −20°C.
The details of the method validation are presented in Supplementary data.
Biomonitoring of PYM in Swedish populations
Environmentally exposed population
In the general population in southern Sweden, 48% of the samples had concentrations of OH–PYM above the LOD of 0.1 ng/mL. All the uncorrected OH–PYM concentrations which were below the LOD were substituted with the lowest (above the LOD) density corrected value divided by the square root of two. The median (density adjusted) concentration was 0.1 ng/mL and the 95th percentile was 4 ng/mL. The highest concentration of OH–PYM measured in the population of 413 participants was 440 ng/mL. The concentrations found in the population were much lower than the maximum urinary concentrations found in the volunteers exposed to a single intake of 50% of ADI in the oral exposure experiment (12 and 10 μg/mL for the female and male volunteer, respectively). Hence, based on current knowledge, the general population in Sweden is much less exposed to PYM than 50% of the ADI.
Occupationally exposed population
Of the 109 urine samples supplied by 18 horticulturists, collected before and after as well as during the apple growth season from April to August, 96% had concentrations of OH–PYM above the LOD. The median (density adjusted) and 95th percentile was 8 ng/mL and 360 ng/mL, respectively, and the highest concentration measured was 1,350 ng/mL. The median concentration found in the apple growers’ samples was close to 100 times higher than in the general population. Hence, it can be concluded that there is a marked occupational exposure to PYM in Swedish apple growers. However, the maximum concentration measured in the occupationally exposed population was lower than the orally exposed volunteers, if the numbers were to be extrapolated to a 100% ADI. Thus, based on current knowledge, health risks for PYM exposed apple growers are probably low (17). Considering the relatively high skin penetration shown in the experimental study, we strongly advocate protective measures to be used, including such that protect the skin.
Conclusions
An LC–MS-MS method was developed for the quantification of OH–PYM, a metabolite of the parent PYM, in human urine. The method showed a good precision and a limit of detection low enough to enable detection of OH–PYM in general populations.
The analytical method was applied for the analysis of urine samples obtained from the experimental exposure of two volunteers. The major urinary metabolite found after experimental exposure to PYM was OH–PYM, most of which was sulfonated. Its urinary excretion seemed to follow first-order kinetics and a two-phase excretion, after both oral and dermal experimental exposure. After the oral exposure, about 80% of the dose was recovered after de-conjugation as OH–PYM and the density adjusted elimination half-lives were 3–5 h (rapid phase) and 15 h (slower phase) in both volunteers. After the dermal exposure, 9–19% of the dose was recovered as OH–PYM and the density adjusted elimination half-lives were 8 h (rapid phase) and 20–30 h (slower phase) in both volunteers. However, the data should be interpreted cautiously considering the limited number of volunteers and the existence of inter-individual differences in metabolism and other characteristics.
Our method was also used for biomonitoring of PYM in environmentally and occupationally exposed populations. In the environmentally exposed population, OH–PYM was detected in urine of approximately 50% of the individuals. In the occupationally exposed population, 96% of the samples were above the detection limit. Thus, OH–PYM is proposed to be a suitable urinary exposure biomarker of PYM.
Ethical approval
The human experimental studies and the investigation of exposure to PYM in the populations of southern Sweden were ethically approved by The Regional Ethical Review Board in Lund, Lund University, Sweden (Dnr463/2005; Dnr2010/41; Dnr2010/465 and Dnr2013/6). All participants had given their written informed consent.
Supplementary Material
Funding
This work was supported by the Swedish Environmental Protection Agency; Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning; The Swedish Agency for International Development Cooperation; The Department for Research Cooperation; Swedish council for working life and social research; Swedish farmers’ foundation for agricultural research; Skåne Regional Council; and the Medical Faculty at Lund University, Sweden.
Abbreviations
ADI, acceptable daily intake; ESI, electro spray ionization; IS, internal standard; LC/MS/MS, liquid chromatography triple quadrupole mass spectrometry; LOD, limit of detection; LOQ, limit of quantification; MS, mass spectrometry; OH-PYM, 4-hydroxypyrimethanil; PIS, product ion scan; PYM, pyrimethanil; QC, quality control; QTOF, quadrupole time of flight; SPE, solid phase extraction; SRM, selected reaction monitoring.
References
- 1. Smilanick J.L., Mansour M.F., Gabler F.M., Goodwine W.R. (2006) The effectiveness of pyrimethanil to inhibit germination of Penicillium digitatum and to control citrus green mold after harvest. Postharvest Biology and Technology, 42, 75–85. [Google Scholar]
- 2. Joint FAO/WHO meeting on pesticide residues., Pesticide residues in food - 2007. Evaluations Part I-Residues, Plant Production and Protection, Paper 191. http://www.fao.org/3/a-a1556e.pdf (last accessed June 2018)
- 3. Joint FAO/WHO meeting on pesticide residues., Pesticide residues in food - 2007. Evaluations Part II: Toxicological. http://whqlibdoc.who.int/publications/2009/9789241665230_eng.pdf (last accessed June 2018)
- 4. Medjakovic S., Zoechling A., Gerster P., Ivanova M.M., Teng Y., Klinge C.M., et al. (2014) Effect of nonpersistent pesticides on estrogen receptor, androgen receptor, and aryl hydrocarbon receptor. Environmental Toxicology, 29, 1201–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hurley P.M., Hill R.N., Whiting R.J. (1998) Mode of carcinogenic action of pesticides inducing thyroid follicular cell tumors in rodents. Environmental Health Perspectives, 106, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ngo M.A., O’Malley M., Maibach H.I. (2010) Percutaneous absorption and exposure assessment of pesticides. Journal of Applied Toxicology, 30, 91–114. [DOI] [PubMed] [Google Scholar]
- 7. Manno M., Viau C., Cocker J., Colosio C., Lowry L., Mutti A., et al. (2010) Biomonitoring for occupational health risk assessment (BOHRA) introduction. Toxicology Letters, 192, 3–16. [DOI] [PubMed] [Google Scholar]
- 8. Mazzachi B.C., Peake M.J., Ehrhardt V. (2000) Reference range and method comparison studies for enzymatic and Jaffe creatinine assays in plasma and serum and early morning urine. Clin Lab, 46, 53–55. [PubMed] [Google Scholar]
- 9. EFSA Scientific Report. (2006) Conclusion on the peer review of pyrimethanil, 61, 1-70. https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2006.61r (last accessed on October 2018)
- 10. Fenske R.A. (2005) State-of-the-art measurement of agricultural pesticide exposures. Scandinavian Journal of Work, Environment & Health, 31, 67–73. [PubMed] [Google Scholar]
- 11. Berthet A., Bouchard M., Vernez D. (2012) Toxicokinetics of captan and folpet biomarkers in dermally exposed volunteers. Journal of Applied Toxicology, 32, 202–209. [DOI] [PubMed] [Google Scholar]
- 12. Wang L., James M.O. (2006) Inhibition of sulfotransferases by xenobiotics. Current Drug Metabolism, 7, 83–104. [DOI] [PubMed] [Google Scholar]
- 13. Barr D.B., Wilder L.C., Caudill S.P., Gonzalez A.J., Needham L.L., Pirkle J.L. (2005) Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environmental Health Perspectives, 113, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suwazono Y., Akesson A., Alfven T., Jarup L., Vahter M. (2005) Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers : Biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals, 10, 117–126. [DOI] [PubMed] [Google Scholar]
- 15. Sauvé J.-F., Lévesque M., Huard M., Drolet D., Lavoué J., Tardiff R., et al. (2015) Creatinine and specific gravity normalization in biological monitoring of occupational exposures. Journal of Occupational and Environmental Hygiene, 12, 123–129. [DOI] [PubMed] [Google Scholar]
- 16. Lindh C.H., Littorin M., Johannesson G., Jönsson B.A.G. (2008) Analysis of ethylenethiourea as a biomarker in human urine using liquid chromatography/triple quadrupole mass spectrometry. Rapid Communications in Mass Spectrometry, 22, 2573–2579. [DOI] [PubMed] [Google Scholar]
- 17. European commission health and consumer protection directorate-general., 23 November 2010, Review report for the active substance pyrimethanil finalised in the standing committee on the food chain and animal health at its meeting on 23 May 2006 in the view of the inclusion of pyrimethanil in annex I of directive 91/414/EEC. http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.ViewReview&id=231 (last accessed June 2018)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.